Abstract
The purpose of this study was to identify barriers to and facilitators of human papillomavirus (HPV) vaccination in children aged 9–17 years across Texas. A literature review informed the development of a web-based survey designed for people whose work involves HPV vaccination in settings serving pediatric patients. The survey was used to examine current HPV vaccine recommendation practices among healthcare providers, barriers to HPV vaccination, reasons for parent/caregiver vaccine refusal, staff and family education practices, utilization of reminder and recall systems and status of vaccine administration (payment, ordering and stocking). 1132 responses were received representing healthcare providers, administrative and managerial staff. Respondents identified perceived barriers to HPV vaccination as parental beliefs about lack of necessity of vaccination prior to sexual debut, parental concerns regarding safety and/or side effects, parental perceptions that their child is at low risk for HPV-related disease, and parental lack of knowledge that the vaccine is a series of three shots. Of responding healthcare providers, 94% (n= 582) reported they recommend the vaccine for 9–12 year olds; however, same-day acceptance of the vaccine is low with only 5% (n=31) of providers reporting the HPV vaccine is “always” accepted the same day the recommendation is made. Healthcare providers and multidisciplinary care teams in pediatric care settings must work to identify gaps between recommendation and uptake to maximize clinical opportunities. Training in methods to communicate an effective HPV recommendation and patient education tailored to address identified barriers may be helpful to reduce missed opportunities and increase on-time HPV vaccinations.
Keywords: HPV vaccination, Barriers, Survey of healthcare professionals, Pediatrics, Vaccine uptake
Introduction
The national uptake rate of human papillomavirus (HPV) vaccination has remained far below the Healthy People 2020 goal of 80%, despite the passage of 10 years since the first HPV vaccine introduction in 2006 [1]. Nationally, the 2015 vaccination rates for completion of the 3-dose vaccine series are still below 40% for girls and 20% for boys [2]. If vaccination uptake rates remain low, HPV-associated cancer rates will continue to rise, along with associated costly treatments, reduced quality of life, and avoidable deaths. [3–5].
The Food and Drug Administration has approved 3 vaccines to prevent mucosal infections from multiple HPV types that cause most HPV-associated cancers and genital warts: Gardasil (June 2006) and Gardasil 9 (December 2014) for male and female patients, and Cervarix (October 2009) for female patients only. The US Advisory Committee on Immunization Practices (ACIP) recommends the HPV vaccine at the 11- or 12-year-old well-child checkup, with catch-up doses administered per recommendations [6, 7]. Two additional vaccines are recommended at the same well-child checkup: the tetanus, diphtheria and pertussis (Tdap) vaccine and the meningococcal vaccine. Uptake rates for Tdap and meningococcal vaccines are estimated to be 87.6% and 79.3% uptake among adolescents in the United States respectively, and 88.2% and 88.6% in Texas in 2014 [2]. Schools in Texas require Tdap and meningococcal vaccines for school entry but do not require HPV vaccination. HPV vaccination remains low compared to other adolescent vaccinations recommended at the same well-child visit.
In February 2014, the President’s Cancer Panel (PCP) published a report titled “Accelerating HPV Vaccination Uptake: Urgency for Action to Prevent Cancer,” which analyzed reasons for the low uptake and outlined critical goals and objectives needed to increase uptake. The panel recommends targeted interventions to improve vaccine uptake, which include reducing missed clinical opportunities for HPV vaccination, increasing public acceptance of the HPV vaccine and improving access to the vaccine. The PCP suggests that if all providers strongly recommend the HPV vaccine to eligible patients at every clinical opportunity, vaccine uptake will increase dramatically. The purpose of this study was to specifically identify barriers to and facilitators of human papillomavirus (HPV) vaccination in children aged 9–17 years across the state of Texas and identify how Texas providers compare nationally in addressing HPV uptake goals.
Methods
Peer-Reviewed Literature
We conducted a review of literature regarding HPV vaccination in Texas to assess research on HPV vaccination interventions and to inform the development of survey questions.
The PubMed, Cumulative Index to Nursing and Allied Health Literature and Cochrane databases were searched for journal articles published between 2009 and 2014 using the key terms “HPV vaccination in Texas” and inclusion criteria related to “pediatric HPV vaccination.” Results of this search yielded 18 articles, which were then abstracted in an Excel spreadsheet and analyzed for key themes. Gray literature, defined as non-peer-reviewed literature, meeting the inclusion criteria was also analyzed. Gray literature was obtained online from the Centers for Disease Control and Prevention (CDC), the Texas Department of State Health Services (DSHS), the Texas Medical Association (TMA), the American Cancer Society (ACS), The Immunization Partnership (TIP), county public health departments, several independent school districts, the Association of Womens’ Health, Obstetric, and Neonatal Nurses, and other organizations.
Stakeholders were identified through existing partnerships and networks, during the review of current literature, and during state immunization educational events. Stakeholders provided feedback during the development of the survey instrument, facilitated the distribution of the survey to their respective networks, and completed the survey when appropriate.
Survey Instrument Development
For survey development, themes discussed in the PCP Report and those described in the literature review were appraised [1]. Questions regarding factors influencing vaccination rates and documentation of immunizations were obtained with permission from a general vaccine survey by TIP, and adjusted to collect information specific to HPV. Questions were designed to assess individual pediatric practice demographics, specific factors impacting HPV vaccination, provider practices regarding HPV vaccination, patient and provider education practices related to HPV, provider opinions regarding HPV vaccination, and any administrative issues with the vaccine, such as ordering, payment, and maintaining stock. Healthcare providers were asked specific questions about their methods of vaccine recommendation, observations of same-day acceptance of the vaccine, immunization documentation, and tools used to increase HPV vaccine uptake. Using the survey we were able to examine current HPV vaccine-related recommendation practices among Texas healthcare providers, reported parent/caregiver reasons for refusal, staff and family education practices, office systems for reminder and recall, and the status of HPV vaccine administration (payment, ordering and stocking). An online survey form was generated using the 2015 Qualtrics platform. A combination of Likert-type scales, closed-ended response choices, and open-ended response choices were used.
Survey Distribution
The University of Texas MD Anderson Cancer Center’s Institutional Review Board approved the study and instrument. A link to the survey was sent by email 4 times over a 6-week period. The link was distributed by several county medical societies; by Texas medical professional organizations, including the Texas Pediatric Society, Texas Academy of Family Practitioners, Texas Association of Obstetricians and Gynecologists, Texas Association of Physician Assistants, and the TMA; and by individual health institution stakeholders. Additionally, immunization coalitions, public health personnel, and academic researchers were also important in distributing the survey link to their respective networks. Figure 1 depicts the geographic reach of the survey.
Figure 1.
Locations of HPV Survey Respondents
Data Collection and Analysis
The online survey was developed using the 2015 Qualtrics platform. Qualtrics is the institution’s preferred web-based survey software and has been vetted by MD Anderson’s compliance, legal, and information security departments for privacy and security assurance. The survey distribution parameters were configured to ensure that responses were anonymous. The survey used an adaptive design and adjusted questions based on the respondent’s “role” selection. Choices of role were “healthcare provider,” “administrative/managerial,” “data entry staff,” and “other.” The survey respondents were not required to answer all questions; therefore, many questions did not have responses equal to the entire sample size. Data was collected through Qualtrics and descriptive statistics are presented in this report.
Results
Sample Characteristics
There were 1,132 respondents. Table 1 summarizes the self-identified roles of the respondents. Table 2 shows the breakdown of the 728 healthcare provider respondents. These respondents worked in a number of care settings including hospitals, clinic systems, federally qualified health centers, private practices, and professional and health service organizations; additional vaccination settings surveyed were identified through stakeholder engagement and included military healthcare facilities, migrant health centers, mobile delivery units, juvenile detention centers, and mental health centers; the responses from these settings made up 13% of the total. Of all respondents, 68% practiced in clinics that participate in the Vaccines for Children (VFC) program.
Table 1.
Self-Identified Roles of 1,132 Survey Respondents
| Respondent Role | Number of Respondents Self- Identifying in Role (%) |
|---|---|
| Healthcare provider | 728 (64.3%) |
| Administrative/managerial | 214 (18.9%) |
| Other | 106 (9.4%) |
| Data entry staff | 84 (7.4%) |
Table 2.
Roles of Healthcare Provider Respondents (n=728)
| Healthcare Provider Role | Number of Respondents Self- identifying in Healthcare Provider Role (%) |
|---|---|
| MD pediatrician | 295 (41.3) |
| Registered nurse | 121 (16.9) |
| MD Family practitioner | 60 (8.4) |
| Physician assistant | 59 (8.3) |
| MD obstetrician/gynecologist | 56 (7.8) |
| Licensed vocational nurse | 51 (7.1) |
| Nurse practitioner | 31 (4.3) |
| MD physician (other specialty) | 29 (4.1) |
| Other | 9 (1.3) |
| Medical assistant | 2 (0.3) |
| Not applicable, I don't provide healthcare | 1 (0.1) |
| No response provided | 14 (0.2) |
Factors Influencing HPV Vaccination
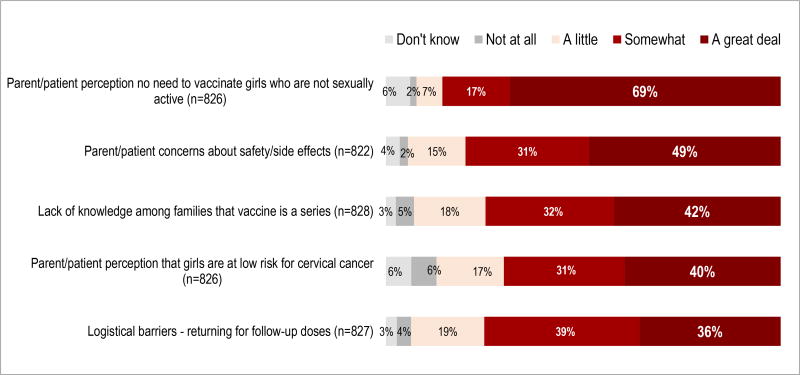
Healthcare providers reported similar barriers to HPV vaccination among both boys and girls. Providers were presented with a list of barriers shown in Table 5 and 6, and asked to describe to which extent each barrier impacts their patients using a likert scale. Figure 2 shows the perceived barriers to HPV vaccination affecting girls, and Figure 3 shows the perceived barriers affecting boys. The most frequently reported barrier was the parental perception that it is not necessary to vaccinate children who are not sexually active: 69% reported that this barrier impacts vaccination of girls “a great deal,” and 67% of provider respondents reported that this factor impacts the vaccination of boys “a great deal.” Other factors that respondents frequently reported to influence HPV vaccination rates included parental concerns regarding vaccine safety and side effects, parental perception that the child is at low risk for HPV-associated cancers and conditions, lack of knowledge that the vaccination is a series, and logistical issues that prevent returning for series completion. Additionally, 37% of providers reported that the parental belief that girls should be the ones to take preventive measures greatly affects HPV vaccination of boys. Lack of provider recommendations for the vaccine among boys and girls was reported as having an impact on vaccination “a great deal” by 27% and 22%, respectively. Less than 22% of responding providers reported that the following factors affect HPV vaccination “a great deal”: lack of routine care for adolescents, vaccine cost, lack of provider knowledge, language barriers, and vaccine availability. When asked to describe other barriers associated with HPV vaccine uptake, providers reported the exclusion of the vaccine from school requirements and parental concerns about the vaccine’s influence on children’s sexual behaviors.
Table 5.
Factors Influencing HPV Vaccination among Girls
| Extent to Which Factor Affects HPV Vaccine Uptake for Girls*
|
|||||
|---|---|---|---|---|---|
| Factor | A great deal |
Somewhat | A little | Not at all |
Don’t know |
| Adolescent girls don’t receive routine medical care (n=818) | 169 (21) | 334 (41) | 219 (27) | 67 (8) | 29 (4) |
| Communication issues due to language differences (n=825) | 75 (9) | 152 (18) | 340 (41) | 229 (28) | 29 (4) |
| Cost of vaccine for patients (n=820) | 142 (17) | 132 (16) | 191 (23) | 285 (35) | 70 (9) |
| Cost of vaccine for providers (n=823) | 113 (14) | 118 (14) | 151 (18) | 332 (40) | 109 (13) |
| Lack of knowledge among providers (n=824) | 80 (10) | 201 (24) | 276 (33) | 230 (28) | 37 (4) |
| Lack of knowledge among families that vaccine is a series of shots (n=828) | 350 (42) | 269 (32) | 150 (18) | 38 (5) | 21 (3) |
| Lack of provider recommendations for vaccine (n=825) | 567 (69) | 141 (17) | 54 (7) | 13 (2) | 51 (6) |
| Lack of vaccines availability among providers (n=824) | 58 (7) | 100 (12) | 159 (19) | 439 (53) | 68 (8) |
| Logistical barriers to returning for series of three shots (n=827) | 296 (36) | 325 (39) | 153 (19) | 30 (4) | 23 (3) |
| Parent/patient concerns about safety or side effects (n=822) | 399 (49) | 257 (31) | 120 (15) | 17 (2) | 29 (4) |
| Parent/patient perception that there is no need to vaccinate girls who are not sexually active (n=826) | 567 (69) | 141 (17) | 54 (7) | 13 (2) | 51 (6) |
| Parent/patient perception that girls are at low risk for cervical cancer (n=826) | 327 (40) | 252 (31) | 141 (17) | 53 (6) | 53 (6) |
Data are numbers of respondents (%).
Data includes responses from individuals that self- identified as the role of “healthcare provider’ and ‘other’.
Table 6.
Factors Influencing HPV Vaccination among Boys
| Extent to Which Factor Affects HPV Vaccine Uptake for Boys†
|
|||||
|---|---|---|---|---|---|
| Factor | A great deal |
Somewhat | A little | Not at all |
Don’t know |
| Adolescent boys don’t receive routine medical care (n=797) | 175 (22) | 298 (37) | 217 (27) | 58 (7) | 49 (6) |
| Communication issues due to language differences (n=796) | 70 (9) | 149 (19) | 309 (39) | 215 (27) | 53 (7) |
| Cost of vaccine for patients (n=793) | 133 (17) | 123 (16) | 173 (22) | 275 (35) | 89 (11) |
| Cost of vaccine for providers (n=798) | 97 (12) | 96 (12) | 159 (20) | 321 (40) | 125 (16) |
| Lack of knowledge among providers (n=794) | 105 (13) | 196 (25) | 214 (27) | 221 (28) | 58 (7) |
| Lack of knowledge among families that vaccine is a series of shots (n=796) | 268 (34) | 252 (32) | 189 (24) | 46 (6) | 41 (5) |
| Lack of provider recommendations for vaccine (n=795) | 211 (27) | 213 (27) | 161 (20) | 155 (19) | 55 (7) |
| Lack of vaccines availability among providers (n=795) | 56 (7) | 92 (12) | 165 (21) | 380 (48) | 102 (13) |
| Logistical barriers to returning for series of three shots (n=798) | 255 (32) | 296 (37) | 170 (21) | 33 (4) | 44 (6) |
| Parent/patient concerns about safety or side effects (n=795) | 334 (42) | 240 (30) | 142 (18) | 23 (3) | 56 (7) |
| Parent/patient perception that there is no need to vaccinate boys who are not sexually active (n=797) | 536 (67) | 146 (18) | 44 (6) | 11 (1) | 60 (8) |
| Parent/patient perception that boys are at low risk for genital warts and cancers caused by HPV (n=797) | 497 (62) | 170 (21) | 55 (7) | 15 (2) | 60 (8) |
| Patient/parent belief that girls and women should be the ones to take preventative steps against cervical cancer (n=794) | 293 (37) | 217 (27) | 103 (13) | 89 (11) | 92 (12) |
Data are numbers of respondents (%).
Data includes responses from individuals that self- identified as the role of “healthcare provider’ and ‘other’.
Figure 2.
Provider-Perceived Factors Impacting HPV Vaccine Uptake among Girls Ages 9–17 Yearsa
a Percentages may not add to 100 because of rounding
Figure 3.
Provider-Perceived Factors Impacting HPV Vaccine Uptake among Boys Ages 9–17 Yearsa
a Percentages may not add to 100 because of rounding
Table 3 shows common reasons cited for HPV vaccine refusal, and Table 4 shows the common ways in which providers respond to refusals. Additional reasons for vaccine refusal reported by respondents included concerns regarding vaccine safety and side effects, parental desire to consult with spouse or partner, concerns regarding increased discomfort or pain, and religious beliefs. Respondents were able to select multiple refusal protocols to describe practice methods; therefore, the total percentage of responses exceeds 100% in Table 4.
Table 3.
Common Reasons Cited for HPV Vaccine Refusal (n=591)
| HPV Vaccine Refusal Reason/Belief | Number of Respondents Citing Reason for Vaccine Refusal (%) |
|---|---|
| Son or daughter too young to be vaccinated for HPV | 179 (30.3) |
| Concerns due to media portrayal of the vaccine | 115 (19.5) |
| Lack of knowledge about diseases caused by HPV infection | 100 (16.9) |
| Consent would lead to riskier sexual behaviors | 94 (15.9) |
| Other (please specify) | 73 (12.4) |
| Inadequate insurance coverage | 30 (5.1) |
Table 4.
Healthcare Providers’ Vaccine Refusal Protocol (n=611)
| Provider Response to Vaccine Refusal | Number of Responses (%) |
|---|---|
| Provide educational materials for patient to consider | 492 (80.1) |
| Document and repeat recommendation at next visit | 471 (77.1) |
| Other | 57 (9.3) |
| Document and do not recommend in future | 31 (5.1) |
Note: Respondents were able to select multiple refusal protocols to describe practice methods; therefore, the total percentage of responses exceeds 100.
Current Practices for HPV Vaccine Recommendation
Eighty-nine percent (n=781) of healthcare providers reported that they “fully support HPV vaccination.” Of the healthcare providers who responded (n= 618), 52% (n=319) begin recommending the HPV vaccine for patients aged 11 years, 29% (n=181) begin recommending the vaccine for patients aged 9 years, and 8% (n=49) begin recommending HPV vaccination for patients 12 years. The providers also differed in the ways in which they recommend the HPV vaccine: 57% (n=294) recommend it with other adolescent vaccines or in a “bundle,” 29% (n=150) use the word “optional” during the recommendation, 12% (n=60) use “other” approaches, and 2% (n=9) of respondents do not recommend the vaccine at all. Healthcare providers reported that older pediatric patients (ages 13–17 years) are more likely to accept the vaccine on the same day as the recommendation was given, 38% of providers reported that patients aged 13–17 years accept the vaccine “very often,” and 28% of providers reported patients aged 9–12 years accept the vaccine “very often.” For both age cohorts, only 5% of healthcare providers reported that patients “always” accept the vaccine.
When respondents were asked which members of the care team are involved in ensuring vaccinations are current, 80% claimed the physician, 57% claimed the medical assistant, and 57% claimed the registered nurse.
Regarding immunization registry participation, 60% (n=461) of providers reported participation in the Texas immunization registry (ImmTrac), 20% did not report participation in ImmTrac, and 20% did not know if their care settings share vaccination data with the state registry.
Reminders
Use of patient reminders for second and third doses or recalls such as mailed cards, phone calls, text messages, and industry-provided products (e.g. refrigerator magnets) were reported by 38% of providers, and 12% of providers stated phone reminders are “very effective.”
HPV Education for Patients and Providers
Of healthcare providers answering the question about whether and how HPV education is provided to patients in their care settings, 64% (n=397) said they provided educational materials regarding HPV-associated cancers. Of these respondents, 75% used materials developed by the CDC, 28% used other materials such as those provided by the vaccine manufacturer, 22% used materials developed by TIP, and 13% used ACS materials.
Only 22% percent of respondents reported that their healthcare settings provided HPV in-service trainings to staff. Seventy-four percent reported that staff meetings were used to deliver provider education, 52% of respondents reported that literature was provided, 39% reported that online education courses were provided, 34% reported that they attended speaker series, 21% reported that they received communication training, and 8% reported other methods.
Vaccine Administration Issues
Respondents were asked to describe challenges associated with maintaining HPV vaccine inventory in their care settings. Seventy-seven percent of providers reported “always” having the HPV vaccine in stock at their care setting. Of 213 respondents, 39% (n=84) reported they experienced no challenges, 22% (n=47) reported vaccine cost as a challenge, 13% (n=29) reported issues or delays with the state VFC supply, and 15% (n=33) chose “not applicable.” The remaining responses included challenges associated with HPV vaccine demand, logistical issues with administration of the vaccine, expiration of vaccines, and shortages of supply from the vaccine manufacturer.
Discussion
Since the HPV vaccine was introduced in 2006, coverage has increased among Texas adolescents, but remains low compared with coverage for other vaccines recommended for adolescents and significantly below the goal of 80% by 2020 [2]. This survey of healthcare providers in Texas indicates that providers perceive barriers such as parental perceptions about HPV, parental knowledge and safety concerns as greatly impacting HPV vaccination rates. This study also indicated that providers are not effectively advocating for HPV vaccinations with strong HPV vaccine recommendations or addressing HPV vaccination at every clinical opportunity. Healthcare providers must be at the forefront of education and advocacy for HPV vaccination, and Texas providers could benefit from increased provider education and vaccine related communication skills to reduce missed opportunities for vaccination.
Two-thirds of providers indicated that the most common barrier for HPV vaccination is associated with parental perceptions about HPV. Parents believe that their children are either too young to be vaccinated against HPV or not at risk for HPV, or parents have concerns about the safety of the vaccine. Parents of boys additionally believe that girls/women should be responsible for prevention against cervical cancer. This parental hesitancy can translate into vaccine refusal with parents refusing vaccines due to belief that their child is too young, concerns over safety or increased promiscuity or lack of knowledge about HPV related disease, These parental beliefs and reasons for refusal are consistent with national data from the Presidents Cancer Panel report on the importance of parental beliefs in vaccine uptake, and indicate that Texas would benefit from education of the lay public [1].
The most common reasons for vaccine refusal in this study were primarily related to misconceptions about HPV related disease or concerns over the vaccine’s safety and societal implications. The PCP demonstrated that the absence of provider recommendation and education resources is a major contributor to the lack of parental knowledge [1]. This suggests that providers are missing opportunities to educate parents about HPV and correct misconceptions to improve vaccination rates. In this study, one quarter of the participants in this study reported that a barrier to vaccination is a lack of provider recommendation. Providers need to be taking advantage of clinical opportunities to recommend and educate the public on HPV, but this study indicates that providers are not effectively communication a recommendation to patients.
An important part of training physicians is instructing them to effectively communicate about HPV and the vaccine. This study found that less than a quarter of provider survey respondents use communication training as an education method for providers, with the majority of education occurring at staff meetings, through review of literature, or through online education. Additionally, almost 33% of the providers surveyed use the word “optional” when offering the HPV vaccine. Eliminating this “optional” focus and instead emphasizing a “same way, same day” approach has been widely recommended by vaccine communication experts [9].
The PCP Report and the CDC suggest that bundling the HPV vaccine recommendation with other recommended adolescent vaccines is the preferred method of recommending the vaccine [1]; specifically, providers are encouraged to say, “Today your son/daughter is due for the Tdap, HPV, and meningococcal vaccine. I strongly recommend you get all three vaccines. Do you have any questions?” [9]. Unfortunately, our survey suggests that many providers do not utilize the approach of bundling the vaccine recommendations and report low rates of same-day HPV vaccine acceptance. Provider education on effective vaccine communication skills, especially skills focused on strong recommendations and responses to inquisitive, poorly informed, or hesitant parents of patients are potential focus areas for future initiatives to increase HPV vaccine uptake.
According to the CDC, missed clinical opportunities are the most common reason that the United States has not achieved high rates of vaccine uptake [1]. In our study, more than 20% of respondents reported that the lack of routine care for adolescents is a barrier to HPV vaccination. This finding is consistent with national results, but it emphasizes the importance of utilizing every clinical visit, including sick visits, as an opportunity for vaccination [10]. In order to reduce missed clinical opportunities, a robust vaccination registry is helpful. However, this study indicated a lack of consistent use of the state registry. While more than 95% of new Texas parents opt in to the registry, there are neither formal incentives for practitioners to use the registry nor disincentives for those who do not use the registry. Though the evidence suggests that use of the state immunization registry is in the best interest of patients and the health of citizens overall, 20% of survey respondents reported that they did not participate in it, and another 20% said they were not certain if they participated. Finding ways to incentivize increased use of electronic health maintenance records and the state immunization registry could decrease missed clinical opportunities for vaccination.
The survey development and results have resulted in the formation of a network of stakeholders that have already begun targeted campaigns in areas notable for low vaccination rates by the National Immunization Survey—Teen [2, 11]. Stakeholders, including the DSHS, the Department of Health and Human Services, the Cancer Prevention Research Institute of Texas, MD Anderson Cancer Center, and professional organizations including the TMA and Texas Pediatric Society will be working together to provide multi-system interventions that include education of providers on effective communication strategies; education of the lay public on the efficacy, safety, and necessity of the vaccine; increased use of vaccine reminder systems; and increased enrollment in and use of the state registry.
Strengths and Limitations
Owing to the method of distribution, with an unknown number of recipients reached, the response rate cannot be determined. Further, the survey sample may not be representative of the Texas healthcare provider population in size and provider type make-up. Additionally, although targeted outreach was conducted across both rural and metropolitan areas, identifying providers and obtaining survey responses from rural counties was challenging, and many rural counties are not well represented. Selection bias from those providers who chose to respond versus those who chose not to respond may also affect results.
This survey study also has strengths, such as a large number of respondents; the inclusion of additional members of the healthcare team such as physician assistants, nurse practitioners, nurses, medical assistants, administrators, and data entry staff; and the attempt to analyze multi-level factors associated with HPV vaccine uptake.
Conclusion
HPV vaccination barriers identified in this study closely align with those identified in the PCP Report. Providers indicated that the most common barrier for HPV vaccination is parental perceptions about HPV and that weak provider recommendations contribute significantly to low uptake. The majority of providers in Texas are offering the HPV vaccine to patients at routine visits; however, our findings support that the recommendation must be strengthened to increase same-day acceptance. A robust immunization tracking system would limit missed opportunities. Effective communication of the recommendation and associated education of both providers and parents of patients is imperative to reduce barriers related to HPV knowledge and increase timely HPV vaccinations.
Acknowledgments
This work was supported in part by the National Institutes of Health through Cancer Center Support Grant (NCI 3 P30 CA016672-39S4).
Footnotes
Compliance with Ethical Standards
The University of Texas MD Anderson Cancer Center’s Institutional Review Board approved the study and instrument.
The authors declare that they have no conflict of interest.
References
- 1.Accelerating HPV vaccine uptake: urgency for action to prevent cancer. A report to the President of the United States from the President’s Cancer Panel. Bethesda, MD: National Cancer Institute; 2014. [Google Scholar]
- 2.Reagan-Steiner S, Yankey D, Jeyarajah J, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13–17 years—United States, 2014. MMWR Morb Mortal Wkly Rep. 2015;64:784–792. doi: 10.15585/mmwr.mm6429a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Age-Adjusted Invasive Cancer Incidence Rates by County in Texas, 2008–2012. Cancer Incidence File, January 2014. Texas Cancer Registry. Cancer-Rates.info. Retrieved Jun 06, 2016, from http://cancer-rates.info/tx/
- 4.HPV-associated cancers statistics. Centers for Disease Control and Prevention. 2014 Retrieved September 03, 2015, from http://www.cdc.gov/cancer/hpv/statistics/index.htm.
- 5.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer JClin. 2014;64(1):9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 6.Strikas RA. Advisory Committee on Immunization Practices recommended immunization schedules for persons aged 0 through 18 years--United States, 2015. MMWR Morb Mortal Wkly Rep. 2015;64(4):93–94. [PMC free article] [PubMed] [Google Scholar]
- 7.Pertussis outbreak trends. Centers for Disease Control and Prevention. 2015 Retrieved August 31, 2015, from http://www.cdc.gov/pertussis/outbreaks/trends.html.
- 8.Holman DM, Benard V, Roland KB, Watson M, Liddon N, Stokley S. Barriers to human papillomavirus vaccination among US adolescents: a systematic review of the literature. JAMA Pediatr. 2014;168(1):76–82. doi: 10.1001/jamapediatrics.2013.2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tips and time-savers for talking with parents about HPV vaccine. Centers for Disease Control and Prevention. Retrieved March 18, 2016, from http://www.kfmc.org/images/docs/HPV/for-hcp-tipsheet-hpv.pdf.
- 10.Nordin JD, Solberg LI, Parker ED. Adolescent primary care visit patterns. Ann Fam Med. 2010;8(6):511–516. doi: 10.1370/afm.1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ashrawi D, Javaid M, Stevens L, Bello R, Ramondetta L. HPV vaccine uptake in Texas pediatric care settings: 2014–2015 environmental scan report. 2015 doi: 10.1007/s10900-016-0228-0. Retrieved March 18, 2016, from http://www.texascancer.info/pdfs/hpvenvironmentalscanreport.pdf. [DOI] [PMC free article] [PubMed]