Abstract
Aims and objectives
The objective of this retrospective study was to evaluate reasons heart failure patients decline study participation, to inform interventions to improve enrollment.
Background
Failure to enrol older heart failure patients (age > 65) and women in studies may lead to sampling bias, threatening study validity.
Design
This study was a retrospective analysis of refusal data from four heart failure studies that enrolled 788 patients in four states.
Methods
Chi-Square and a pooled t-test were computed to analyse refusal data (n = 300) obtained from heart failure patients who were invited to participate in one of the four studies but declined.
Results
Refusal reasons from 300 patients (66% men, mean age 65 33) included: not interested (n = 163), too busy (n = 64), travel burden (n = 50), too sick (n = 38), family problems (n = 14), too much commitment (n = 13) and privacy concerns (n = 4). Chi-Square analyses showed no differences in frequency of reasons (p > 0 05) between men and women. Patients who refused were older, on average, than study participants.
Conclusions
Some reasons were patient-dependent; others were study-dependent. With ‘not interested’ as the most common reason, cited by over 50% of patients who declined, recruitment measures should be targeted at stimulating patients’ interest. Additional efforts may be needed to recruit older participants. However, reasons for refusal were consistent regardless of gender.
Relevance to clinical practice
Heart failure researchers should proactively approach a greater proportion of women and patients over age 65. With no gender differences in type of reasons for refusal, similar recruitment strategies can be used for men and women. However, enrolment of a representative proportion of women in heart failure studies has proven elusive and may require significant effort from researchers. Employing strategies to stimulate interest in studies is essential for recruiting heart failure patients, who overwhelmingly cited lack of interest as the top reason for refusal.
Keywords: cardiovascular, heart disease, older, research, women
Introduction
Heart failure (HF) affects 5 1 million Americans over age twenty (Go et al. 2014) and is the leading cause of hospitalisation among older adults in the USA (Jencks et al. 2009). By 2030, the incidence of HF is projected to increase 46%, resulting in over 8 million adults with HF (Go et al. 2014). Among those diagnosed with HF, approximately 50% will die within five years (Go et al. 2014). In addition to the high mortality rate, symptoms of HF are often severe and debilitating. Common symptoms include fatigue, shortness of breath, pain, trouble sleeping, depression, anxiety and difficulty with concentration and memory, among others. The symptom burden of HF is distressing and detrimental to health-related quality of life (Zambroski et al. 2005).
Background
Compared with other populations of healthy persons or persons with less serious medical conditions, HF patients may be challenging to enrol in research studies (Pressler et al. 2008). Recruitment of HF patients is complicated by frequent hospitalisations and high symptom burden. Severe fatigue is a common symptom of HF that deters potential participants. For example, severe fatigue is a common symptom of HF that deters potential participants (Pressler et al. 2008). With the addition of inclusion criteria for specific HF type or stage, the pool of eligible participants becomes smaller, and recruiting a sample of HF patients typically involves screening a large number of people (Pressler et al. 2008).
As a consequence, participants recruited for HF studies may not be representative of the general population of HF patients. In a review of 59 randomised-controlled trials, the characteristics of participants in HF studies differed from characteristics of HF patients in the community (Heiat et al. 2002). Compared to the general population of HF patients, study participants were younger in age and more likely to be white or male. Although HF is most prevalent in people 80 years of age or older (Go et al. 2014), the average age of participants enrolled in HF studies was 61.4 years (Heiat et al. 2002). Among the 59 studies, the age distribution of participants was poorly documented, and only four trials reported enrolling patients older than 80 years of age (Heiat et al. 2002).
Although incidence of HF is similar among women and men (Go et al. 2014), women are underrepresented in HF studies (Pressler 2014). At age 40, lifetime risk for developing HF is 20% for both men and women, and in 2010 women represented approximately 47% of HF patients (Go et al. 2014). However, women with HF have been difficult to enrol in studies (Harris & Douglas 2000, Pressler 2014). In a study of cardiovascular clinical trials funded by the National Heart, Lung, and Blood Institute (NHLBI) between 1965–1998, women were found to have consistently low enrolment in HF studies, with a mean enrolment of 23% (Harris & Douglas 2000). Despite policies mandating the inclusion of women in clinical research, the proportion of women enrolled in HF studies has changed little over time. In a review of 264 HF studies published in 2013 in eleven major medical and nursing journals, the mean percentage of women enrolled in studies using primary data sources was 32% and the median percentage of women enrolled in studies using primary and existing data sources was 29% (Pressler 2014). Homogeneous samples of primarily men limit the ability of researchers to analyse and report data for men and women separately (Institute of Medicine, 2010).
Previous studies have described the challenges of recruiting HF patients for clinical research. In a randomised clinical trial of behavioural therapy for HF patients, Chang and colleagues evaluated both motivating factors and reasons for refusal among patients approached at the Veterans Affairs Boston Healthcare System (n = 541, 99% male) (Chang et al. 2004). The most common reason for refusal among patients approached in the clinic was ‘lives too far away,’ while the most common reason among patients recruited by phone was ‘no interest’ (Chang et al. 2004). Among patients who did participate, the most common motivating factors were a perceived benefit to himself or herself, a desire to help others or help the VA, encouragement from trusted professionals, an attractive or persuasive recruiter and monetary compensation (Chang et al. 2004). It is worth nothing that the sample was almost exclusively male (Chang et al. 2004). In another recruitment study, Pressler et al. (2008) examined reasons for refusal among clinic patients who declined participation in a study to evaluate cognition in patients with HF (n = 29, 55% male). The most common reasons for refusal were ‘lack of interest,’ ‘no time’ and ‘too sick’ (Pressler et al. 2008). These studies were informative; however, they included few women and did not examine potential age effects or gender differences in the type of reasons cited for refusal.
Overall, failure to enrol is a major source of bias due to inadequate or nonrepresentative samples. This imbalance threatens study validity and limits the ability to generalise findings. However, enrolment of a sufficiently large and representative sample of HF patients is a challenge. Therefore, the purpose of this retrospective study was to categorise HF patients’ reasons for refusal, identify differences in reasons for refusal based on gender, and examine age differences in patients who participated in research vs. patients who declined. This study expands upon the results reported by Pressler et al. (2008), with a larger sample consisting of refusal data from the previous study in addition to refusal data from three other HF studies. The ultimate objective is to use this knowledge to design recruitment strategies to improve enrolment and reduce sampling bias in HF studies. The research questions were: (1) What were the reasons that HF patients refused to participate? (2) What were the differences in reasons for refusal by gender? (3) Did average age or gender ratio in the patients who declined participation differ from average age or gender ratio of the patients who participated in the four studies?
Methods
Study design and procedures
In this retrospective study, Chi-Square and a pooled t-test were computed to analyse data (n = 300) obtained from four studies conducted in four states. Refusal data were obtained from HF patients who were invited to participate in one of four different studies at sites in Indiana, Ohio, Kentucky and Michigan but declined (see Table 1 for study descriptions). One study used comparative cross-sectional design, one used prospective design and two used experimental intervention design (Lennie et al. 2011; Pressler et al. 2010; Pressler et al. 2011; Pressler et al. 2015). All studies had IRB approval and required signed informed consent. Patients were recruited through outpatient HF clinics and one general medicine practice. Study team members and clinic staff who participated in recruitment for the studies recorded the study site, gender, age and reason for refusal for patients who met eligibility criteria, but declined to participate. Data from refusal sheets for each study were entered into an SPSS database and verified (n = 300).
Table 1.
Heart failure study descriptions
| Study | Design | Sample | Measure |
|---|---|---|---|
| Three gramme sodium intake is associated with longer event-free survival only in patients with advanced heart failure (Lennie et al. 2011) | Prospective observational study measuring dietary sodium intake and 12-month event-free survival while controlling for other clinical variables. Patients were divided into two groups using a 3-g urine sodium cutpoint and stratified by NYHA Class (I/II vs. III/IV) | 302 HF patients recruited from outpatient clinics associated with six large community hospitals and academic medical centres in Kentucky, Georgia, Indiana and Ohio | 24-hour urine sodium to indicate sodium intake; event-free survival for 12 months as determined by patient or family interviews and medical record review. Differences in cardiac event-free survival determined by Kaplan–Meier survival curve with log-rank test and Cox hazard regression. Included questionnaires to assess HRQL, adherence, functional status and self-care |
| Cognitive deficits in chronic heart failure (Pressler et al. 2010) | Comparative design used to evaluate cognitive deficits among three groups (HF group, healthy participants group and other medical conditions group) and an explanatory correlational design to evaluate the relationships between HF severity, age, and comorbidity and cognitive deficits in the HF patients (Pressler et al. 2010) | 414 total participants (249 HF patients, 63 healthy and 102 medical participants) recruited from HF clinics and a general medicine practice in Indiana | Series of neuropsychological tests administered to all participants designed to measure the following cognitive domains most likely to be impaired in vascular cognitive disorders: global cognitive function, premorbid intellect, language, working memory, verbal memory, visuospatial ability, psychomotor speed and executive function |
| Nurse-enhanced memory intervention in heart failure: the MEMOIR study (Pressler et al. 2011) | 12-week randomised interventional study designed to determine efficacy of Brain Fitness, a computerised cognitive training intervention, for heart failure (HF) patients. Half the participants were randomly assigned to the Brain Fitness intervention group, and the other half was received a health education intervention | 40 patients recruited from HF clinic in Michigan | Series of neuropsychological tests and questionnaires administered at baseline, 8, and 12 weeks to measure memory, working memory, psychomotor speed, executive function and performance of cognitive activities and instrumental activities of dialling living (IADLs) |
| Cognitive training to improve memory in heart failure (MEMOIR-2) (Pressler et al. 2015) | 12-week randomised interventional study designed to build upon the MEMOIR-1 study by determining the efficacy of the Brain Fitness cognitive training intervention for HF patients. Half the participants were randomly assigned to the Brain Fitness intervention group, and the other half received a health education intervention | 31 HF patients recruited from HF clinics in Michigan | Series of neuropsychological tests and questionnaires administered at baseline, 8, and 12 weeks to measure recall and working memory, psychomotor speed, executive function, IADL performance, HRQL, depressive symptoms, Timed Up and Go Test. Also measured gene and serum BDNF levels |
Sample
The sample of 300 HF patients who declined participation consisted of 191 men and 97 women (gender data were unavailable for 12 people), ranging in age from 33 to 91 years, with an overall mean age of 65 33 (SD = 12 85) and a median age of 67 years. Race and ethnicity were not available for the majority of people in this sample.
Statistical analysis
Frequencies were calculated for each reason provided. To analyse differences in reasons for refusal by gender, patients whose gender was not available were excluded, resulting in a sample size of 288. Chi-Square analyses were used to compare frequencies of reasons for refusal between men and women. To analyse differences in participation by age, patients whose age was not available were excluded, resulting in a sample size of 271. Weighted means of the refusal group and participant group ages were calculated for all four studies combined. A pooled t-test was conducted to compare the age of the refusal group with the study participants group. The Pearson Chi-Square test was applied to determine if gender ratio in the refusal group differed from gender ratio in the participant group. Significance level was set at p < 0 05 for all analyses (Table 2).
Table 2.
Age and gender analysis of patients who did and did not participate
| Study | Participants
|
People who refused
|
||
|---|---|---|---|---|
| Age – Mean (SD) | Sample size | Age – Mean (SD) | Sample size | |
| THINK | 62.9 (14 6) | 249 | 65 (12) | 88 |
| BMI | 62 (12) | 302 | 67 (13) | 134 |
| MEMOIR1 | 57.8 (13.1) | 40 | 65 (11) | 19 |
| MEMOIR2 | 61 (13) | 31 | 62 (12) | 59 |
| Overall | 62.0 (8.3) | 788 | 65.3 (7.2) | 271 |
|
| ||||
| %Men; %Women | Sample size | %Men; %Women | Sample size | |
|
| ||||
| THINK | 63.5; 36.5 | 249 | 55.7; 44.3 | 88 |
| BMI | 67.2; 32.8 | 302 | 70.1; 29.9 | 134 |
| MEMOIR1 | 70; 30 | 40 | 68.4; 31.6 | 19 |
| MEMOIR2 | 77.4; 22.6 | 31 | 74.5; 25.5 | 47 |
| Overall | 66%M; 34%W | 788 | 66%M; 34%W | 288 |
Results
Reasons for refusal were clustered into seven categories: not interested (n = 163), too busy (n = 64), travel is a burden (n = 50), too sick (n = 38), family problems (n = 14), the study involves too much commitment (n = 13) and privacy concerns (n = 4). A total of 45 people cited multiple reasons for refusal. Additional reasons reported less often included ‘doing well right now’ (n = 1), ‘too nervous’ (n = 1), ‘refused due to blood draw’ (n = 1), ‘daughter doesn’t approve’ (n = 1), ‘intervention requires too much computer time’ (n = 1) and ‘cannot focus and complete the intervention’ (n = 1). Percentages of men and women citing each of the top four reasons for refusal are shown in Fig. 1.
Figure 1.
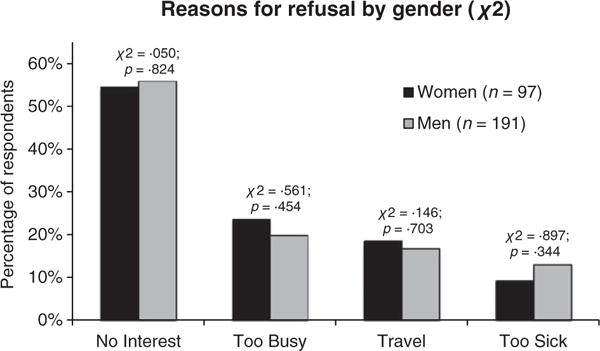
Four most common reasons for refusal by gender.
A Chi-Square analysis of the reasons with sufficient sample sizes showed no significant differences (p < 0 05) between men and women for any of the top four reasons for refusal (not interested: χ2 (df = 1, n = 288) = 0.050, p = 0.824; too busy: χ2 (df = 1, n = 288) = 0.561, p = 0.454; travel is a burden: χ2 (df = 1, n = 288) = 0.146, p = 0 703; and too sick: χ2 (df = 1, n = 288)=0.897, p = 0.344).
Results of the pooled t-test showed a significant age difference (t(920) = 5.81, p < 0.001) between patients who declined participation and patients who participated in the study. The refusal group was slightly older (mean age 65.3; SD 7.2), on average, compared with the participant group (mean age 62.0; SD 8.3).
A Chi-Square analysis comparing gender ratios between the refusal group and study participant group showed no difference in gender ratio (χ2 (1) = 0.001, p = 0.981), with 66% men in each group.
Discussion
These results provide important insight into the most common factors that deter HF patients from participating in research studies. Older patients were more likely to decline participation. The analysis did not show significant differences by gender in the type of reasons for refusal reported by patients. According to these results, the top four most common factors that deter HF patients from participating in studies are consistent among men and women: (1) no interest in participation; (2) too busy; (3) too sick and (4) travel to the study site is a burden. Therefore, strategies that address these reasons could be developed to encourage participation in studies for all patients, but additional efforts may be needed to recruit older participants. In addition, although women and men did not differ in their rates of refusal, both the refusal group and the study participants group consisted of only 34% women.
Although women did not have a higher rate of refusal than men in this analysis, enrolment of a representative proportion of women in heart failure studies has proven elusive and may require significant effort from researchers. Approaching a greater number of women for HF studies is important but may not be sufficient. In a clinical trial examining depression in patients with coronary heart disease, researchers enrolled diverse recruitment staff to match recruiters to the gender and ethnicity of patients who were asked to participate. Although this strategy required extreme effort, they managed to enrol a sample with 44% women (ENRICHD Investigators, 2001). In another clinical trial enrolling women for long-term cardiac rehabilitation, researchers used individualised ‘orientation sessions’ to introduce women to the clinical trial, using motivational interviewing techniques to examine patients’ reasons for ambivalence and address their concerns about participation (Beckie et al. 2009). Kim and Menon (2009) recommend questionnaires to assess women’s attitudes towards research during the consent process for clinical trials.
To increase the proportion of older participants in studies, modifications to the study design or recruitment procedures may be necessary. Study materials may need larger font size or adaptations for patients who are hard of hearing. Older patients may prefer telephone-based interventions rather than home visits or travelling to the study site. In an interventional study for stroke patients and their caregivers, many participants preferred to receive follow-up support by telephone after the initial face-to-face education sessions (Hoffmann et al. 2013). The researchers found no significant differences in methods when the outcome data were collected by telephone vs. face-to-face meetings (Hoffmann et al. 2013). When possible, telephone-based communication reduces the time and travel burden of the study. Focus groups or qualitative studies with older patients may be helpful to determine what accommodations would encourage them to participate.
Low health literacy, another barrier that disproportionately affects older adults and patients with limited English proficiency (Berkman et al. 2011), may deter some patients from participating in studies. All recruitment materials and study materials should be below the sixth grade reading level (Berkman et al. 2011). Piloting study materials in small patient focus groups prior to recruitment may be beneficial to identify sources of confusion, particularly for intervention studies that involve learning self-management behaviours (DeWalt et al. 2004).
Some reasons for refusal were patient-dependent (not interested, too busy, too sick, family problems), while others were study-dependent (travel is a burden, too much commitment involved, privacy concerns) and could be modified in the study design. These results are consistent with and expand upon a past study examining recruitment issues in HF patients (Pressler et al. 2008). People who cited travel as a burden may find home-based or phone-based interventions more appealing. If in-person visits are necessary, scheduling interviews or data collection at the same time as regular clinic appointments can reduce travel burden. Similarly, in consideration of patients who thought participating in studies was too much commitment, researchers need to condense the number of sessions and study instruments when possible, being mindful of the burden for patients. Some patients were also concerned about privacy. In these cases, physicians, nurses and other health care providers can be a valuable resource for recruitment. Patients are encouraged to participate when someone they trust takes the time to explain the study and address their concerns. Patient-provider conversations are often the crucial moment when a patient decides whether to participate in a study (Parreco et al. 2012). Developing a best practices guide may be helpful to avoid these common barriers to recruitment in future studies.
The finding that ‘not interested’ was the most common reason for refusal, cited by over 50% of patients who declined, calls for specific recruitment measures that target stimulating patients’ interest. Innovative methods such as media outreach may be necessary to recruit patients with a particular condition such as HF. Although online recruitment efforts may not be effective to reach some ageing adults who do not use the Internet, the number of Internet users over age 65 is growing rapidly. According to the Pew Research Center, 59% of Americans over age 65 reported using the Internet in 2014, compared to just 35% in 2008 (Smith 2015). Online recruitment has become an increasingly viable method to increase participation in studies, both in the USA and internationally. Tong et al. describe the increasing use of media advertisement to recruit patients for biomedical studies in Singapore, with adoption of strategies used in the USA and Europe, including advertisement on online support groups to recruit participants with a particular disease or condition (Tong et al. 2010). An example is armyofwomen.org, a website created with the goal of recruiting one million women worldwide for a breast cancer cohort study (Army of Women, 2014). A similar type of registry for heart failure clinical trials can be found on the World Health Organization International Clinical Trials Registry website. A search filtered by condition or intervention will locate clinical trials currently recruiting patients (World Health Organization, 2014).
Digital recruitment, a method in which potential participants are identified via online surveys, is another strategy to stimulate patient interest in studies. The nature of some conditions may make recruitment exceptionally difficult. Digital recruitment has recently been used to recruit clinical trial participants for prodromal Alzheimer’s disease, an early stage of the disease during which many people experience symptoms but have not yet been diagnosed with the condition (Hughes et al. 2014). Online surveys act as a screening tool to identify a pool of eligible participants based on their symptoms, resulting in a more targeted group brought to the study site for formal screening. This strategy not only reduces the study team’s workload but also gives researchers access to a group of people they may not otherwise have been able to reach (Hughes et al. 2014).
Patients who express ‘no interest’ in studies may be reluctant to participate due to uncertainty about what participation will entail. Yates et al. (2009) trialed a new informed consent process that used a flipchart to explain a hypothetical clinical trial to cardiac rehabilitation patients. The purpose of this visual presentation format was to explain the objectives of the study while preventing misunderstandings about its implications for participants and their treatment. After the flipchart presentation, 54% of patients said they would participate in the hypothetical study, compared to 22% who agreed in a similar hypothetical study that did not use the flipcharts. A visual aid such as the flipchart can help convey the importance of the study while addressing patients’ concerns about the details of participation (Yates et al. 2009).
To encourage patients’ interest and participation in studies, the best time to begin patient engagement in clinical research may be during the study design process. With inadequate patient enrolment threatening the success of clinical trials in the USA and internationally, patient education and engagement in clinical research must become a priority for researchers (Tong et al. 2010). Engaging a variety of stakeholders, including patients, has become increasingly common in comparative effectiveness research. The Center for Medical Technology Policy has begun recruiting patients to participate in working groups to design comparative effectiveness studies, including studies for cardiology treatments (Hoffman et al. 2010). Researchers have identified technical jargon as a barrier to patient participation. Presenting information in layperson’s terms has allowed patients to make more meaningful contributions to discussion (Hoffman et al. 2010). To help researchers engage patients in their own health care on a larger scale, the American Institutes for Research have created a ‘Roadmap for Patient and Family Engagement in Healthcare Practice and Research’ (Carman et al. 2014). Developed with the collaboration of interdisciplinary stakeholders, including clinicians, researchers, patients and families, the Roadmap consists of eight strategies to improve health care and patient outcomes at lower costs. One of the strategies, ‘Patient and Family Preparation,’ calls for development of training programmes to engage patients and families as members of the research team. With training to introduce the research process and explain their role within the team, patients and families are better prepared to help researchers design studies relevant to their needs (Carman et al. 2014). Continued engagement of patients throughout the research process keeps researchers focused on the issues that matter most to patients and, in turn, encourages patients to participate in studies.
Finally, in addition to patient and study-related barriers to recruitment, institutional and regulatory barriers may exist. To maximise the yield of recruitment efforts, Sullivan-Bolyai et al. (2007) have identified common barriers to recruiting patients for studies, as well as strategies to overcome these barriers. Since 2003, HIPAA regulations have become stricter, requiring researchers to contact potential participants through health care providers, rather than calling them directly. To facilitate access to patients who have previously been recruited, patients can sign a waiver allowing researchers to contact them for future studies (Sullivan-Bolyai et al. 2007). In addition to strict regulations, clinicians are busy and may be reluctant to add study recruitment to their workload. With limited time and resources to recruit patients for studies, clinicians must perceive the study to be important and meaningful for their patient population. Researchers may also need to make contributions in return for clinicians’ assistance, such as offering staff and patient education opportunities at the clinical site. When possible, involving clinicians as study team members from an early stage, such as the research proposal process, can keep them engaged throughout the duration of the study (Sullivan-Bolyai et al. 2007).
For future studies, the high rate of patients who responded ‘no interest’ in participation requires further investigation. The recruitment strategies described in the literature may not fully address some patients’ reluctance to participate in research. Qualitative interviews may be necessary to better understand heart failure patients’ motivation for participating or declining to participate in research. Although patients who decline participation in a study would likely decline to participate in qualitative interviews, a few short qualitative survey questions may be informative. Patients who cite ‘no interest’ in research may be deterred from participating for various reasons. The benefits of participation may be unclear, and patients may view research as ‘experimental’ and therefore unappealing. Further exploration is warranted to clarify the reasons for patients’ reported lack of interest in clinical studies.
Limitations
A limitation of this study was variation in the way refusal data were collected. The circumstances under which the data were collected and the way the questions were phrased may have differed by study and site. The total number of people screened across the four studies is unknown. In some cases, clinic nurses or other staff assisted with recruiting patients and did not maintain refusal records for every patient screened. Refusal data were not available for all participants screened for these studies due to variations in recruiting methods at the sites, which was difficult for the researchers to control. Another potential limitation was the uneven ratio of males to females in the records examined. Males comprised 66% of the patients whose refusal data were recorded and analysed. In addition, the pooling of four studies with different aims and designs may limit the generalisability of these results. However, due to historical difficulties in recruiting heart failure patients for any type of research study, representation of heart failure patients recruited for a variety of study types in diverse geographic regions was desirable.
Records of refusal data are often too vague for researchers to modify recruitment methods based on the results (Pressler et al. 2008). Collecting systematic data about people who do not enrol and reporting it in study results can help researchers identify barriers to enrolment, allowing for ongoing improvements in recruitment strategies and study design. A minimum data set to collect should include patient age, gender, race/ethnicity, study site and a specific reason for refusal, as well as the name and role of the person recruiting. If the patient does not provide a specific reason for refusal, the recruiter may have to ask for more details. In addition, collecting data on reasons patients do choose to participate may help identify factors that motivate them to join studies. For some patients, altruism and desire to advance the science may act as a motivating factor. Sources of motivation may differ for women and men. A simple question at the end of the interview about why the person decided to participate may be informative for future studies.
Conclusions
The results of this analysis can be used to identify strategies for study recruitment by addressing the most common reasons HF patients decline participation, which appear to be consistent regardless of gender. Employing strategies to stimulate patient interest in studies is essential for recruiting HF patients, who overwhelmingly cited lack of interest as the top reason for refusal. However, qualitative interviews may be necessary to better understand why so many patients reported a lack of interest in clinical research. In addition, with an apparent bias towards younger, male participants in HF studies (Heiat et al. 2002, Pressler 2014), researchers should proactively approach a greater proportion of women and patients over age 65. Older patients may be more likely to decline participation, so a greater emphasis on recruitment of this age group is necessary to obtain a representative sample. Developing a best practices guide to increase recruitment of patients can reduce this source of bias. In future studies, a standardised checklist of reasons for refusal may give researchers more insight into why patients declined participation and subsequently a greater understanding of how study-based incentives can be used to increase recruitment. Finally, engaging clinicians and patients as members of the study team helps to ensure that the study is relevant and meaningful for patients with HF. Patients have significant contributions to make in clinical studies, both as study participants and study team members, and engaging them in the research process is critical to maximising the impact of clinical research.
What does this paper contribute to the wider global clinical community?
Among heart failure patients who refuse to participate in research studies, no significant differences in reasons for refusal were found between men and women. However, people who refused were older, on average, than study participants.
With ‘not interested’ as the most common reason, cited by over 50% of patients who declined, recruitment measures should be targeted at stimulating patients’ interest through innovative methods such as media outreach.
To reduce sampling bias in heart failure studies, recruitment of older patients and women should be emphasised. With no gender differences in the type of reasons for refusal cited, similar recruitment strategies can be used for men and women. However, enrolment of a representative proportion of women in heart failure studies has proven elusive and may require significant effort from researchers. Developing a best practices guide could be used to increase recruitment of heart failure patients, reducing this source of bias.
Acknowledgments
The BMI study (Lennie et al. 2011) was supported in part by a grant from NIH NINR R01 NR009280; General Clinical Research Centers at University of Kentucky: M01RR02602; Emory University: M01RR0039; PHS Grant UL1 RR025008 from the Clinical and Translational Science Award program, National Center for Research Resources, and the Atlanta Veterans Administration Medical Center; Indiana University: MO1 RR000750; and NIH, NINR Center grant 1P20NR010679 to the University of Kentucky College of Nursing. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NINR, NCRR or the Veterans Administration. The THINK study (Pressler et al. 2008) was funded by the NINR (R01 NR008147). The MEMOIR study (Pressler et al. 2011) was funded by NINR research grants R01 NR008147 and P30 NR009000. MEMOIR-2 (Pressler 2014, epub ahead of print) was funded by MCubed Project of the University of Michigan and supported in part by the Michigan Institute for Clinical Research (2UL1TR000433). For both MEMOIR studies, Brain Fitness software was donated by PositScience and Heart Insight magazines were donated by the American Heart Association.
Footnotes
Contributions
Study design: JMH, MJ, SJP; Data collection and analysis: JMH, MJ, TAL, DKM, SBD, DLR, PLR, SJP; Manuscript preparation: JMH, MJ, TAL, DKM, DGS, SBD, TKM, BG, SJP.
Contributor Information
Jordan M Harrison, School of Nursing, University of Michigan, Ann Arbor, MI.
Miyeon Jung, Postdoctoral Fellow, School of Nursing, Indiana University, Indianapolis, IN.
Terry A Lennie, Professor, Associate Dean for Graduate Faculty Affairs, College of Nursing, University of Kentucky, Lexington, KY.
Debra K Moser, Professor and Gill Endowed Chair, College of Nursing, University of Kentucky, Lexington, KY.
Dean G Smith, Dean, Louisiana State University Health Sciences Center School of Public Health, New Orleans, LA.
Sandra B Dunbar, Charles Howard Candler Professor of Cardiovascular Nursing, Associate Dean for Academic Advancement, Nell Hodgson Woodruff School of Nursing, Emory University, Atlanta, GA.
David L Ronis, Research Scientist, School of Nursing, University of Michigan, Ann Arbor, MI.
Todd M Koelling, Professor, Department of Internal Medicine, University of Michigan, Ann Arbor, MI.
Bruno Giordani, Professor, Department of Psychiatry, University of Michigan, Ann Arbor, MI.
Penny L Riley, Clinical Assistant Professor, School of Nursing, University of Michigan, Ann Arbor, MI.
Susan J Pressler, Professor and Sally Reahard Chair, Director, Center for Enhancing Quality of Life in Chronic Illness, School of Nursing, Indiana University, Indianapolis, IN, USA.
References
- Army of Women. A Program of the Dr Susan Love Research Foundation. 2014 Available at: http://www.armyof-women.org (accessed 1 April 2015)
- Beckie TM, Mendonca MA, Fletcher GF, Schocken DD, Evans ME, Banks SM. Examining the challenges of recruiting women into a cardiac rehabilitation clinical trial. Journal of Cardiopulmonary Rehabilitation and Prevention. 2009;29:13. doi: 10.1097/HCR.0b013e31819276cb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkman ND, Sheridan SL, Donahue KE, Halpern DJ, Viera A, Crotty K, Holland A, Brasure M, Lohr KN, Harden E, Tant E, Wallace I, Viswanathan M. Health literacy interventions and outcomes: An updated systematic review. (AHRQ Publication Number 11-E006).Evidence Report/Technology Assessment No 199. 2011 [PMC free article] [PubMed] [Google Scholar]
- Carman KL, Dardess P, Maurer ME, Workman T, Ganachari D, Pathak-Sen E. A Roadmap for Patient and Family Engagement in Healthcare Practice and Research. Gordon and Betty Moore Foundation; Palo Alto, CA: 2014. Prepared by the American Institutes for Research under a grant from the Gordon and Betty Moore Foundation, Dominick Frosch, Project Officer and Fellow; Susan Baade, Program Officer. Available at: www.patientfamilyengagement.org (accessed 1 April 2015) [Google Scholar]
- Chang B, Hendricks AM, Slawsky MT, Locastro JS. Patient recruitment to a randomized clinical trial of behavioral therapy for chronic heart failure. BMC Medical Research Methodology. 2004;4:8. doi: 10.1186/1471-2288-4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeWalt DA, Pignone M, Malone R, Rawls C, Kosnar MC, George G, Bryant B, Rothman RL, Angel B. Development and pilot testing of a disease management program for low literacy patients with heart failure. Patient Education & Counseling. 2004;55:78–86. doi: 10.1016/j.pec.2003.06.002. [DOI] [PubMed] [Google Scholar]
- ENRICHD Investigators. Enhancing recovery in coronary heart disease (ENRICHD): baseline characteristics. American Journal of Cardiology. 2001;88:316–322. doi: 10.1016/s0002-9149(01)01652-6. [DOI] [PubMed] [Google Scholar]
- Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, Turner MB, et al. American Heart Association Statistics Committee and Stroke Statistics Subcommittee Heart disease and stroke statistics 2014 update: a report from the American heart association. Circulation. 2014;129:e28–e292. doi: 10.1161/01.cir.0000441139.02102.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris DJ, Douglas PS. Enrollment of women in cardiovascular clinical trials funded by the national heart, lung, and blood institute. New England Journal of Medicine. 2000;343:475–480. doi: 10.1056/NEJM200008173430706. [DOI] [PubMed] [Google Scholar]
- Heart Failure-Clinical Trials. World Health Organization International Clinical Trials Registry Platform Web Site. 2014 Available at: http://www.nhs.uk/Conditions/Heart-failure/Pages/clinical-trial.aspx (accessed April 2015)
- Heiat A, Gross CP, Krumholz HM. Representation of the elderly, women, and minorities in heart failure clinical trials. Archives of Internal Medicine. 2002;162:1682–1688. doi: 10.1001/archinte.162.15.1682. [DOI] [PubMed] [Google Scholar]
- Hoffman A, Montgomery R, Aubry W, Tunis SR. How to best engage patients, doctors, and other stakeholders in designing comparative effectiveness studies. Health Affairs. 2010;29:1834–1841. doi: 10.1377/hlthaff.2010.0675. [DOI] [PubMed] [Google Scholar]
- Hoffmann T, Worrall L, Read S, Wong A. Randomised controlled trial of an education and support package for stroke patients and their carers. British Medical Journal Open. 2013;3(5):e002538. doi: 10.1136/bmjopen-2012-002538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes L, Vanbelle C, Hayduk R. Innovative Patient Recruitment Strategies in prodromal Alzheimer’s Disease Trials. Quintiles. 2014 Available at: http://www.quintiles.com/library/white-papers/innovative-digital-patient-recruitment-strategies-in-prodromal-alzheimers-disease-trials (accessed 1 April 2015)
- Institute of Medicine. Women’s Health Research Progress Pitfalls, and Promise. 2010 Available at: http://www.iom.edu/Reports/2010/Womens-Health-Research-Progress-Pitfalls-and-Promise.aspx (accessed 1 April 2015)
- Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the medicare fee-for-service program. New England Journal of Medicine. 2009;360:1418–1428. doi: 10.1056/NEJMsa0803563. [DOI] [PubMed] [Google Scholar]
- Kim ESH, Menon V. Status of women in cardiovascular clinical trials. Arteriosclerosis, Thrombosis, and Vascular Biology. 2009;29:279–283. doi: 10.1161/ATVBAHA.108.179796. [DOI] [PubMed] [Google Scholar]
- Lennie TA, Song EK, Wu JR, Chung ML, Dunbar SB, Pressler SJ, Moser DK. Three gram sodium intake is associated with longer event-free survival only in patients with advanced heart failure. Journal of Cardiac Failure. 2011;17:325–330. doi: 10.1016/j.cardfail.2010.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parreco LK, DeJoice RW, Massett HA, Padberg RM, Thakkar SS. Power of an effective clinical trial conversation: improving accrual into clinical trials. Journal of Oncology Practice. 2012;8:282–286. doi: 10.1200/JOP.2011.000478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pressler SJ. Women with heart failure are disproportionately studied as compared to prevalence: a review of published studies from 2013. Journal of Cardiovascular Nursing. 2014 doi: 10.1097/JCN.0000000000000212. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Pressler SJ, Subramanian U, Shaw RM, Meyer LE, Stoudemire K, Gradus-Pizlo I. Research in patients with heart failure: challenges in recruitment. American Journal of Critical Care. 2008;17:198–203. [PubMed] [Google Scholar]
- Pressler SJ, Subramanian U, Kareken D, Perkins SM, Gradus-Pizlo I, Sauve MJ, Ding Y, Kim JS, Sloan R, Jaynes H, Shaw RM. Cognitive deficits in chronic heart failure. Nursing Research. 2010;59:127–139. doi: 10.1097/NNR.0b013e3181d1a747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pressler SJ, Therrien B, Riley PL, Chou CC, Ronis DL, Koelling TM, Smith DG, Sullivan BJ, Frankini AM, Giordani B. Nurse-enhanced memory intervention in heart failure: the MEMOIR study. Journal of Cardiac Failure. 2011;17:832–843. doi: 10.1016/j.cardfail.2011.06.650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pressler SJ, Titler M, Koelling TM, Riley PL, Jung M, Hoyland-Domenico L, Ronis DL, Smith DG, Bleske BE, Dorsey SG, Giordani B. Nurse-enhanced computerized cognitive training increases serum brain-derived neurotrophic factor levels and improves working memory in heart failure. Journal of Cardiac Failure. 2015;21(8):630–641. doi: 10.1016/j.cardfail.2015.05.004. [DOI] [PubMed] [Google Scholar]
- Smith A. US Smartphone Use in 2015. Pew Research Centers Internet American Life Project RSS. 2015 Available at: http://www.pewinternet.org/2015/04/01/us-smartphone-use-in-2015/ (accessed 1 April 2015)
- Sullivan-Bolyai S, Bova C, Deatrick JA, Knafl K, Grey M, Leung K, Trudeau A. Barriers and strategies for recruiting study participants in clinical settings. Western Journal of Nursing Research. 2007;29:486–500. doi: 10.1177/0193945907299658. [DOI] [PubMed] [Google Scholar]
- Tong SC, Tin AS, Lim JF, Chow WL. Innovative proven clinical-research strategies for participant recruitment and retention. Proceedings of Singapore Healthcare. 2010;19:64–68. [Google Scholar]
- World Health Organization Clinical Trials Registry Platform. 2015 Available at: http://www.who.int/ictrp/en/ (accessed 1 April 2015)
- Yates BC, Dodendorf D, Lane J, LaFramboise L, Pozehl B, Duncan K, Knodel K. Testing an alternate informed consent process. Nursing Research. 2009;58:135–139. doi: 10.1097/NNR.0b013e31818c3df5. [DOI] [PubMed] [Google Scholar]
- Zambroski CH, Moser DK, Bhat G, Ziegler C. Impact of symptom prevalence and symptom burden on quality of life in patients with heart failure. European Journal of Cardiovascular Nursing. 2005;4:198–206. doi: 10.1016/j.ejcnurse.2005.03.010. [DOI] [PubMed] [Google Scholar]


