Abstract
Background
Corticosteroids increase the expression of β2-adrenoceptors (β2-ARs) and protect them against down-regulation. Conversely, β2-AR agonists improve the anti-inflammatory action of corticosteroids. Nevertheless, it is still uncertain whether adding a long-acting β2-AR agonist (LABA) to an inhaled corticosteroid (ICS) results in an additive effect, or there is true synergy.
Therefore, the aim of this study was to pharmacologically characterize the interaction between the ICS beclomethasone diproprionate (BDP) and the LABA formoterol fumarate (FF) in a validated human ex vivo model of bronchial asthma.
Methods
Human medium and small airways were stimulated by histamine and treated with different concentrations of BDP and FF, administered alone and in combination at concentration-ratio reproducing ex vivo that of the currently available fixed-dose combination (FDC; BDP/FF 100:6 combination-ratio). Experiments were performed in non-sensitized (NS) and passively sensitized (PS) airways. The pharmacological interaction was assessed by using Bliss Independence and Unified Theory equations.
Results
BDP/FF synergistically increased the overall bronchorelaxation in NS and PS airways (+ 15.15% ± 4.02%; P < 0.05 vs. additive effect). At low-to-medium concentrations the synergistic interaction was greater in PS than in NS bronchioles (+ 16.68% ± 3.02% and + 7.27% ± 3.05%, respectively). In PS small airways a very strong synergistic interaction (Combination Index: 0.08; + 20.04% ± 2.18% vs. additive effect) was detected for the total concentrations of BDP/FF combination corresponding to 10.6 ng/ml.
Conclusion
BDP/FF combination synergistically relaxed human bronchi; the extent of such an interaction was very strong at low-to-medium concentrations in PS small airways.
Trial registration
Not applicable.
Electronic supplementary material
The online version of this article (10.1186/s12931-018-0770-7) contains supplementary material, which is available to authorized users.
Keywords: ICS/LABA FDC, Asthma, Interaction, Medium bronchi, Small airways
Background
Combining an inhaled corticosteroid (ICS) with a long-acting β2-adrenoceptor (β2-AR) agonist (LABA) is the cornerstone for the treatment of adult patients with asthma symptoms when a medium dose of ICS alone fails to achieve control of asthma [1].The scientific rationale for inhaled combination therapy with β2-AR agonists and corticosteroids has been debated for a long time [2]. Already in the early 2000s, it was widely recognized that the addition of a LABA to an ICS provides the optimal control of asthma in most patients, and ICS/LABA fixed-dose combinations (FDCs) represent effective controllers in patients with persistent asthma [3]. There is clear evidence that ICS/LABA FDC is superior to either drug given as monotherapy in the clinical management of moderate to severe asthma [1, 4].
Previous works elucidated some mechanisms behind the additive ant-inflammatory effect of adding a LABA to a corticosteroid in the treatment of asthma [5–9]. Nevertheless, to date there is still a large gap in the knowledge on how these two agents delivered in combination lead to superior clinical efficacy. In this regard, recent advances in multiscale modelling by using quasi-3D model may provide the opportunity of investigating how ICSs and LABAs interact each other with respect to their absorption, transport and retention into the lung at the level of lining liquid, epithelium, interstitium, airway smooth muscle (ASM), immune cells, and endothelium [10].
Overall, β2-AR agonists reduce the contractile tone of ASM, prevent plasma exudation, and inhibit the release of mediators from inflammatory cells and activation of sensory nerves. Conversely, corticosteroids reduce chronic inflammation and bronchial hyperresponsiveness (BHR) [3]. Taken together, these effects allow achieving an adequate asthma control. However, the intimate interaction between the activation of membrane β2-AR and intracellular glucocorticoid receptor (GR) is complex and not fully understood. Unquestionably, corticosteroids enhance the expression of β2-AR and protect these receptors against down-regulation in response to chronic activation at the level of ASM cells, whereas β2-AR agonists may increase the anti-inflammatory effects of corticosteroids at the level of inflammatory cells [11].
Although each class of drug enhances beneficial actions induced by the other class, the doubts raised by Barnes and Giembycz [3, 4] more than a decade ago on whether adding a LABA to an ICS results in an additive effect, or there is true synergy, are still current. In fact, little information is available on the real pharmacological characterization of ICS/LABA combination [4].
Indeed, the concept of synergy is appealing and extensively used, as confirmed by the last update of the understanding how LABAs enhance the clinical efficacy of ICS in asthma [12]. The increasing recognition that asthma and chronic obstructive pulmonary disease (COPD) are heterogeneous disorders has lead the attention to the needs of a group of patients with clinical features of both asthma and COPD, the so called asthma–COPD overlap syndrome (ACOS) [13]. Nevertheless, to date it is widely recognized that it is premature to recommend the designation of ACOS as a disease entity in both primary and specialist care [14]. In fact, more research is needed to adequately characterize patients and to obtain a validated definition of ACOS that would be based on markers that best predict treatment response in individual patients [15]. Thus, in agreement with the current international recommendations for the diagnosis and treatment of asthma and COPD [1, 16], ICS/LABA FDCs remain the cornerstone therapy for most asthmatic patients rather then COPD patients.
Nevertheless, to date only two studies [5, 17] have assessed the interaction between a β2-AR agonist and a corticosteroid by applying correct pharmacological models. These original researches [5, 17] were carried out in murine models of allergic lung inflammation, and not in human airways. While the synergism between bronchodilator agents has been confirmed in clinical studies [18, 19] by using specific pharmacological modeling of drug interaction, to date no clinical trials aimed to assess the potential clinical synergy between ICSs and LABAs have been carried out by using these models, namely the Bliss Independence equation and the Unified Theory [20].
Therefore, the aim of this study was to pharmacologically characterize the impact of beclomethasone dipropionate (BDP) on the bronchorelaxant effect of formoterol fumarate (FF), administered at the concentration-ratio delivered by the currently available FDC in the marked, in human medium bronchi and small airways by using a validated ex vivo model of bronchial asthma.
Methods
Tissue collection and preparation
Regions of macroscopically normal lungs were taken from uninvolved areas resected from 16 patients undergoing lobectomy surgery for lung cancer, but without a history of chronic airway disease. Detailed demographic and metric characteristics of patients are reported in Table 1.
Table 1.
Demographic characteristics of human subjects and normal ranges in agreement with GOLD and GINA [1, 16]
| Characteristics | Value | Normal range |
|---|---|---|
| Gender (male/female) | 8/8 | / |
| Age (years) | 50.0 ± 3.0 | / |
| Height (cm) | 164.8 ± 2.0 | / |
| Weight (Kg) | 68.3 ± 3.0 | / |
| Smoking status: | ||
| current | 4 | / |
| former | 5 | / |
| never | 7 | / |
| IgE (IU/ml) | 55.8 ± 5.7 | < 100 |
| Pack years | 24.4 ± 5.6 | / |
| FEV1 (L) | 2.71 ± 0.28 | / |
| FEV1 (% predicted) | 93.1 ± 4.1 | > 80 |
| FEV1 reversibility (%) | 4.8 ± 1.3 | < 12% |
| FVC (L) | 3.34 ± 0.36 | / |
| FEV1/FVC | 0.81 ± 0.01 | > 0.7 |
FEV1 forced expiratory volume in 1 s, FVC forced vital capacity, GINA Global Initiative for Asthma, GOLD Global Initiative for Chronic Obstructive Lung Disease, IgE immunoglobulin E, IU international units
Tissues were placed in Krebs-Henseleit buffer solution (KH) as previously described [21–23] and transported to the Laboratory of Respiratory Clinical Pharmacology at the University of Rome Tor Vergata (Italy) from a nearby hospital. None of the patients had been chronically treated with inhaled bronchodilators or glucocorticosteroids. Serum immunoglobulin E (IgE) levels determined on the day of surgery were in the normal range (< 100 IU/ml) [24]. Preoperative lung function parameters were generally normal and there were no signs of respiratory infections.
In the laboratory, the airways were cut into rings (sub-segmental bronchi: thickness 1–2 mm, diameter 4–6 mm) and transferred into a 10-ml High Tech 8 Channels Manual Compact Organ Bath system (Panlab Harvard Apparatus, Spain) containing KH buffer solution (37 °C) and aerated with O2/CO2 (95:5%). Tissues were allowed to equilibrate and the KH buffer solution was constantly changed.
Airways, which were studied in videomorphometry, were cut into precision cut lung slices (PCLS) (bronchioles: thickness < 500 μm, diameter < 1 mm) by a Motorised Advance Vibroslice equipped with ceramic blades (Campden Instruments, UK). Slices were processed without the complications related to the use of confounding agarose gel to inflate the lung or complex parenchymal sections that have numerous contracting elements [25–27]. PCLSs were then mounted into a Visual Imaging and Patching Chamber connected to a Proportional Integral Derivative Temperature Controller with dual thermistor feedback CI7800 (Campden Instruments, UK), containing KH buffer solution (37 °C) and continuously aerated with O2/CO2 (95:5%).
Passive sensitization
Isolated airways were rotated overnight at room temperature in tubes containing KH buffer solution in the presence of 10% vol− 1 sensitizing serum (passively sensitized bronchi) or 10% vol− 1 non-sensitizing serum collected from non-atopic donors (non-sensitized bronchi). Sera were prepared by centrifugation from the whole blood of patients suffering from atopic asthma (total IgE 1000 U ml− 1 specific against common aeroallergens) during exacerbation and non-atopic subjects [28, 29], providing signed consent for serum donation. Sera were frozen at − 80 °C in 250 μl aliquots until required. The next morning bronchial tissues were transferred into the isolated organ bath system or videomorphometry chamber containing KH buffer solution (37 °C) and continuously gassed with O2/CO2 (95:5%).
The passive sensitization is a model that closely mimics important functional characteristics of non-specific bronchial hyperresponsiveness (BHR) in asthmatic patients, as previously reported [28–32].
Preparation of drugs
The following drugs were used: acetylcholine, beclomethasone dipropionate (BDP), formoterol fumarate (FF), histamine, papaverine. All compounds were obtained by Sigma-Aldrich (Milan, Italy). All products were dissolved in adequate diluents such as distilled water, ethanol and dimethyl sulfoxide (DMSO). The maximum amount of ethanol and DMSO did not influence isolated tissue response [33, 34]. Compounds were stored in small aliquots at − 80 °C until their use.
Contraction measurement
Isolated bronchi
Bronchial rings were connected to isometric force transducers Fort25 (WPI, UK). PowerLab 8/36 and Octal Bridge Amp system (ADInstruments, UK), recorded and analyzed with the LabChart 7 interface software (ADInstruments, UK). Tissues were mounted on hooks, and attached with thread to a stationary rod and the other tied with thread to an isometric force displacement transducer. Airways were allowed to equilibrate by flushing with fresh KH buffer solution. Passive tension was determined by gentle stretching of tissue (0.5–1.0 g) during equilibration. The isometric change in tension was measured by the transducer and the tissue vitality assessed by transmural stimulation (also called electrical field stimulation, EFS) at 25 Hz; when the passive contractile tone reached the plateau, rings were washed three times with KH buffer solution and allowed to equilibrate for 45 min [35, 36].
Videomorphometry
Bronchial contraction was evaluated by a stereo microscope Zenith SZR-10 and a digital Optikam-B5 managed by OptikaView7 software (Optika Microscopes, Italy). Airways were allowed to equilibrate by flushing with fresh KH buffer solution until the luminal area was stable. The area into the lumen was measured by the Image Processing and Analysis software ImageJ (http://rsbweb.nih.gov/ij/index.html). The tissue vitality was assessed by acetylcholine (0.3 μM) in order to produce an area reduction of at the least 25% [25, 37–41]. After that, rings were washed three times with KH buffer solution and allowed to equilibrate for 45 min.
Study design
Following equilibration of non-sensitized and passively sensitized tissues, bronchial rings/slices were sub-maximally contracted by histamine (70% of maximal contraction, EC70). After the plateau was reached, semi-logarithmic concentration-response curves (CRCs) to FF were constructed. Each CRC was obtained by the cumulative addition of the drug at intervals of 5–15 min to reach a stable level of relaxation before the next dose administration.
In order to investigate the pharmacological interaction between BDP and FF in the ex vivo model of asthma, non-sensitized and passively sensitized airways were also treated overnight with different concentrations of BDP. After that, the CRCs to FF were constructed on the sub-maximal contractile tone induced by histamine, as described above. BDP and FF were tested at a ratio of concentrations (100:6 weight/weight combination-ratio) reproducing that of the FDC currently approved for the treatment of adult asthmatic patients (BDP/FF 100:6 combination-ratio) [42]. Thus, the range of concentrations of BDP to be combined with FF were chosen in agreement with the concentrations of FF that elicited a detectable bronchorelaxant response.
In the control groups, cumulative concentrations of vehicle was administered and used as time control. At the end of the experiments, papaverine (100 μM) was added to the baths to determine the maximal relaxant response achievable for each isolated airway. These experiments were carried out in both isolated organ bath and PCLS systems, in order to evaluate the relaxant response associated with the ASM strength and related to the increasing of intra-luminal bronchial area [43, 44].
Analysis
Pharmacological analysis
The contractile relaxation of isolated bronchial rings/slices was expressed as a percentage of the maximal relaxation (Emax, strength/luminal area) induced by papaverine (100 μM) on the submaximal contractile plateau induced by histamine (70% maximal contractility). Appropriate curve-fitting to a sigmoidal models was used to calculate the effect, the maximal response, the concentration inducing 50% maximal effect and the dose inducing 70% maximal effect (E, Emax, EC50 and EC70 respectively). The equation used was: log (agonist) vs. response, Variable slope, expressed as Y=Bottom+(Top-Bottom)/{1 + 10^[(LogEC50-X)*HillSlope]}. For the statistical analysis of the potency, pEC50 values were used, where pEC50 = -LogEC50) [45]. If necessary, bell-shaped curves were constructed by fitting models of biological data using nonlinear regressions [45]. For every seven bronchial rings mounted in the isolated organ bath system, one was used as a time control [46].
Interaction analysis
The interaction between BDP and FF was tested by applying the Bliss Independence (BI) theory, one of the most commonly used models to study combined effects of substances in vivo, ex vivo and in vitro. This method provides results on the statistical significance of the difference between the expected additive response and the observed effect in CRCs of drug combinations [47–50]. The main assumption of the BI theory is that two or more agents act independently from one another. In particular, if fulfilling the criterion, the mode, and possibly also the site of action of the compounds in the mixture, always differ. The BI theory for two agents is expressed by the following equation “E(x,y) = Ex+Ey-(Ex*Ey)”, where E is the fractional effect, and x and y are the concentrations of compounds in a combination experiment [51]. If the combination effect is higher than the expected value from the above equations, the interaction is synergistic, while if this effect is lower, the interaction is antagonistic. Otherwise, the effect is additive and there is no interaction [51–57]. In this protocol, the BI equation was used to investigate the interaction between BDP and FF administered in combination.
In addition to BI method, also the Unified Theory analysis was carried out, as proposed by Chou and colleagues [49, 58], in order to adequately quantify the magnitude of synergism through the Combination Index outcome and to produce results that are easy to be interpreted through the isobologram representation of data. The Unified Theory is represented by the Median-Effect equation that includes four major biochemical and biophysical equations (Henderson-Hasselbalch, Michaelis-Menten, Hill, and Scatchard), leading to the Combination Index theorem and to the isobologram equation for multiple drug combinations. Thus, the Combination Index is effect-oriented and quantifies the synergism or antagonism, where values < 1, =1, and > 1 indicate synergism, additive effect and antagonism, respectively, whereas the isobologram is concentration-oriented and it is expressed as a graph with equipotency sum of the doses [57, 58].
Statistical analysis
Values are presented as mean ± SEM of n = 5 bronchi from different subjects. The statistical significance was assessed by the t-test and analysis of variance (ANOVA), where necessary. The level of statistical significance was defined as P < 0.05. All data analysis was performed using computer software GraphPad Prism 5 (La Jolla, CA, USA) and CompuSyn (Paramus, NJ. USA).
Results
Baseline characteristics of isolated airways
The baseline characteristics of non-sensitized and passively sensitized isolated airways used in this study are reported in Additional file 1.
Pharmacological characteristics of BDP and FF administered as monocomponents on the histaminergic contractility
FF completely relaxed both non-sensitized and passively sensitized medium bronchi submaximally pre-contracted by histamine, although the potency of FF was significantly (P < 0.001) greater in passively sensitized than in non-sensitized bronchi. FF partially relaxed non-sensitized PCLS and completely relaxed passively sensitized PCLS submaximally pre-contracted by histamine. The potency of FF was significantly (P < 0.05) greater in passively sensitized than in non-sensitized PCLS. Overall, FF was ≃1.25 logarithm more potent in medium bronchi than in small airways.
After overnight incubation, BDP induced a very weak and not significant (P > 0.05 vs. plateau induced by histamine) reduction of the histaminergic contractile tone of both medium bronchi and PCLS, in both non-sensitized and passively sensitized tissues.
Detailed pharmacological characteristics of BDP and FF are reported in Table 2 and specific results of each CRC are shown in Additional file 1: Fig. S1.
Table 2.
Pharmacological characteristics of BDP and FF administered as monocomponents on the histaminergic contractile tone of medium bronchi and small airways (PCLS) in non-sensitized and passively sensitized tissues
| BDP | FF | |||||||
|---|---|---|---|---|---|---|---|---|
| Medium bronchi | Bronchioles | Medium bronchi | Bronchioles | |||||
| Emax | pEC50 | Emax | pEC50 | Emax | pEC50 | Emax | pEC50 | |
| Non-sensitized | < 20% | NC | < 20% | NC | 97.00 ± 2.77% | 8.26 ± 0.07 | 72.03% ± 4.34# | 7.13 ± 0.11# |
| Passively sensitized | < 20% | NC | < 20% | NC | 94.96 ± 1.22% | 8.89 ± 0.04*** | 105.30%*** | 7.52 ± 0.09*# |
*P < 0.05 and ***P < 0.001 vs. non-sensitized airways of equal diameter, #P < 0.001 vs. medium bronchi undergoing the same treatment (statistical significance assessed by t-test). BDP Beclomethasone dipropionate, EC50 The concentration inducing 50% Emax, Emax maximal effect, FF Formoterol fumarate, NC Not calculable, PCLS Precision cut lung slices, pEC50 -LogEC50
Pharmacological interaction between BDP and FF in non-sensitized and passively sensitized medium isolated bronchi
The observed effect induced by increasing concentrations of BDP plus FF combined at 100:6 combination-ratio in both non-sensitized and passively sensitized bronchi pre-contracted by histamine was significantly (P < 0.01 determined by two-way ANOVA) greater than the expected additive effect resulting from the BI model (Fig. 1a and b). The maximal synergistic interaction was detected for the drug mixture corresponding to BDP/FF 10/0.6 ng/ml in non-sensitized airways (percentage change: + 28.73% ± 7.25% vs. additive effect; P < 0.001), whereas in passively sensitized tissues the extent of synergistic interaction remained constant for BDP/FF combinations administered from 1/0.06 ng/ml to 100/6 ng/ml (overall percentage change: + 12.74% ± 4.62% vs. additive effect; P < 0.01) (Fig. 1c and d).
Fig. 1.
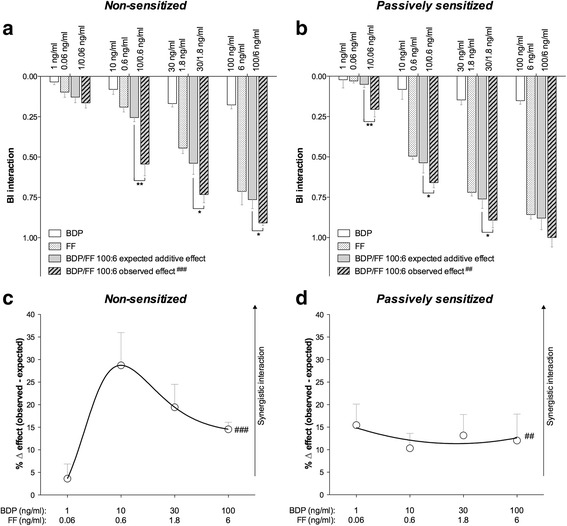
Bliss Independence analysis (a and b) and delta effect between the observed and expected relaxant response (c and d) induced by BDP plus FF administered at 100:6 combination-ratio in non-sensitized (a and c) and passively sensitized (b and d) human medium bronchi submaximally contracted by histamine. *P < 0.05 and **P < 0.01 vs. the expected additive relaxant effect as predicted by BI equation (statistical significance assessed by Student’s t test); ##P < 0.01 and ###P < 0.001 vs. the expected additive relaxant effect as predicted by BI equation (statistical significance assessed by two-way ANOVA). Points represent the mean ± SEM of n = 5 sub-segmental bronchi from different subjects. BI: Bliss independence; BDP: beclomethasone dipropionate; FF: formoterol fumarate
Synergism was confirmed by the Unified Theory analysis. The logarithmic Combination Index plot showed that the effect of BDP/FF 100:6 combination-ratio was substantially in the area of synergistic interaction (Log10 of Combination Index < 0) when the drugs mixtures were tested in both non-sensitized and passively sensitized bronchi (Fig. 2a and b). The isobologram analysis reported that BDP/FF 100:6 combination-ratio was a balanced combination inducing synergistic effect from low to high concentrations, in both non-sensitized and passively sensitized bronchi (Fig. 2c and d).
Fig. 2.
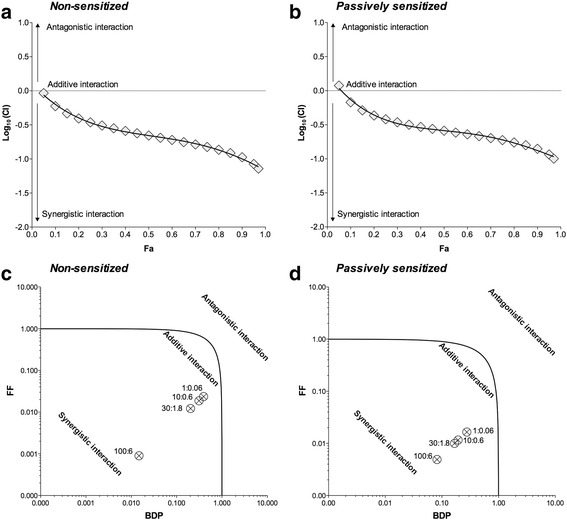
Graphical representation of Unified Theory analysis for BDP/FF combination administered at 100:6 concentration-ratio reporting the logarithmic Combination Index plot (a and b) and the normalized isobologram (c and d) in non-sensitized (a and c) and passively sensitized (b and d) human medium bronchi submaximally contracted by histamine. Points represent the mean ± SEM of n = 5 sub-segmental bronchi from different subjects; the labels of points report the weight/weight ratio (ng/ng) between BDP and FF. BDP: beclomethasone dipropionate; CI: Combination Index; Fa: fraction affected; FF: formoterol fumarate. Fu: fraction unaffected
Pharmacological interaction between BDP and FF in non-sensitized and passively sensitized small airways
Combining increasing concentrations of BDP plus FF elicited a significant (P < 0.001 determined by two-way ANOVA) synergistic relaxant effect of both non-sensitized and passively sensitized bronchioles pre-contracted by histamine (Fig. 3a and b). The maximal synergistic interaction was detected for BDP/FF combined at 1/0.06 μg/ml in non-sensitized small airways (+ 20.41% ± 4.10% vs. additive effect; P < 0.001), and for BDP/FF combined at 10/0.6 ng/ml in passively sensitized bronchioles (+ 20.04% ± 2.18% vs. additive effect; P < 0.01) (Fig. 3c and d).
Fig. 3.
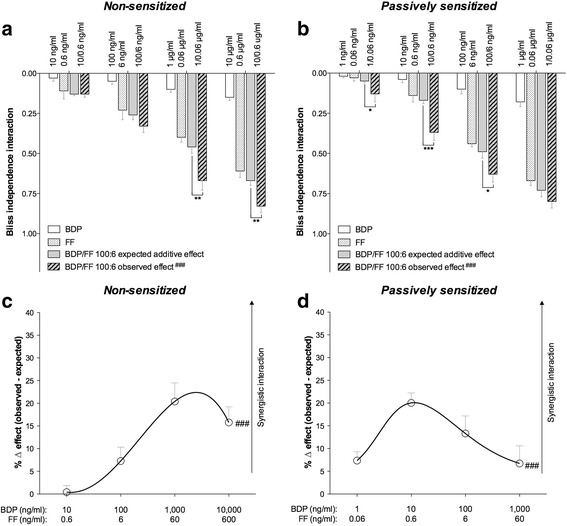
Bliss Independence analysis (a and b) and delta effect between the observed and expected relaxant response (c and d) induced by BDP plus FF administered at 100:6 combination-ratio in non-sensitized (a and c) and passively sensitized (b and d) human small airways (PCLS) submaximally contracted by histamine. *P < 0.05, **P < 0.01 and ***P < 0.001 vs. the expected additive relaxant effect as predicted by BI equation (statistical significance assessed by Student’s t test); ###P < 0.001 vs. the expected additive relaxant effect as predicted by BI equation (statistical significance assessed by two-way ANOVA). Points represent the mean ± SEM of n = 5 bronchioles from different subjects. BI: Bliss independence; BDP: beclomethasone dipropionate; FF: formoterol fumarate; PCLS: precision cut lung slices
The Unified Theory approach confirmed the synergy between BDP and FF at the level of small airways. The logarithmic Combination Index plot indicated that the synergy of BDP/FF 100:6 combination-ratio was substantially greater at higher concentrations than at lower concentrations in non-sensitized PCLS (Fig. 4a). On the other hand, in passively sensitized small airways we detected a reverse trend, characterized by a greater synergistic interaction at lower concentrations compared with that at higher concentrations (Fig. 4b). Similarly, the isobologram analysis reported that the extent of the synergistic interaction between BDP and FF was directly related with the concentrations of drugs mixture in non-sensitized bronchioles (Fig. 4c). On the other hand, the extent of interaction was inversely related to the concentrations of BDP/FF combinations in passively sensitized small airways (Fig. 4d).
Fig. 4.
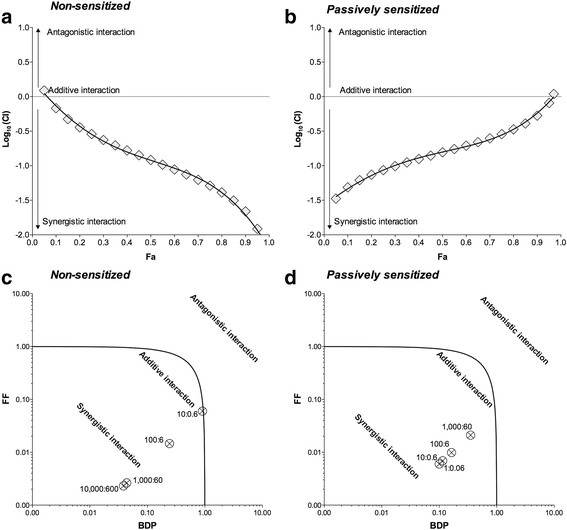
Graphical representation of Unified Theory analysis for BDP/FF combination administered at 100:6 concentration-ratio reporting the logarithmic Combination Index plot (a and b) and the normalized isobologram (c and d) in non-sensitized (a and c) and passively sensitized (b and d) human small airways (PCLS) submaximally contracted by histamine. Points represent the mean ± SEM of n = 5 bronchioles from different subjects; the labels of points report the weight/weight ratio (ng/ng) between BDP and FF. BDP: beclomethasone dipropionate; CI: Combination Index; Fa: fraction affected; FF: formoterol fumarate. Fu: fraction unaffected; PCLS: precision cut lung slices
Magnitude of synergistic interaction in medium and small airways
Overall, BDP/FF combination elicited synergistic relaxant response of ASM submaximally contracted by histamine.
In medium bronchi synergism (overall Combination Index: 0.425) was detected at low concentrations inducing ≤25% Emax, and strong synergism (overall Combination Index: 0.178) from concentrations eliciting ≥50% Emax.
In non-sensitized small airways the extent of synergism was strong (overall Combination Index: 0.205) at low concentrations inducing from 25% to 50% Emax, and very strong (overall Combination Index: 0.035) at higher concentrations.
Very strong synergistic interaction (overall Combination Index: 0.070) was detected in passively sensitized small airways yet at very low concentrations inducing 15–25% Emax, and the extent of interaction remained strong (overall Combination Index: 0.215) up to concentrations eliciting submaximal bronchorelaxant effect (75% Emax).
Table 3 summarizes the magnitude of the pharmacological interaction between BDP and FF administered at 100:6 combination-ratio in non-sensitized and passively sensitized human medium and small airways.
Table 3.
Magnitude of the pharmacological interaction between BDP and FF administered at 100:6 combination-ratio in non-sensitized and passively sensitized human medium and small airways (PCLS)
| Medium bronchi | Small airways | |||||||
|---|---|---|---|---|---|---|---|---|
| Non-sensitized | Passively sensitized | Non-sensitized | Passively sensitized | |||||
| Relaxant effect (% Emax) | Combination Index | Interaction magnitude | Combination Index | Interaction magnitude | Combination Index | Interaction magnitude | Combination Index | Interaction magnitude |
| 15 | 0.47 | +++ | 0.51 | +++ | 0.47 | +++ | 0.06 | +++++ |
| 25 | 0.34 | +++ | 0.38 | +++ | 0.29 | ++++ | 0.08 | +++++ |
| 50 | 0.22 | ++++ | 0.26 | ++++ | 0.12 | ++++ | 0.15 | ++++ |
| 75 | 0.15 | ++++ | 0.20 | ++++ | 0.05 | +++++ | 0.28 | ++++ |
| 90 | 0.10 | ++++ | 0.14 | ++++ | 0.02 | +++++ | 0.52 | +++ |
The smaller the Combination Index, the greater the synergistic interaction. +++: synergism; ++++: strong synergism; +++++: very strong synergism. BDP Beclomethasone dipropionate, FF formoterol fumarate, PCLS Precision cut lung slices
Discussion
The results of this study confirmed that FF is a potent and effective bronchorelaxant agent at the level of medium bronchi, as previously reported by studies carried out in 1990s [59, 60]. Although in a previous study we documented that FF is able to abolish the contraction of small airways induced by acetylcholine [47], in the present research FF did not completely relax bronchioles submaximally pre-contracted by histamine. However, we have provided for the first time the evidence that FF is ≃0.5 logarithm more potent in passively sensitized than in non-sensitized airways and completely abolishes the histaminergic tone of passively sensitized PCLS. These evidences indicate that FF has a beneficial bronchorelaxant impact especially in human hyperresponsive airways. On the contrary, the results indicated that the overnight incubation with BDP did not modulate the bronchial contractility induced by histamine in both medium and small airways, even after passive sensitization procedure.
Conversely, combining BDP with FF at 100:6 concentration-ratio not only improved the effectiveness of the β2-AR agonist, but elicited a synergistic bronchorelaxant effect in both medium and small airways, either non-sensitized or passively sensitized.
The BI analysis showed that the in non-sensitized tissues the concentration of drugs mixture necessary to induce the maximal synergistic interaction in bronchioles was ≃2 logarithms higher than that necessary to elicit the greatest synergism in medium bronchi. The total concentration of BDP/FF combination required to induce submaximal relaxation of non-sensitized medium bronchi was in the order of magnitude of ng/ml, whereas in small airways the order of magnitude to elicit the same effect was in the range of μg/ml. On the other hand, in passively sensitized tissues low concentrations of BDP/FF combination, in the order of magnitude of ng/ml, were sufficient to submaximally relax both medium and small airways. These findings prove that passively sensitized small airways are more sensitive to the beneficial synergistic interaction induced by adding BDP to FF, compared to non-sensitized bronchioles.
The BI approach showed that the BDP/FF 100:6 combination-ratio induced statistically significant synergism, and permitted to quantify the overall extent of bronchodilation and compare the observed synergism with the expected additive effect. Nevertheless, the BI equation did not allow assessing what is the real magnitude of synergism occurring between BDP and FF. At concentrations of drugs mixture corresponding to EC50 or below it is not difficult to detect synergism, whereas a synergistic interaction may be more difficult to be detected at higher concentrations producing a submaximal effect. Therefore, after have performed the statistical analysis of the synergism between BDP and FF by using the BI model, we have carried out a further evaluation by applying the Unified Theory in order to quantify the magnitude of the interaction [57].
The analysis of the logarithmic Combination Index plots and isobolograms evidenced that in medium bronchi, either non-sensitized or passively sensitized, BDP/FF 100:6 combination-ratio elicited a certain level of bronchorelaxant synergism, even at high concentrations. In non-sensitized small airways low concentrations induced additive effect, and very high concentrations were necessary to elicit synergistic interaction. Surprisingly, in passively sensitized small airways, even very small total concentrations of BDP/FF combination, ranging between 1.06 ng/ml and 10.6 ng/ml, were effective in producing a marked synergistic relaxation of ASM. Certainly the increased acute bronchorelaxant effect of FF in isolated airways incubated overnight with BDP can be prevalently due to the genomic effect of the corticosteroid [61, 62], although investigating this matter was beyond the scope of our study.
The findings of this study are undoubtedly interesting, considering the potential translational implications. In fact, we have previously demonstrated that the relaxant effect of bronchodilator agents detected ex vivo in human medium isolated airways was related with their impact in vivo on the changes in forced expiratory volume in 1 s (FEV 1) [63]. Furthermore, the luminal area of small airways studied by using PCLS with an internal diameter < 2 mm is related with the flow in small airways that, in turn, seems to be associated with the forced expiratory flow between 25% and 75% of vital capacity (FEF 25–75) [64, 65]. Overall, the flow resistance in medium and small bronchi contributes to the total airway resistance in asthmatic patients, which may influence also the alveolar moiety as demonstrated by the measurement of nitric oxide at different flow rates [66–68].
Lower airways significantly contribute to the severity of chronic obstructive pulmonary disorders, such as asthma and COPD [69]. The dysfunction of bronchioles has been also demonstrated in specific asthma phenotypes, namely nocturnal asthma, exercise-induced asthma, and allergic asthma [70, 71]. Thus, delivering an adequate amount of inhaled ICS/LABA combination to distal airways represents a central target to treat asthmatic patients. However, although the new generation of inhaler devices emitting extrafine formulations have lead to an improved lung deposition of drugs mixture, and a more effective aerosol penetration into the lung periphery [70, 72], in asthmatic patients approximately two-thirds of extrafine formulation of BDP/FF FDC are deposited in the central lung region, and only one-third reaches the peripheral lung [73, 74]. Therefore, it is crucial that the amount of drugs mixture that reaches small airways is effective at concentrations lower than those detectable in larger airways, and that the extent of effectiveness remains sustained also at the low concentrations that are present in the airways immediately before the next dose is inhaled.
In this regard, the very strong synergistic interaction elicited by low concentrations of BDP/FF administered at 100:6 combination-ratio in hyperresponsive small airways may explain the superiority of BDP/FF FDC (400/24 μg daily) delivered via an extrafine formulation in improving asthma control, compared to the combination of the same drugs formulated as larger non-extrafine agents administered at equipotent doses [75]. Furthermore, considering lung functional parameters related with peripheral airway dysfunction, extrafine BDP/FF FDC treatment was superior to an equipotent dose of the non-extrafine fluticasone propionate/salmeterol combination in improving air trapping in moderate to severe asthmatic patients [76]. The beneficial impact of BDP/FF FDC (400/24 μg daily) on small airways in asthma was further confirmed by a pilot study that demonstrated an improvement in closing capacity after 12 weeks of treatment [71]. Intriguingly, in this study [71] the authors also evidenced that BDP/FF FDC was effective in decreasing the BHR of larger airways. This finding indirectly confirms the results of our study, with regard to the evidence that combining an ICS with a LABA can target both medium and small hyperresponsive airways, leading to bronchorelaxant synergistic interaction.
Although the data of the present study result from a widely validated human model of non-specific BHR typical of bronchial asthma [23, 36, 44, 77–80], this research remains an ex vivo study characterized by intrinsic limitations [27, 57]. The findings of ex vivo studies aimed to assess the pharmacological interaction between drugs characterized by different mechanisms of action [81] have been generally confirmed by a translational approach in clinical trials [18, 19], and vice versa [82, 83]. Nevertheless, we must highlight that, although the responses of human isolated ASM strictly resembles those elicited in vivo, results obtained from ex vivo studies need to be confirmed by clinical trials specifically designed to detect pharmacological interaction of drugs mixture [57, 82].
One of the major objectives of having synergistic drug combinations is to reduce the dose of the drugs used, thereby reducing the risk of adverse events, while optimizing the efficacy [57, 58]. In this respect, our data support the delivery of BDP/FF 100:6 combination-ratio via extrafine formulation to reduce the total dose of the monocomponents, improve the distribution in the lung, and optimize the effectiveness, compared to non-extrafine formulations. This approach permits to reduce the deposition of drugs mixture in the oropharynx, resulting in decreased systemic absorption through the gastrointestinal tract and obvious beneficial consequences on the safety profile [84].
A recent and extensive review of Newton and Giembycz [12] attempted to explain how LABAs enhance the clinical efficacy of ICS in asthma. The authors reported that combining an ICS with a LABA might produce profound synergy at the level of genes and proteins expression involved in ASM contractility and airway inflammation [12]. Unfortunately, the original studies [61, 85–88] cited in that review [12] to support the positive interaction between ICSs and LABAs provided arbitrary interpretation of synergy because no methods were used to adequately analyze the real drug interaction. Furthermore, the functional impact of combining a corticosteroid with a β2-ARs was indirectly assessed by using cytosolic surrogate of ASM contractility [86]. In any case, even assuming that an ICS/LABA combination can modulate the expression of genes and proteins in asthmatic ASM, it is not implicit that such an interaction may lead to functional synergy [62]. Therefore, at the best of our knowledge, the present study provides for the first time the evidence-based pharmacological characterization of the synergism between an ICS and a LABA, at least with regard to the beneficial impact against BHR in human airways.
Conclusions
This study indicates that BDP/FF administered at 100:6 combination-ratio induces synergistic bronchorelaxant effect in human medium bronchi and small airways, and that the magnitude of this synergy is greater in hyperresponsive airways than in non-sensitized tissues. Further research is needed to characterize the intimate mechanism/s leading to such an extensive interaction, and assess if combining an ICS with a LABA may lead also to synergistic anti-inflammatory effect in human airways.
Additional file
Baseline characteristics of bronchial tissue used in the study and CRCs to BDP and FF. (DOCX 431 kb)
Acknowledgements
We thank miss Beatrice Ludovica Ritondo (Department of Experimental Medicine and Surgery, University of Rome Tor Vergata, Rome, Italy) for her support in the laboratory activities.
Authorship contribution
LC, MGM, FF, MC and PR have made substantial contributions to conception and design, acquisition of data, and analysis and interpretation of data; were involved in drafting the manuscript or revising it critically for important intellectual content; gave final approval of the version to be published. Each author participated sufficiently in the work to take public responsibility for appropriateportions of the content; and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Funding
Chiesi Farmaceutici has provided unconditional support and funding in this research.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- ANOVA
analysis of variance
- ASM
airway smooth muscle
- BDP
beclomethasone dipropionate
- BHR
bronchial hyperresponsiveness
- BI
Bliss Independence
- CRC
concentration-response curves
- E
effect
- ECn
n% Emax
- ECn
n% Emax
- Emax
maximal effect
- FDC
fixed-dose combination
- FEV1
forced expiratory volume in 1 s
- FF
formoterol fumarate
- FVC
forced vital capacity
- GR
glucocorticoid receptor
- ICS
inhaled corticosteroid
- IgE
immunoglobulin E
- KH
Krebs-Henseleit
- LABA
long-acting β2-AR agonist
- PCLS
precision cut lung slices
- pEC50
potency, -LogEC50
- β2-AR
β2-adrenoceptor
Ethics approval and consent to participate
Ethical approval (RS 233.16, 2016; Independent Ethical Committee, Fondazione PTV Policlinico Tor Vergata) and informed consent were consistent with the 2009 National Committee of Bioethics, National Committee of Bio-safety, Biotechnology and Sciences (Italy) recommendations on the collection of biological samples for research purposes, the 2010 Italian ethical and legal recommendations concerning the biobank and the research biorepository (Istituto Nazionale dei Tumori – Independent Ethics Committee, 2010), and the Comitato Nazionale per la Biosicurezza, le Biotecnologie e le Scienze per la Vita (Raccolta di campioni biologici a fini di ricerca, consenso informato, 2009; available at: http://www.governo.it/bioetica/gruppo_misto/Consenso_Informato_allegato_Petrini_2009.pdf).
Consent for publication
Not applicable.
Competing interests
LC has participated as advisor in scientific meetings under the sponsorship of Boehringer Ingelheim and Novartis, received non-financial support by AstraZeneca, received a research grant partially funded by Chiesi Farmaceutici, Boehringer Ingelheim, Novartis, and Almirall, and is or has been a consultant to ABC Farmaceutici, Edmond Pharma, Zambon, Verona Pharma, and Ockham Biotech. His department was funded by Almirall, Boehringer Ingelheim, Novartis, Zambon and Chiesi Farmaceutici.
MGM has participated as a lecturer and advisor in scientific meetings and courses under the sponsorship of Almirall, AstraZeneca, Boehringer Ingelheim, Chiesi Farmaceutici, GlaxoSmithKline and Novartis, and has been a consultant to Chiesi Farmaceutici.
FF has no conflict of interest to declare.
MC has participated as a lecturer and advisor in scientific meetings and courses under the sponsorship of Almirall, AstraZeneca, Biofutura, Boehringer Ingelheim, Chiesi Farmaceutici, GlaxoSmithKline, Menarini Group, Lallemand, Mundipharma, Novartis, Pfizer, Verona Pharma, and Zambon, and has been a consultant to ABC Farmaceutici, Edmond Pharma, Chiesi Farmaceutici, Lallemand, Novartis, Verona Pharma, and Zambon. His department was funded by Almirall, Boehringer Ingelheim, Novartis, and Zambon.
PR participated as a lecturer and advisor in scientific meetings and courses under the sponsorship of Almirall, AstraZeneca, Biofutura, Boehringer Ingelheim, Chiesi Farmaceutici, GlaxoSmithKline, Menarini Group, Mundipharma, and Novartis. Her department was funded by Almirall, Boehringer Ingelheim, Novartis, Zambon and Chiesi Farmaceutici.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Footnotes
Electronic supplementary material
The online version of this article (10.1186/s12931-018-0770-7) contains supplementary material, which is available to authorized users.
Contributor Information
Luigino Calzetta, Email: luigino.calzetta@uniroma2.it.
Maria Gabriella Matera, Email: mariagabriella.matera@unicampania.it.
Francesco Facciolo, Email: francesco.facciolo@ifo.gov.it.
Mario Cazzola, Email: mario.cazzola@uniroma2.it.
Paola Rogliani, Phone: +39 06 2090 4656, Email: paola.rogliani@uniroma2.it.
References
- 1.GINA: Global Initiative for Asthma (GINA). Global strategy for asthma management and prevention. 2017. Available at: http://www.ginasthma.org. Accessed 24 Nov 2017.
- 2.Barnes PJ. Scientific rationale for using a single inhaler for asthma control. Eur Respir J. 2007;29:587–595. doi: 10.1183/09031936.00080306. [DOI] [PubMed] [Google Scholar]
- 3.Barnes PJ. Scientific rationale for inhaled combination therapy with long-acting beta2-agonists and corticosteroids. Eur Respir J. 2002;19:182–191. doi: 10.1183/09031936.02.00283202. [DOI] [PubMed] [Google Scholar]
- 4.Giembycz MA, Kaur M, Leigh R, Newton R, Holy Grail A. Of asthma management: toward understanding how long-acting beta(2)-adrenoceptor agonists enhance the clinical efficacy of inhaled corticosteroids. Br J Pharmacol. 2008;153:1090–1104. doi: 10.1038/sj.bjp.0707627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wyss D, Bonneau O, Trifilieff A. Synergistic effect of formoterol and mometasone in a mouse model of allergic lung inflammation. Br J Pharmacol. 2007;152:83–90. doi: 10.1038/sj.bjp.0707381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Razzetti R, Bergamaschi M, Villetti G, Bolzoni P, Civelli M, Berti F, Rossoni G. Formoterol and beclomethasone dipropionate interact positively in antagonising bronchoconstriction and inflammation in the lung. Pharmacol Res. 2007;55:426–432. doi: 10.1016/j.phrs.2007.01.018. [DOI] [PubMed] [Google Scholar]
- 7.Profita M, Gagliardo R, Di Giorgi R, Pompeo F, Gjomarkaj M, Nicolini G, Bousquet J, Vignola AM. Biochemical interaction between effects of beclomethasone dipropionate and salbutamol or formoterol in sputum cells from mild to moderate asthmatics. Allergy. 2005;60:323–329. doi: 10.1111/j.1398-9995.2005.00702.x. [DOI] [PubMed] [Google Scholar]
- 8.Spoelstra FM, Postma DS, Hovenga H, Noordhoek JA, Kauffman HF. Additive anti-inflammatory effect of formoterol and budesonide on human lung fibroblasts. Thorax. 2002;57:237–241. doi: 10.1136/thorax.57.3.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pauwels R. Additive effects of inhaled formoterol and budesonide in reducing asthma exacerbations. Allergy. 1998;53:20–23. doi: 10.1111/j.1398-9995.1998.tb04889.x. [DOI] [PubMed] [Google Scholar]
- 10.Kannan RR, Singh N, Przekwas A. A Compartment-Quasi3D multiscale approach for drug absorption, transport, and retention in the human lungs. Int J Numer Method Biomed Eng. 2017; 10.1002/cnm.2955. [DOI] [PMC free article] [PubMed]
- 11.Pelaia G, Muzzio CC, Vatrella A, Maselli R, Magnoni MS, Rizzi A. Pharmacological basis and scientific rationale underlying the targeted use of inhaled corticosteroid/long-acting beta2-adrenergic agonist combinations in chronic obstructive pulmonary disease treatment. Expert Opin Pharmacother. 2015;16:2009–2021. doi: 10.1517/14656566.2015.1070826. [DOI] [PubMed] [Google Scholar]
- 12.Newton R, Giembycz MA. Understanding how long-acting beta2 -adrenoceptor agonists enhance the clinical efficacy of inhaled corticosteroids in asthma - an update. Br J Pharmacol. 2016;173:3405–3430. doi: 10.1111/bph.13628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bateman ED, Reddel HK, van Zyl-Smit RN, Agusti A. The asthma-COPD overlap syndrome: towards a revised taxonomy of chronic airways diseases? Lancet Respir Med. 2015;3:719–728. doi: 10.1016/S2213-2600(15)00254-4. [DOI] [PubMed] [Google Scholar]
- 14.Cazzola M, Rogliani P. Do we really need asthma-chronic obstructive pulmonary disease overlap syndrome? J Allergy Clin Immunol. 2016;138:977–983. doi: 10.1016/j.jaci.2016.04.028. [DOI] [PubMed] [Google Scholar]
- 15.Postma DS, Rabe KF. The asthma-COPD overlap syndrome. N Engl J Med. 2015;373:1241–1249. doi: 10.1056/NEJMra1411863. [DOI] [PubMed] [Google Scholar]
- 16.GOLD: Global Initiative for Chronic Obstructive Lung Disease. Global strategy for diagnosis, management, and prevention of COPD – 2018 Report (accessed March 26, 2018). 2018:Available at http://goldcopd.org/wp-content/uploads/2017/2011/GOLD-2018-v2016.2010-FINAL-revised-2020-Nov_WMS.pdf.
- 17.Chapman RW, Curran AK, House A, Richard J, Salisbury B, Hunter JC, Anthes JC, Phillips JE. Effect of mometasone furoate (MF)/formoterol fumarate (F) combination (MF/F) on late-phase responses in allergen-challenged Brown Norway rats. Pulm Pharmacol Ther. 2011;24:67–73. doi: 10.1016/j.pupt.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 18.Cazzola M, Calzetta L, Ora J, Puxeddu E, Rogliani P, Matera MG. Searching for the synergistic effect between aclidinium and formoterol: from bench to bedside. Respir Med. 2015;109:1305–1311. doi: 10.1016/j.rmed.2015.08.005. [DOI] [PubMed] [Google Scholar]
- 19.Cazzola M, Calzetta L, Segreti A, Facciolo F, Rogliani P, Matera MG. Translational study searching for synergy between Glycopyrronium and Indacaterol. COPD. 2015;12:175–181. doi: 10.3109/15412555.2014.922172. [DOI] [PubMed] [Google Scholar]
- 20.Calzetta L, Matera MG, Cazzola M. Pharmacological mechanisms leading to synergy in fixed-dose dual bronchodilator therapy. Curr Opin Pharmacol. 2018;40:39–45. doi: 10.1016/j.coph.2018.03.011. [DOI] [PubMed] [Google Scholar]
- 21.Cazzola M, Calzetta L, Page CP, Rinaldi B, Capuano A, Matera MG. Protein prenylation contributes to the effects of LPS on EFS-induced responses in human isolated bronchi. Am J Respir Cell Mol Biol. 2011;45:704–710. doi: 10.1165/rcmb.2010-0306OC. [DOI] [PubMed] [Google Scholar]
- 22.Calzetta L, Cazzola M, Page CP, Rogliani P, Facciolo F, Matera MG. Pharmacological characterization of the interaction between the dual phosphodiesterase (PDE) 3/4 inhibitor RPL554 and glycopyrronium on human isolated bronchi and small airways. Pulm Pharmacol Ther. 2015;32:15–23. doi: 10.1016/j.pupt.2015.03.007. [DOI] [PubMed] [Google Scholar]
- 23.Matera MG, Calzetta L, Peli A, Scagliarini A, Matera C, Cazzola M. Immune sensitization of equine bronchus: glutathione, IL-1beta expression and tissue responsiveness. Respir Res. 2005;6:104. doi: 10.1186/1465-9921-6-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cosio BG, Soriano JB, Lopez-Campos JL, Calle-Rubio M, Soler-Cataluna JJ, de-Torres JP, Marin JM, Martinez-Gonzalez C, de Lucas P, Mir I, et al. Defining the asthma-COPD overlap syndrome in a COPD cohort. Chest. 2016;149:45–52. doi: 10.1378/chest.15-1055. [DOI] [PubMed] [Google Scholar]
- 25.van Lunteren E, Moyer M. Auxotonic contractile responses of rat tracheal and bronchial airway smooth muscle. Pulm Pharmacol Ther. 2001;14:443–453. doi: 10.1006/pupt.2001.0308. [DOI] [PubMed] [Google Scholar]
- 26.Wohlsen A, Uhlig S, Martin C. Immediate allergic response in small airways. Am J Respir Crit Care Med. 2001;163:1462–1469. doi: 10.1164/ajrccm.163.6.2007138. [DOI] [PubMed] [Google Scholar]
- 27.Calzetta L, Rogliani P, Cazzola M, Matera MG. Advances in asthma drug discovery: evaluating the potential of nasal cell sampling and beyond. Expert Opin Drug Discov. 2014;9:595–607. [DOI] [PubMed]
- 28.Rabe KF. Mechanisms of immune sensitization of human bronchus. Am J Respir Crit Care Med. 1998;158:S161–S170. doi: 10.1164/ajrccm.158.supplement_2.13tac130. [DOI] [PubMed] [Google Scholar]
- 29.Watson N, Bodtke K, Coleman RA, Dent G, Morton BE, Ruhlmann E, Magnussen H, Rabe KF. Role of IgE in hyperresponsiveness induced by passive sensitization of human airways. Am J Respir Crit Care Med. 1997;155:839–844. doi: 10.1164/ajrccm.155.3.9117014. [DOI] [PubMed] [Google Scholar]
- 30.Mitchell RW, Rabe KF, Magnussen H, Leff AR. Passive sensitization of human airways induces myogenic contractile responses in vitro. J Appl Physiol (1985) 1997;83:1276–1281. doi: 10.1152/jappl.1997.83.4.1276. [DOI] [PubMed] [Google Scholar]
- 31.Schmidt D, Ruehlmann E, Branscheid D, Magnussen H, Rabe KF. Passive sensitization of human airways increases responsiveness to leukotriene C4. Eur Respir J. 1999;14:315–319. doi: 10.1034/j.1399-3003.1999.14b13.x. [DOI] [PubMed] [Google Scholar]
- 32.Schaafsma D, Zuidhof AB, Nelemans SA, Zaagsma J, Meurs H. Inhibition of rho-kinase normalizes nonspecific hyperresponsiveness in passively sensitized airway smooth muscle preparations. Eur J Pharmacol. 2006;531:145–150. doi: 10.1016/j.ejphar.2005.12.043. [DOI] [PubMed] [Google Scholar]
- 33.Freas W, Hart JL, Golightly D, McClure H, Muldoon SM. Contractile properties of isolated vascular smooth muscle after photoradiation. Am J Phys. 1989;256:H655–H664. doi: 10.1152/ajpheart.1989.256.3.H655. [DOI] [PubMed] [Google Scholar]
- 34.Hatake K, Wakabayashi I. Ethanol suppresses L-arginine-induced relaxation response of rat aorta stimulated with bacterial lipopolysaccharide. Nihon Arukoru Yakubutsu Igakkai Zasshi. 2000;35:61–68. [PubMed] [Google Scholar]
- 35.Calzetta L, Spina D, Cazzola M, Page CP, Facciolo F, Rendina EA, Matera MG. Pharmacological characterization of adenosine receptors on isolated human bronchi. Am J Respir Cell Mol Biol. 2011;45:1222–31. [DOI] [PubMed]
- 36.Matera MG, Calzetta L, Passeri D, Facciolo F, Rendina EA, Page C, Cazzola M, Orlandi A. Epithelium integrity is crucial for the relaxant activity of brain natriuretic peptide in human isolated bronchi. Br J Pharmacol. 2011;163:1740–1754. doi: 10.1111/j.1476-5381.2011.01339.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kott KS, Pinkerton KE, Bric JM, Plopper CG, Avadhanam KP, Joad JP. Methacholine responsiveness of proximal and distal airways of monkeys and rats using videomicrometry. J Appl Physiol. 2002;92:989–996. doi: 10.1152/japplphysiol.00415.2001. [DOI] [PubMed] [Google Scholar]
- 38.Martin C, Uhlig S, Ullrich V. Videomicroscopy of methacholine-induced contraction of individual airways in precision-cut lung slices. Eur Respir J. 1996;9:2479–2487. doi: 10.1183/09031936.96.09122479. [DOI] [PubMed] [Google Scholar]
- 39.Bergner A, Sanderson MJ. Acetylcholine-induced calcium signaling and contraction of airway smooth muscle cells in lung slices. J Gen Physiol. 2002;119:187–198. doi: 10.1085/jgp.119.2.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kummer W, Wiegand S, Akinci S, Wessler I, Schinkel AH, Wess J, Koepsell H, Haberberger RV, Lips KS. Role of acetylcholine and polyspecific cation transporters in serotonin-induced bronchoconstriction in the mouse. Respir Res. 2006;7:65. doi: 10.1186/1465-9921-7-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wohlsen A, Martin C, Vollmer E, Branscheid D, Magnussen H, Becker WM, Lepp U, Uhlig S. The early allergic response in small airways of human precision-cut lung slices. Eur Respir J. 2003;21:1024–1032. doi: 10.1183/09031936.03.00027502. [DOI] [PubMed] [Google Scholar]
- 42.Kanniess F, Scuri M, Vezzoli S, Francisco C, Petruzzelli S. Extrafine beclomethasone/formoterol combination via a dry powder inhaler (NEXThaler((R))) or pMDI and beclomethasone monotherapy for maintenance of asthma control in adult patients: a randomised, double-blind trial. Pulm Pharmacol Ther. 2015;30:121–127. doi: 10.1016/j.pupt.2014.07.006. [DOI] [PubMed] [Google Scholar]
- 43.Matera MG, Calzetta L, Rogliani P, Bardaro F, Page CP, Cazzola M. Evaluation of the effects of the R- and S-enantiomers of salbutamol on equine isolated bronchi. Pulm Pharmacol Ther. 2011;24:221–226. doi: 10.1016/j.pupt.2010.12.008. [DOI] [PubMed] [Google Scholar]
- 44.Matera MG, Calzetta L, Parascandolo V, Curradi G, Rogliani P, Cazzola M. Relaxant effect of brain natriuretic peptide in nonsensitized and passively sensitized isolated human bronchi. Pulm Pharmacol Ther. 2009;22:478–482. doi: 10.1016/j.pupt.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 45.Motulsky H, Christopoulos A. Fitting models to biological data using linear and nonlinear regression : a practical guide to curve fitting. Oxford: Oxford University Press; 2004. [Google Scholar]
- 46.Mercier FJ, Naline E, Bardou M, Georges O, Denjean A, Benhamou D, Advenier C. Relaxation of proximal and distal isolated human bronchi by halothane, isoflurane and desflurane. Eur Respir J. 2002;20:286–292. doi: 10.1183/09031936.02.00275702. [DOI] [PubMed] [Google Scholar]
- 47.Cazzola M, Calzetta L, Page CP, Rogliani P, Facciolo F, Gavaldà A, Matera MG. Pharmacological characterization of the interaction between aclidinium bromide and formoterol fumarate on human isolated bronchi. Eur J Pharmacol. 2014;745:135–143. doi: 10.1016/j.ejphar.2014.10.025. [DOI] [PubMed] [Google Scholar]
- 48.Cazzola M, Calzetta L, Segreti A, Facciolo F, Rogliani P, Matera MG. Translational study searching for synergy between Glycopyrronium and Indacaterol. COPD. 2014;12:175–81. [DOI] [PubMed]
- 49.Calzetta L, Page CP, Spina D, Cazzola M, Rogliani P, Facciolo F, Matera MG. Effect of the mixed phosphodiesterase 3/4 inhibitor RPL554 on human isolated bronchial smooth muscle tone. J Pharmacol Exp Ther. 2013;346:414–423. doi: 10.1124/jpet.113.204644. [DOI] [PubMed] [Google Scholar]
- 50.Rogliani P, Calzetta L, Rendina EA, Massullo D, Dauri M, Rinaldi B, Capuano A, Matera MG. The influence of propofol, remifentanil and lidocaine on the tone of human bronchial smooth muscle. Pulm Pharmacol Ther. 2013;26:325–331. doi: 10.1016/j.pupt.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 51.Goldoni M, Johansson C. A mathematical approach to study combined effects of toxicants in vitro: evaluation of the bliss independence criterion and the Loewe additivity model. Toxicol in Vitro. 2007;21:759–769. doi: 10.1016/j.tiv.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 52.Boik JC, Newman RA, Boik RJ. Quantifying synergism/antagonism using nonlinear mixed-effects modeling: a simulation study. Stat Med. 2008;27:1040–1061. doi: 10.1002/sim.3005. [DOI] [PubMed] [Google Scholar]
- 53.Boucher AN, Tam VH. Mathematical formulation of additivity for antimicrobial agents. Diagn Microbiol Infect Dis. 2006;55:319–325. doi: 10.1016/j.diagmicrobio.2006.01.024. [DOI] [PubMed] [Google Scholar]
- 54.Greco WR, Bravo G, Parsons JC. The search for synergy: a critical review from a response surface perspective. Pharmacol Rev. 1995;47:331–385. [PubMed] [Google Scholar]
- 55.Lee SI. Drug interaction: focusing on response surface models. Korean J Anesthesiol. 2010;58:421–434. doi: 10.4097/kjae.2010.58.5.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Meletiadis J, Mouton JW, Meis JF, Verweij PE. In vitro drug interaction modeling of combinations of azoles with terbinafine against clinical Scedosporium prolificans isolates. Antimicrob Agents Chemother. 2003;47:106–117. doi: 10.1128/AAC.47.1.106-117.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Calzetta L, Matera MG, Cazzola M. Pharmacological interaction between LABAs and LAMAs in the airways: optimizing synergy. Eur J Pharmacol. 2015;761:168–173. doi: 10.1016/j.ejphar.2015.05.020. [DOI] [PubMed] [Google Scholar]
- 58.Chou TC. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol Rev. 2006;58:621–681. doi: 10.1124/pr.58.3.10. [DOI] [PubMed] [Google Scholar]
- 59.Nials AT, Ball DI, Butchers PR, Coleman RA, Humbles AA, Johnson M, Vardey CJ. Formoterol on airway smooth muscle and human lung mast cells: a comparison with salbutamol and salmeterol. Eur J Pharmacol. 1994;251:127–135. doi: 10.1016/0014-2999(94)90392-1. [DOI] [PubMed] [Google Scholar]
- 60.Naline E, Zhang Y, Qian Y, Mairon N, Anderson GP, Grandordy B, Advenier C. Relaxant effects and durations of action of formoterol and salmeterol on the isolated human bronchus. Eur Respir J. 1994;7:914–920. [PubMed] [Google Scholar]
- 61.Joshi T, Johnson M, Newton R, Giembycz MA. The long-acting β2-adrenoceptor agonist, indacaterol, enhances glucocorticoid receptor-mediated transcription in human airway epithelial cells in a gene-and agonist-dependent manner. Br J Pharmacol. 2015;172:2634–2653. doi: 10.1111/bph.13087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pascoe CD, Swyngedouw NE, Seow CY, Pare PD. Gene expression in asthmatic airway smooth muscle: a mixed bag. Can J Physiol Pharmacol. 2015;93:137–143. doi: 10.1139/cjpp-2014-0390. [DOI] [PubMed] [Google Scholar]
- 63.Rogliani P, Calzetta L, Ora J, Lipsi R, Segreti A, Matera MG, Cazzola M. Pharmacological assessment of the onset of action of aclidinium and glycopyrronium versus tiotropium in COPD patients and human isolated bronchi. Eur J Pharmacol. 2015;761:383–390. doi: 10.1016/j.ejphar.2015.04.042. [DOI] [PubMed] [Google Scholar]
- 64.Hogg JC, McDonough JE, Suzuki M. Small airway obstruction in COPD: new insights based on micro-CT imaging and MRI imaging. Chest. 2013;143:1436–1443. doi: 10.1378/chest.12-1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Martin C, Frija J, Burgel PR. Dysfunctional lung anatomy and small airways degeneration in COPD. Int J Chron Obstruct Pulmon Dis. 2013;8:7–13. doi: 10.2147/COPD.S28290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lipworth B. Targeting the small airways asthma phenotype: if we can reach it, should we treat it? Ann Allergy Asthma Immunol. 2013;110:233–239. doi: 10.1016/j.anai.2013.02.009. [DOI] [PubMed] [Google Scholar]
- 67.Corren J. Small airways disease in asthma. Curr Allergy Asthma Rep. 2008;8:533–539. doi: 10.1007/s11882-008-0097-4. [DOI] [PubMed] [Google Scholar]
- 68.Condorelli P, Shin H-W, Aledia AS, Silkoff PE, George SC. A simple technique to characterize proximal and peripheral nitric oxide exchange using constant flow exhalations and an axial diffusion model. J Appl Physiol. 2007;102:417–425. doi: 10.1152/japplphysiol.00533.2006. [DOI] [PubMed] [Google Scholar]
- 69.Kannan RR, Singh N, Przekwas A. A quasi-3D compartmental multi-scale approach to detect and quantify diseased regional lung constriction using spirometry data. Int J Numer Method Biomed Eng. 2018; [DOI] [PMC free article] [PubMed]
- 70.Lavorini F, Pedersen S, Usmani OS. Aerosol drug management improvement T: dilemmas, confusion, and misconceptions related to small airways directed therapy. Chest. 2017;151:1345–1355. doi: 10.1016/j.chest.2016.07.035. [DOI] [PubMed] [Google Scholar]
- 71.Scichilone N, Battaglia S, Sorino C, Paglino G, Martino L, Paterno A, Santagata R, Spatafora M, Nicolini G, Bellia V. Effects of extra-fine inhaled beclomethasone/formoterol on both large and small airways in asthma. Allergy. 2010;65:897–902. doi: 10.1111/j.1398-9995.2009.02306.x. [DOI] [PubMed] [Google Scholar]
- 72.Rogliani P, Calzetta L, Coppola A, Cavalli F, Ora J, Puxeddu E, Matera MG, Cazzola M. Optimizing drug delivery in COPD: the role of inhaler devices. Respir Med. 2017;124:6–14. doi: 10.1016/j.rmed.2017.01.006. [DOI] [PubMed] [Google Scholar]
- 73.Corradi M, Chrystyn H, Cosio BG, Pirozynski M, Loukides S, Louis R, Spinola M, Usmani OS. NEXThaler, an innovative dry powder inhaler delivering an extrafine fixed combination of beclometasone and formoterol to treat large and small airways in asthma. Expert Opin Drug Deliv. 2014;11:1497–1506. doi: 10.1517/17425247.2014.928282. [DOI] [PubMed] [Google Scholar]
- 74.Nicolini G, Scichilone N, Bizzi A, Papi A, Fabbri LM. Beclomethasone/formoterol fixed combination for the management of asthma: patient considerations. Ther Clin Risk Manag. 2008;4:855–864. doi: 10.2147/TCRM.S3126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Huchon G, Magnussen H, Chuchalin A, Dymek L, Gonod FB, Bousquet J. Lung function and asthma control with beclomethasone and formoterol in a single inhaler. Respir Med. 2009;103:41–49. doi: 10.1016/j.rmed.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 76.Papi A, Paggiaro P, Nicolini G, Vignola AM, Fabbri LM. Group ISs: beclomethasone/formoterol vs fluticasone/salmeterol inhaled combination in moderate to severe asthma. Allergy. 2007;62:1182–1188. doi: 10.1111/j.1398-9995.2007.01493.x. [DOI] [PubMed] [Google Scholar]
- 77.Rogliani P, Calzetta L, Capuani B, Facciolo F, Cazzola M, Lauro D, Matera MG. Glucagon-like peptide 1 receptor: a novel pharmacological target for treating human bronchial Hyperresponsiveness. Am J Respir Cell Mol Biol. 2016;55:804–814. doi: 10.1165/rcmb.2015-0311OC. [DOI] [PubMed] [Google Scholar]
- 78.Cazzola M, Calzetta L, Rogliani P, Puxeddu E, Facciolo F, Matera MG. Interaction between corticosteroids and muscarinic antagonists in human airways. Pulm Pharmacol Ther. 2016;36:1–9. doi: 10.1016/j.pupt.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 79.Calzetta L, Soggiu A, Roncada P, Bonizzi L, Pistocchini E, Urbani A, Rinaldi B, Matera MG. Propofol protects against opioid-induced hyperresponsiveness of airway smooth muscle in a horse model of target-controlled infusion anaesthesia. Eur J Pharmacol. 2015;765:463–471. doi: 10.1016/j.ejphar.2015.09.007. [DOI] [PubMed] [Google Scholar]
- 80.Calzetta L, Passeri D, Kanabar V, Rogliani P, Page C, Cazzola M, Matera MG, Orlandi A. Brain natriuretic peptide protects against hyperresponsiveness of human asthmatic airway smooth muscle via an epithelial cell-dependent mechanism. Am J Respir Cell Mol Biol. 2014;50:493–501. doi: 10.1165/rcmb.2013-0119OC. [DOI] [PubMed] [Google Scholar]
- 81.Cazzola M, Calzetta L, Puxeddu E, Ora J, Facciolo F, Rogliani P, Matera MG. Pharmacological characterisation of the interaction between glycopyrronium bromide and indacaterol fumarate in human isolated bronchi, small airways and bronchial epithelial cells. Respir Res. 2016;17:70. doi: 10.1186/s12931-016-0386-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Calzetta L, Rogliani P, Facciolo F, Rendina E, Cazzola M, Matera MG. Pharmacological characterization of the interaction between umeclidinium and vilanterol in human bronchi. Eur J Pharmacol. 2017;812:147–154. doi: 10.1016/j.ejphar.2017.07.026. [DOI] [PubMed] [Google Scholar]
- 83.Donohue JF, Singh D, Munzu C, Kilbride S, Church A. Magnitude of umeclidinium/vilanterol lung function effect depends on monotherapy responses: results from two randomised controlled trials. Respir Med. 2016;112:65–74. doi: 10.1016/j.rmed.2016.01.001. [DOI] [PubMed] [Google Scholar]
- 84.Scichilone N, Benfante A, Morandi L, Bellini F, Papi A. Impact of extrafine formulations of inhaled corticosteroids/long-acting beta-2 agonist combinations on patient-related outcomes in asthma and COPD. Patient Relat Outcome Meas. 2014;5:153–162. doi: 10.2147/PROM.S55276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Greer S, Page CW, Joshi T, Yan D, Newton R, Giembycz MA. Concurrent agonism of adenosine A2B and glucocorticoid receptors in human airway epithelial cells cooperatively induces genes with anti-inflammatory potential: a novel approach to treat chronic obstructive pulmonary disease. J Pharmacol Exp Ther. 2013;346:473–485. doi: 10.1124/jpet.113.206284. [DOI] [PubMed] [Google Scholar]
- 86.Holden NS, Bell MJ, Rider CF, King EM, Gaunt DD, Leigh R, Johnson M, Siderovski DP, Heximer SP, Giembycz MA. β2-adrenoceptor agonist-induced RGS2 expression is a genomic mechanism of bronchoprotection that is enhanced by glucocorticoids. Proc Natl Acad Sci. 2011;108:19713–19718. doi: 10.1073/pnas.1110226108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Holden NS, George T, Rider CF, Chandrasekhar A, Shah S, Kaur M, Johnson M, Siderovski DP, Leigh R, Giembycz MA. Induction of regulator of G-protein signaling 2 expression by long-acting β2-adrenoceptor agonists and glucocorticoids in human airway epithelial cells. J Pharmacol Exp Ther. 2014;348:12–24. doi: 10.1124/jpet.113.204586. [DOI] [PubMed] [Google Scholar]
- 88.BinMahfouz H, Yan D, Borthakur B, George T, Giembycz MA, Newton R: Superiority of combined PDE3/PDE4 inhibition over PDE4 inhibition alone on glucocorticoid-and long-acting β2-adrenoceptor agonist-induced gene expression in human airway epithelial cells. Mol Pharmacol 2014:mol. 114.093393. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Baseline characteristics of bronchial tissue used in the study and CRCs to BDP and FF. (DOCX 431 kb)
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.


