Abstract
Spaceflight results in bone loss like that associated with osteoporosis or decreased weight-bearing (for example, high-energy trauma such as explosive injuries and automobile accidents). Thus, the unique spaceflight laboratory on the International Space Station presents the opportunity to test bone healing agents during weightlessness. We are collaborating with NASA and the US Army to study bone healing in spaceflight. Given the unique constraints of spaceflight, study design optimization was required. Male mice were selected primarily because their femur is larger than females’, allowing for more reproducible surgical outcomes. However, concern was raised regarding male mouse aggression. In addition, the original spaceflight study design included cohousing nonoperated control mice with mice that had undergone surgery to create a segmental bone defect. This strategy prompted the concern that nonoperated mice would exhibit aggressive behavior toward vulnerable operated mice. We hypothesized that operated and nonoperated male mice could be cohoused successfully when they were cagemates since birth and underwent identical anesthetic, analgesic, preoperative, and postoperative conditions. Using quantitative behavioral scoring, body weight, and organ weight analyses (Student t test and ANOVA), we found that nonoperated and operated C57BL/6 male mice could successfully be housed together. The male mice did not exhibit aggressive behavior toward cagemates, whether operated or nonoperated, and the mice did not show evidence of stress, as indicated by veterinary assessment, or change in body or proportional organ weights. These findings allowed our mission to proceed (launched February 2017) and may inform future surgical study designs, potentially increasing housing flexibility.
Abbreviations: ISS, International Space Station
Advances in orthopedics have increasingly been centered on optimizing biologic activity related to bone healing. Despite these advances, many scenarios remain in which bone healing is difficult or impossible. One such scenario is that of a segmental defect in which a portion of a bone is lost during an orthopedic injury.5,9,23 Oncologic procedures often produce segmental defects in long bones which are bridged with metal endoprostheses or allografts, both of which are associated with complications.2,6 Furthermore, high-energy trauma can produce segmental defects with the added complication of a compromised soft-tissue envelope.22 Current surgical fixation techniques, such as compression plating and intramedullary nails, provide adequate mechanical constructs, but fail to address the biologic milieu needed for bone healing.23 The osteoinductive protein bone morphogenetic protein 2 has successfully augmented bone formation; however, the use of this factor has been markedly decreased due to recent safety concerns, particularly a link to cancer.3,4 The need for osteoinductive agents remains, and our laboratory has recently identified thrombopoietin as a potential candidate. Thrombopoietin is the main megakaryocyte growth factor and results in rapid increases in circulating megakaryocyte numbers. Among other actions, these megakaryocytes stimulate osteoblast proliferation resulting in increased bone mass in mice.15 Based on these observations, our group is investigating the possibility that locally delivered thrombopoietin might augment healing of segmental defects by stimulating osteoblasts through megakaryocyte recruitment at the injury site.
In cooperation with NASA and the US Army, our laboratory is comparing the ability of bone morphogenetic protein 2 and thrombopoietin to heal femoral segmental bone defects. NASA has an interest in the augmentation of bone healing, given that prolonged spaceflight leads to bone atrophy, which might be considerable after prolonged missions (that is, expeditions to Mars). Indeed, astronauts lose approximately 1% to 3% of their bone mineral density each month, whereas osteoporotic patients lose 1% of their bone mineral density annually.7,12,20 This bone loss can increase the risk of fracture and is compounded by a decreased ability to heal fractures.16 Very little is known about segmental bone healing in microgravity because of the technical challenges and expense associated with spaceflight. Current Earth-based models inadequately mimic the clinical scenario: in our experience, mice, rats, and pigs ambulate on their operated limbs as soon as they recover from anesthesia, whereas humans are placed on strict bedrest or have limited weight-bearing before undergoing a supervised rehabilitation program. Therefore, bone healing in an animal model in a weightless environment more closely resembles the physiologic effects associated with the prolonged, nonweight-bearing typical in human patients with severe musculoskeletal injuries that require skeletal reconstruction (that is, blast injuries, motor vehicle collisions, oncologic reconstructions). Therefore, these studies are relevant not only to astronauts but also to patients suffering from poor bone-healing outcomes on Earth.
Although superior to ground-based studies in many ways, spaceflight introduces unique constraints regarding study design, the most common of which is physical space. Designs that require singly housed animals severely limit the number of mice used in spaceflight experiments, thus limiting statistical power and the scope of questions that can be asked. Related to this, only female mice have successfully been cohoused during spaceflight studies, given their less aggressive behavior. But, just as the primary use of male mice has been recognized to introduce bias in studies on Earth, the exclusive use of female mice in space similarly introduces undesirable sex-associated bias. For example, mice (and humans) have sex-specific differences in bone physiology, such as mass and response to sex steroids.26 In addition, considering that most astronauts in space are male and that most of the military personnel with blast injuries are male, it is important to develop the ability to conduct spaceflight studies using male mice. Ideally, studies would use male and female mice, but this practice is impractical given the environmental size constraints that would effectively diminish the statistical power a study could provide. In addition, the larger femur of male mice allows for more consistent results from a difficult orthopedic surgery, given the small size of mouse femurs. Therefore, in our experiments, we use male mice to perform the segmental bone defect surgeries.
One of the unavoidable differences between spaceflight and ground-based studies is the necessity to temporarily maintain mice at increased housing density (that is, mice per cm2). This constraint occurs prior to launch and several days thereafter. During this time, mice are in a Transporter unit (that is, mouse caging on SpaceX Dragon commercial resupply service vehicle) until being offloaded at the ISS.20 The SpaceX Dragon can accommodate only 2 Transporters (each has 2 sides, with a maximum of 10 mice per side), whereas the ISS has space for 4 Rodent Habitat units (each unit has 2 sides and can hold 5 mice on each side).20
Another important consideration of spaceflight investigation is the limited quantity of spaceflight hardware for rodents and the location of the spaceflight hardware. For example, temperature variations, secondary to inadvertent placement next to a high-output heat source, might affect the ambient temperature of the animals, introducing an unforeseen variable into the study. Furthermore, concentrating all members of a treatment group into a single Habitat hardware unit is risky because of the unlikely—but possible—failure of that unit. Therefore, to minimize experimental variables, improve statistical reliability, and decrease the risk of losing an entire experimental or control group (due to catastrophic hardware failure), we deemed it important to cohouse experimental and control groups, thus requiring the mixture of operated and nonoperated mice. To evaluate the feasibility of cohousing these animals in spaceflight, we determined that an analogous ground-based study should be conducted in which operated and nonoperated mice were housed together. That study, described herein, followed the temporal and hardware constraints experienced during spaceflight. We hypothesized that operated and nonoperated male mice could be cohoused successfully—provided that they had been cagemates since birth and experienced identical anesthetic, analgesic, preoperative, and postoperative conditions.
Completion of this ground-based study was one requirement for NASA approval of our Rodent Research 4 Mission, which successfully launched February 19, 2017, on SpaceX CRS-10.
Materials and Methods
Ethics statement.
All animal experiments were approved by the IACUC of the Indiana University School of Medicine and were performed in facilities accredited by the AAALAC. Importantly, for spaceflight studies, 2 separate IACUC (housed at NASA Kennedy Space Center and NASA Ames Research Center) oversee the welfare of all animals. Even though this study was ground-based, we worked closely with the NASA IACUC to mimic the unique transport and environmental conditions associated with spaceflight, in preparation for spaceflight investigations. Due to limitations associated with the current spaceflight transport mechanism, mice have to be housed at a density that is higher than that recommended in the Guide for Care and Use of Laboratory Animals (the Guide).14 However, note that recommendations in the Guide are based on the surface area of the floor. NASA's spaceflight hardware has wire grids on 5 of the 6 surfaces, thereby allowing mice to climb and explore most areas of the cage. In addition, the Guide acknowledges that “there is no ideal formula for calculating an animal's space needs,”14 and individual studies require review and modification in coordination with the IACUC. Therefore, for the ground-based studies described here, mice were temporarily housed at higher densities than recommended in the Guide, as detailed later.
Another difference between our spaceflight and Earth-based studies was the utilization of environmental enrichment. In these ground-based studies, nesting material was removed to simulate the spaceflight environment where nesting material was not used as it will float and clog air filters, and potentially interfere with access to food and water. Regarding hard enrichment, the spaceflight hardware allowed for climbing, and the water assembly unit provided a partially enclosed area for the mice to huddle. The mice were observed to congregate in this area, which was similar to what was observed in a standard shoebox or N10 cage containing a hut on Earth. NASA has successfully completed similarly designed experiments with females after passing stringent IACUC review. For future studies, NASA has developed a prototype hut, which is currently undergoing testing.
Animals and housing.
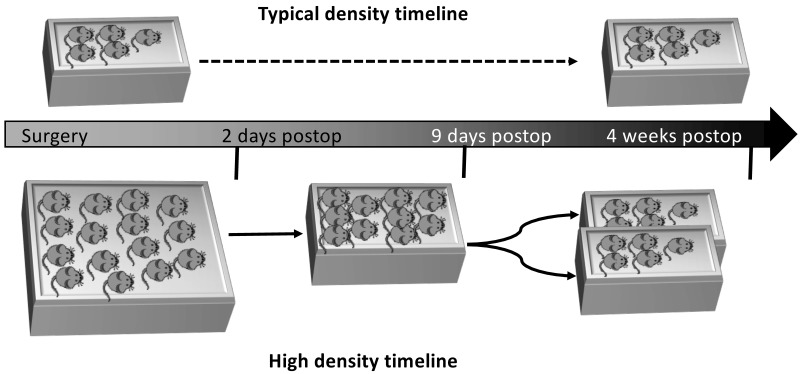
For this study, we purchased male, allo-reared C57BL/6 mice (n = 200; age, 8 wk; Jackson Laboratories, Bar Harbor, ME). Specifically, groups of 5 or 15 male mice were allo-reared, meaning they were maintained in same-sex groups since the time of weaning. Some of the mice were maintained in N40 mouse cages (48.26 cm × 26.67 cm × 15.56 cm; polycarbonate, Ancare, Bellmore, NY) with 15 mice per cage and were allowed to acclimate at the Indiana University School of Medicine laboratory animal resource center for 1 wk after arrival (termed ‘high-density cages’ in Figure 1). Other mice were housed in groups of 5, according to standard procedures in standard shoebox cages (polycarbonate, 19.05 cm × 29.21 cm × 12.70 cm; N10, Ancare; termed ‘typical-density cages’ in Figure 1). On arrival to the animal resource center, mice were provided with one bedding pack (EnviroPAK, WF Fisher and Son, Somerville, NJ) per 5 mice as soft enrichment.
Figure 1.
Experimental timelines and caging schemes of mice housed at typical or high density.
Cages of mice were randomly assigned into 2 groups: all operated or combined (that is, both operated and nonoperated mice in a single cage). Of the 200 mice recruited into the study, 150 were housed in N40 cages (10 cages of 15 mice each), and the remaining 50 mice were housed in N10 cages (10 cages of 5 mice each). Of the 10 N40 cages, 4 cages contained both nonoperated and operated mice (1:4 ratio), and in the remaining 6 cages, all mice underwent surgery. Of the 10 N10 cages, 6 cages contained both nonoperated and operated mice (1:4 ratio), and in the remaining 4 cages, all mice received surgery. Therefore, surgery was performed on 182 of the 200 mice. In addition, mice randomly assigned as operated or nonoperated mice. Specifically, for cages containing both operated and nonoperated mice, the first cage assigned as a combination cage had the first mouse selected (N10) or first 3 mice selected (N40) to serve as nonoperated controls. The second combination cage had the second mouse selected (N10) or second set of 3 mice selected (mice 4, 5, and 6; N40) to serve as nonoperated controls. This process was repeated for the remaining 4 N40 cages and the 6 N10 cages. For the 6th N10 cage, the first combination cage randomization was repeated (that is, the first mouse selected served as nonoperated control).
Mice were maintained on a 12:12-h light:dark cycle. The macroenvironment was maintained at 22 °C and 30% to 70% relative humidity. Mice were moved to clean cages 3 times each week at 0915, just after videotaping was completed. All mice had unrestricted access to water and Teklad Rodent Diet (8604, Envigo, Indianapolis, IN). The health of the colony was determined by using indirect sentinels that were screened quarterly. At the time of this study, the colony was free of the following pathogens: ectromelia virus, rotavirus A, lymphocytic choriomeningitis virus, Mycoplasma pulmonis, murine hepatitis virus, minute virus of mice, mouse parvovirus, murine polyoma virus, murine pneumovirus, mammalian orthreovirus, Sendai virus, theilovirus, Haantan virus, and internal and external parasites.
After 1 wk of acclimation, a raised wire floor containing 3 openings per 2.54 cm (N10SSRWF [for N10 cages] and N40SSRWF [for N40 cages], Ancare) was placed on the floor of all cages to simulate the structure of cages used for spaceflight. After 1 wk on the wire flooring, mice were randomly assigned to operated or nonoperated groups (as detailed earlier), and surgery was performed on operated mice. Resting boards (7.62 cm × 15.24 cm; catalog no. K3392, Rest Stops, Bio-Serve, Flemington, NJ)—flat, polycarbonate boards that provide a surface on which animals can walk and rest to ease the transition to ambulating after surgery—were placed in the cages during recovery from surgery. Resting boards and EnviroPAK material were removed 2 d after surgery. At this time, the 15 mice housed in N40 cages were then reduced to 10 per cage by removing and euthanizing mice that were aggressive or had poor postoperative outcomes, such as overt gait abnormalities (lack of weight-bearing, limping, inability to flex or extend the leg or foot). Specifically, 17 of the 182 operated mice had poor surgical outcomes and thus were euthanized; no mice were removed from study due to aggressive behavior. If no selection criteria were met, mice were removed at random until 10 mice remained. The study design mandated that, to inform the spaceflight experiment, a cage had to contain either 10 (N40) or 5 (N10) mice at 2 d postoperatively to continue in the study. In particular, complications with anesthesia (2 mice from 1 N10 cage) and hardware (8 mice from 1 N40 cage) led to the loss of 1 N10 and 1 N40 cage for failing to meet the required numbers of mice at 2 d postoperatively. For the other animals, 10 mice from the N40 cage were then placed into new N10 cages and were designated as high-density housing, as detailed in the timeline shown in Figure 1. This density was meant to simulate that of the Transporter unit used aboard the SpaceX Dragon rocket. At this time, all nesting material was removed to simulate the spaceflight environment, where nesting material is not used. After 1 wk, the 10 experimental mice were randomly assigned to groups of 5 and were placed in standard N10 mouse cages (containing a raised wire floor), where they remained for the duration of the study (4 wk after surgery). This arrangement was meant to simulate the density of the Habitat unit used aboard the ISS.
The typical-density mice, which were initially in groups of 5 per N10 cage, remained in the N10 cage throughout the experiment. Like the mice in the N40 cages, after 1 wk of acclimation, a raised wire floor was placed in the bottom of the cage for the duration of the study. After 1 wk of acclimation to the wire floor, mice were randomly assigned to operated or nonoperated groups, and surgery was performed on mice in the operated group. Resting boards were placed in the cages during surgical recovery. Again, resting boards and nesting materials were removed at 2 d after surgery, and then the mice remained in the N10 cages with raised wire flooring for the remainder of the study (4 wk after surgery).
All mice were checked daily for evidence of fighting. According to our protocol, when evidence of fighting was observed, mice identified as aggressors (when possible) were removed from the cages. Although no mice were removed from the primary study due to fighting, in our preliminary studies whereby the video reader assessments were validated (see Data Collection), one mouse was removed from the highly aggressive cage during this scoring validation phase (see Results). Mice were weighed weekly as part of general health assessment. A weight loss of greater than 15% triggered removal from the study (no mice lost 15% or more of their body weight during this study). When the group size fell below 8 (for N40 cages) or 4 (for N10 cages) animals per cage, the cage was considered a failure (due to large change in density and a loss of statistical power). After completion of the study, mice were euthanized (through CO2 inhalation, delivery of 100% CO2 at a rate of 30% volume displacement per minute, followed by cervical dislocation) for tissue collection.
Segmental defect surgery.
Mice were anesthetized with ketamine–xylazine (125 and 20 mg/kg, respectively, IP; Patterson Veterinary, Charlotte, NC), ophthalmic ointment was applied to each eye, and the right hindlimb was shaved and prepped with ethanol and povidone–iodine. A 1-cm incision was made laterally over the right thigh. The femur was exposed by using blunt dissection, at which point the muscle was bluntly stripped of soft tissue attachments in the diaphyseal region. Next, the knee was flexed and the patellar tendon split by using a 27-gauge needle, which was then manually advanced retrograde between the femoral condyles into the femoral intramedullary canal. A sterile rotary cutting tool was used to remove a 2-mm intercalary segment from the femoral diaphysis, and the needle was advanced through a 2-mm synthetic graft and through the greater trochanter. The tip of the needle was bent inferiorly back on itself, and the needle was pulled taunt against the greater trochanter in an anterograde direction. Next, a collagen sponge treated with either normal saline (negative control), 5 µg thrombopoietin (experimental group, Peprotech, Rocky Hill, MJ), or 5 µg bone morphogenic protein 2 (positive control; Medtronic, Charlotte, NC) was wrapped around a synthetic graft and sutured into place by using 3-0 polyglycolic acid suture (J215H, Ethicon, Somerville, NJ). Although comparing the bone-healing attributes of these proteins was not the aim of this study, we used them to recapitulate the conditions of the spaceflight study for which we were preparing. In addition, this practice allowed for evaluating the effect of these growth factors on behavior. The muscle fascia was closed by using 3-0 polyglycolic acid suture and the skin closed by using standard 7-mm wound clips (RF7CS, Braintree Scientific, Braintree, MA).
The nonoperated mice were treated as follows. Each mouse was anesthetized in the same manner as the operated mice. The right hindlimb was shaved and prepped with ethanol and povidone–iodine. The same skin clips were then placed over an imaginary incision, similar to the operated mice. Both operated and nonoperated mice within the same cage recovered from anesthesia together. Cages were placed half on heating pads until all mice had recovered from anesthesia. Respiratory rate, body warmth, and signs of discomfort were monitored until mice ambulated within the cage. Once all animals in the cage were ambulating independently, the cage was returned to the animal facility, and research personnel or veterinary staff monitored mice twice daily for the first 48 h after surgery and daily thereafter.
The day prior to surgical procedures animals received sustained-release buprenorphine (3.0 to 3.5 mg/kg SC, 72 h of analgesic coverage per injection; Patterson Veterinary). Animals received a second dose of sustained-release buprenorphine at 48 h after completion of the procedure, to achieve a total of 5 d of analgesic coverage. When research personnel or veterinary staff (at least daily monitoring) identified animals that were in pain or distress, the mice received additional analgesia.
Data collection.
All cages of mice were videorecorded for 2 h each during the daytime (light cycle) and nighttime (dark cycle) for a total of 4 h, by using security cameras with infrared night-vision capability (Lorex, Elkridge, MD). Videorecording occurred during the 2 h immediately after the light cycle changes (at 0700 and 1900), which are known to cause additional stress, because we were interested in identifying potential triggers of aggressive behavior.8,13 Videorecordings were analyzed every 12 h for the first 5 d after surgery and weekly thereafter (consecutive daytime and nighttime recordings) for the experimental duration. Cages were placed in similar positions relative to each camera. The recordings were scored for interactions between conspecifics in the cage (that is, fighting, pursuit). Three independent readers (from a pool of 7 readers) scored each 2-h video for each cage to obtain a numerical value that corresponded to the level of activity. The scoring system was as follows: numerical scores were assigned to each instance of behavior observed, and points were totaled at the end of each recording to result in a final individual cage score for each video. In consultation with veterinary staff, we differentiated between increased normal activity and aggressive behaviors. As detailed in Figure 2, in increasing order, the following categories were scored: pursuit (3 points), anogenital contact (7 points), mutual upright threat posture (7 points), and offensive sideways threat posture (7 points). In addition, 10 points were added for each cluster of aggressive behaviors with incorporated tumbling to denote fighting or increased likelihood that injury has occurred. Aggressive behaviors were previously described, and ethograms included threat posture, urogenital contact, rear-biting, horizontal threat jumps, and escalated jumping or tumbling.18 This scoring system was validated for acceptable interobserver reliability.
Figure 2.
Scores for and examples of different mouse behaviors.
Readers were trained in person by inhouse veterinary staff to recognize the behaviors and postures in the ethogram.18 Readers were blinded by denying access to prior scores for any given cage or any corresponding scores from coreaders. The design of the study did not allow blinding for treatment, however, because differences in housing densities between groups were readily apparent. Veterinary staff and research personnel checked mice daily for evidence of fighting, evaluated them by using standard Mouse Health Check Forms (assessing activity, extremities, coat or fur, skin, respiration, eyes, and nose), and weighed them twice each week throughout the study. At the end of the experiment, weights for adrenal gland, spleen, thymus, and whole body were obtained to evaluate changes corresponding to stress. Understanding that lack of aggression does not necessarily indicate a stress free-environment, we concluded that an aggression-limited environment likely correlated to decreased stress, provided that veterinary assessment supported that animals were healthy.
Statistics.
Unless otherwise indicated, all data are presented as the mean ± SEM. Student t tests were performed when only 2 groups were compared; ANOVA was used for comparisons of more than 2 groups. All analyses were performed by using the Statistical Package for Social Sciences (SPSS 19, IBM, Armonk, NY) software package and were 2-tailed, with the level of significance set at 0.05.
A pretest power analysis of our primary endpoint (operated compared with combined) indicated that a sample size of 8 was required for this study (assuming power of 0.8 and α = 0.05). Because the subject of the study was cages of mice, 8 cages per group were required. Given the speculative nature of this calculation, we examined 10 cages of mice for each group.
Results
Validation of activity assessment scores.
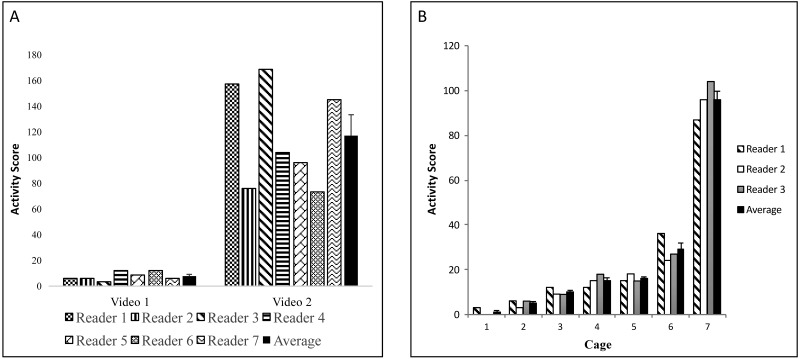
We used a pool of 7 total readers to achieve 3 independent scores of activity levels per video. To validate our scoring system, we first sought to determine interreader reproducibility. All 7 readers scored activity in 2 different 2-h videos, which appeared to contain low and high activity based on a cursory viewing. The readers were unaware of the assigned activity level of the teaching videos prior to viewing. Figure 3 A shows each reader's scores for each video and the average for all readers. The results clearly indicated that our scoring method distinguishes high and low activity states (Student t test, P < 0.001). Furthermore, ANOVA of individual scores did not reveal statistically significant differences between readers for each video analyzed. Importantly, our method successfully captured increases in activity that correlated with physical findings. In particular, we registered increased activity (fighting with incorporated tumbling that warranted 10 points) and noted evidence of fighting (bite marks, scarring, scratches) on 4 of the 5 mice (Figure 3 B); the aggressive mouse was immediately removed from the cage. According to the veterinary staff, the offending aggressor was likely the animal that lacked bite marks, scarring, and scratches. This association was confirmed when the wounding ceased after the suspected aggressor was removed and placed in a separate cage.
Figure 3.
(A) Interreader variability. Readers independently scored mouse activity at random time intervals. (B) Interreader variability by cage. For each cage, 3 readers independently scored mouse activity for a 2-h recording. The last bar (black bar) for each cage reviewed represents the mean ± SEM.
Next, we analyzed the consistency among 3 randomly assigned readers. This assessment was necessary because of the sheer amount of data (more than 300 videos, each of which was 2 h in duration). As shown in Figure 3 B, activity score averages for all cage activity demonstrated a consistent trend tracked well. None of the readers was consistently higher or lower than the mean. Reader 1 was higher than the mean for 4 readings and lower for 3 readings. Reader 2 was higher twice and lower for 4 readings (with 1 reading at exactly the mean). And, reader 3 was higher than the mean 3 times and lower 4 times. Again, ANOVA did not reveal significant differences among readers. Thus, we have validated that our scheme of assigning random readers to score video segments captures both high and low activity and is reproducible.
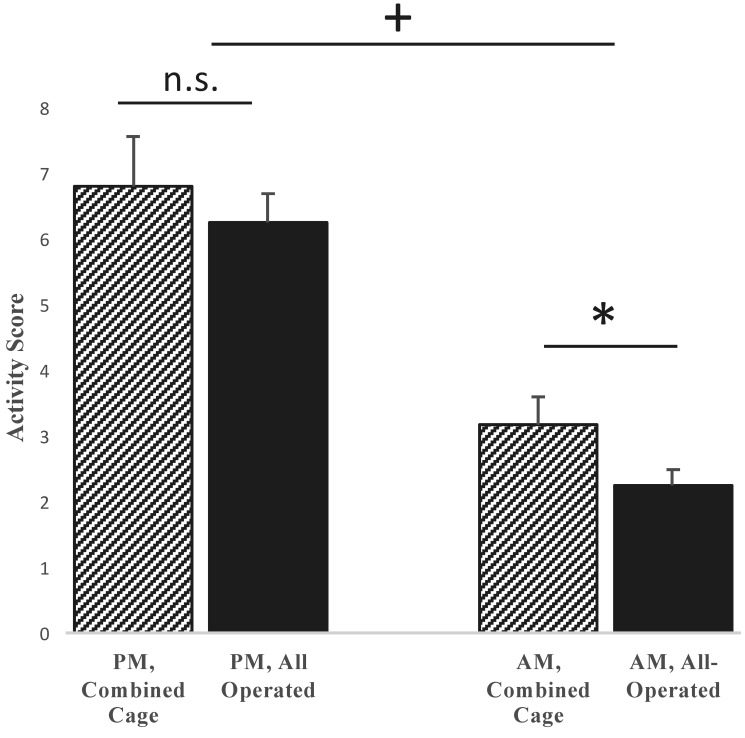
Finally, we validated activity assessments according to the time of the recordings. Specifically, because mice are nocturnal, we expected they would have higher activity at night. As shown in Figure 4, activity scores were markedly higher during the nighttime recordings as compared with the daytime readings. From these results, we conclude that our technique is sufficiently sensitive to capture expected increases in nighttime activity.
Figure 4.
Comparison of nighttime (PM) and daytime (AM) activity scores across the entire study. Nighttime scores were not significantly different (n.s.) between cages in which all mice underwent surgery (all operated) and those containing mice that had surgery and those that did not (combined). However, daytime scores differed (*, P < 0.05) between combined and all-operated cages, but the magnitude was small, and neither group demonstrated high activity or aggression. As expected, combined night activity was greater than combined day activity (+, P < 0.05).
Effect of cohousing nonoperated and operated mice on activity scores.
We sought to understand the effects of cohousing presumed healthy nonoperated mice with mice that had undergone a major orthopedic surgery, to evaluate the activity level and indicators of stress. Specifically, we were interested in understanding whether nonoperated mice behaved aggressively toward the more vulnerable population that underwent surgery. In regard to activity (Figure 4), the mean overall (all daytime and nighttime readings combined) activity score was 5.0 ± 0.4 for cages with a combination of operated and nonoperated animals, compared with a score of 4.2 ± 0.3 in cages with only operated animals (Student t test, P > 0.05). When nighttime recordings alone were examined, the averages increased to 6.8 ± 0.8 for combined cages and 6.3 ± 0.5 for operated-only cages, with no significant difference between the 2 groups (P = 0.5). However, when daytime recordings alone were assessed, the mean activity score was 3.2 ± 0.4 for combined cages and 2.3 ± 0.2 for operated only cages (P < 0.05).
Effect of cohousing nonoperated and operated mice on body and organ weights.
Next, we examined whether cohousing nonoperated mice with operated mice had an effect on body weight or adrenal gland, thymic, or splenic weight. These weights are known to change as a physiologic response to stress.10 As shown in Table 1, we observed a small (approximately 5%) but statistically significant decrease in final body weight between all animals in the operated-only cages (25.0 ± 0.2 g) compared with those contained in the combined cages (26.2 ± 0.2 g; P < 0.05). However, one cohort of mice (and used in the combined cages) weighed significantly more than other cohorts on arrival from Jackson Laboratories. When change in body weight was examined instead of final body weight, no significant differences were detected (data not shown, P > 0.05). Similarly, organ weights did not differ between groups when corrected for body weight (Table 1, P > 0.05). We also evaluated whether surgery itself led to a change in body weight at the end of the experimental period. As shown in Table 2, the final body weight of all operated mice (that is, regardless of housing type) did not differ from that of nonoperated mice (data not shown, P > 0.05).
Table 1.
Body and proportional organ weights (mean ± SEM) of mice in all-operated or combined cages
| (Organ weight / body weight) × 1000 |
|||||
| Group | n | Body weight (g) | Adrenal gland | Thymus | Spleen |
| All-operated | 68 | 25.0 ± 0.2a | 0.28 ± 0.03 | 1.7 ± 0.1 | 3.6 ± 0.1 |
| Combined | 46 | 26.2 ± 0.2 | 0.35 ± 0.02 | 1.8 ± 0.1 | 3.7 ± 0.1 |
Significant (P < 0.05) difference between operated and combined groups
Table 2.
Body and proportional organ weights of mice that underwent surgery compared with nonoperated mice
| (Organ weight / body weight) × 1000 |
|||||
| Group | n | Body weight (g) | Adrenal gland | Thymus | Spleen |
| Operated | 100 | 25.0 ± 0.2 | 0.32 ± 0.02 | 1.7 ± 0.1 | 3.6 ± 0.1 |
| Nonoperated | 14 | 26.4 ± 0.4 | 0.27 ± 0.04 | 1.6 ± 0.2 | 3.5 ± 0.2 |
No significant differences detected.
Discussion
Spaceflight offers a unique laboratory to study complex physiologic changes associated with microgravity. At the same time, unique challenges must be overcome to maximize what can be learned from these expensive and technically challenging experiments. The focus of our work with NASA is to study bone regeneration during spaceflight. As part of the preparation for this experiment, we sought to establish housing conditions that minimized potentially aggressive behavior among cohoused male mice. The experimental design and physical space constraints of the SpaceX Dragon rocket and the Transporter unit required housing mice at high density for transport to the ISS and at lower, typical housing density for the experimental period onboard the ISS. The current recommendation in the Guide is that mice smaller than 25 g are allocated 96.7 cm2 of floor space. Several recent studies indicate that higher housing densities do not lead to aggressive behavior in male mice,19,21 but this topic remains under debate.11,25,27 Consequently, confirming an acceptably low level of aggression in our housing scheme was of utmost importance, given the high stakes associated with spaceflight experimentation. Very few studies in space have used male mice. One recent launch of the Russian Bion-M 1 biosatellite housed male mice in groups of 3, with training that consisted of handling and acclimation to a specialized food delivery system.1 These researchers suggested that male aggression was low under these housing conditions. However, only 16 of the 45 mice survived the spaceflight, and 25% of the survivors had limb injuries and 38% had tail injuries. Therefore, whether male mice can be cohoused successfully during spaceflight remains unknown, and clarifying this point was one of our overall goals. For the current work, we were interested specifically in cohousing allo-reared male mice at a high density for a short time (1 wk) and then moving these mice to caging at a lower, typical housing density (which also changes the hierarchical structure in the cage). In addition, our unique study design included a major orthopedic surgery, the effect of which on mouse behavior and associated healing has yet to be studied in outer space.
To objectively compare the activity and aggression in the various cages, we created a scoring method. The goal of the system was to accurately assess various postures and activities as aggressive or nonaggressive and to assign a single score to each to simply portray the atmosphere of the cage at a given time point. To confirm that the method was reproducible, we assessed scores across 7 readers for cages with low activity compared with cages with an aggressive environment. Figure 3 shows that although variability is present between readers, the scoring method is reproducible. In addition to reproducibility, the scoring method differentiates between high- and low-activity environments. For example, for cage 7, we registered increased activity and noted evidence of fighting, such as bite marks. Likewise, we found the expected significantly higher activity at night as compared with daytime readings (Figure 4). Encountered limitations primarily involved camera capabilities and cage placement. Video definition varied between recordings taken when rooms were lighted compared with those taken in the dark. For example, although postures could be easily assessed and differentiated during the day, these same postures were more challenging to differentiate at night. Nevertheless, we found very good interreader reproducibility, and no evidence of fighting occurred that was not captured on video.
Of critical importance for this study, activity did not differ between cages in which all mice had undergone surgery compared with cages in which some mice had undergone surgery and others had not. In addition, further analysis of the nocturnal (higher activity) readings revealed no significant difference in activity between the 2 groups (cages containing all operated mice compared with cages containing a combination of operated and nonoperated mice). In contrast, the daytime activity (low activity) readings were significantly different between the 2 groups. However, the activity scores were quite low, and averages for cages containing operated mice only and those containing both operated and nonoperated mice were still lower than corresponding nighttime readings. Given that trained veterinary staff assessed mice assessed regularly in regard to their wellbeing and that body and proportional organ weights did not differ between groups, the mice apparently were not unduly stressed in these studies. These key findings enabled the use of an optimized acclimation and housing scheme during our studies aboard the ISS and have implications regarding cohousing healthy nonoperated and operated mice in terrestrial laboratories.
Acknowledgments
We thank Ms Jane Han for her technical assistance with this project. We also thank the entire Rodent Research 4 team at the NASA Ames Research Center and the US Army Center for Environmental Health Research for their assistance with developing these studies. This work was supported in part by the Medical Student Affairs Summer Research Program in Academic Medicine, Indiana University School of Medicine, funded in part by NIH NIAMS T32 AR065971 (JDR), a postdoctoral NIH T32 Training Grant in Hematopoiesis, T32 DK007519-31 (PC), and the Department of Orthopaedic Surgery, Indiana University School of Medicine (MAK). This work was supported in part by a grant from the Orthopaedic Trauma Association (MAK) and the Ralph W and Grace M Showalter Research Trust Fund (MAK). In addition, research reported in this publication was supported in part by the following grants: NIH NIAMS R01 AR060863 (MAK) and GA-2015-217 from the Center for the Advancement of Sciences in Space (CASIS, MAK). The views, opinions, and findings contained in this report are those of the author(s) and should not be construed as official Department of the Army, NIH, NASA, or CASIS position, policy, or decision, unless so designated by other official documentation.
References
- 1.Andreev-Andrievskiy A, Popova A, Boyle R, Alberts J, Shenkman B, Vinogradova O, Dolgov O, Anokhin K, Tsvirkun D, Soldatov P, Nemirovskaya T, Ilyin E, Sychev V. 2014. Mice in Bion-M1 space mission: training and selection. PLoS One 9:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aponte-Tinao L, Farfalli GL, Ritacco LE, Ayerza MA, Muscolo DL. 2011. Intercalary femur allografts are an acceptable alternative after tumor resection. Clin Orthop Relat Res 470:728–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carragee EJ, Chu G, Rohatgi R, Hurwitz EL, Weiner BK, Yoon ST, Comer G, Kopjar B. 2013. Cancer risk after use of recombinant bone morphogenetic protein 2 for spinal arthrodesis. J Bone Joint Surg Am 95:1537–1545. [DOI] [PubMed] [Google Scholar]
- 4.Carragee EJ, Hurwitz EL, Weiner BK. 2011. A critical review of recombinant human bone morphogenetic protein 2 trials in spinal surgery: emerging safety concerns and lessons learned. Spine J 11:471–491. [DOI] [PubMed] [Google Scholar]
- 5.Cook SD, Baffes GC, Wolfe MW, Sampath TK, Rueger DC, Whitecloud TS., 3rd 1994. The effect of recombinant human osteogenic protein 1 on healing of large segmental bone defects. J Bone Joint Surg Am 76:827–838. [DOI] [PubMed] [Google Scholar]
- 6.Damron TA, Sim FH, Shives TC, An KN, Rock MG, Pritchard DJ. 1996. Intercalary spacers in the treatment of segmentally destructive diaphyseal humeral lesions in disseminated malignancies. Clin Orthop Relat Res 324:233–243. [DOI] [PubMed] [Google Scholar]
- 7.Demontis GC, Germani MM, Caiani EG, Barravecchia I, Passino C, Angeloni D. 2017. Human pathophysiological adaptations to the space environment. Front Physiol 8:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dudley CA, Erbel-Sieler C, Estill SJ, Reick M, Franken P, Pitts S, McKnight SL. 2003. Altered patterns of sleep and behavioral adaptability in NPAS2-deficient mice. Science 301:379–383. [DOI] [PubMed] [Google Scholar]
- 9.Einhorn TA, Lane JM, Burstein AH, Kopman CR, Vigorita VJ. 1984. The healing of segmental bone defects induced by demineralized bone matrix. A radiographic and biomechanical study. J Bone Joint Surg Am 66:274–279. [PubMed] [Google Scholar]
- 10.Everds NE, Snyder PW, Bailey KL, Bolon B, Creasy DM, Foley GL, Rosol TJ, Sellers T. 2013. Interpreting stress responses during routine toxicity studies: a review of the biology, impact, and assessment. Toxicol Pathol 41:560–614. [DOI] [PubMed] [Google Scholar]
- 11.Foltz C, DeLong D. 2010. The response of C57BL/6J and BALB/cJ mice to increased housing density. J Am Assoc Lab Anim Sci 49:136–137. [PMC free article] [PubMed] [Google Scholar]
- 12.Garcia HD, Hays SM, Tsuji JS. 2013. Modeling of blood lead levels in astronauts exposed to lead from microgravity-accelerated bone loss. Aviat Space Environ Med 84:1229–1234. [DOI] [PubMed] [Google Scholar]
- 13.Hascoët M, Bourin M, Nic Dhonnchadha BA. 2001. The mouse light–dark paradigm: a review. Prog Neuropsychopharmacol Biol Psychiatry 25:141–166. [DOI] [PubMed] [Google Scholar]
- 14.Institute for Laboratory Animal Research 2011. Guide for the care and use of laboratory animals, 8th ed. Washington (DC): National Academies Press. [Google Scholar]
- 15.Kacena MA, Shivdasani RA, Wilson K, Xi Y, Troiano N, Nazarian A, Gundberg CM, Bouxsein ML, Lorenzo JA, Horowitz MC. 2004. Megakaryocyte-osteoblast interaction revealed in mice deficient in transcription factors GATA1 and NF-E2. J Bone Miner Res 19:652–660. [DOI] [PubMed] [Google Scholar]
- 16.Kahn J, Liverman CT, McCoy MA. 2014. Health standards for long duration and exploration spaceflight: ethics principles, responsibilities, and decision framework. Washington (DC): National Academies Press. [PubMed] [Google Scholar]
- 17.LeBlanc A, Schneider V, Shackelford L, West S, Oganov V, Bakulin A, Voronin L. 2000. Bone mineral and lean tissue loss after long duration space flight. J Musculoskelet Neuronal Interact 1:157–160. [PubMed] [Google Scholar]
- 18.Miczek KA, O'Donnell JM. 1978. Intruder-evoked aggression in isolated and nonisolated mice: effects of psychomotor stimulants and L-dopa. Psychopharmacology (Berl) 57:47–55. [DOI] [PubMed] [Google Scholar]
- 19.Morgan JL, Svenson KL, Lake JP, Zhang W, Stearns TM, Marion MA, Peters LL, Paigen B, Donahue LR. 2014. Effects of housing density in 5 inbred strains of mice. PLoS One 9:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.National Aeronautics and Space Administration. [Internet] 2017. Rodent research hardware system. [Cited 1 July 2017]. Available at: https://www.nasa.gov/ames/research/space-biosciences/rodent-research-hardware.
- 21.Nicholson A, Malcolm RD, Russ PL, Cough K, Touma C, Palme R, Wiles MV. 2009. The response of C57BL/6J and BALB/cJ mice to increased housing density. J Am Assoc Lab Anim Sci 48:740–753. [PMC free article] [PubMed] [Google Scholar]
- 22.Pape HC, Tornetta P, 3rd, Tarkin I, Tzioupis C, Sabeson V, Olson SA. 2009. Timing of fracture fixation in multitrauma patients: the role of early total care and damage control surgery. J Am Acad Orthop Surg 17:541–549. [DOI] [PubMed] [Google Scholar]
- 23.Penn-Barwell JG, Bennett PM, Fries CA, Kendrew JM, Midwinter MJ, Rickard RF. 2013. Severe open tibial fractures in combat trauma: management and preliminary outcomes. Bone Joint J 95-B:101–105. [DOI] [PubMed] [Google Scholar]
- 24.Reichert JC, Saifzadeh S, Wullschleger ME, Epari DR, Schutz MA, Duda GN, Schell H, van Griensven M, Redl H, Hutmacher DW. 2009. The challenge of establishing preclinical models for segmental bone defect research. Biomaterials 30:2149–2163. [DOI] [PubMed] [Google Scholar]
- 25.Smith AL, Mabus SL, Muir C, Woo Y. 2005. Effects of housing density and cage floor space on 3 strains of young adult inbred mice. Comp Med 55:368–376. [PubMed] [Google Scholar]
- 26.Syed F, Khosla S. 2005. Mechanisms of sex steroid effects on bone. Biochem Biophys Res Commun 328:688–696. [DOI] [PubMed] [Google Scholar]
- 27.Whittaker AL, Howarth GS, Hickman DL. 2012. Effects of space allocation and housing density on measures of wellbeing in laboratory mice: a review. Lab Anim 46:3–13. [DOI] [PubMed] [Google Scholar]