Abstract
Purpose
Telephone disclosure of genetic test results can improve access to services. To date, studies of its impact have focused on return of Mendelian risk information, principally hereditary cancer syndromes.
Methods
In a multisite trial of Alzheimer’s disease genetic risk disclosure, asymptomatic adults were randomized to receive test results in-person or via telephone. Primary analyses examined patient outcomes 12 months after disclosure.
Results
Data from 257 participants showed that telephone disclosure occurred 7.4 days sooner and were 30% shorter, on average, than in-person disclosure (both p<0.001). Anxiety and depression scores were well below cutoffs for clinical concern across protocols. Comparing telephone and in-person disclosure protocols, 99% CIs of mean differences were within non-inferiority margins on scales assessing anxiety, depression, and test-related distress, but inconclusive about positive impact. No differences were observed on measures of recall and subjective impact. Sub-analyses supported non-inferiority on all outcomes among APOE ε4-negative participants. Sub-analyses were inconclusive for APOE ε4-positive participants, although mean anxiety and depression scores were still well below cutoffs for clinical concern.
Conclusion
Telephone disclosure of APOE results and risk for Alzheimer’s disease is generally safe and helps providers meet demands for services, even when results identify an increased risk for disease.
Keywords: Alzheimer’s disease, APOE, genetics, genomics, risk assessment, personalized medicine, information recall, telephone, genetic test results
INTRODUCTION
Face-to-face disclosure of genetic test results has been a long-standing practice,1 but the demand for services is outpacing the capacity of most clinics.2,3 Some individuals, particularly those in rural areas, find it challenging to meet with genetic service providers in person.4 To expedite timely disclosure of genetic test results to as many patients as possible, genetic specialists are increasingly providing results via telephone.5–7
Analyses of telephone disclosure to date are encouraging. Many patients prefer it to in-person or mailed disclosure,8 and studies of its use during testing for hereditary cancer syndromes have shown comparable levels of patient understanding and satisfaction after telephone and in-person disclosure.9–13 Two randomized trials demonstrated non-inferiority of telephone disclosure on a variety of outcomes, showing that differences from in-person disclosure on scales of test-related distress, knowledge and overall satisfaction were not clinically meaningful.13–15 To date, however, studies of telephone disclosure have almost exclusively focused on hereditary cancer syndromes.
Genetic testing to determine risk of Alzheimer’s disease (AD) provides a rich context for examining disclosure of genetic risk information for a common, complex conditions. The ε4 allele of apolipoprotein E (APOE) is a prevalent and robust genetic risk factor for AD.16 but is neither necessary nor sufficient for disease. The Risk Evaluation and Education for Alzheimer’s Disease (REVEAL) Study is a series of multi-center randomized clinical trials that have examined the impact of AD genetic susceptibility testing using APOE genotyping. Previous analyses have shown that testing does not increase risks for psychological harm, even among individuals who are ε4-positive;17–19 and that the majority of participants accurately recall test results.20–22 Here, we report on the third REVEAL Study trial, hypothesizing that mean scores on a variety of patient outcomes would be no worse following telephone disclosure than following in-person disclosure.
METHODS
Study Population
We recruited cognitively normal adults using mailings to research registries, postings on institutional research web sites, referrals from collaborating physicians, and advertisements in local newspapers at study sites in Boston, Cleveland, Ann Arbor, and Washington, DC. We established targets to enroll equal numbers of adults over and under the age of 60, equal numbers of men and women, and a 1:3 ratio of subjects with zero and one AD-affected first-degree relative (FDR), respectively. Screening per prespecified criteria was conducted initially by phone, and again more extensively by study clinicians during the first in-person appointment. Individuals were excluded if they had histories suggestive of hereditary AD (2 or more AD-affected FDRs, family members with average AD onset under age 60), or scored below an education-adjusted 87 on the Modified Mini-Mental State Examination (3MS).23 We also screened out individuals with severe anxiety or depression per validated scales (defined below).
Study Design
A multidisciplinary team designed the study protocol, approved by institutional review boards at each study site and an independent external Ethics and Safety Board, and the study was registered at ClinicalTrials.gov (NCT00462917). The study protocol, statistical code, and data set are available from the authors upon request. Briefly, participants who completed a phone interview and written questionnaire received educational materials developed in a prior trial.17 Participants then met with a genetic counselor and provided a blood sample for APOE genotyping at a CLIA-certified laboratory. Participants provided informed consent by telephone prior to the initial phone interview, then again in person prior to the blood draw for genotyping. Disclosure sessions were scheduled at the discretion of individual sites. Genetic counselors disclosed APOE genotypes and AD risk information using a script that allowed participants to raise any questions or concerns they had. Numeric estimates of lifetime (cumulative incidence from birth to the age of 85 years. Range: 6% to 73%) and remaining risk for AD (cumulative incidence from current age to the age of 85 years) were accompanied by graphs of AD risk curves. The methods for calculating risk estimates and generating risk curves are published previously.24,25 A written summary of the risk assessments were given to participants at the end of in-person disclosure sessions or mailed to participants following telephone disclosure.
A 2×2 factorial design determined whether participants received risk assessments in-person or via telephone, and whether or not participants additionally learned about an association between the ε4 allele of APOE and an increased risk for coronary artery disease, reported separately.19 Participants were randomized equally within strata, in blocks of size four, into “AD-only, in-person disclosure,” “AD-only, telephone disclosure,” “AD+CAD, in-person disclosure,” and “AD+CAD, telephone disclosure” arms. Randomization strata were defined by site, age (<60 vs ≥60), family history of AD, and gender. Serially-numbered envelopes concealed randomization statuses until the initial in-person visit. Randomization occurred prior to this appointment, which included confirmation of study eligibility, to allow consent forms customized to AD+CAD or AD-only randomization status to be mailed before in-person review.
Outcomes were assessed through questionnaires administered 6 weeks, 6 months, and 12 months after genotype and genetic risk disclosure. At the end of 6 week and 6 month follow-up appointments and after surveys were completed, genetic counselors verbally reminded participants about their genotypes, risk estimates, and about APOE-CAD associations (if appropriate). For safety purposes, subjects whose anxiety or depression scores exceeded standard cutoffs for severe mood disorders or increased by more than 15 points from baseline were immediately interviewed by a genetic counselor.
Measures
Outcome Variables
Outcomes of interest are bolded and included anxiety, depression, test-related distress (assessed via two scales), positive impact, recall of results, and subjective impact at 6 weeks, 6 months, and 1 year after results disclosure, all assessed via self-administered questionnaires. Outcomes were selected based on concerns about how telephone disclosure may impact the communication of information.26
Psychological outcomes included measures of general anxiety, general depression, two scales assessing test-related distress, and positive impact. Anxiety was assessed using the Beck Anxiety Inventory (BAI),27 with scores ranging from 0–63 (>8: mild, >15: moderate, >25: severe). Depression was assessed using the 20-item Center for Epidemiological Studies-Depression Scale (CES-D),28 with scores ranging from 0–60 (>10: mild, >16: moderate, >26: severe).29 Test-related distress was assessed with the 15-item Impact of Event Scale (IES),30 with scores ranging from 0–75 (≥20: significant distress) and the distress subscale of Impact of Genetic Testing for Alzheimer’s disease instrument (IGT-AD distress), with higher scores on a 0–60 scale indicating more negative feelings.31 Positive impact was assessed with the positive subscale of the IGT-AD, where items were reverse-scored such that lower scores on a 0–20 scale indicated more positive feelings.31
Recall of results was measured as the sum of correct responses when participants report back their results: 1) number of AD risk increasing alleles, 2) genotype, 3) lifetime AD risk estimate, and 4) remaining AD risk estimate. The risk allele and genotype items were assessed via multiple choice and included a “don’t remember” option. Risk estimate items asked participants to provide their lifetime and remaining risk percentages and were considered correct if responses were within 5% of communicated results, as used in prior analyses.20–22 Item prompts encouraged participants to provide their best guess if they did not remember their estimate. An additional open-ended item asked, “What other disease did we tell you is associated with the APOE gene?”, but is analyzed separately from other recall items because it was administered only to participants randomized to AD+CAD disclosure.
Subjective impact was assessed by asking participants to rate the “overall impact” that the risk information had on a 5-point scale (“very negative” to “very positive”).
Other Variables
Participants reported demographic information during the phone interview and on the baseline written questionnaire. Personal and family history of AD, cardiovascular disease, and other medical conditions were assessed during the blood draw appointment, as was numeracy using a validated scale with scores ranging from 0–8 based on the number of items a participant answers correctly.32 Participants also completed a validated 4-item version of a scale assessing self-reported comfort with numbers, with scores ranging from 1 to 6 based on mean ratings across items.33 After disclosure sessions, genetic counselors completed a chart note and indicated if topics discussed addressed any 12 issues, including the accuracy of results or preventive measures. Disclosure session length was calculated in minutes by comparing the start and finish times recorded on chart notes.
Statistical Analysis
A priori goals to enroll 280 participants and achieve 256 disclosures were set to power original hypotheses to compare disclosure of AD and CAD risk information against disclosure of only AD risk information, the main focus of the trial.19 Here we focused on the comparison between telephone and in-person disclosure. We used t-tests and chi-squared tests to compare demographics of the randomization arms and to analyze who dropped out after randomization. We used chi-squared tests to compare dropout rates of study arms after randomization, but before results disclosure. We used t-tests of log-transformed times to compare how long after the blood draw that disclosure sessions occurred and to compare the length of disclosure sessions, and used chi-squared tests to determine whether topics discussed during disclosure sessions varied by randomization status.
Telephone disclosure was intended to streamline service delivery rather than improve patient responses. Therefore, we used a non-inferiority framework to test whether outcomes were no worse after telephone disclosure than after in-person disclosure. We asserted non-inferiority of telephone disclosure on measures of anxiety, depression, test-related distress, and positive impact if the upper limit for the confidence interval (CI) of mean differences between telephone and in-person randomization arms was below a predefined margin.34
We used longitudinal analyses for all outcomes, including all observed data and imputing data for the few missing observations. We used generalized linear models fit with generalized estimating equations. For analyses of BAI, CES D, IES, IGT-AD distress and IGT-AD positive scores, we used a log link and Gamma distribution to compare outcomes by phone versus in-person randomization status, as these measures were very skewed and had a high proportion with zeros. For analyses of ordinal variables, recall was dichotomized to compare full recall (all items recalled correctly) against less than full recall, and subjective impact was dichotomized to compare responses of “very” and “somewhat positive” against responses of “neutral” and “somewhat” or “very negative.” Analyses of recall and subjective impact used a logit link and binomial distribution. We used an autoregressive working correlation structure with robust standard errors to account for the repeated measures within participant. A value of one was added to BAI, CES-D, IES, and IGT measures to shift their distributions away from zero. Models included terms for phone or in-person disclosure randomization status, time as a categorical variable, interaction between time and randomization arm, and the corresponding baseline psychological measure where applicable. Analyses on the IGT-AD distress subscale included a term to account for an interaction between telephone/in-person randomization status and AD-only/AD+CAD randomization status (p<0.05). Additional analyses were conducted to compare arms in APOE ε4-positive and APOE ε4-negative participants, as well as to further adjust for age, gender, education, race, family history of AD, AD+CAD or AD-only information disclosure, numeracy and self-reported comfort in analyses of information recall.20 We also conducted analyses where missing data were not imputed to minimize potential biases in non-inferiority analyses.34 These adjusted analyses and available case analyses are omitted from this report, because unadjusted models using imputed data were considered conservative in comparison (i.e., we report non-inferiority only when it was demonstrated in all analyses). We used contrasts to compare randomization arms at specific time points and overall for a time-averaged comparison.
Consistent with analyses of prior REVEAL Study trials,17,19 outcomes at 12 months were considered primary, while outcomes at 6 months and 6 weeks were considered secondary. Data for AD+CAD and AD-only disclosure arms were pooled in analyses because interactions between AD-only/AD+CAD randomization status and in-person/telephone disclosure randomization status were not observed (p-values for two-way tests of interaction between the two treatment arms and three-way interactions between the treatment arms and time were all greater than 0.05 except for analyses of IGT-AD distress scores, as noted earlier). The margin of non-inferiority for BAI, CES-D, and IES scores was 5 points, as used in prior REVEAL Study trials, indicative of medium to large effect sizes (Cohen’s d = 1.03, 0.64, and 0.66, respectively).17,18 This criterion was a more conservative margin than 5 to 10-point definitions of “clinically meaningful” differences used in other studies that are based on the intervals between cutoffs for minimal, mild, moderate, and severe anxiety and depression.35–37 For IGT-AD scores where prior studies have not identified a meaningful difference, we set margins following the strategy used in a related non-inferiority analysis:13 three points, representing a shift from “sometimes” to “never” on a single item and indicative of medium effect sizes (Cohen’s d = 0.43 and 0.48 for distress and positive subscales, respectively). Non-inferiority margins were not established for recall or subjective impact measures.
We used 99% CIs for primary analyses (12 month outcomes) to account for testing of seven outcomes (anxiety, depression, test-related distress per the IES and IGT-AD, positive impact, and recall of results). These CIs are more conservative than a Bonferroni correction, given guidelines to use one-sided tests.34 To be consistent across outcomes, we used 99% CIs on all secondary analyses. Additional secondary analyses used 99% CIs of 2-group tests of proportions to compare recall rates on individual recall items by randomization status and McNemar tests to compare whether participants were more likely to recall specific items more often than others.
Analyses included only participants receiving genetic risk information (genotype data for participants who provided blood but dropped out of the study before the disclosure session was destroyed per the IRB-approved protocol). We assumed data were missing at random and imputed missing values using multiple imputation (Markov Chain Monte Carlo procedures with 40 imputed data sets). All analyses were conducted using SAS, version 9.3 (SAS Institute, Cary, North Carolina).
RESULTS
Of 290 randomized participants, 257 (88.6%) received genetic risk disclosure (Figure 1) with no observed differences in dropout rates between telephone and in-person disclosure arms (∆=0.1%, p=0.99). Twenty four randomized participants withdrew before disclosure for the following non-exclusive reasons: concerns about potential emotional responses (8), study demands (4), personal or family health problems (4), concerns about privacy, confidentiality, or discrimination (4), lack of AD prevention options (3), moved (2), no longer interested (1), concerns about test limitations (1), and desire for higher remuneration (1). One participant passed away, and four were lost to follow-up. Four others did not meet eligibility criteria due to low 3MS scores, high CES-D scores, questionable family history of AD, and failure to attend study appointments.
Figure 1.
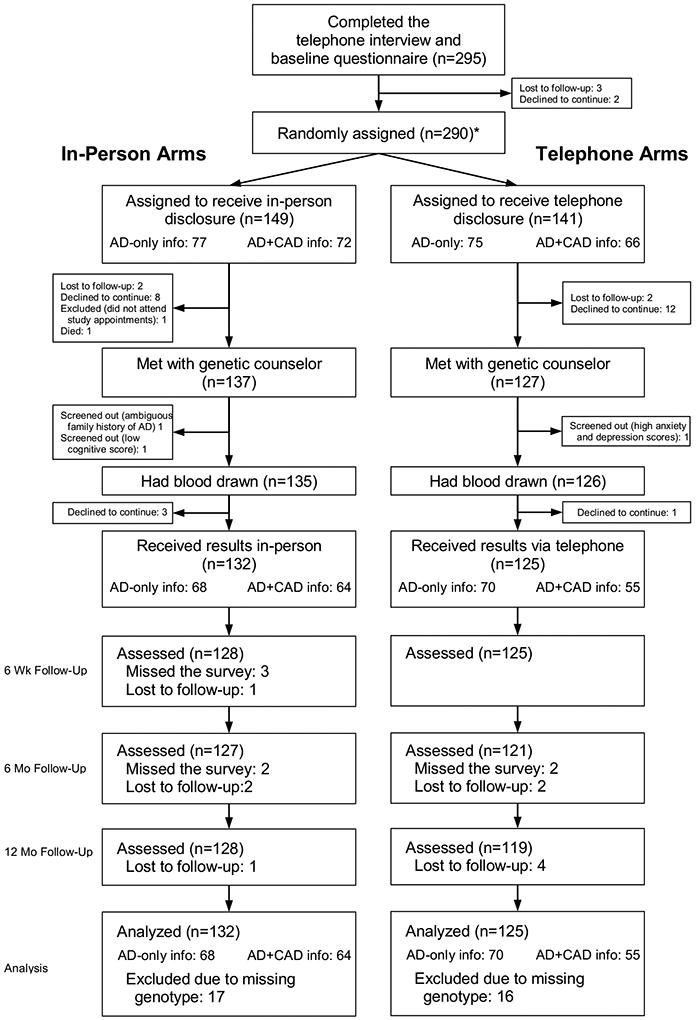
Enrollment flow chart
* Second randomization occurred at this point to determine whether participants received only AD risk information or whether they additionally received risk information about CAD.
Demographic characteristics of participants who received genetic risk information did not vary by disclosure method (Table 1) and were similar to those of the second REVEAL Study trial except for the inclusion of individuals without an AD-affected FDR.17 On average, telephone disclosure occurred 7.4 days sooner than in-person disclosure (27.8 vs 35.2 days after the blood draw, respectively; p=0.002). Disclosure sessions ranged from 6 to 40 minutes in length for telephone disclosure and 5 to 50 minutes in length for in-person disclosure, and telephone disclosure sessions were 6.6 minutes shorter than in-person disclosure sessions, on average (p<0.001). Disclosure session chart notes showed that participants were more likely to discuss preventive options during in-person disclosure than telephone disclosure (26% vs 14%, respectively, p=0.015). Disclosure sessions did not vary in length by APOE status (p=0.15 in bivariate analyses, p=0.16 in analyses controlling for telephone/in-person randomization status).
Table 1.
Characteristics of subjects who received genetic risk disclosure
| Randomization Arm | |||
|---|---|---|---|
| Characteristic | In-Person (n=132) |
Telephone (n=125) |
P |
| Age: yrs | |||
| Mean ± SD | 58.2 ± 12.9 | 58.1 ± 13.0 | 0.95 |
| Range | 21–83 | 22–82 | |
| Female sex: n (%) | 73 (55) | 68 (54) | 0.88 |
| African American race: n (%) | 21 (16) | 17 (14) | 0.60 |
| Education: yrs | |||
| Mean ± SD | 16.6 ± 2.4 | 17.0 ± 2.2 | 0.18 |
| Range | 10–20 | 12–20 | |
| Currently married: n (%) | 78 (59) | 75 (60) | 0.88 |
| Site: n (%) | 0.99 | ||
| Boston | 40 (30) | 38 (30) | |
| Cleveland | 32 (25) | 32 (25) | |
| Ann Arbor, MI | 35 (27) | 33 (26) | |
| Washington, DC | 25 (19) | 22 (18) | |
| Parent or sibling with AD: n (%) | 89 (67) | 89 (71) | 0.51 |
| Pleiotropic disclosure: n (%) | 64 (48) | 55 (44) | 0.47 |
| Has ε4 allele: n (%) | 0.86 | ||
| One copy | 37 (28) | 34 (27) | |
| Two copies | 7 (5) | 5 (4) | |
| Numeracy: mean ± SD | 7.1 ± 1.3 | 7.4 ± 1.3 | 0.06 |
| Comfort with numbers: mean ± SD | 4.5 ± 1.0 | 4.7 ± 0.9 | 0.10 |
| BAI score: mean ± SD | 3.4 ± 3.5 | 3.6 ± 3.5 | 0.55 |
| CES-D score: mean ± SD | 5.9 ± 5.0 | 5.5 ± 5.2 | 0.56 |
One year after disclosure and across randomization arms, participants remembered 3.0 of 4 items correctly, on average, and scored well below cutoffs for clinical concern on anxiety and depression scales (see Table 2). Overall, 24% of participants receiving in-person disclosure and 23% of participants receiving telephone disclosure reported moderate anxiety, depression, or test-related distress at one or more follow-up time points, with no differences observed by disclosure method (p=0.61). The majority of participants (65.6%) rated the subjective impact of their risk assessment as positive, while 7.9% rated it negative and 26.5% rated it neutral. Mean scores on measures of general anxiety and depression were well below cutoffs for clinical concern at all time points, and positive impact scores increased over time (p<0.001). With the exception of IGT-AD positive scores, CIs for mean differences between telephone and in-person disclosure arms were below prespecified margins of non-inferiority for all scales at all time points in analyses that were not stratified by APOE status (Table 2) and in analyses of ε4-negative participants (Table 3). However, analyses in ε4-positive participants supported non-inferiority of telephone disclosure at 12 months only on anxiety. Furthermore, upper limits of 99% CIs exceed margins for non-inferiority on IES and IGT-AD distress scores at the six-week and six-month time points, and upper limits of 99% CIs exceeded margins for non-inferiority on the IGT-AD positive scale at six weeks.
Table 2.
Mean outcome scores by randomization group and time after APOE genotype disclosure.*
| Variable | In person (n=132) |
Phone (n=125) |
Difference (99% CI) |
|---|---|---|---|
| 12-mo outcomes | |||
| BAI | 3.6 | 3.5 | −0.2 (−1.5 to 1.2) |
| CES-D | 6.2 | 7.4 | 1.1 (−1.2 to 3.4) |
| IES | 3.1 | 3.7 | 0.5 (−2.1 to 3.1) |
| IGT distress | 4.4 | 4.2 | −0.3 (−2.7 to 2.2) |
| IGT positive | 13.1 | 14.3 | 1.2 (−1.3 to 3.8) |
| Full recall | 37.3% | 50.1% | 1.7 (0.8 to 3.4)† |
| Positive subjective impact | 68.7% | 63.8% | 0.8 (0.4 to 1.7)† |
| 6-mo outcomes | |||
| BAI | 3.1 | 2.8 | −0.3 (−1.4 to 0.9) |
| CES-D | 5.3 | 6.1 | 0.8 (−1.2 to 2.8) |
| IES | 3.8 | 4.0 | 0.2 (−2.4 to 2.7) |
| IGT distress | 4.1 | 4.6 | 0.5 (−1.7 to 2.7) |
| IGT positive | 11.3 | 11.7 | 0.4 (−2.0 to 2.8) |
| Full recall | 46.6% | 55.4% | 1.4 (0.7 to 2.8)† |
| Positive subjective impact | 71.8% | 64.7% | 0.7 (0.4 to 1.5)† |
| 6-wk outcomes | |||
| BAI | 2.9 | 3.1 | 0.3 (−0.7 to 1.3) |
| CES-D | 4.8 | 6.5 | 1.7 (−0.2 to 3.6) |
| IES | 3.9 | 4.4 | 0.5 (−2.1 to 3.1) |
| IGT distress | 4.6 | 5.1 | 0.5 (−1.8 to 2.7) |
| IGT positive | 9.2 | 10.4 | 1.1 (−1.1 to 3.4) |
| Full recall | 61.6% | 61.9% | 1.0 (0.5 to 2.0)† |
| Positive subjective impact | 63.9% | 56.8% | 0.7 (0.4 to 1.5)† |
| Time-averaged outcomes | |||
| BAI | 3.2 | 3.1 | 0.0 (−0.9 to 0.9) |
| CES-D | 5.4 | 6.6 | 1.0 (−0.4 to 2.8) |
| IES | 3.6 | 4.0 | 0.4 (−1.9 to 2.7) |
| IGT distress | 4.4 | 4.6 | 0.2 (−1.9 to 2.3) |
| IGT positive | 11.1 | 12.0 | 0.9 (−1.1 to 3.0) |
| Full recall | 44.4% | 52.4% | 1.4 (0.8 to 2.4)† |
| Positive subjective impact | 65.5% | 58.3% | 0.8 (0.4 to 1.3)† |
Scores were estimated using generalized estimating equations with log link and γ distribution for continuous measures and with logit link and binomial distribution for dichotomized measures, with adjustment for corresponding baseline values (where applicable) and the genetic counselor providing disclosure.
Differences for dichotomized outcomes, full recall and positive subjective impact, represent odds ratios (i.e, the odds of full recall or positive subjective impact following telephone disclosure compared to in-person disclosure).
Table 3.
Mean outcome scores, stratified by APOE status.*
| APOE ε4 Noncarriers | APOE ε4 Carriers | |||||
|---|---|---|---|---|---|---|
| In person (n=88) |
Phone (n=86) |
Difference (99% CI) |
In person (n=44) |
Phone (n=39) |
Difference (99% CI) |
|
| 12-mo outcomes | ||||||
| BAI | 3.4 | 3.1 | −0.3 (−1.8 to 1.2) | 4.1 | 4.3 | 0.2 (−2.8 to 3.1) |
| CES-D | 6.0 | 6.5 | 0.6 (−1.8 to 2.9) | 6.8 | 9.2 | 2.4 (−2.6 to 7.4) |
| IES | 2.8 | 2.0 | −0.8 (−3.0 to 1.4) | 3.7 | 7.1 | 3.4 (−2.8 to 9.7) |
| IGT distress | 3.8 | 3.1 | −0.7 (−3.3 to 1.8) | 5.6 | 6.4 | 0.8 (−4.2 to 5.8) |
| IGT positive | 12.0 | 13.5 | 1.5 (−1.3 to 4.4) | 15.0 | 15.9 | 0.9 (−2.8 to 4.6) |
| Full recall | 28.6% | 44.4% | 2.1 (0.9 to 5.0)† | 47.4% | 52.4% | 1.2 (0.4 to 3.7)† |
| Positive subjective impact | 76.1% | 72.2% | 0.8 (0.3 to 2.0)† | 47.4% | 37.5% | 0.6 (0.2 to 2.2)† |
| 6-mo outcomes | ||||||
| BAI | 3.2 | 2.5 | −0.6 (−1.9 to 0.6) | 2.9 | 3.4 | 0.5 (−1.8 to 2.7) |
| CES-D | 5.1 | 5.9 | 0.8 (−1.4 to 3.0) | 5.7 | 6.6 | 0.9 (−3.0 to 4.8) |
| IES | 3.0 | 2.6 | −0.4 (−2.9 to 2.2) | 5.5 | 6.8 | 1.3 (−4.0 to 6.6) |
| IGT distress | 3.5 | 3.3 | −0.2 (−2.3 to 2.0) | 5.5 | 7.5 | 2.0 (−2.6 to 6.6) |
| IGT positive | 9.7 | 11.1 | 1.4 (−1.3 to 4.2) | 14.4 | 12.8 | −1.5 (−5.0 to 1.9) |
| Full recall | 41.2% | 54.5% | 1.7 (0.8 to 3.8)† | 47.4% | 47.4% | 1.1 (0.3 to 3.4)† |
| Positive subjective impact | 81.5% | 76.2% | 0.7 (0.3 to 2.0)† | 44.4% | 33.3% | 0.6 (0.2 to 2.2)† |
| 6-wk outcomes | ||||||
| BAI | 2.8 | 2.9 | 0.0 (−1.2 to 1.2) | 2.9 | 3.8 | 0.9 (−0.9 to 2.6) |
| CES-D | 4.9 | 6.7 | 1.8 (−0.6 to 3.0) | 4.4 | 5.9 | 1.6 (−1.3 to 4.4) |
| IES | 2.7 | 3.3 | 0.6 (−1.8 to 3.0) | 6.3 | 6.6 | 0.3 (−5.4 to 6.0) |
| IGT distress | 3.8 | 4.0 | 0.3 (−2.0 to 2.5) | 6.4 | 7.3 | 0.9 (−3.8 to 5.6) |
| IGT positive | 7.7 | 8.8 | 1.1 (−1.4 to 3.5) | 12.1 | 13.8 | 1.6 (−2.0 to 5.2) |
| Full recall | 60.0% | 63.0% | 1.1 (0.5 to 2.6)† | 50.0% | 50.0% | 1.0 (0.3 to 3.1)† |
| Positive subjective impact | 73.0% | 68.8% | 0.8 (0.3 to 2.0)† | 41.2% | 23.1% | 0.4 (0.1 to 1.5)† |
| Time-averaged outcomes | ||||||
| BAI | 3.1 | 2.8 | −0.3 (−1.8 to 1.2) | 4.1 | 4.3 | 0.2 (−2.6 to 3.1) |
| CES-D | 5.3 | 6.4 | 1.0 (−0.7 to 2.8) | 5.6 | 7.1 | 1.6 (−1.4 to 4.6) |
| IES | 2.8 | 2.6 | −0.2 (−2.2 to 1.7) | 5.0 | 6.8 | 1.8 (−3.5 to 7.0) |
| IGT distress | 3.7 | 3.5 | −0.2 (−2.3 to 1.9) | 5.9 | 7.0 | 1.2 (−3.2 to 5.6) |
| IGT positive | 9.6 | 11.0 | 1.3 (−0.9 to 3.6) | 13.8 | 14.1 | 0.3 (−2.6 to 3.2) |
| Full recall | 44.4% | 54.5% | 1.6 (0.8 to 3.1)† | 47.4% | 50.0% | 1.1 (0.4 to 2.8)† |
| Positive subjective impact | 76.7% | 72.2% | 0.8 (0.4 to 1.7)† | 44.4% | 33.3% | 0.6 (0.2 to 1.5)† |
Scores were estimated using generalized estimating equations with log link and γ distribution for continuous measures and with logit link and binomial distribution for dichotomized measures, with adjustment for corresponding baseline values and the genetic counselor providing disclosure.
Differences for dichotomized outcomes, full recall and positive subjective impact, represent odds ratios (i.e, the odds of full recall or positive subjective impact following telephone disclosure compared to in-person disclosure).
Additional analyses suggested that telephone disclosure outperformed in-person disclosure on some components of recalling results (Table 4). Participants were more likely to correctly recall lifetime AD risk estimates at 6 months (∆=17.6%, 99%CI: 4.6% to 30.6%) and 12 months (∆=15.8%, 99%CI: 2.1% to 29.6%) after telephone disclosure compared to in-person disclosure. Independent of disclosure method or genotype, recall scores were higher at 6 weeks than at 6 months (∆=0.3, p<0.001) or 12 months (∆=0.4, p<0.001). At 12 months, ε4-positive participants were more likely than ε4-negative participants to correctly recall their genotype (74.0% vs 56.3%, p=0.008). Across genotypes and randomization arms, data for individual recall items were highly correlated (r>0.57 in all pairwise comparisons), although participants were less likely to recall their specific genotypes compared to the number of risk alleles they had or their lifetime or remaining AD risk estimates (all pairwise comparisons p<0.001). Participants who did not accurately recall numeric AD risk estimates tended to provide estimates lower than what were reported during disclosure sessions: 64.2% of participants with inaccurate lifetime risk recall provided estimates lower than those reported, and 61.5% of participants with inaccurate remaining risk recall provided estimates lower than those reported.
Table 4.
Percentages recalling specific genetic test results correctly by disclosure method and time point, adjusted for APOE genotype.
| Information | In-Person (n=132) |
Telephone (n=125) |
Difference (99% CI) |
|---|---|---|---|
| 1 Year | |||
| Number of risk alleles | 80.5% | 79.8% | −0.7% (−13.5% to 12.2%) |
| Presence/absence of a risk allele | 81.9% | 83.8% | 1.9% (−10.2% to 14.0%) |
| Genotype | 59.3% | 65.0% | 5.7% (−9.9% to 21.2%) |
| Lifetime AD risk estimate (±5%) | 66.4% | 82.2% | 15.8% (2.1% to 29.6%) |
| Remaining AD risk estimate (±5%) | 71.6% | 83.6% | 12.0% (−1.2% to 25.3%) |
| Additional disease association* | 78.1% | 87.3% | 9.2% (−8.5% to 26.8%) |
| 6 Months | |||
| Number of risk alleles | 85.8% | 83.8% | −2.0% (−13.5% to 9.6%) |
| Presence/absence of a risk allele | 86.8% | 85.6% | −1.2% (−12.3% to 9.9%) |
| Genotype | 61.4% | 66.0% | 4.6% (−10.8% to 20.1%) |
| Lifetime AD risk estimate (±5%) | 69.1% | 86.7% | 17.6% (4.6% to 30.6%) |
| Remaining AD risk estimate (±5%) | 77.0% | 80.3% | 3.3% (−9.9% to 16.4%) |
| Additional disease association* | 84.4% | 87.3% | 2.9% (−13.6% to 19.4%) |
| 6 Weeks | |||
| Number of risk alleles | 86.9% | 82.4% | −4.5% (−16.0% to 7.1%) |
| Presence/absence of a risk allele | 90.3% | 86.4% | −3.9% (−14.2% to 6.4%) |
| Genotype | 70.9% | 79.2% | 8.3% (−5.5% to 22.2%) |
| Lifetime AD risk estimate (±5%) | 82.9% | 86.0% | 3.2% (−8.5% to 14.8%) |
| Remaining AD risk estimate (±5%) | 86.3% | 91.9% | 5.6% (−4.3% to 15.6%) |
| Additional disease association* | 78.1% | 83.6% | 5.5% (−13.0% to 24.0%) |
Item was administered to participants randomized to AD+CAD disclosure, only.
DISCUSSION
On average, telephone disclosure of genetic risk information about AD was safe and did not increase psychological risks. Although we were unable to demonstrate statistical non-inferiority for some outcomes within the subset of individuals who learned that they were APOE ε4-positive, their mean anxiety and depression scores were still well below cutoffs for clinical concern. These findings are notable because risk disclosure for AD has been used as an example of potentially distressing information, given the lack of proven preventative strategies. Anticipation of telephone disclosure did not appear to affect participants’ willingness to receive a genetic risk assessment, and wait times for disclosure sessions were shorter when results were disclosed by telephone, with the disclosure sessions themselves briefer than in-person disclosure sessions.
These findings are encouraging given that many genetic service providers are already disclosing test results via phone. Telephone disclosure is a long-standing practice in prenatal settings given time-sensitive implications for pregnancies, and it is now in wider use in other settings when results suggest no carrier or disease risks.6 Our data are consistent with findings from research on hereditary breast and ovarian cancer syndromes, showing that telephone disclosure does not increase risks for misremembering results or psychological harms.11–13 In fact, telephone disclosure seemed to improve participants’ abilities to retain numeric risk estimates. This improvement may be because participants received information at two distinct time points, first during phone disclosure sessions, then again when participants received the information via mail. The time savings noted in our study were also comparable to the time savings observed in the BRCA1/2 telephone disclosure studies, although our overall session lengths in our trial were often shorter. The comparably greater time needed for disclosing BRCA1/2 test results may be attributable to time needed to discuss medical management decisions and implications for other family members.
Genotype-specific analyses of our data suggest that genetic service providers should be mindful of the potential for additional distress when disclosing results indicating increased risk via telephone. Non-inferiority was supported on all psychological outcomes except positive impact at 12 months among ε4-negative participants. However, non-inferiority of telephone disclosure was not demonstrated on some 12 month outcomes among ε4-positive participants. Possible explanations for this include the reduced ability of providers to read non-verbal cues since genetic counselors are trained to use and respond to body language to encourage patients to engage with information and to reduce discomfort.1 Telephone conversations are often more succinct than those that are face-to-face,38 and participants or genetic counselors in our study may have felt pressure to be parsimonious about what they addressed on the phone. Our telephone disclosure protocol did not allow participants to view reports while genetic counselors were discussing results, and made it difficult for participants to have a support person present during disclosure. Whatever the reasons, genetic service providers may need to be selective about when to disclose potentially distressing genetic risk information via telephone. Pre-disclosure anxiety and depression scores are the strongest predictors of post-disclosure outcomes. Therefore, telephone disclosure of ε4-positive results to individuals with significant anxiety or depression may not be optimal.18 One strategy that many clinics have already implemented is to have an in-person consultation following telephone disclosure when patients have an increased genetic risk.10,11
Our study also evaluated recall of genetic test results one year after disclosure. Typically, studies have examined recall immediately after or within 1–2 months of disclosure. Even after reiterating information 6 weeks and 6 months after disclosure, we observed a steady decrease in recall over time. Of note, this trial had higher percentages of correct recall, even at six weeks, than the first REVEAL Study trial,21 perhaps due to the study population (e.g., more self-referred participants, individuals without AD-affected FDRs) or due to modifications in disclosure protocols. Data from this trial also reinforced findings from prior trials, showing that individuals who did not recall their lifetime and remaining risks correctly tended to underestimate their risk estimates, and that ε4-positive individuals were more likely to correctly recall their genotype than ε4-negative individuals.21 It is likely that individuals who were APOE ε4-positive saw a greater need to remember this information given their increased risk status.
Limitations
Study participants had higher education and numeracy than the general population. Measures were self-administered, and we could not ascertain whether participants reviewed written summaries during recall assessments. For questions about lifetime and remaining AD risk estimates, participants were not given a “don’t remember” option, and participants may have been categorized as correctly recalling information after guessing. Results may not generalize to situations in which results might be disclosed by healthcare providers without genetics expertise. Post-hoc calculations based on the differences that we observed suggested that we only had approximately 17% power to confirm noninferiority among ε4-positive participants on the IES, where differences were closest to noninferiority margins; and that we would need approximately 106 ε4-positive participants in each arm to achieve 80% power. Our sample size also limited our ability to examine interactions between telephone disclosure and factors such as education and race.9 Our study did not assess potential benefits such as increasing access to testing, decreasing costs to patients, or opening up clinic slots.5,9 We also did not assess the impact of telephone disclosure on outcomes such as risk perceptions, health behaviors and advance planning, although we have reported on the impact of genetic risk disclosure for AD on these outcomes previously.19,39–41 Such analyses may be important given differences we observed between randomization arms in the likelihood of discussing prevention during disclosure sessions.
Conclusion
Important challenges still remain to telephone disclosure of genetic test results, including reimbursement policies that encourage in-person visits, but payers are increasingly willing to pay for telephone consultations,42 and companies are now offering telephone genetic counseling. In addition, efforts are already underway to improve the timely disclosure of test results using patient portals associated with electronic medical record systems, mailing negative results, web-based disclosure, and video conferencing. AD risk disclosure is likely to grow in importance as prediction algorithms improve by incorporating polygenic, clinical, and environmental risk factors.43 Our results regarding telephone disclosure are encouraging given the need for efficient and effective approaches for conveying risk information and test results for common, complex diseases.
Supplementary Material
Acknowledgments
Work was supported by NIH grants HG002213, HG006500, HG009173, HD077671, HG009173, AG013846, RR000533, RR010284, and TR001102.
Footnotes
Conflict of interest notification page
Dr. Green is compensated for speaking or advisory services from AIA, GenePeeks, Helix, Illumina, Prudential and Veritas, and is co-founder and advisor to Genome Medical, Inc. The other authors have no conflicts of interest to disclose.
References
- 1.Uhlmann WR, Schuette JL, Yashar BM. A Guide to Genetic Counseling. 2. Hoboken: Wiley-Blackwell; 2009. [Google Scholar]
- 2.Taylor MRG, Edwards JG, Ku L. Lost in transition: challenges in the expanding field of adult genetics. Am J Med Genet C Semin Med Genet. 2006;142C(4):294–303. doi: 10.1002/ajmg.c.30105. [DOI] [PubMed] [Google Scholar]
- 3.Carroll J. Genetic testing: counselors desperately needed. Biotechnol Healthc. 2009;6(2):14–22. [PMC free article] [PubMed] [Google Scholar]
- 4.Koil CE, Everett JN, Hoechstetter L, Ricer RE, Huelsman KM. Differences in physician referral practices and attitudes regarding hereditary breast cancer by clinical practice location. Genet Med. 2003;5(5):364–369. doi: 10.1097/01.gim.0000086477.00766.c9. [DOI] [PubMed] [Google Scholar]
- 5.Bradbury AR, Patrick-Miller L, Fetzer D, et al. Genetic counselor opinions of, experiences with telephone communication of BRCA1/2 test results. Clin Genet. 2011;79(2):125–131. doi: 10.1111/j.1399-0004.2010.01540.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wham D, Vu T, Chan-Smutko G, Kobelka C, Urbauer D, Heald B. Assessment of clinical practices among cancer genetic counselors. Fam Cancer. 2010;9(3):459–468. doi: 10.1007/s10689-010-9326-9. [DOI] [PubMed] [Google Scholar]
- 7.Baumanis L, Evans J, Callanan N, Susswein L. Telephoned BRCA1/2 genetic test results: prevalence, practice, and patient satisfaction. J Genet Couns. 2009;18(5):447–463. doi: 10.1007/s10897-009-9238-8. [DOI] [PubMed] [Google Scholar]
- 8.O'Shea R, Meany M, Carroll C, et al. Predictive genetic testing and alternatives to face to face results disclosure: a retrospective review of patients preference for alternative modes of BRCA 1 and 2 results disclosure in the Republic of Ireland. J Genet Couns. 2016;25(3):422–431. doi: 10.1007/s10897-015-9887-8. [DOI] [PubMed] [Google Scholar]
- 9.Patrick-Miller L, Egleston BL, Daly M, et al. Implementation and outcomes of telephone disclosure of clinical BRCA1/2 test results. Patient Educ Couns. 2013;93(3):413–419. doi: 10.1016/j.pec.2013.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Klemp JR, O’Dea A, Chamberlain C, Fabian CJ. Patient satisfaction of BRCA1/2 genetic testing by women at high risk for breast cancer participating in a prevention trial. Fam Cancer. 2005;4(4):279–284. doi: 10.1007/s10689-005-1474-y. [DOI] [PubMed] [Google Scholar]
- 11.Doughty Rice C, Ruschman J, Martin L, Manders J, Miller E. Retrospective comparison of patient outcomes after in-person and telephone results disclosure counseling for BRCA1/2 genetic testing. Fam Cancer. 2010;9(2):203–212. doi: 10.1007/s10689-009-9303-3. [DOI] [PubMed] [Google Scholar]
- 12.Jenkins J, Calzone K, Dimond E, et al. Randomized comparison of phone versus in-person BRCA1/2 predisposition genetic test result disclosure counseling. Genet Med. 2007;9(8):487–495. doi: 10.1097/gim.0b013e31812e6220. [DOI] [PubMed] [Google Scholar]
- 13.Schwartz MD, Valdimarsdottir HB, Peshkin BN, et al. Randomized noninferiority trial of telephone versus in-person genetic counseling for hereditary breast and ovarian cancer. J Clin Oncol. 2014;32(7):618–626. doi: 10.1200/JCO.2013.51.3226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kinney AY, Butler KM, Schwartz MD, et al. Expanding access to BRCA1/2 genetic counseling with telephone delivery: a cluster randomized trial. J Natl Cancer Inst. 2014;106(12) doi: 10.1093/jnci/dju328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kinney AY, Steffen LE, Brumbach BH, et al. Randomized noninferiority trial of telephone delivery of BRCA1/2 genetic counseling compared with in-person counselling: 1-year follow-up. J Clin Oncol. 2016;34(24):2914–2924. doi: 10.1200/JCO.2015.65.9557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Farrer LA, Cupples LA, Haines JL, et al. Effects of age, sex and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease: a meta-analysis. JAMA. 1997;278:1349–1356. [PubMed] [Google Scholar]
- 17.Green RC, Christensen KD, Cupples LA, et al. A randomized noninferiority trial of condensed protocols for genetic risk disclosure of Alzheimer's disease. Alzheimers Dement. 2015;11(10):1222–1230. doi: 10.1016/j.jalz.2014.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Green RC, Roberts JS, Cupples LA, et al. Disclosure of APOE genotype for risk of Alzheimer's disease. N Engl J Med. 2009;361(3):245–254. doi: 10.1056/NEJMoa0809578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Christensen KD, Roberts JS, Whitehouse PJ, et al. Disclosing pleiotropic effects during genetic risk assessment for Alzheimer disease. A randomized trial. Ann Intern Med. 2016;164(3):155–163. doi: 10.7326/M15-0187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Besser AG, Sanderson SC, Roberts JS, et al. Factors affecting recall of different types of personal genetic information about Alzheimer's disease risk: the REVEAL Study. Public Health Genomics. 2015;18(2):78–86. doi: 10.1159/000368888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eckert SL, Katzen H, Roberts JS, et al. Recall of disclosed apolipoprotein E genotype and lifetime risk estimate for Alzheimer's disease: the REVEAL Study. Genet Med. 2006;8(12):746–751. doi: 10.1097/01.gim.0000250197.44245.a3. [DOI] [PubMed] [Google Scholar]
- 22.Roberts JS, Chen CA, Uhlmann WR, Green RC. Effectiveness of a condensed protocol for disclosing APOE genotype and providing risk education for Alzheimer disease. Genet Med. 2012;14(8):742–748. doi: 10.1038/gim.2012.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Teng EL, Chui HC. The Modified Mini-Mental State (3MS) Examination. J Clin Psychiatry. 1987;48(8):314–318. [PubMed] [Google Scholar]
- 24.Christensen KD, Roberts JS, Royal CDM, et al. Incorporating ethnicity into genetic risk assessment for Alzheimer disease: the REVEAL Study experience. Genet Med. 2008;10(3):207–214. doi: 10.1097/GIM.0b013e318164e4cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cupples LA, Farrer LA, Sadovnick AD, Relkin N, Whitehouse P, Green RC. Estimating risk curves for first-degree relatives of patients with Alzheimer's disease: the REVEAL Study. Genet Med. 2004;6(4):192–196. doi: 10.1097/01.gim.0000132679.92238.58. [DOI] [PubMed] [Google Scholar]
- 26.Ormond KE, Haun J, Cook L, Duquette D, Ludowese C, Matthews AL. Recommendations for Telephone Counseling. J Genet Couns. 2000;9(1):63–71. doi: 10.1023/A:1009433224504. [DOI] [PubMed] [Google Scholar]
- 27.Beck AT, Epstein N, Brown G, Steer RA. An inventory for measuring clinical anxiety: psychometric properties. J Consult Clin Psychol. 1988;56(6):893–897. doi: 10.1037//0022-006x.56.6.893. [DOI] [PubMed] [Google Scholar]
- 28.Radloff LS. The CES-D scale: A self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 29.Santor DA, Zuroff DC, Ramsay JO, Cervantes P, Palacios J. Examining scale discriminability in the BDI and CES-D as a function of depressive severity. Psychol Assess. 1995;7:131–139. [Google Scholar]
- 30.Horowitz M, Wilner N, Alvarez W. Impact of Event Scale: a measure of subjective stress. Psychosom Med. 1979;41(3):209–218. doi: 10.1097/00006842-197905000-00004. [DOI] [PubMed] [Google Scholar]
- 31.Chung WW, Chen CA, Cupples LA, et al. A new scale measuring psychologic impact of genetic susceptibility testing for Alzheimer disease. Alzheimer Dis Assoc Disord. 2009;23(1):50–56. doi: 10.1097/wad.0b013e318188429e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lipkus IM, Samsa G, Rimer BK. General performance on a numeracy scale among highly educated samples. Med Decis Making. 2001;21(1):37–44. doi: 10.1177/0272989X0102100105. [DOI] [PubMed] [Google Scholar]
- 33.Fagerlin A, Zikmund-Fisher BJ, Ubel PA, Jankovic A, Derry H, Smith DM. Measuring numeracy without a math test: development of the Subjective Numeracy Scale (SNS) Med Decis Making. 2007;27(5):672–680. doi: 10.1177/0272989X07304449. [DOI] [PubMed] [Google Scholar]
- 34.Piaggio G, Elbourne DR, Altman DG, Pocock SJ, Evans SW. Reporting of noninferiority and equivalence randomized trials: an extension of the consort statement. JAMA. 2006;295(10):1152–1160. doi: 10.1001/jama.295.10.1152. [DOI] [PubMed] [Google Scholar]
- 35.Cooley K, Szczurko O, Perri D, et al. Naturopathic care for anxiety: a randomized controlled trial ISRCTN78958974. PLoS ONE. 2009;4(8):e6628. doi: 10.1371/journal.pone.0006628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pearson-Fuhrhop KM, Dunn EC, Mortero S, et al. Dopamine genetic risk score predicts depressive symptoms in healthy adults and adults with depression. PLoS ONE. 2014;9(5):e93772. doi: 10.1371/journal.pone.0093772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McEvoy PM, Nathan P. Effectiveness of cognitive behavior therapy for diagnostically heterogeneous groups: a benchmarking study. J Consult Clin Psychol. 2007;75(2):344–350. doi: 10.1037/0022-006X.75.2.344. [DOI] [PubMed] [Google Scholar]
- 38.Locatis C, Williamson D, Gould-Kabler C, et al. Comparing in-person, video, and telephonic medical interpretation. J Gen Intern Med. 2010;25(4):345–350. doi: 10.1007/s11606-009-1236-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Christensen KD, Roberts JS, Zikmund-Fisher BJ, et al. Associations between self-referral and health behavior responses to genetic risk information. Genome Med. 2015;7(1):10. doi: 10.1186/s13073-014-0124-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vernarelli JA, Roberts JS, Hiraki S, Chen CA, Cupples LA, Green RC. Effect of Alzheimer disease genetic risk disclosure on dietary supplement use. Am J Clin Nutr. 2010;91(5):1402–1407. doi: 10.3945/ajcn.2009.28981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Roberts JS, Christensen KD, Green RC. Using Alzheimer's disease as a model for genetic risk disclosure: implications for personal genomics. Clin Genet. 2011;80(5):407–414. doi: 10.1111/j.1399-0004.2011.01739.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Robeznieks A. 'The right direction'. Mod Healthc. 2013;43(28):6–7. [PubMed] [Google Scholar]
- 43.Chatterjee N, Shi J, Garcia-Closas M. Developing and evaluating polygenic risk prediction models for stratified disease prevention. Nat Rev Genet. 2016;17(7):392–406. doi: 10.1038/nrg.2016.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


