Abstract
Enhancer of zeste homolog 2 (EZH2) shows upregulated expression in tumors and is an important driver of tumor development and progression. However, the mechanism underlying the mediation of tumor aggressiveness by EZH2 remains unclear. We here investigated the levels of EZH2 in various normal and tumorous dog tissues and compared these patterns with those of the corresponding human tissues. Immunohistochemical analysis showed positive staining for EZH2 in 76 of 82 cases of canine tumors, whereas low or negligible staining occurred in normal tissues and other canine tumors, including hepatocellular adenoma and lipoma. In particular, canine lymphoma, melanoma, basal cell tumors, squamous cell carcinoma, and prostate cancer all show EZH2 overexpression, as do their human counterparts. Given the similarities of spontaneous canine tumors to human cancers, we believe that these canine tumors can be used as animal models in future research and clinical trials in the development of EZH2 inhibitors.
Abbreviations: EZH2, enhancer of zeste homolog 2; PRC2, polycomb repressive complex 2; TBS, TRIS-buffered saline
Enhancer of zeste homolog 2 (EZH2) is an enzymatic subunit of polycomb repressive complex 2 (PRC2), which induces the methylation of lysine 27 in histone H3 and silences specific gene transcription.40 In particular, PRC1 and PRC2 suppress gene expression through covalent modification of selected histones, thereby reducing the expression of tumor suppressor proteins. EZH2 is often upregulated in tumors and is an important driver of tumor development and progression that is often correlated with poor prognosis.3,10,11 EZH2 acts as a transcriptional repressor that maintains the homeostatic balance between gene expression and repression, and the disruption of this function may lead to oncogenesis.35 In addition, EZH2 appears to be associated with tumor aggressiveness in several tumor types.3,7,11 Therefore, EZH2 and EZH2-mediated deregulation of signaling play important roles in human tumors, making it an attractive molecular marker for targeted therapy or personalized treatment of human cancers.16,24,37
EZH2 is deregulated in several human tumors, including breast, prostate, urinary bladder, ovarian, liver, lung, and gastric cancer, as well as renal cell carcinoma, lymphoma, melanoma, myeloid leukemia, endometrial cancer, squamous cell carcinoma, medulloblastoma, and glioblastoma.3,4,7,11-13,18,21,23,28,29,33,34,36,41-43 Recently, small-molecule inhibitors of EZH2 histone methyltransferase activity have been shown to decrease global histone H3 lysine 27 trimethylation levels, resulting in the reactivation of silenced target genes and inhibition of proliferation in lymphomas.23,30 High-throughput screening of different inhibitors based on the SET (Su[var]3-9, Enhancer of zeste, and Trithorax) domain of EZH2 has revealed several additional specific and potent inhibitors.30 In addition, 2 other clinical trials in subjects with lymphoma (trials NCT02395601 and NCT02082977) are currently recruiting participants.
In our previous study, we noted that the EZH2 protein was overexpressed in canine mammary carcinomas, as in human breast cancer.6 In the present study, we investigated the expression level of EZH2 in various normal or tumorous tissues in dogs and compared those patterns of expression with those in their human counterparts. To investigate the roles of EZH2 in canine carcinogenesis, we examined expression profiles of canine EZH2 in clinical samples of various types of cancer and found that EZH2 levels were significantly upregulated compared with those in corresponding normal tissues.35 The data presented herein indicate that canine tumors share molecular features with human cancers and are likely to serve as effective models during investigation into novel therapeutic approaches.
Materials and Methods
Canine tissue samples.
Tumor samples were collected from 82 domestic dogs that had been seen at a veterinary clinic because of a mass. The lesions were surgically removed, fixed in 10% neutral buffered formalin at the time of resection, and were submitted to the University of Ulsan College of Medicine between June 2014 and November 2016. All the tissues were processed, trimmed, embedded in paraffin, sectioned (thickness, 4 to 6 µm), and stained with hematoxylin and eosin for microscopic examination. Normal dog tissues were obtained from the paraffin-embedded blocks (n = 10 each) of skin, spleen, lymph node, liver, epididymis, testis, uterus, cervix, and ovary from control beagles in another study, to avoid euthanizing additional animals.
Human tissue samples.
Fresh tumor samples were obtained from patients during surgical procedures performed at Asan Medical Center from 2005 through 2015, according to procedures approved by the Institutional Review Board (Asan Medical Center, 2016–1209). At the time of exploratory laparotomy or surgical resection, samples were examined grossly by a surgical pathologist for tumor infiltration and were frozen in liquid nitrogen. Samples were stored at –70 °C. Informed consent could not be obtained, because research was performed by using samples in a retrospective tissue database; consequently, no waiver was requested from the ethical committee. We examined 52 normal samples and 48 tumor tissues, which were resected from breast, colon, gallbladder, kidney, liver, lung, ovary, pancreas, stomach, thyroid gland, tonsil, uterus, and cervix.
Fresh tissue preparation.
Frozen tissue specimens were embedded in optimal cutting temperature compound (OCT, Sakura Finetek, Torrance, CA) for thin sectioning in a cryostat microtome at –80 °C. Sections (thickness, 4 to 6 μm) were cut and placed on coated slides (catalog no. 5116- 20F, Silane Coated Slide, Muto-Glass, Tokyo, Japan). Sections were air dried for 1 min at room temperature and acetone-fixed for 5 min at room temperature, followed by an additional 60 min of air drying before staining.
Histologic evaluation.
Microscopic evaluations of all dog samples were performed by at least 2 veterinary pathologists. In addition, all samples were classified according to their morphologic origin. All 86 human cases had been diagnosed by surgical pathologists at Asan Medical Center.
Immunohistochemistry.
Sections (3 μm) from paraffin-embedded tissue blocks of canine tumor tissues were mounted on glass slides (catalog no. 5116-20F, Silane Coated Slide, Muto-Glass). Immunohistochemistry of the sections was performed by using an automated slide preparation system (Benchmark XT, Ventana Medical Systems, Tucson, AZ). Deparaffinization, epitope retrieval, and immunostaining were performed according to the manufacturer's instructions. Epitope retrieval was performed by using cell-conditioning solutions (catalog no. 950-124, Ventana Medical Systems) at 100 °C for 60 min. For immunostaining, the BMK UltraView Universal DAB Detection Kit (catalog no. 760-500, Ventana Medical Systems) was used. Tumor sections were stained with EZH2 (dilution, 1:100; catalog no. ab109398, Abcam, Cambridge, MA) at 37 °C for 36 min. Ultraview HRP Universal Multimer (included in the UltraVIEW DAB Detection Kit) was used as a secondary antibody at 37 °C for 8 min. Positive signals were amplified by using UltraVIEW DAB and UltraVIEW Copper (included in the UltraVIEW DAB Detection Kit) at 37 °C for 4 min, and sections were counterstained with hematoxylin and bluing reagent at 37 °C for 4 min each.
Sections (6 μm) from tissue blocks of human tissues were mounted on glass slides. Immunohistochemistry of the frozen sections was performed by using a semiautomated slide preparation system (model S3800 Autostainer Plus, DakoCytomation, Glostrup, Denmark). Rabbit polyclonal antibody to EZH2 was used (dilution, 1:100; catalog no. ab84989, Abcam). Slides were mounted on the Autostainer, treated with methanol for 1 min, and blocked with peroxidase blocking reagent (catalog no. S2023, Dako, Carpinteria, CA) for 10 min and with protein blocking solution (catalog no. X0909, Dako) for 1 h. After that, the slides were incubated with primary antibody to EZH2 (dilution, 1:100; catalog no. ab84989, Abcam) for 1 h and then with antirabbit HRP-labeled polymer (K4003, Dako) as a secondary antibody for 30 min. The marker was visualized with DAB chromogen substrate (catalog no. K3468, Dako). The sections were removed from the stainer and counterstained with Mayer hematoxylin (catalog no. S3309, Dako), and then the slides were rinsed, dehydrated, and mounted. All procedures were performed at room temperature.
Immunohistochemical evaluation of EZH2.
EZH2 expression was evaluated on the slides by using a previously described semiquantitative scoring system, with modifications.6 Samples were evaluated for staining intensity (0, none; 1, weakly positive; 2, moderately positive; 3, strongly positive; and 4, markedly positive). The distribution of nuclear area positively stained for EZH2 was scored as follows: 0, 0%; 1, 1% to 25%; 2; greater than 25% to 50%; 3; greater than 50% to 75%; and 4, greater than 75%.
SDS-PAGE and Western blotting.
Approximately 10 mg canine tumor tissue was homogenized by using TissueLyser II (Qiagen, Valencia, CA); suspended in sample buffer (62 mmol/L Tris-Cl, pH 6.8; 2% SDS; 10% glycerol, and 0.01% bromophenol blue with 5% 2-mercaptoethanol), incubated for 5 min at 100 °C, and then electrophoretically separated in a 12% polyacrylamide minigel. Electrophoresis was performed in Tris-buffered saline (TBS) at a constant current of 60 mA for 2 h. Molecular weight standards (catalog no. P8502–050, GenDEPOT, Barker, TX) were run simultaneously. The gel was stained with Coomassie blue. A parallel SDS-PAGE gel was run as described, and the separated proteins were transferred directly by tank blotting onto a polyvinyl difluoride transfer membrane (Bio-Rad, Hercules, CA) for 90 min at a constant current of 80 mA. After saturation of nonspecific sites by incubating the membrane in 5% nonfat milk in TBS overnight at 4 °C, the proteins were probed by incubating the blocked membrane in a 1:500 dilution of rabbit antiEZH2 antibody (catalog no. ab186006, Abcam) overnight at 4 °C. The blot was then washed in 20 mM Tris-HCl (pH 7.5) and 0.14 mM NaCl containing 0.5% Tween 20and incubated for 2 h at room temperature in TBS-Tween containing antirabbit HRP-conjugated IgG antibody (dilution, 1:1000; catalog no. SC-2004, Santa Cruz Biotechnology, Santa Cruz, CA). The immunoblot was exposed to an enhanced chemiluminescence immunoassay substrate reagent (catalog no. DG-WP250, DoGen, Seoul, Korea) for 1 min to detect signals, and the membrane was exposed to X-ray film for 5 min. Band intensity on the exposed film was semiquantified by using ImageJ software (NIH, Bethesda, MD).
Statistical analysis.
Data are expressed as mean ± 1 SD. Because our data were not normally distributed in the Kolmogorov–Smirnov test, we compared the data by using the Kruskal–Wallis test, a nonparametric method (SPSS version 21, IBM, Armonk, NY). Paired samples were compared by using the Mann–Whitney test. Bonferroni correction was applied to correct for multiple comparisons of the primary end point. Statistically significant difference between mean scores was defined as a P value less than 0.05.
Results
Histologic evaluation.
We described the clinical and morphologic features of the 82 canine cases (Table 1). These 82 cases represented various tumor morphologies that displayed a range of benign to malignant features. In addition, 86 human cases were diagnosed and included each type of tumor tissue paired with its normal counterpart (Table 2).
Table 1.
Summary of canine tumor cases
| Lymphoma | Melanocytoma | Melanoma | Mast cell tumor | Histiocytoma | Hemangioma | Hemangiosarcoma | Hepatocellular carcinoma | Prostate adenocarcinoma | Sebaceous gland tumor | Hepatoid gland tumor | Squamous cell carcinoma | Trichoblastoma | Basal cell tumor | Lipoma | Ameloblastoma | Seminoma | Granulosa cell tumor | |
| No. of cases | 10 | 3 | 6 | 9 | 5 | 3 | 3 | 3 | 1 | 6 | 6 | 3 | 4 | 4 | 4 | 4 | 4 | 4 |
| Distribution | 10 | 3 | 6 | 9 | 5 | 3 | 3 | 3 | 1 | 6 | 6 | 3 | 4 | 4 | 4 | 4 | 4 | 4 |
| 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 0 |
| 1 | 0 | 2 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 2 | 0 | 0 | 0 | 1 | 1 | 1 | 1 |
| 2 | 4 | 0 | 1 | 3 | 0 | 1 | 1 | 0 | 1 | 3 | 3 | 1 | 2 | 0 | 0 | 2 | 1 | 2 |
| 3 | 4 | 0 | 4 | 4 | 3 | 1 | 1 | 0 | 0 | 2 | 1 | 1 | 2 | 2 | 0 | 1 | 2 | 1 |
| 4 | 2 | 0 | 1 | 1 | 2 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 2 | 0 | 0 | 0 | 0 |
| Mean | 2.80 | 0.67 | 3.00 | 2.56 | 3.40 | 2.00 | 3.00 | 0.33 | 2.00 | 2.17 | 1.83 | 3.00 | 2.75 | 3.25 | 0.25 | 2.00 | 2.25 | 2.00 |
| 1 SD | 0.79 | 0.58 | 0.63 | 0.88 | 0.55 | 1.00 | 1.00 | 0.58 | — | 0.75 | 0.75 | 1.00 | 0.96 | 0.50 | 0.50 | 0.82 | 0.96 | 0.82 |
| Intensity | 10 | 3 | 6 | 9 | 5 | 3 | 3 | 3 | 1 | 6 | 6 | 3 | 4 | 4 | 4 | 4 | 4 | 4 |
| 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 0 |
| 1 | 1 | 2 | 0 | 2 | 0 | 1 | 0 | 1 | 0 | 2 | 2 | 1 | 0 | 0 | 1 | 1 | 1 | 1 |
| 2 | 4 | 0 | 2 | 3 | 1 | 1 | 1 | 0 | 0 | 2 | 3 | 0 | 2 | 0 | 0 | 3 | 2 | 3 |
| 3 | 3 | 0 | 4 | 4 | 4 | 1 | 1 | 0 | 1 | 2 | 1 | 1 | 1 | 3 | 0 | 0 | 1 | 0 |
| 4 | 2 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 |
| Mean | 2.60 | 0.67 | 2.67 | 2.22 | 2.80 | 2.00 | 3.00 | 0.33 | 3.00 | 2.00 | 1.83 | 2.67 | 2.50 | 3.50 | 0.25 | 1.75 | 2.00 | 1.75 |
| 1 SD | 0.97 | 0.58 | 0.52 | 0.83 | 0.45 | 1.00 | 1.00 | 0.58 | — | 0.89 | 0.75 | 1.53 | 0.58 | 0.58 | 0.50 | 0.50 | 0.82 | 0.50 |
Distribution (positively stained nuclear EZH2 area): 0, 0%; 1, 1% to 25%; 2; >25% to 50%; 3; >50% to 75%; and 4, >75%.
Intensity: 0, none; 1, weakly positive; 2, moderately positive; 3, strongly positive; and 4, markedly positive.
Table 2.
Mean EZH2 scores in human normal tissues
| Breast | Colon | Gallbladder | Kidney | Liver | Lung | Ovary | Pancreas | Stomach | Thyroid gland | Tonsil | Uterus | Cervix | |
| Number of cases | 4 | 6 | 2 | 5 | 5 | 5 | 4 | 3 | 4 | 5 | 3 | 3 | 3 |
| EZH2 distribution | 0.00 | 2.00 | 1.00 | 1.80 | 2.60 | 1.80 | 0.75 | 2.00 | 4.00 | 0.60 | 0.33 | 1.00 | 0.00 |
| EZH2 intensity | 0.00 | 1.33 | 0.50 | 1.40 | 2.40 | 1.20 | 0.50 | 1.00 | 2.00 | 0.60 | 0.33 | 1.00 | 0.00 |
See Table 1 for definitions.
Immunohistochemistry of canine tissue.
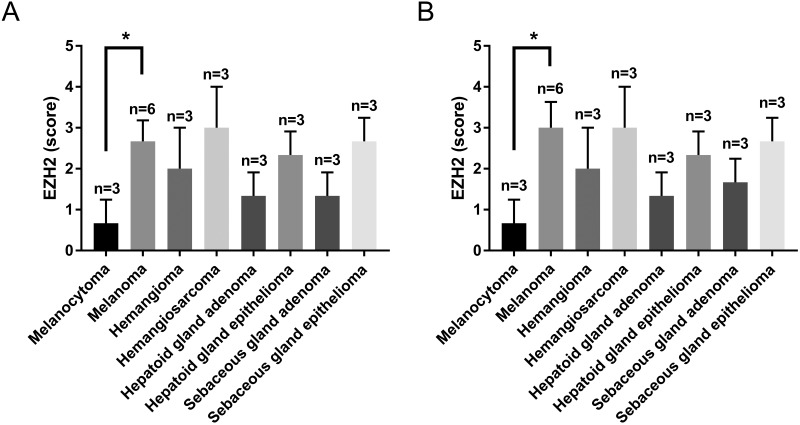
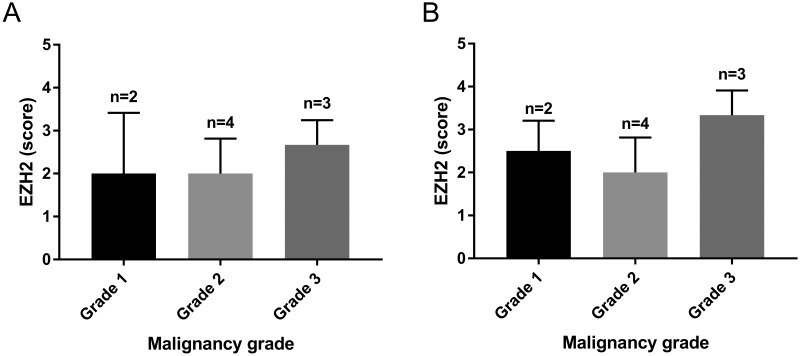
We were interested in determining whether EZH2 expression is dysregulated in canine tumors that are similar to human tumors. We identified 10 normal canine tissues and 82 cases of canine tumors. In immunohistochemical analyses, normal and nonneoplastic tissues showed negative or weak nuclear staining for EZH2. Nuclear–cytoplasmic staining for EZH2 was present in 76 of the 82 canine tumors (Figure 1). Positive staining was widely distributed in cases of canine basal cell tumor, histiocytoma, melanoma, squamous cell carcinoma, hemangiosarcoma, lymphoma, sebaceous gland epithelioma, and mast cell tumor. The intensity of the positive pattern was particularly strong in basal cell tumor, prostate adenocarcinoma, hemangiosarcoma, trichoblastoma, histiocytoma, melanoma, squamous cell carcinoma, sebaceous gland epithelioma, and lymphoma. These tumors showed a higher level of EZH2 expression than normal tissues, and the EZH2 expression level was associated with the aggressiveness of the tumor. For example, melanoma, hemangiosarcoma, hepatoid gland adenoma, and sebaceous gland adenoma had higher EZH2 levels and malignancy grades than melanocytoma, hemangioma, hepatoid epithelioma, and sebaceous gland epithelioma, respectively (Figure 2). Within mast cell tumors, grade 3 represented the highest grade of intensity and distribution (Figure 3).
Figure 1.
Representative canine tumor tissues. (A) Melanoma, hematoxylin and eosin. (B) Melanoma, EZH2 immunohistochemistry. The EZH2 distribution score 3, and the intensity score is 2. (C) Lymphoma, hematoxylin and eosin. (D) Lymphoma, EZH2 immunohistochemistry. The distribution score is 3, and the intensity score is 4. (E) Squamous cell carcinoma, hematoxylin and eosin. (F) Squamous cell carcinoma, EZH2 immunohistochemistry. The distribution score is 4 and the intensity score is 4. (G) Mast cell tumor, hematoxylin and eosin. (H) Mast cell tumor, EZH2 immunohistochemistry. The distribution score is 4, and the intensity score is 3. Magnification, 200×.
Figure 2.
Immunohistochemical scores of EZH2 expression in various canine tumors. (A) EZH2 distribution scores. (B) EZH2 intensity scores. *, P < 0.05. Data are expressed as mean ± 1 SD.
Figure 3.
Immunohistochemical intensity scores of EZH2 according to the grade of malignancy in canine mast cell tumors. (A) Distribution score within mast cell tumor. (B) Intensity score within mast cell tumor. Data are expressed as mean ± 1 SD.
Immunohistochemistry of human tissue.
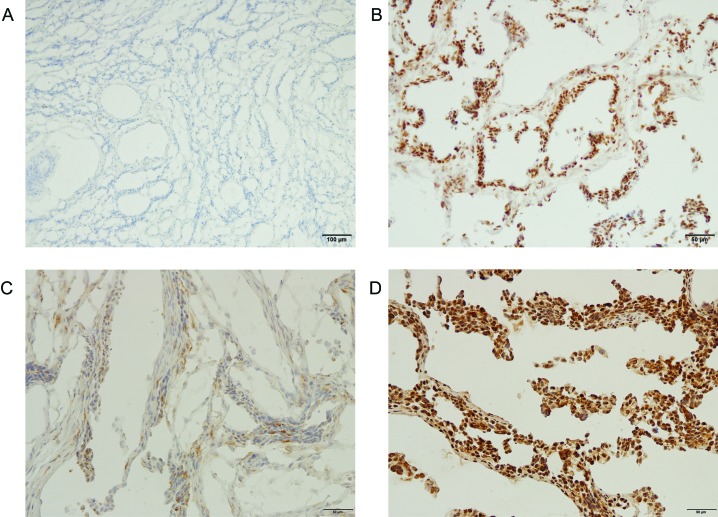
In all, 48 cases of human tumors and 52 samples of normal tissues were evaluated. Negative or weak nuclear staining for EZH2 was observed in most normal tissues, including breast, colon, gallbladder, kidney, lung, ovary, pancreas, thyroid gland, tonsil, uterus, and cervix, (Figure 4). However, EZH2 staining intensity or distribution in normal liver and stomach tissues was similar to or greater than that in tumors derived from each location (Table 2). According to our criteria, human tumors of the colon, gallbladder, kidney, lung, ovary, pancreas, thyroid, tonsil, uterus, and cervix had elevated levels of EZH2 expression (Table 3). In addition, tumors of the colon, ovary, gallbladder, and uterus showed high EZH2 staining intensity, and ovary, gallbladder, colon, and uterus tissues demonstrated a widely distributed nuclear pattern of EZH2 staining.
Figure 4.
EZH2 immunohistochemistry of representative human tissues. (A) Normal cervix; magnification, 100×. No positive staining. (B) Cervical adenocarcinoma; magnification, 200×. The EZH2 distribution score is 3, and the intensity score is 4. (C) Normal ovary; magnification, 100×. Weak positive staining is present. The distribution score is 2, and the intensity score is 1. (D) Ovarian papillary serous cystadenocarcinoma; magnification, 200×. The distribution score is 4, and the intensity score is 4.
Table 3.
Mean EZH2 scores in human tumors
| Breast | Colon | Gallbladder | Kidney | Liver | Lung | Ovary | Pancreas | Stomach | Thyroid gland | Tonsil | Uterus | Cervix | |
| Number of cases | 3 | 4 | 3 | 4 | 5 | 4 | 4 | 3 | 4 | 3 | 5 | 3 | 3 |
| EZH2 distribution | 1.33 | 3.00 | 3.33 | 2.50 | 2.60 | 2.50 | 3.50 | 2.67 | 3.00 | 2.33 | 2.40 | 3.00 | 1.33 |
| EZH2 intensity | 1.33 | 3.50 | 3.00 | 2.50 | 2.20 | 2.50 | 3.25 | 2.67 | 2.50 | 2.33 | 2.80 | 3.00 | 2.00 |
See Table 1 for definitions.
Western blotting.
We used immunoblotting to compare the results of immunohistochemistry with molecular data. Western blot analysis showed a higher expression of EZH2 in canine tumor tissues than in control, nonneoplastic tissues. In particular, lymphoma, mast cell tumors, and histiocytomas had markedly higher levels of EZH2 expression than samples of normal tissue from the corresponding organs (Figure 5).
Figure 5.
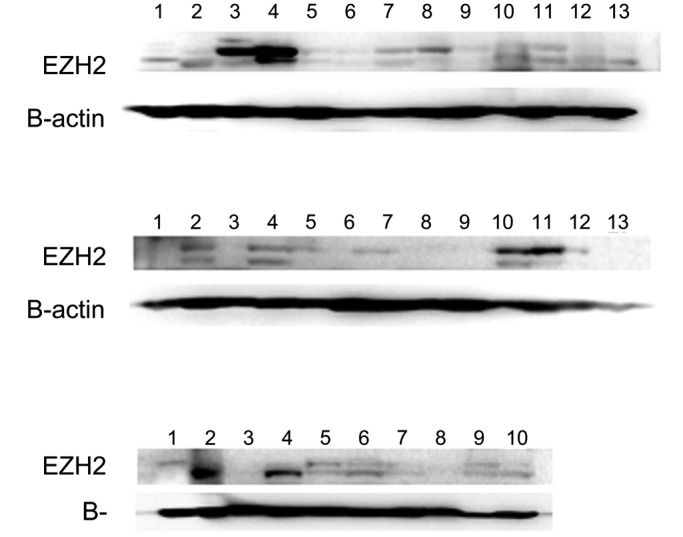
Immunoblots of canine tissues for EZH2. First row: lane 1, normal lymph node; 2–4, lymphoma; 5, normal spleen; 6–8, lymphoma; 9 and 10, hemangioma; and 11–13, hemangiosarcoma. Second row: lane 1, normal skin; 2–4, melanoma; 5–7, squamous cell carcinoma; 8–10, mast cell tumor (grade 2); and 11–13, histiocytoma. Third row: lane 1, normal skin; 2–4, trichoblastoma; 5–7, sebaceous gland adenoma; 8, sebaceous gland epithelioma; 9, normal liver; and 10, hepatocellular carcinoma. EZH2 expression was higher in the lymphoma, mast cell tumors, and histiocytoma samples than the counterpart normal tissues.
Discussion
Recently, EZH2 has emerged as a potential target for anticancer drugs, and this line of thinking is prompting the development of inhibitory strategies for EZH2. Although increased expression of EZH2 has been observed in aggressive tumors in humans, the mechanism involved in the mediation of tumor aggressiveness by EZH2 remains unclear. In our current study, we characterized the expression pattern of EZH2 in canine tumors by using immunohistochemical staining and immunoblot analysis. In our immunohistochemical analysis, tumor cells exhibited nuclear–cytoplasmic staining patterns with various expression levels. We found that EZH2 protein was more highly expressed in several canine tumors than in normal-tissue counterparts.
In our present analysis, canine lymphoma demonstrated high expression of EZH2; this result corresponds with those for human lymphoma. The hyperactivation of PRC2 due to mutation or overexpression of EZH2 has been identified as a frequent genetic event among B-cell lymphomas.19,20,22 In particular, EZH2 is upregulated when B cells proliferate and during immunoglobulin affinity maturation.
In humans, EZH2 is an important driver of melanoma progression. Its upregulation in melanoma is associated with more aggressive tumor subtypes and decreased survival.3,17 Although we have no prognostic data for melanoma in dogs, our present data showed that the EZH2 expression level is higher in malignant melanoma than in benign melanocytoma, thus suggesting correlation between the EZH2 level and the aggressiveness of the tumor. We further found a high level of EZH2 expression in canine basal cell tumors, squamous cell tumors, trichoblastomas, sebaceous gland tumors, and hepatoid gland adenomas. These results are consistent with observations in humans, in that EZH2 overexpression has been reported in human basal cell tumors and squamous cell tumors.1,4,25 In addition, EZH2 expression was elevated in canine granulosa cell tumors, seminomas, and prostate adenocarcinomas, again similar to human data and indicating EZH2 overexpression in tumors of male and female reproductive organs.3,9,11,34 In the present study, we compared human ovary, cervix, and breast tumors with normal tissue and found increased tumor expression of EZH2. Furthermore, hemangiosarcoma showed particularly high EZH2 expression, which implies a correlation between EZH2 overexpression and tumor aggressiveness.
Neither normal nor tumor tissue samples from canine liver showed high EZH2 expression. This low level of EZH2 expression in normal dog tissue was similar to that in normal human samples, but the expression level in canine tumors did not match that of human tumors. In contrast, increased positive expression was noted in both normal and tumor tissues among our human samples, and these levels did not significantly differ between the two types of samples. Although EZH2 overexpression in liver has been reported,2,5,32,39 in the human protein database, 5 of 11 human liver cancer samples showed low or no EZH2 staining.32 Integrating these data, the expression level of EZH2 in liver cancer seems to be diverse, and we do not recommend using a canine clinical model for this type of tumor.
There were some positive signals in normal dog and human tissues as well. Our results indicate that in dogs, cell populations in the skin (particularly the basal layer and hair follicle), immune system (including lymph nodes and the spleen), and ovary show strong positive staining patterns for EZH2. These findings correlate with those in the counterpart human samples. Especially in the skin, EZH2 expression appears to reflect the functions of this protein. It has been reported that Polycomb group proteins (including EZH2) control gene expression during the differentiation of stem cells.14,15,31 Repression mediated by Polycomb proteins controls the timing of the differentiation of epidermal precursor cells.8,10,38 Similar to embryonic stem cells, basal cells have EZH2 and other Polycomb group proteins. Therefore, the staining pattern in skin probably arose because EZH2 plays vital roles in stem cell maintenance and lineage differentiation.
In summary, our current results show that canine lymphoma, melanoma, basal cell tumors, squamous cell carcinoma, and—despite its low incidence—prostate cancer demonstrate EZH2 overexpression. Because these features correspond to those of the human tumor counterparts, these naturally occurring canine tumors might be used as clinical models. Although mast cell tumors, histiocytomas, sebaceous gland tumors, hepatoid gland tumors, trichoblastomas, and ameloblastomas are more frequent in or specific to dogs, these tumors are still worth using as models for the development of drugs for veterinary indications. In addition, the patterns of EZH2 expression in normal tissues might give insights into the target organ or location of possible toxicity due to EZH2 inhibition.
EZH2 inhibitors are under development and testing, and 3 of these compounds have moved to clinical trials. Although our data are based on a rather limited number of samples, our results constitute a foundation for further research regarding the role of EZH2 in humans and dogs and lend credence to the view that spontaneous canine tumors are valuable models for studies on EZH2 and other factors.26,27 Further research into the mechanisms of the EZH2 deregulation in canine tumors is necessary to develop specific targeted treatment methods.
Acknowledgments
This study was supported by the Technology Innovation Program (grant number 10067737: Establishment of risk management platform with the aim of reducing the attrition of new drugs and their service) funded by the Ministry of Trade, Industry, and Energy (Korea) and the Korean Health Technology Research and Development Project funded by the Ministry of Health and Welfare, Republic of Korea (grant number HI15C0972).
References
- 1.Adhikary G, Grun D, Balasubramanian S, Kerr C, Huang JM, Eckert RL. 2015. Survival of skin cancer stem cells requires the Ezh2 Polycomb group protein. Carcinogenesis 36:800–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Au SLK, Wong CCL, Lee JMF, Fan DNY, Tsang FH, Ng IOL, Wong CM. 2012. Enhancer of zeste homolog 2 epigenetically silences multiple tumor suppressor microRNAs to promote liver cancer metastasis. Hepatology 56:622–631. [DOI] [PubMed] [Google Scholar]
- 3.Bachmann IM, Halvorsen OJ, Collett K, Stefansson IM, Straume O, Haukaas SA, Salvesen HB, Otte AP, Akslen LA. 2005. EZH2 expression is associated with high proliferation rate and aggressive tumor subgroups in cutaneous melanoma and cancers of the endometrium, prostate, and breast. J Clin Oncol 24:268–273. [DOI] [PubMed] [Google Scholar]
- 4.Banerjee R, Mani RS, Russo N, Scanlon CS, Tsodikov A, Jing X, Cao Q, Palanisamy N, Metwally T, Inglehart RC, Tomlins S, Bradford C, Carey T, Wolf G, Kalyana-Sundaram S, Chinnaiyan AM, Varambally S, D'Silva NJ. 2011. The tumor suppressor gene rap1GAP is silenced by miR101-mediated EZH2 overexpression in invasive squamous cell carcinoma. Oncogene 30:4339–4349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen Y, Lin MCM, Wang H, Chan CY, Jiang L, Ngai SM, Yu J, He ML, Shaw PC, Yew DT, Sung JJ, Kung HF. 2007. Proteomic analysis of EZH2 downstream target proteins in hepatocellular carcinoma. Proteomics 7:3097–3104. [DOI] [PubMed] [Google Scholar]
- 6.Choi HJ, Jang S, Ryu JE, Lee HJ, Lee HB, Ahn WS, Kim HJ, Lee HJ, Lee HJ, Gong GY, Son WC. 2016. Significance of EZH2 expression in canine mammary tumors. BMC Vet Res 12:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Collett K, Eide GE, Arnes J, Stefansson IM, Eide J, Braaten A, Aas T, Otte AP, Akslen LA. 2006. Expression of enhancer of zeste homologue 2 is significantly associated with increased tumor cell proliferation and is a marker of aggressive breast cancer. Clin Cancer Res 12:1168–1174. [DOI] [PubMed] [Google Scholar]
- 8.Ezhkova E, Pasolli HA, Parker JS, Stokes N, Su IH, Hannon G, Tarakhovsky A, Fuchs E. 2009. Ezh2 orchestrates gene expression for the stepwise differentiation of tissue-specific stem cells. Cell 136:1122–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hinz S, Magheli A, Weikert S, Schulze W, Krause H, Schrader M, Miller K, Kempkensteffen C. 2009. Deregulation of EZH2 expression in human spermatogenic disorders and testicular germ cell tumors. World J Urol 28:631–635. [DOI] [PubMed] [Google Scholar]
- 10.Hock H. 2012. A complex Polycomb issue: the 2 faces of EZH2 in cancer. Genes Dev 26:751–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim KH, Roberts CWM. 2016. Targeting EZH2 in cancer. Nat Med 22:128–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ler LD, Ghosh S, Chai XR, Thike AA, Heng HL, Siew EY, Dey S, Koh LK, Lim JQ, Lim WK, Myint SS, Loh JL, Ong P, Sam XX, Huang DC, Lim T, Tan PH, Nagarajan S, Cheng CWS, Ho H, Ng LG, Yuen J, Lin PH, Chuang CK, Chang YH, Weng WH, Rozen SG, Tan P, Creasy CL, Pang ST, McCabe MT, Poon SL, Teh BT. 2017. Loss of tumor suppressor KDM6A amplifies PRC2-regulated transcriptional repression in bladder cancer and can be targeted through inhibition of EZH2. Sci Transl Med 9:eaai8312. [DOI] [PubMed] [Google Scholar]
- 13.Li H, Cai Q, Godwin AK, Zhang RG. 2010. Enhancer of zeste homolog 2 promotes the proliferation and invasion of epithelial ovarian cancer cells. Mol Cancer Res 8:1610–1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li J, Xi Y, Li W, McCarthy RL, Stratton SA, Zou W, Li W, Dent SY, Jain AK, Barton MC. 2017. TRIM28 interacts with EZH2 and SWI/SNF to activate genes that promote mammosphere formation. Oncogene 36:2991–3001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Margueron R, Reinberg D. 2011. The polycomb complex PRC2 and its mark in life. Nature 469:343–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McCabe MT, Ott HM, Ganji G, Korenchuk S, Thompson C, Van Aller GS, Liu Y, Graves AP, Della Pietra A, Diaz E, LaFrance LV, Mellinger M, Duquenne C, Tian XR, Kruger RG, McHugh CF, Brandt M, Miller WH, Dhanak D, Verma SK, Tummino PJ, Creasy CL. 2012. EZH2 inhibition as a therapeutic strategy for lymphoma with EZH2-activating mutations. Nature 492:108–112. [DOI] [PubMed] [Google Scholar]
- 17.McHugh JB, Fullen DR, Ma L, Kleer CG, Su LD. 2007. Expression of Polycomb group protein EZH2 in nevi and melanoma. J Cutan Pathol 34:597–600. [DOI] [PubMed] [Google Scholar]
- 18.Mohammad F, Weissmann S, Leblanc B, Pandey DP, Højfeldt JW, Comet I, Zheng C, Johansen JV, Rapin N, Porse BT, Tvardovskiy A, Jensen ON, Olaciregui NG, Lavarino C, Suñol M, de Torres C, Mora J, Carcaboso AM, Helin K. 2017. EZH2 is a potential therapeutic target for H3K27M-mutant pediatric gliomas. Nat Med 23:483–492. [DOI] [PubMed] [Google Scholar]
- 19.Morin RD, Johnson NA, Severson TM, Mungall AJ, An JH, Goya R, Paul JE, Boyle M, Woolcock BW, Kuchenbauer F, Yap D, Humphries RK, Griffith OL, Shah S, Zhu H, Kimbara M, Shashkin P, Charlot JF, Tcherpakov M, Corbett R, Tam A, Varhol R, Smailus D, Moksa M, Zhao YJ, Delaney A, Qian H, Birol I, Schein J, Moore R, Holt R, Horsman DE, Connors JM, Jones S, Aparicio S, Hirst M, Gascoyne RD, Marra MA. 2010. Somatic mutations altering EZH2 (Tyr641) in follicular and diffuse large B-cell lymphomas of germinal-center origin. Nat Genet 42:181–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morin RD, Mendez-Lago M, Mungall AJ, Goya R, Mungall KL, Corbett RD, Johnson NA, Severson TM, Chiu R, Field M, Jackman S, Krzywinski M, Scott DW, Trinh DL, Tamura-Wells J, Li S, Firme MR, Rogic S, Griffith M, Chan S, Yakovenko O, Meyer IM, Zhao EY, Smailus D, Moksa M, Chittaranjan S, Rimsza L, Brooks-Wilson A, Spinelli JJ, Ben-Neriah S, Meissner B, Woolcock B, Boyle M, McDonald H, Tam A, Zhao YJ, Delaney A, Zeng T, Tse K, Butterfield Y, Birol I, Holt R, Schein J, Horsman DE, Moore R, Jones SJM, Connors JM, Hirst M, Gascoyne RD, Marra MA. 2011. Frequent mutation of histone-modifying genes in nonHodgkin lymphoma. Nature 476:298–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oki S, Sone K, Oda K, Nishijima A, Takeuchi M, Agapiti C, Asada K, Makii C, Kawana K, Osuga Y, Fuji T. 2016. The histone methyltransferase EZH2 is a novel target of anticancer therapy in endometrial cancer. Cancer Res 76 14 Suppl:969A. [Google Scholar]
- 22.Okosun J, Bodor C, Wang J, Araf S, Yang CY, Pan CY, Boller S, Cittaro D, Bozek M, Iqbal S, Matthews J, Wrench D, Marzec J, Tawana K, Popov N, O'Riain C, O'Shea D, Carlotti E, Davies A, Lawrie CH, Matolcsy A, Calaminici M, Norton A, Byers RJ, Mein C, Stupka E, Lister TA, Lenz G, Montoto S, Gribben JG, Fan YH, Grosschedl R, Chelala C, Fitzgibbon J. 2013. Integrated genomic analysis identifies recurrent mutations and evolution patterns driving the initiation and progression of follicular lymphoma. Nat Genet 46:176–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peker D, Roman-Holba S, Kwon Y, Gordetsky J, Mehta A, Forero A, Costa LJ, Reddy V, Yang Y, Chakravarthi BVSK, Varambally S. 2016. EZH2 upregulation is associated with unfavorable prognosis in diffuse large B-cell lymphoma through potential RUNX3 downregulation. Blood 128:5301. [Google Scholar]
- 24.Qi W, Chan HM, Teng L, Li L, Chuai SN, Zhang RP, Zeng J, Li M, Fan H, Lin Y, Gu J, Ardayfio O, Zhang JH, Yan XX, Fang JL, Mi Y, Zhang M, Zhou T, Feng G, Chen ZJ, Li GB, Yang T, Zhao KH, Liu XH, Yu ZT, Lu CX, Atadja P, Li E. 2012. Selective inhibition of EZH2 by a small molecule inhibitor blocks tumor cells proliferation. Proc Natl Acad Sci USA 109:21360–21365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rao RC, Chan MP, Andrews CA, Kahana A. 2016. EZH2, proliferation rate, and aggressive tumor subtypes in cutaneous basal cell carcinoma. JAMA Oncol 2:962–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Riccardo F, Aurisicchio L, Impellizeri JA, Cavallo F. 2014. The importance of comparative oncology in translational medicine. Cancer Immunol Immunother 64:137–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saba C, Paoloni M, Mazcko C, Kisseberth W, Burton JH, Smith A, Wilson-Robles H, Allstadt S, Vail D, Henry C, Lana S, Ehrhart EJ, Charles B, Kent M, Lawrence J, Burgess K, Borgatti A, Suter S, Woods P, Gordon I, Vrignaud P, Khanna C, LeBlanc AK. 2016. A comparative oncology study of iniparib defines its pharmacokinetic profile and biological activity in a naturally occurring canine cancer model. PLoS One 11:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sakurai T, Bilim VN, Ugolkov AV, Yuuki K, Tsukigi M, Motoyama T, Tomita Y. 2012. The enhancer of zeste homolog 2 (EZH2), a potential therapeutic target, is regulated by miR101 in renal cancer cells. Biochem Biophys Res Commun 422:607–614. [DOI] [PubMed] [Google Scholar]
- 29.Sasaki M, Ikeda H, Itatsu K, Yamaguchi J, Sawada S, Minato H, Ohta T, Nakanuma Y. 2008. The overexpression of Polycomb group proteins BMI1 and EZH2 is associated with the progression and aggressive biological behavior of hepatocellular carcinoma. Lab Invest 88:873–882. [DOI] [PubMed] [Google Scholar]
- 30.Shahabipour F, Caraglia M, Majeed M, Derosa G, Maffioli P, Sahebkar A. 2017. Naturally occurring anticancer agents targeting EZH2. Cancer Lett 400:325–335. [DOI] [PubMed] [Google Scholar]
- 31.Shen X, Liu YC, Hsu YJ, Fujiwara Y, Kim J, Mao XH, Yuan GC, Orkin SH. 2008. EZH1 mediates methylation on histone H3 Lysine 27 and complements EZH2 in maintaining stem cell identity and executing pluripotency. Mol Cell 32:491–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sudo T, Utsunomiya T, Mimori K, Nagahara H, Ogawa K, Inoue H, Wakiyama S, Fujita H, Shirouzu K, Mori M. 2005. Clinicopathological significance of EZH2 mRNA expression in patients with hepatocellular carcinoma. Br J Cancer 92:1754–1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Urayama S, Habib A, Pan W, Alzofon N, Wang S. 2016. Potential targets and role of EZH2 in pancreatic cancer. Pancreas 45:1543–1544. [Google Scholar]
- 34.Varambally S, Dhanasekaran SM, Zhou M, Barrette TR, Kumar-Sinha C, Sanda MG, Ghosh D, Pienta KJ, Sewalt RGAB, Otte AP, Rubin MA, Chinnaiyan AM. 2002. The Polycomb group protein EZH2 is involved in progression of prostate cancer. Nature 419:624–629. [DOI] [PubMed] [Google Scholar]
- 35.Völkel P, Dupret B, Le Bourhis X, Angrand PO. 2015. Diverse involvement of EZH2 in cancer epigenetics. Am J Transl Res 7:175–193. [PMC free article] [PubMed] [Google Scholar]
- 36.Wang H, Albadine R, Magheli A, Guzzo TJ, Ball MW, Hinz S, Schoenberg MP, Netto GJ, Gonzalgo ML. 2012. Increased EZH2 protein expression is associated with invasive urothelial carcinoma of the bladder. Urol Oncol 30:428–433. [DOI] [PubMed] [Google Scholar]
- 37.Xiao Y. 2011. Enhancer of zeste homolog 2: a potential target for tumor therapy. Int J Biochem Cell Biol 43:474–477. [DOI] [PubMed] [Google Scholar]
- 38.Xie H, Xu J, Hsu JH, Nguyen M, Fujiwara Y, Peng C, Orkin SH. 2014. Polycomb repressive complex 2 regulates normal hematopoietic stem cell function in a developmental-stage–specific manner. Cell Stem Cell 14:68–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xu B, Konze KD, Jin J, Wang GG. 2015. Targeting EZH2 and PRC2 dependence as novel anticancer therapy. Exp Hematol 43:698–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yamaguchi H, Hung MC. 2014. Regulation and role of EZH2 in cancer. Cancer Res Treat 46:209–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yamamoto I, Nosho K, Kanno S, Igarashi H, Kurihara H, Ishigami K, Ishiguro K, Mitsuhashi K, Maruyama R, Koide H, Okuda H, Hasegawa T, Sukawa Y, Okita K, Takemasa I, Yamamoto H, Shinomura Y, Nakase H. 2017. EZH2 expression is a prognostic biomarker in patients with colorectal cancer treated with antiEGFR therapeutics. Oncotarget 8:17810–17818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang H, Qi J, Reyes JM, Li L, Rao PK, Li FG, Lin CY, Perry JA, Lawlor MA, Federation A, De Raedt T, Li YY, Liu Y, Duarte MA, Zhang YX, Herter-Sprie GS, Kikuchi E, Carretero J, Perou CM, Reibel JB, Paulk J, Bronson RT, Watanabe H, Brainson CF, Kim CF, Hammerman PS, Brown M, Cichowski K, Long H, Bradner JE, Wong KK. 2016. Oncogenic deregulation of EZH2 as an opportunity for targeted therapy in lung cancer. Cancer Discov 6:1006–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhu Q, Zhang L, Li X, Chen F, Jiang L, Yu G, Wang Z, Yin C, Jiang X, Zhong Q, Zhou H, Ding B, Wang C, Meng F. 2016. Higher EZH2 expression is associated with extramedullary infiltration in acute myeloid leukemia. Tumour Biol 37:11409–11420. [DOI] [PubMed] [Google Scholar]