Abstract
Mesenchymal stem cells (MSCs) are of particular interest for the treatment of immune-related diseases owing to their immunosuppressive properties. In this study, we aimed to identify the effect of interferon (IFN)-γ priming on immunomodulation by MSCs and elucidate the possible mechanism underlying their properties for the clinical treatment of allogeneic conflicts. Infusion of MSCs primed with IFN-γ significantly reduced the symptoms of graft-versus-host disease (GVHD) in NOD-SCID mice, thereby increasing survival rate when compared with naïve MSC-infused mice. However, infusion of IFN-γ-primed MSCs in which indoleamine 2,3-dioxygenase (IDO) was downregulated did not elicit this effect. The IDO gene was expressed in MSCs via the IFN-γ-Janus kinase (JAK)-signal transducer and activator of transcription 1 (STAT1) pathway, and the infusion of IDO-over-expressing MSCs increased survival rate in an in vivo GVHD model, similar to infusion of IFN-γ-primed MSCs. These data indicate that IFN-γ production by activated T-cells is correlated with the induction of IDO expression in MSCs via the IFN-γ-JAK-STAT1 pathway, which in turn results in the suppression of T-cell proliferation. Our findings also suggest that cell therapy based on MSCs primed with IFN-γ can be used for the clinical treatment of allogeneic conflicts, including GVHD.
Keywords: Mesenchymal stem cell; Interferon-γ; Indoleamine 2,3-dioxygenase; Graft-versus-host disease; Cell therapy
Abbreviations: BM, bone marrow; MSCs, mesenchymal stem cells; NK, natural killer; IL, interleukin; IDO, indoleamine 2,3-dioxygenase; HSCT, hematopoietic stem cell transplantation; GVHD, graft-versus-host disease; HLA, human leukocyte antigen; IFN, Interferon; JAK, Janus kinase; STAT, signal transducer and activator of transcription; CB, cord blood; AT, adipose tissue; WJ, Wharton's jelly; hPBMCs, human peripheral blood-derived mononuclear cells; TNF, tumor necrosis factor; IRF, interferon regulatory factor; CXCL, chemokine (C-X-C motif) ligand; CCL, chemokine (C-C motif) ligand; TLR, Toll-like receptor.
Highlights
-
•
IFN-γ priming enhances the immunosuppressive properties of human MSCs in in vitro and in vivo models.
-
•
IFN-γ priming induces IDO expression in MSCs via the JAK/STAT1 signaling pathway, but TLR3 activation does not.
-
•
Cell therapy using MSCs primed with IFN-γ could be highly effective in treating allogeneic diseases, including GVHD.
It is necessary to improve the function of mesenchymal stem cells (MSCs) to maximize their treatment potential beyond what is currently achieved in cell therapy studies using naïve heterogeneous MSCs. The preclinical study of a candidate cell therapy based on MSCs primed with interferon-γ as reported in this study, could lay the foundation for the use of cell therapy for the treatment of graft-versus-host disease (GVHD), and is very important for the initiation of clinical trials. Our findings also suggest that cell therapy based on functionally improved MSCs could be used for the clinical treatment of allogeneic conflicts.
1. Introduction
The marrow stromal cells that provide growth factors, cell-to-cell interactions, matrix proteins, are derived from common precursor cells that reside in the bone marrow (BM) microenvironment, and are referred to as mesenchymal stem cells (MSCs) (Caplan, 1991, Prockop, 1997). MSCs also have the capacity to differentiate into a variety of cell types including osteoblasts, adipocytes, and chondrocytes (Barry and Murphy, 2004, Pittenger et al., 1999). MSCs can be used to help reconstitute a host BM microenvironment that has been damaged by chemotherapy or irradiation, or can serve as a vehicle for gene therapy (Baksh et al., 2004). A number of studies have revealed that following their mobilization and migration to sites of injury, MSCs contribute not only to the repair of damaged tissues but also have an immunomodulatory function (Ankrum et al., 2014, Wang et al., 2014). In this latter regard, MSCs inhibit the activation, proliferation, and function of a variety of immune cells including T-cells, B-cells, natural killer (NK) cells, and antigen-presenting cells (Nauta and Fibbe, 2007). MSC-mediated immunosuppression involves cell contact-dependent mechanisms through such proteins as programmed death-ligand 1 (PDL-1, also known as CD274 or B7 homolog 1) (Augello et al., 2005), and soluble factors such as interleukin (IL)-10 (Soleymaninejadian et al., 2012), transforming growth factor-β (Soleymaninejadian et al., 2012), nitric oxide (Sato et al., 2007, Soleymaninejadian et al., 2012), indoleamine 2,3-dioxygenase (IDO) (Meisel et al., 2004, Soleymaninejadian et al., 2012, Spaggiari et al., 2008), and prostaglandin E2 (Soleymaninejadian et al., 2012, Spaggiari et al., 2008).
Allogeneic hematopoietic stem cell transplantation (HSCT) has been widely used to treat various malignant and non-malignant hematologic diseases, autoimmune diseases, primary immunodeficiency diseases, and inborn errors of metabolism (Ringdén et al., 2006a). However, graft-versus-host disease (GVHD) remains a major cause of post-transplant morbidity and mortality, even in patients who receive a graft from a human leukocyte antigen (HLA)-identical donor (Qian et al., 2013, Ringdén et al., 2006a). GVHD is caused by donor T-cells that are activated by host antigen-presenting cells, which then migrate to target tissues (e.g., skin, gut, and liver), and cause target organ dysfunction (Bucher and Passweg, 2012). The standard first-line treatment for GVHD is a course of corticosteroids (Ruutu et al., 2012). However, about 50% of patients do not respond to first-line treatment, and those with steroid-refractory GVHD generally show a high mortality rate (Bürgler et al., 2014). Since there is no established second-line treatment for steroid-refractory GVHD, there is an urgent need for new therapies in patients suffering from severe GVHD (Medinger et al., 2013).
Interferon (IFN) γ, is a potent pro-inflammatory cytokine that is produced by multiple cell types including activated T-cells, NK cells, NKT cells, and macrophages, and plays important and complex roles in both innate and adaptive immune responses, and is considered to be a pathogenic factor related to acute GVHD. IFN-γ negatively regulates alloreactive T-cells by inhibiting cell division and promoting cell death, and prevents tissue damage through a direct interaction with recipient parenchymal cells (Asavaroengchai et al., 2007, Wang et al., 2009). Although the role of IFN-γ in activating MSCs has been previously reported in vitro (Le Blanc et al., 2003, Polchert et al., 2008, Ryan et al., 2007), little is known about its effect on the immunomodulatory effect of MSCs in vivo when used for the treatment of GVHD. The first pilot study using MSCs to treat GVHD after allogeneic HSCT showed that MSCs is very promising treatment for acute GVHD (Ringdén et al., 2006b). The infusion of MSCs with therapeutic intent is feasible and safe (Koç et al., 2000). However, the development of a cell therapy based on MSC has been hindered by the heterogeneity found in MSCs derived from various donors and various tissues (Phinney, 2007, Phinney, 2012, Reyes et al., 2001), as well as an absence of standardized protocols and culture methods used to produce such cells (Sekiya et al., 2002, Sotiropoulou et al., 2006, Vogel et al., 2003). To overcome this, it is necessary to improve the function of MSCs to maximize their treatment potential beyond what is currently achieved in cell therapy studies using naïve heterogeneous MSCs.
This study employed a microarray approach to analyze in detail the influence of IFN-γ priming on the gene expression profile related to the immunomodulatory functions of MSCs. In addition, the possible mechanism by which MSCs contribute to the control of immune conflicts was investigated using both in vitro and in vivo studies. Herein, we show that proliferation of T-cells was suppressed after co-culture with IFN-γ-primed MSCs, and that these cells increase the survival rate of GVHD mice. IFN-γ stimulation induced IDO expression, which is a key modulator in the immunosuppressive pathway in MSCs, through the IFN-γ-Janus kinase (JAK)-signal transducer and activator of transcription (STAT) 1 pathway. The results of this study therefore provide a useful and practical method for obtaining functionally qualified MSCs that can appropriately modulate the immune response, and which could be used for the treatment of immune-related disorders, including GVHD.
2. Materials and Methods
2.1. Isolation and Culture of MSCs Derived from Human Tissues
The Institutional Review Board of Samsung Medical Center approved this study (IRB No. 2015-10-025), and all samples were obtained with informed consent.
2.1.1. BM-MSCs and CB-MSCs
MSCs were isolated and cultured as described previously (Lee et al., 2004). Briefly, mononuclear cells were isolated from normal BM aspirates or term umbilical cord blood (CB) from newborns using a Ficoll-Hypaque density gradient centrifugation (Histopaque-1077; Sigma-Aldrich, St. Louis, MO). Cells were seeded at a density of 3 × 105 cells/cm2 in low-glucose Dulbecco's modified Eagle's medium (LG-DMEM; Invitrogen-Gibco, Rockville, MD) containing 10% fetal bovine serum (FBS; Invitrogen-Gibco) and 100 U/mL penicillin-streptomycin (PS; Invitrogen-Gibco). Cells were incubated in a humidified atmosphere at 37 °C with 5% CO2, and the medium was changed every 3–4 days until adherent fibroblast-like cells reached confluency. Adherent cells were then resuspended in 0.05% trypsin-EDTA (ethylenediaminetetraacetic acid; Invitrogen-Gibco) and reseeded at a density of 2 × 103 cells/cm2.
2.1.2. AT-MSCs
Adipose tissue (AT) MSCs were isolated and cultured according to a previously reported protocol (Zuk et al., 2001). Briefly, lipoaspirates were washed extensively with equal volumes of phosphate-buffered saline (PBS; Biowest, Nuaille, France), and the extracellular matrix was digested with 0.075% collagenase A (Roche Applied Science, Penzberg, Germany) at 37 °C for 30 min. Enzyme activity was neutralized with LG-DMEM containing 10% FBS and 100 U/mL PS, and samples were then centrifuged at 1200g for 10 min. After being washed with PBS and filtered through a 100 μm nylon mesh (Cell strainer; BD Biosciences, Franklin Lakes, NJ), cells were incubated in a humidified atmosphere at 37 °C with an atmosphere containing 5% CO2. Adherent cells were resuspended in 0.05% trypsin-EDTA and reseeded at a density of 2 × 103 cells/cm2.
2.1.3. WJ-MSCs
Wharton's jelly (WJ) MSCs were isolated using an explant culture method (Mitchell et al., 2003). Umbilical cords were washed in PBS to remove blood components and were then cut into small pieces (0.5–1 cm). Vessels were removed to avoid endothelial cell contamination. The WJ region of the cord was cut into 0.5–1 cm3 pieces and placed directly into culture wells for culture expansion in LG-DMEM containing 10% FBS and 100 U/mL PS. After colonies of cells appeared, and reached 70% confluency, cells were resuspended in 0.05% trypsin-EDTA and reseeded at a density of 2 × 103 cells/cm2.
2.2. T-Cell Proliferation: BrdU Incorporation Assay
MSCs were seeded at a density of 1.25 × 104 cells/mL in 96-well plates in high-glucose DMEM (Invitrogen-Gibco) supplemented with 10% FBS. After 24 h, 10 μg/mL mitomycin-C (Sigma-Aldrich) was added to inhibit cell proliferation. Treated cells were incubated for an additional 2 h at 37 °C and then washed five times with culture media. Following this, 1 × 105 human peripheral blood-derived mononuclear cells (hPBMCs) were isolated by gradient centrifugation, added to each well, and stimulated with 1 μg/mL phytohemagglutinin (PHA; Sigma-Aldrich) to stimulate T-cell proliferation. PHA-activated hPBMCs were then cultured on differentially conditioned MSCs for 3–4 days before the addition of 5-bromo-2-deoxyuridine (BrdU; Roche Applied Science). Proliferation levels were assessed after 18 h using a BrdU incorporation assay kit from Roche Applied Science.
2.3. Establishing an Animal GVHD Model
This study was approved by the Institutional Animal Care and Use Committee of Samsung Medical Center. NOD-SCID mice (8–9 weeks old; Jackson Laboratories, Bar Harbor, ME) received 300 cGy of total body irradiation 24 h before intravenous injection of hPBMCs. On the day of transplantation, 2 × 107 hPBMCs were administered to each mouse along with 1 × 106 MSCs. The same number of MSCs was injected again on day 7.
2.4. Analysis of Cytokines: Luminex Assay
MSCs were seeded at a density of 1 × 104 cells/cm2 in 6-well plates in 2 mL of LG-DMEM. After 48 h, supernatants were collected and frozen at − 70 °C. A multiplex human cytokine, chemokine, and growth factor detection kit (Upstate, Waltham, MA) was used to measure the production of IL-1α, IL-1β, IL-2, IL-3, IL-4, IL-5, IL-6, IL-7, IL-8, IL-9, IL-10, IL-12 (p40), IL-12 (p70), IL-13, IL-15, eotaxin, granulocyte-macrophage colony-stimulating factor (GM-CSF), IFN-γ, IFN-α2, IFN-γ-inducible protein (IP)-10, monocyte chemoattractant protein (MCP)-1, macrophage inflammatory protein (MIP)-1α, MIP-1β, regulated upon activation normal T-cell expressed and secreted (RANTES), tumor necrosis factor (TNF)-α, TNF-β, fibroblast growth factor (FGF), platelet-derived growth factor (PDGF)-AA, PDGF-BB, epidermal growth factor (EGF), Flt-3 ligand, and vascular endothelial growth factor (VEGF) in the culture supernatants. In addition, supernatants were collected from hPBMCs co-cultured with MSCs and stored at − 70 °C, and the production of the secretory factors listed above was assessed using the same multiplex human cytokine, chemokine, and growth factor detection kit according to the manufacturer's instructions.
2.5. RNA Isolation and Microarray Analysis
Total RNA was isolated from MSCs for gene expression profiling. Cultured MSCs were collected by treatment with 0.05% trypsin-EDTA. Total cellular RNA was extracted from pelleted cells and purified using a QIAGEN RNeasy Mini Kit (QIAGEN, Valencia, CA) according to the manufacturer's protocol. RNA quality was determined by denaturing gel electrophoresis, the OD 260/280 ratio, and analysis on an Agilent 2100 Bioanalyzer (Agilent Technologies, Palo Alto, CA). Biotinylated complementary RNA was prepared and hybridized to the Illumina Human HT-12 Expression BeadChip (Illumina, San Diego, CA). The arrays were scanned and analyzed with Illumina Genome Studio v2009.2 software (Gene Expression Module v1.5.4, Illumina). The false discovery rate was controlled by adjusting P-values using the Benjamini-Hochberg algorithm, followed by performance of gene set enrichment analysis and a one-tailed Fisher's exact test. Microarray data from 24,526 probes were filtered by applying two criteria for significance, P < 0.05 and fold change (FC) > 2.
2.6. qRT-PCR Analysis
Complementary DNA (cDNA) was produced using the Superscript reverse transcription (RT)-polymerase chain reaction (PCR) System (Invitrogen-Gibco) according to the manufacturer's recommendations for oligo (dT)20-primed cDNA synthesis. Quantitative real-time (qRT)-PCR was performed in 384-well microtiter plates using gene-specific TaqMan probes and primer sets (Assays-on-Demand, Applied Biosystems, Foster City, CA) and an ABI PRISM 7900HT Sequence Detection System (Applied Biosystems). Template cDNA was added to the reaction mixture, and amplifications were initiated with a 10 min template denaturation step at 95 °C, followed by 40 cycles of 95 °C for 15 s and 60 °C for 1 min. All samples were amplified in triplicate. Data were analyzed with Sequence Detector Software (Applied Biosystems).
2.7. RT-PCR Analysis
MSCs were cultured in 6-well plates in the presence or absence of 200 IU/mL IFN-γ (R&D Systems, Minneapolis, MN) and concurrent treatment with 100 μg/mL poly I:C (Invitrogen-Gibco) or 10 ng/mL TNF-α (R&D Systems) for 24 h. Total RNA was isolated from MSCs using a QIAGEN RNeasy Mini Kit and used to perform semi-quantitative RT-PCR assays with a commercial kit (PrimeScript™ first strand cDNA synthesis kit; TaKaRa Shuzo, Shiga, Japan). The sequences of the PCR primers are provided in Table S1. The band intensity of each PCR product was measured using NIH image/ImageJ (http://rsb.info.nih.gov/nih-image/) and normalized against that of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA.
2.8. Immunoblotting
MSCs were washed with cold PBS and lysed in 300 μL of cold RIPA buffer (Thermo Fisher Scientific, Rockford, IL) containing 1 × protease inhibitor cocktail (Thermo Fisher Scientific). Cell lysates were centrifuged at 3000g for 15 min at 4 °C. The supernatant was collected, and protein concentrations were analyzed using a bicinchoninic acid protein assay kit (Thermo Fisher Scientific). For electrophoresis, samples containing 30–50 μg of protein, were dissolved in sample buffer (Invitrogen-Gibco), boiled for 5 min, and separated on a 4–12% gradient sodium dodecyl sulfate reducing gel (Invitrogen-Gibco). Separated proteins were transferred onto polyvinylidenedifluoride membranes (GE Healthcare, Buckinghamshire, UK) using a trans-blot system (Invitrogen-Gibco). Blots were blocked for 1 h at room temperature in Tris-buffered saline (TBS; Biosesang, Korea) containing 5% nonfat dry milk (BD Biosciences), washed three times with TBS, and incubated at 4 °C overnight with primary antibodies diluted in TBST (TBS containing 0.1% Tween 20 (Sigma-Aldrich)) containing 3% nonfat dry milk. Primary antibodies specific for phospho-JAK1/2 (#3771), STAT1 (#9172), phospho-STAT1 (#9171), and β-Actin (#4967) were obtained from Cell Signaling Technology (Danvers, MA). Antibodies specific for IDO (sc-25808) and interferon regulatory factor (IRF) 1 (sc-13041) were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). The next day, the blots were washed three times with 0.3% TBST and incubated for 1 h at room temperature with secondary antibodies diluted in TBST containing 3% nonfat dry milk. After three washes with 0.3% TBST, proteins were visualized with an enhanced chemiluminescence detection system (GE Healthcare).
2.9. Inhibition of JAK and STAT1 Activities
To inhibit the activity of JAK, an intracellular domain of the IFN-γ receptor, MSCs were incubated with 100 ng/mL of an anti-IFN-γ antibody (BD Biosciences) or 1 μM of the JAK inhibitor AG490 (CalBiochem, San Diego, CA) for 24 h before IFN-γ priming. The culture medium was changed, prior to use of cells in experiments. To inhibit the expression of STAT1, MSCs were transfected with a small interfering RNA (siRNA) targeting STAT1 (sc-44123) or with a scrambled siRNA (sc-37007), both purchased from Santa Cruz Biotechnology. Cells were plated 24 h before siRNA transfection such that they reached 50% confluency on the day of transfection. Cells were incubated with siRNA-Lipofectamine 2000 (Invitrogen-Gibco) complexes for 12 h at 37 °C. The medium was then changed, and transfected cells were incubated for an additional 12 h until the target gene was effectively down-regulated.
2.10. Establishment of Genetically Modified MSCs
Pre-made human IDO short hairpin RNA (shRNA) lentiviral particles for the inhibition of IDO expression and lentiviral particles expressing an inducible form of human IDO were purchased from Santa Cruz Biotechnology (sc-45,939-V) and GenTarget Inc. (LVP302) (San Diego, CA), respectively. To transfect BM-MSCs with the inducible IDO lentiviral particles, cells were infected with lentiviral particles at a multiplicity of infection (MOI) of 10 in LG-DMEM containing 10% FBS, incubated for 72 h in a humidified atmosphere at 37 °C with 5% CO2, and washed twice with PBS, after which medium was added. Infected MSCs were selected by incubation for 7 days in MSC medium containing 5 μg/mL puromycin (Sigma-Aldrich), which was confirmed by the expression of red fluorescent protein (RFP)-derived red fluorescence and immunoblotting. To transfect BM-MSCs with IDO-down-regulating lentiviral particles, cells were pretreated with 5 μg/mL polybrene (Santa Cruz Biotechnology) prepared in LG-DMEM containing 10% FBS and then treated with lentiviral particles at a MOI of 10. After incubation for 24 h in a humidified atmosphere at 37 °C with 5% CO2, cells were washed twice with PBS and the medium was added. Infected MSCs were selected by incubation for 7 days in MSC medium containing 5 μg/mL puromycin, which was confirmed by immunoblotting.
2.11. Immunocytochemistry
MSCs were placed in a fixative solution (4% paraformaldehyde prepared in PBS) for 30 min at room temperature in the dark, and then washed three times with PBS. To detect intracellular proteins, cells were permeabilized with 0.25% Triton X-100 prepared in PBS for 5 min at room temperature in the dark. Cells were washed three times and then incubated in blocking solution (5% FBS prepared in PBS) for 1 h at room temperature. After another washing step, antibodies against IDO (1:100) were added and cells were incubated for 1 h at room temperature. Cells were washed three times and incubated with Alexa Fluor 488-conjugated secondary goat anti-mouse IgG (Invitrogen-Gibco) for 1 h at room temperature. Following this, cells were washed three times and mounted in DAPI (4′,6-diamidino-2-phenylindole)-containing an anti-fade mounting solution (Vectashield; Vector Lab., Burlingame, CA). Fluorescence imaging of cells was performed using a Carl Zeiss LSM 700 confocal microscope system (Jena, Germany) with a 25 mW laser diode tuned to 405 nm for DAPI staining of cell nuclei, a 30 mW argon laser tuned to 488 nm for visualization of Alexa Fluor 488-labeled IDO, and a 10 mW HeNe laser tuned to 555 nm for visualization of RFP-expressed MSCs. Confocal microscopy results were analyzed using LSM 700 Zen software (Jena).
2.12. Statistical Analysis
All results were expressed as means ± standard deviation (SD). Differences between experimental conditions were analyzed with a t-test or by analysis of variance. A P-value of < 0.05 was considered statistically significant.
3. Results
3.1. Isolation and Characterization of MSCs Derived from Human Tissues
Human MSCs were obtained from four human tissues namely BM, AT, CB, and WJ. The morphology of the adherent cells varied between the cells derived from different sources. Cells obtained from BM, CB, and WJ were fibroblastic in shape, whereas AT-derived cells were small and spindle-shaped (Fig. S1a). These cells were uniformly positive for CD73, CD90, CD105, and CD166 expression, but were negative for the hematopoietic lineage markers CD14, CD34, CD45, and HLA-DR, as confirmed by a flow cytometry analysis of expressed surface antigens (Fig. S1b). To assess the ability of MSCs from each tissue to differentiate into mesenchymal derivatives, cells were grown in osteogenic, adipogenic, or chondrogenic induction media for 14–21 days, and probed for alkaline phosphatase activity, deposition of neutral lipid vacuoles, and accumulation of chondrocyte matrix, respectively. All MSCs, irrespective of their origin, showed osteogenic, adipogenic, and cartilage-like phenotypes after induction of differentiation with the appropriate media (Fig. S1c).
3.2. Immunosuppressive Properties of naïve MSCs in In Vitro and In Vivo Models
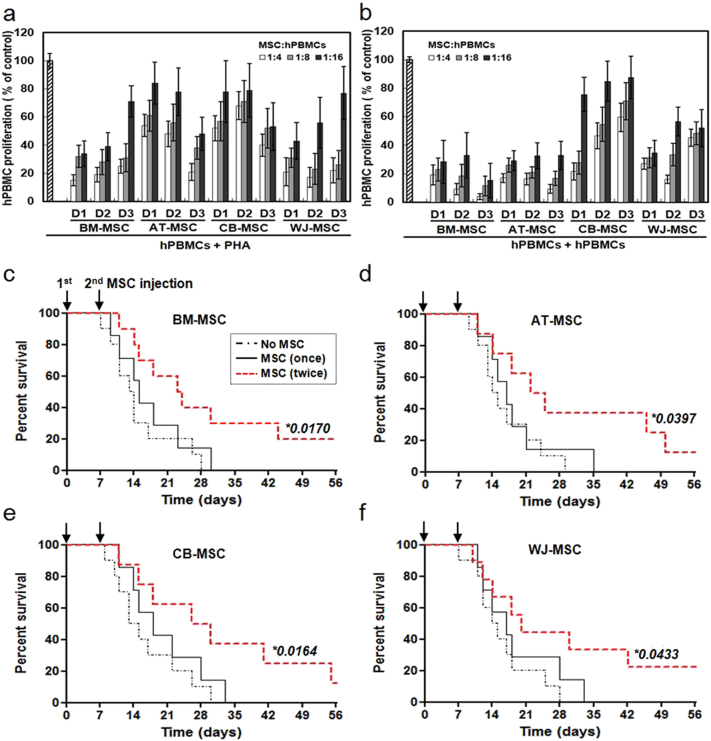
The immunomodulatory properties of naïve MSCs were evaluated using a mixed lymphocyte reaction (MLR). When PHA-activated PBMCs were incubated on a layer of MSCs obtained from BM, AT, CB, or WJ, T-cell proliferation decreased significantly, and this effect was dependent on the number of MSCs in the co-culture (Fig. 1a). There were no significant differences in the immunomodulatory properties of MSCs derived from different tissues. These data demonstrate that AT-, CB-, and WJ-MSCs, were as efficient at suppressing T-cell proliferation as BM-MSCs. Similar results were obtained when T-cell proliferation was induced using a two-way culture with PBMCs from two different donors (Fig. 1b). To assess whether the inhibition by MSCs was mediated by direct contact or by soluble factors, we used a transwell system in which PBMCs were co-cultured with MSCs placed in transwell inserts. The data shown in Fig. S2 demonstrates that MSCs from each tissue inhibited hCD3+ CD8+ T-cell proliferation in response to PHA stimulation, even in the absence of direct cell-cell contact, although there appeared to be a slight reduction in the degree of inhibition compared to that seen with direct cell-cell contact. These findings indicate that not only cell-cell contact, but also soluble factors, contribute to the immunomodulatory effects of MSCs. To confirm the immunosuppressive function of MSCs, mice were injected with hPBMCs and the MSCs derived from the four different tissues. At 8 weeks after transplantation, all mice transplanted with hPBMCs alone, or once with a combination of hPBMC and MSCs, had died, while about 20% of the mice transplanted twice with MSCs survived (Fig. 1c–f). To elucidate the contribution of soluble factors to immunosuppression, the supernatants from mitogen-activated T-cells cultured in the absence or presence of MSCs were examined. The secretion of various cytokines, chemokines, and growth factors was increased when PBMCs were activated. In the presence of MSCs, the levels of pro-inflammatory cytokines, such as IFN-γ and TNF-α, were reduced, irrespective of the origin of the MSCs. In contrast, secretion of Th2 cytokines such as IL-2, IL-4, and IL-5 was the same in cultures either in the presence or absence of MSCs (Table 1).
Fig. 1.
Immunosuppressive properties of MSCs derived from different tissues. MSCs derived from four different tissues (BM-, AT-, CB-, and WJ-MSC) and three different donors (D1–D3) were used. (a) PHA-induced hPBMC proliferation in the absence or presence of different numbers of MSCs. hPBMC proliferation was evaluated on day 3 as the percentage of BrdU+ cells. (b) hPBMC proliferation using a mixed lymphocyte reaction in the absence or presence of different numbers of MSCs. Data are expressed as the percentage of hPBMC proliferation in the absence of MSCs and represent the mean ± SD of three separate experiments. Increased survival of GVHD mice in response to BM-MSCs (c), AT-MSCs (d), CB-MSCs (e), and WJ-MSCs (f). MSCs were administered once (black solid line), or twice (red dotted line) with a 7-day interval, via intravenous injection, and the survival rates of GVHD mice were determined. The survival rate increased when MSCs were administered twice. (c) No MSC (n = 10), MSC (once) (n = 7), and MSC (twice) (n = 10). (d) No MSC (n = 10), MSC (once) (n = 7), and MSC (twice) (n = 8). (e) No MSC (n = 10), MSC (once) (n = 7), and MSC (twice) (n = 8). (f) No MSC (n = 10), MSC (once) (n = 7), and MSC (twice) (n = 9). *, P-value of the MSC (twice) group versus the No MSC group.
Table 1.
Secretory factor levels in MSCs co-cultured with hPBMCs or PHA-activated hPBMCs.
| BM-MSC |
AT-MSC |
CB-MSC |
WJ-MSC |
No MSC |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| hPBMCs |
− |
+ |
+ |
− |
+ |
+ |
− |
+ |
+ |
− |
+ |
+ |
+ |
+ |
| PHA | − | − | + | − | − | + | − | − | + | − | − | + | − | + |
| IL-1α | − | − | + | − | − | + | − | − | + | − | − | + | − | + |
| IL-1β | − | − | − | − | − | + | − | − | − | − | − | + | − | + |
| IL-2 | + | + | ++ | + | + | ++ | + | + | ++ | + | + | ++ | + | ++ |
| IL-3 | − | − | + | − | − | + | − | − | + | − | − | + | − | + |
| IL-4 | − | − | +/++ | − | − | +/++ | − | − | +/++ | − | − | +/++ | − | + |
| IL-5 | − | − | + | − | − | + | − | − | + | − | − | + | − | + |
| IL-6 | ++ | +++ | +++ | ++ | +++ | +++ | ++ | +++ | +++ | +++ | +++ | +++ | + | +++ |
| IL-7 | − | − | + | − | − | + | − | − | + | − | − | − | − | −/+ |
| IL-8 | + | ++ | +++ | +/++ | ++ | +++ | + | +++ | +++ | +++ | +++ | +++ | ++ | +++ |
| IL-9 | − | − | + | − | − | + | − | − | + | − | − | + | − | +/++ |
| IL-10 | − | − | ++ | − | − | ++ | − | − | ++ | − | − | ++ | − | ++ |
| IL-12(p40) | − | ++ | ++ | − | ++ | ++ | − | ++ | ++ | + | ++ | ++ | ++ | ++ |
| IL-12(p70) | − | − | − | − | − | − | − | − | − | − | − | − | − | + |
| IL-13 | − | − | ++ | − | − | +/++ | − | − | ++ | − | − | ++ | − | +/++ |
| IL-15 | − | − | −/+ | − | − | −/+ | + | − | −/+ | + | − | −/+ | − | −/+ |
| Eotaxin | ++ | + | + | ++ | + | + | ++ | + | + | ++ | + | + | + | + |
| GM-CSF | −/+ | + | +/++ | − | + | ++ | −/+ | + | +/++ | + | + | +/++ | + | +/++ |
| IFN-γ | − | − | ++ | − | − | ++ | − | − | ++ | − | − | ++ | − | ++/+++ |
| IFN-α2 | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| IP-10 | +/− | + | +++ | +/− | ++ | +++ | +/− | ++ | +++ | + | + | +++ | ++ | +++ |
| MCP-1 | + | ++ | +++ | + | ++ | ++/+++ | ++ | +++ | +++ | ++ | +++ | +++ | ++ | ++/+++ |
| MIP-1α | −/+ | + | ++/+++ | −/+ | + | ++/+++ | −/+ | + | +++ | −/+ | ++ | ++/+++ | +/++ | ++/+++ |
| MIP-1β | + | +/++ | +++ | −/+ | ++ | +++ | + | ++ | +++ | + | ++ | +++ | ++ | +++ |
| RANTES | − | ++ | ++/+++ | − | ++ | ++ | − | + | ++ | + | ++ | ++ | ++ | ++ |
| TNF-α | − | − | −/+ | − | − | −/+ | − | − | +/++ | − | − | −/+ | − | ++ |
| TNF-β | − | − | −/+ | − | − | −/+ | − | − | −/+ | − | − | − | − | +/++ |
| FGF | + | + | +/++ | + | + | +/++ | + | + | +/++ | + | + | +/++ | + | + |
| PDGF-AA | −/+ | + | +/++ | −/+ | + | + | ++ | ++ | ++ | + | +/++ | +/++ | + | +/++ |
| PDGF-BB | − | − | + | − | −/+ | + | − | + | + | − | + | + | + | +/++ |
| EGF | −/+ | + | + | −/+ | + | + | −/+ | + | + | −/+ | + | + | + | + |
| Flt-3 | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| VEGF | +/++ | ++ | ++ | + | ++ | ++ | ++ | ++ | ++ | − | − | − | − | − |
A multiplex human cytokine detection kit was utilized to measure secretory factor levels in cell culture supernatants from MSCs co-cultured with hPBMCs or PHA-activated hPBMCs. –, value < 50 pg/mL; +, 50 pg/mL ≤ value < 500 pg/mL; ++, 500 pg/mL ≤ value < 5000 pg/mL; +++, 5000 pg/mL ≤ value.
3.3. Gene Expression Profile of MSCs Primed with IFN-γ
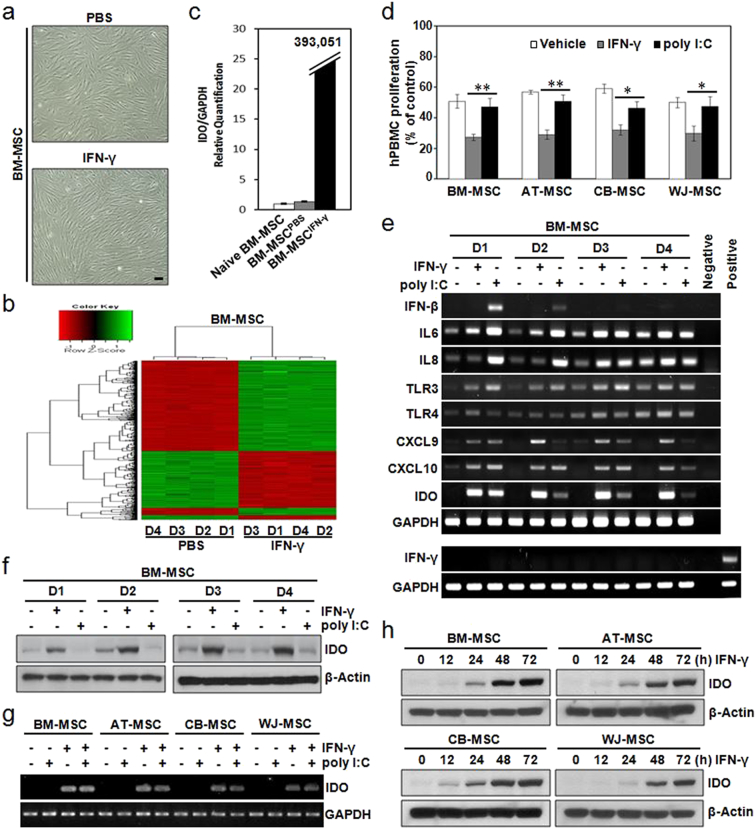
To determine the effect of IFN-γ on gene expression in MSCs, gene expression profiles were compared before and after IFN-γ stimulation. BM-MSCs did not differ markedly in terms of their morphology when they were primed with IFN-γ (Fig. 2a). However, their gene expression profiles were significantly altered by IFN-γ priming (Fig. 2b). In fact, 512 genes were up-regulated in IFN-γ-primed MSCs (Table S2), and as shown in Table 2, four of these genes were potentially involved in the immunosuppressive functions of MSCs. The mRNA expression levels of chemokine (C-X-C motif) ligand (CXCL) 9, CXCL10, chemokine (C-C motif) ligand (CCL) 8, and IDO, which were found to be highly up-regulated in IFN-γ-primed MSCs, were confirmed to be up-regulated by qRT-PCR (Figs. 2c and S3).
Fig. 2.
IFN-γ priming commonly induces IDO expression in MSCs, but TLR3 activation rarely does. (a–c) BM-MSCs were treated with 200 IU/mL IFN-γ for 24 h prior to harvest. (a) Morphological appearance of BM-MSCs, with or without, IFN-γ priming. (b) Microarray data were filtered by applying two criteria for significant changes, i.e. P < 0.05 and a fold change > 2, between PBS-treated and IFN-γ-primed BM-MSCs. Hierarchical cluster analysis of genes differentially expressed between PBS-treated and IFN-γ-primed MSCs. Four samples (D1–D4) were analyzed per culture condition. (c) Quantitative real-time (qRT)-PCR analysis of IDO mRNA, which was up-regulated in IFN-γ-primed BM-MSCs. (d) PHA-induced hPBMC proliferation in the presence of MSCs pretreated with PBS (vehicle), 200 IU/mL IFN-γ, or 100 μg/mL poly I:C. hPBMC proliferation was evaluated on day 3 and is expressed as the percentage of BrdU+ cells. Data are expressed as the percentage of hPBMC proliferation in the absence of MSCs and represent the mean ± SD of three separate experiments. *P < 0.05, **P < 0.01. (e–f) MSCs derived from four different donors (D1–D4) were treated with 200 IU/mL IFN-γ or 100 μg/mL poly I:C for 24 h. (e) mRNA levels of the indicated genes were determined by semi-quantitative RT-PCR. PHA-treated hPBMCs were used as a positive control. (f) Protein levels of IDO were determined by immunoblotting. (g–h) MSCs derived from four different tissues (BM-, AT-, CB- and WJ-MSC) were used. (g) MSCs were treated with 200 IU/mL IFN-γ and/or 100 μg/mL poly I:C for 24 h. IDO mRNA levels were determined by semi-quantitative RT-PCR. (h) MSCs were treated with 200 IU/mL IFN-γ for the indicated amounts of time. IDO protein levels were determined by immunoblotting. IDO protein expression increased in a time-dependent manner in IFN-γ-primed MSCs. GAPDH and β-Actin were used as loading controls for PCR and western blotting, respectively.
Table 2.
Genes highly expressed in BM-MSCs primed with IFN-γ.
| Symbol | Full Name | *Fold change | Adjusted P-value | Biological function | Gene ontology category |
|---|---|---|---|---|---|
| CXCL9 | C-X-C motif chemokine ligand 9 | 312.27 | < 1.00E − 04 | A type of T-cell chemoattractant induced by IFN-γ | GO:0006935 _ chemotaxis GO:0006955 _ immune response |
| CXCL10 | C-X-C motif chemokine ligand 10, also known as IP-10 | 218.98 | < 1.00E − 04 | Involved in chemoattraction for immune cells | GO:0006935 _ chemotaxis GO:0006955 _ immune response |
| CCL8 | C-C motif chemokine ligand 8, also known as MCP-2 | 204.78 | < 1.00E − 04 | Associated with survival rate of acute GVHD | GO:0006935 _ chemotaxis GO:0006955 _ immune response |
| IDO | Indoleamine-pyrrole 2,3-dioxygenase | 203.53 | < 1.00E − 04 | Causes depletion of tryptophan to halt growth of microbes and T-cells | GO:0006569 _ tryptophan catabolic process GO:0033754 _ indoleamine 2,3-dioxygenase activity |
A microarray analysis was performed to evaluate the effect of IFN-γ pretreatment on MSCs. *Up-regulated genes in MSC cultures primed with IFN-γ were sorted based on gene expression profiles (P-value < 0.01 and fold change > 200) and classified according to their related biological processes based on Gene Ontology terms using DAVID Bioinformatics Resources 6.7.
3.4. IFN-γ Priming Induces IDO Expression in MSCs, but TLR3 Activation Does Not
Several previous studies have suggested that Toll-like receptor (TLR) 3 activation induces IDO expression in MSCs (Waterman et al., 2012, Waterman et al., 2010, Opitz et al., 2009). Thus, we compared the immunosuppressive properties between TLR3-activated and IFN-γ-primed MSCs. The proliferation of hPBMCs was similarly suppressed when they were co-cultured with naïve or TLR3-activated MSCs, while it was further suppressed upon co-culture with IFN-γ-primed MSCs, irrespective of the origin of the MSCs (Fig. 2d). The percentage of CFSElow hCD3+ cells was lower in co-cultures with IFN-γ-primed MSCs than in co-cultures with PBS-treated MSCs (Fig. S4). In addition, IDO expression was marginal when they were treated with poly I:C or TNF-α, although other function-related genes such as CXCL10, IL6, and IL8 were highly expressed in all BM-MSCs (D1–D4) following TLR3 activation (Figs. 2e and S5). Interestingly, induction of IFN-γ expression was not observed in BM-MSCs following either IFN-γ priming or TLR3 activation (Fig. 2e). Furthermore, IDO expression was increased when BM-MSCs from different donors (D1–D4) were stimulated with IFN-γ (Fig. 2e and f), and this induction of IDO was uniformly seen in all MSCs isolated from different tissues (Fig. 2g and h). These results indicate that ex vivo IFN-γ priming induces IDO expression in MSCs to allow them to express their immunosuppressive properties, but TLR3 activation does not.
3.5. IFN-γ Induces IDO Expression in MSCs Via the JAK-STAT1 Signaling Pathway
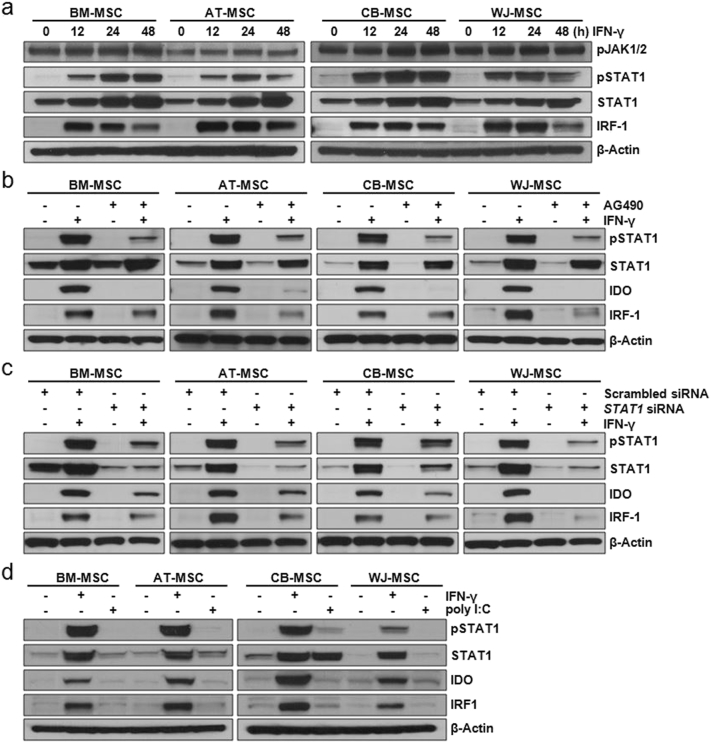
Using an immunoblot analysis, we found that both JAK1/2 and STAT1 in MSCs were activated following IFN-γ-stimulation (Fig. 3a). To determine the correlation between the IFN-γ-JAK-STAT1 pathway and IDO expression, immunoblot analysis was performed after the addition of an anti-IFN-γ antibody, a JAK inhibitor AG490, or following transfection with a STAT1-targeted siRNA, respectively. IDO expression was minimal or absent in the presence of an anti-IFN-γ antibody, the JAK inhibitor AG490 or a STAT1-targeted siRNA (Fig. S6 and 3b and c), indicating that the induction of IDO expression by IFN-γ occurs through the JAK-STAT1 pathway, irrespective of the origin of the MSCs. In contrast, STAT1 activation and expression of IDO were not detected in TLR3-activated MSCs (Fig. 3d).
Fig. 3.
IDO expression in IFN-γ-primed MSCs via a JAK-STAT1 signaling pathway. MSCs derived from four different tissues (BM-, AT-, CB-, and WJ-MSC) were used. (a) MSCs were incubated with 200 IU/mL IFN-γ for the indicated amounts of time. The expression levels of phospho-JAK1/2, phospho-STAT1, STAT1, and IRF-1 in these MSCs were detected by immunoblotting. (b) To inhibit the activity of JAK, an intracellular domain of the IFN-γ receptor, MSCs were incubated with 1 μM AG490 (a JAK inhibitor) for 24 h before IFN-γ priming. The expression levels of phospho-STAT1, STAT1, IDO, and IRF-1 in AG490-treated MSCs were detected by immunoblotting. AG490 treatment induced the down-regulation of STAT1 activity and IDO expression. (c) To down-regulate STAT1 activity, MSCs were transfected with a scrambled siRNA or with an siRNA targeting STAT1. The expression levels of phospho-STAT1, STAT1, IDO, and IRF-1 in these transfected MSCs were detected by immunoblotting. Down-regulation of STAT1 activity effectively induced a decrease in IDO expression in IFN-γ-primed MSCs. (d) MSCs were treated with 200 IU/mL IFN-γ or 100 μg/mL poly I:C for 24 h. The expression levels of phospho-STAT1, STAT1, IDO, and IRF-1 in these MSCs were detected by immunoblotting. β-Actin was used as a loading control for all western blots.
3.6. IFN-γ Priming Enhances the Immunosuppressive Properties of Human MSCs
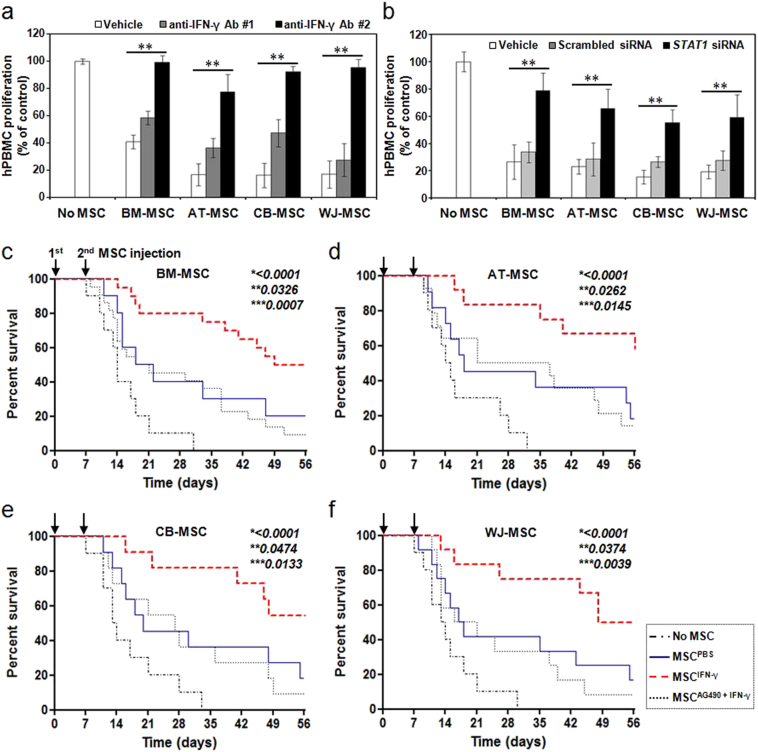
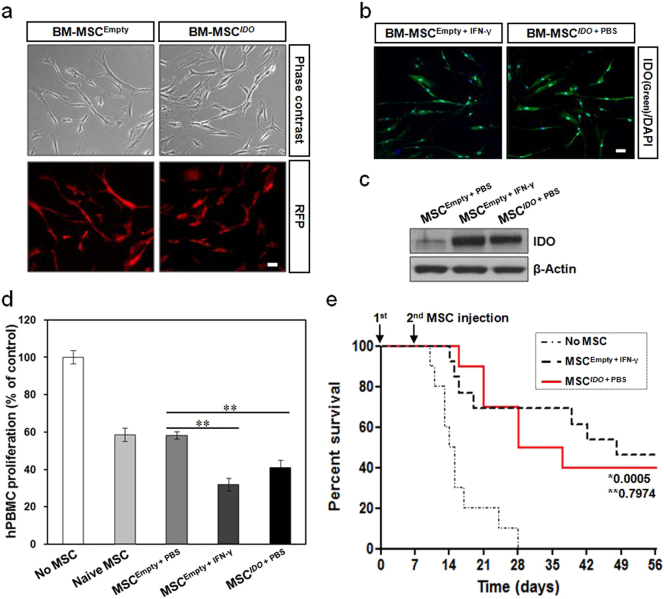
The proliferation of hPBMCs was suppressed when they were co-cultured with naïve MSCs; proliferation was further suppressed after co-culture with IFN-γ-primed MSCs, as shown in Fig. 2d. These effects disappeared upon the addition of an anti-IFN-γ antibody (Fig. 4a), as well as when signaling downstream of IFN-γ was blocked using a STAT1-targeted siRNA (Fig. 4b), indicating that IFN-γ secreted by activated immune cells, including T-cells, has an important role in the immunomodulatory properties of MSCs by increasing their expression of IDO. In addition, hPBMC-transplanted mice were administered twice with IFN-γ-primed MSCs, with a 7-day interval, in order to evaluate whether these MSCs further ameliorate GVHD. The survival rate of mice transplanted with hPBMCs alone was compared with that of mice transplanted with a combination of hPBMCs and MSCs, IFN-γ-primed MSCs, or IFN-γ-primed MSCs pretreated with AG490, a JAK inhibitor. Mice co-transplanted with hPBMCs and IFN-γ-treated MSCs showed an improved survival rate compared to mice transplanted with hPBMCs alone (Fig. 4c–f). Furthermore, there appeared to be a trend toward a survival benefit in mice co-transplanted with hPBMCs and IFN-γ-primed MSCs, compared to mice co-transplanted with hPBMCs and naïve MSCs. Pretreating MSCs with AG490 resulted in a loss of the survival benefit obtained by IFN-γ-priming. A clinical scoring (Table S3) and histological analysis (Fig. S7 and Table S4) also showed that co-transplantation with IFN-γ-primed MSCs effectively decreased clinical symptoms and immune cell infiltration into the skin and small intestine of GVHD mice, indicating that IFN-γ priming enhances the immunosuppressive properties of MSCs in vivo. To evaluate whether the transplanted MSCs could be detected in the tissues of GVHD mice, confocal images were taken after infusion of CM-DiI-stained MSCs. We could successfully identify CM-DiI-stained MSCs infiltrating the tissues (Fig. S8a–d). Induction of IDO in MSCs was also confirmed in the tissues of GVHD mice when IFN-γ-treated MSCs were transplanted (Fig. S8a–d). In addition, when mice were transplanted with MSCs pretreated with AG490 along with IFN-γ, the degree of MSC infiltration and IDO expression was less than that seen in mice tissues after transplantation of MSCs pretreated with IFN-γ alone (Fig. S8a and b).
Fig. 4.
Enhanced immunosuppressive properties of IFN-γ-primed MSCs. (a–b) 200 IU/mL IFN-γ was added to MSCs, and the cells were incubated for 24 h. PHA-stimulated hPBMCs were incubated in the absence or presence of PBS-treated, or IFN-γ-primed MSCs. (a) Pretreatment with an anti-IFN-γ antibody (#1, once; #2, twice) before IFN-γ priming significantly decreased the suppressive effect of MSCs on PHA-induced T-cell proliferation. (b) Down-regulation of STAT1 activity using an siRNA before IFN-γ priming significantly decreased the suppressive effect of MSCs on PHA-induced T-cell proliferation. hPBMC proliferation was evaluated on day 3 and is expressed as the percentage of BrdU+ cells. Data are expressed as the percentage of hPBMC proliferation in the absence of MSCs and represent the mean ± SD of three separate experiments. **P < 0.01. (c–f) Increase in the survival rates of GVHD mice after infusion of IFN-γ-primed BM-MSCs (c), AT-MSCs (d), CB-MSCs (e), and WJ-MSCs (f). MSCs pretreated with or without IFN-γ were intravenously administered MSCs twice, with a 7-day interval. The survival rate was increased more when IFN-γ-primed MSCs were administered than when PBS-treated MSCs were administered. There was no difference in the survival rate of mice between the group co-transplanted with hPBMCs and PBS-treated MSCs, and the group co-transplanted with hPBMCs and the JAK inhibitor AG490- plus IFN-γ-primed MSCs. (c) No MSC (n = 10), MSCPBS (n = 10), MSCIFN-γ (n = 20), and MSCAG490 + IFN-γ (n = 22). (d) No MSC (n = 10), MSCPBS (n = 11), MSCIFN-γ (n = 12), and MSCAG490 + IFN-γ (n = 14). (e) No MSC (n = 10), MSCPBS (n = 11), MSCIFN-γ (n = 11), and MSCAG490 + IFN-γ (n = 11). (f) No MSC (n = 10), MSCPBS (n = 12), MSCIFN-γ (n = 12), and MSCAG490 + IFN-γ (n = 12). *, P-value of MSCIFN-γ group versus No MSC group; **, P-value of MSCIFN-γ group versus MSCPBS group; and ***, P-value of MSCIFN-γ group versus MSCAG490 + IFN-γ group.
3.7. Potential Involvement of IDO in the Immunosuppressive Properties of Human MSCs
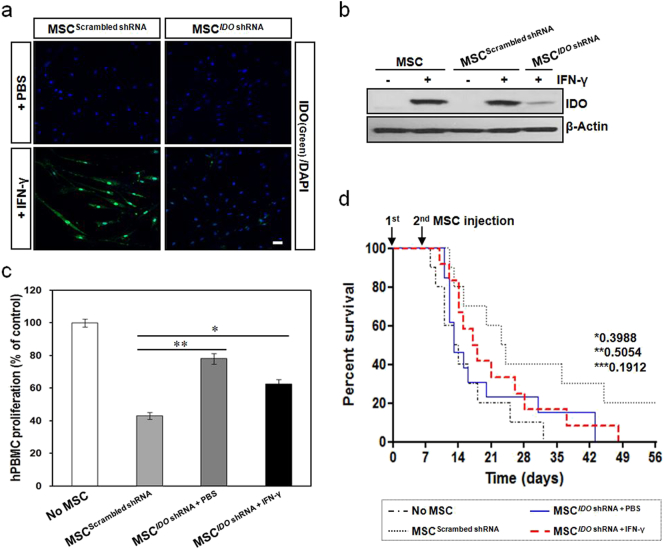
To further evaluate the involvement of IDO in the enhanced immunosuppressive properties of MSCs, we established MSCs having stable IDO expression by transduction with a lentiviral vector expressing both IDO and RFP. The expression of RFP and IDO could easily be observed in these IDO-overexpressing MSCs (Fig. 5a–c). To confirm the immunosuppressive properties of IDO-overexpressing MSCs, we tested the performance of these MSCs in in vitro and in vivo models. PHA-induced hPBMC proliferation was significantly inhibited when hPBMCs were co-cultured with IDO-overexpressing MSCs, similar to the result obtained when hPBMCs were co-cultured with IFN-γ-primed MSCs (Fig. 5d). In addition, the survival rate in the IDO-overexpressing MSC group was as high as that seen in the IFN-γ-primed MSC group of GVHD mice (Fig. 5e). Correlating with these survival outcomes, immunofluorescence images showed the presence of IDO in IDO-overexpressing MSCs in the small intestine and skin from GVHD mice (Fig. S9). Following this, shRNA was employed to silence IDO gene expression in order to evaluate its role in the immunomodulatory function of MSCs. The expression of IDO in IFN-γ-primed MSCs was successfully inhibited by an IDO-targeted shRNA (Fig. 6a and b). PHA-induced hPBMC proliferation was significantly restored when hPBMCs were co-cultured with PBS-treated or IFN-γ-primed MSCs in which IDO was inhibited by the IDO-targeted shRNA, whereas the inhibitory effect was unaffected when hPBMCs were co-cultured with scrambled shRNA-transduced MSCs (Fig. 6c). In addition, IDO-down-regulated MSCs were prepared, with or without IFN-γ priming, and intravenously administered twice, with a 7-day interval, into GVHD mice. There was no statistically significant difference in the survival rate between the group of mice co-transplanted with hPBMCs plus IDO-down-regulated MSCs and the group transplanted with hPBMCs only (Fig. 6d). Correlating with these survival outcomes, immunofluorescence images showed a low level of IDO in IDO-down-regulated MSCs in the small intestine and skin obtained from GVHD mice (Fig. S10). These findings demonstrate that IFN-γ-primed MSCs can home to tissues with GVHD, and exert their immunosuppressive function through the induction of IDO.
Fig. 5.
Enhancement of immunosuppressive properties in IDO-overexpressing MSCs. A lentiviral vector carrying IDO and RFP was transduced into BM-MSCs. (a) MSCs with stable IDO expression were established and RFP expression was observed under a fluorescence microscope. Scale bar: 50 μm. (b) Immunocytochemistry showing the expression of IDO in IDO-overexpressing MSCs. IFN-γ-primed MSCs were used as a positive control. Scale bar: 50 μm. (c) Immunoblot analysis of IDO protein expression in IDO-overexpressing MSCs. (d) PHA-induced hPBMC proliferation in the presence of IDO-overexpressing MSCs. hPBMC proliferation was evaluated on day 3 and is expressed as the percentage of BrdU+ cells. Data are expressed as the percentage of hPBMC proliferation in the absence of MSCs and represent the mean ± SD of three separate experiments. **P < 0.01. (e) Survival rates of GVHD mice after infusion of IDO-overexpressing MSCs. MSCs were intravenously administered twice, with a 7-day interval, into GVHD mice. The survival rate in the IDO-overexpressing MSC group was as high as that in the IFN-γ-primed MSC group. *, P-value of MSCIDO + PBS group versus No MSC group; **, P-value of MSCIDO + PBS group versus MSCEmpty + IFN-γ group. No MSC (n = 10), MSCIDO + PBS (n = 10), and MSCEmpty + IFN-γ (n = 13).
Fig. 6.
Decrease in the immunosuppressive properties of MSCs after down-regulation of IDO expression. BM-MSCs were transduced with lentiviral particles containing an IDO-targeting shRNA or a scrambled shRNA. (a) Immunocytochemistry showing low expression of IDO in IDO-down-regulated MSCs. Scale bar: 50 μm. (b) Immunoblot analysis of IDO protein expression in IDO-down-regulated MSCs. (c) PHA-induced hPBMC proliferation in the presence of IDO-down-regulated MSCs that were primed by IFN-γ. hPBMC proliferation was evaluated on day 3 and is expressed as the percentage of BrdU+ cells. Data are expressed as the percentage of hPBMC proliferation in the absence of MSCs and represent the mean ± SD of three separate experiments. *P < 0.05, **P < 0.01. (d) Survival rates for GVHD mice after infusion of IDO-down-regulated MSCs. MSCs were prepared with or without IFN-γ pretreatment and were intravenously administered twice, with a 7-day interval, into GVHD mice. There was no statistically significant difference in the survival rate of mice between the group co-transplanted with hPBMCs plus IDO-down-regulated MSCs and the group transplanted with hPBMCs only. *, P-value of MSCIDO shRNA + PBS group versus No MSC group; **, P-value of MSCIDO shRNA + PBS group versus MSCIDO shRNA + IFN-γ group; ***, P-value of MSCIDO shRNA + IFN-γ group versus MSCScrambled shRNA group. No MSC (n = 10), MSCScrambled shRNA (n = 10), MSCIDO shRNA + PBS (n = 13), and MSCIDO shRNA + IFN-γ (n = 12).
4. Discussion
In this study, our data have shown that activated T-cells secrete a greater amount of IFN-γ than quiescent T-cells, and that IFN-γ levels are significantly reduced when activated T-cells are co-cultured with MSCs. This is indicative of an IFN-γ autocrine-paracrine loop. It is believed that MSCs need a ‘license’ to exert their immunosuppressive function, and IFN-γ is known as a key cytokine capable of providing MSCs with that license (Le Blanc et al., 2003, Polchert et al., 2008). In this context, we postulated that priming MSCs with IFN-γ might produce more potent MSCs that are better able to control immune dysregulation. Polchert et al. (2008) and Ryan et al. (2007) have previously suggested that many cytokines in addition to IFN-γ, such as TNF-α, IL-1α, and IL-1β, are involved in the initiation and efficacy of the immunosuppressive activities of MSCs (Chan et al., 2006, English et al., 2010, Meirelles et al., 2009, Ren et al., 2008). In this study, we analyzed gene expression profiles to specifically compare the expression levels of a variety of genes that could be potentially associated with immunosuppressive functions between IFN-γ-primed and PBS-treated MSCs. As a result, 512 up-regulated genes were observed in IFN-γ-primed MSCs, including CXCL9, CXCL10, CCL8, and IDO. Chemokines such as CXCL9, CXCL10, and CCL8 may play important roles in the recruitment of leukocytes leading to various immune responses (Fallarino and Grohmann, 2011, Müller et al., 2010, Stec et al., 2012, Tan and Bharath, 2009, Yamamoto et al., 2011), but their precise roles in IFN-γ-primed MSCs remains to be defined.
Unlike CXCL9, CXCL10, and CCL8, IDO is known to be more directly involved in the immunosuppressive properties of MSCs and in the suppression of antigen-driven proliferation of T-cells (Fallarino and Grohmann, 2011, Munn et al., 2004, Spaggiari et al., 2008). In this study, IDO, one of the most well-known IFN-γ-induced genes, was found to be highly up-regulated in IFN-γ-primed MSCs. We also confirmed that the increased expression of IDO in MSCs stimulated by IFN-γ is a common phenomenon that occurred in all MSCs tested, irrespective of their origin. Our data showed that IDO expression was increased in IFN-γ-primed MSCs through the JAK-STAT1 signaling pathway. The immunosuppressive activities of IDO are mediated by its ability to degrade tryptophan, an amino acid that is essential for T-cell proliferation (Fallarino and Grohmann, 2011, Spaggiari et al., 2008, Tan and Bharath, 2009). Thus, IDO and a paucity of tryptophan have received a special emphasis in many immune-related disorders.
Here, we further explored the signaling pathways involved in the up-regulation of IDO in response to TLR signaling. In a previous study, Opitz et al. (2009) showed that the signaling pathway, leading from either TLR3 or TLR4 activation to the induction of functional IDO, involves autocrine IFN-β signaling and activation of STAT1 and IRF-1. Active STAT1 is directly involved in the induction of IDO by binding to the IDO gene regulatory region, and indirectly by inducing the production of IRF-1 (Fallarino and Grohmann, 2011, Munn et al., 2004, Spaggiari et al., 2008), in accordance with this study. While another group showed that TLR3 stimulation of human MSCs supports their established immunosuppressive properties (MSC2), TLR4 activation of human MSCs more consistently provides a pro-inflammatory signature (MSC1) (Waterman et al., 2012, Waterman et al., 2010). They proposed that short-term, low-level exposure with TLR4 agonists polarizes human MSCs toward a pro-inflammatory MSC1 phenotype important for early injury responses. By contrast, the downstream consequences of TLR3 agonist exposure of human MSCs are their polarization toward an immunosuppressive MSC2 phenotype, essential for later anti-inflammatory responses that help resolve the tissue injury. In this study, however, we confirmed that stimulation of TLR3 in human MSCs rarely induced IFN-β and/or IDO expression, indicating that TLR signaling is not a major pathway in the immunosuppressive functions of human MSCs. In addition, IFN-γ priming highly induced IDO expression in all MSCs tested. Thus, we strongly suggest that direct IFN-γ priming is a powerful tool for the improvement of the function of MSCs to treat immune-related disorders.
A number of clinical trials employing MSCs are currently in progress worldwide; however, unfortunately, protocols and methods, including the specific optimal culture conditions for the harvest of culture-expanded MSCs, have not yet been standardized (Chen et al., 2006, Dazzi and Krampera, 2011, Krampera, 2011), which may reflect MSC heterogeneity (Phinney, 2007, Phinney, 2012, Reyes et al., 2001), as well as differences between culture conditions (Sekiya et al., 2002, Sotiropoulou et al., 2006, Vogel et al., 2003). Therefore, further efforts should be made to obtain better functioning MSCs that are suitable for therapeutic purposes. Although a number of clinical trials have shown the beneficial effects of MSCs in ameliorating steroid-refractory GVHD, a significant proportion of patients with severe GVHD do not benefit from MSC treatment. Polchert et al. (2008) have observed that MSCs can significantly increase the survival rate of recipient mice only when IFN-γ levels are at their peak, with their efficacy dependent on the presence of IFN-γ in the environment. These results may be of interest in patients for whom treatment is ineffective. In this study, we demonstrated that GVHD mice injected with IFN-γ-primed MSCs expressed IDO, even though the increased IDO levels were more readily observed in vitro than in vivo. Therefore, the enhanced immunosuppressive activities of MSCs seemed to be highly associated with IFN-γ pretreatment, likely due in part to IDO induction. Moreover, it is notable that better immunomodulatory effects of IFN-γ primed MSCs were successfully demonstrated in our GVHD mouse model, whereas clinical attempts using naïve MSCs directed toward immunoregulation have shown limited therapeutic impact in the amelioration of GVHD (Chen et al., 2006, Dazzi and Krampera, 2011, Krampera, 2011). Although it will be difficult to adapt MSCs as a first-line treatment for established GVHD due to the high cost and lack of successful clinical data (Phinney, 2007, Phinney, 2012), future clinical trials using MSCs primed with IFN-γ may provide a solution to overcoming steroid-refractory severe GVHD.
In conclusion, we have shown that IFN-γ priming can have a profound influence on the immunomodulatory properties of MSCs, which extends their utility to the promotion of engraftment and the amelioration of GVHD after HSCT. A lack of standards for the preparation of MSCs has hampered the comparison of results among different studies. We have also shown that MSCs exhibit IFN-γ-inducible IDO activity, and this mechanism contributes to the inhibition of T-cells mediated by MSCs. Given that the incidence of GVHD exceeds 50% following allogeneic HSCT, and few therapeutic strategies are available for steroid-refractory diseases, new approaches to overcome severe GVHD, and its significant morbidity and mortality, are urgently needed. Our data may provide a useful way to obtain functionally qualified MSCs that can be more readily adapted for further clinical uses. Ameliorating GVHD using activated MSCs, via IFN-γ priming, may therefore help to improve the outcome of allogeneic HSCT. In addition, these functionally augmented MSCs might be applicable to various autoimmune diseases associated with immune deregulation.
Funding Sources
This research was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI) funded by the Ministry of Health & Welfare, Republic of Korea (Grant numbers: HI14C3484 and HI15C2963). These funding sources had no role in the study design, data collection, data analysis, interpretation, or writing of this manuscript.
Conflict of Interest Statement
The authors declare that they have no competing interests.
Author Contributions
This study was designed and supervised by MWL and KHY. Experiments were conducted by DSK, IKJ, MWL, and YJK. Data analysis was conducted by DSK, IKJ, MWL, DHL, JWL, KWS, HHK, and KHY. Funding was obtained by HHK and KHY. The manuscript was written by DSK, IKJ, MWL, and KHY. The manuscript was revised by DSK, IKJ, MWL, and KHY. All authors have read and approved the final manuscript.
Acknowledgements
Not applicable.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ebiom.2018.01.002.
Contributor Information
Myoung Woo Lee, Email: mwlee77@hanmail.net.
Ki Woong Sung, Email: kwsped@skku.edu.
Hong Hoe Koo, Email: hhkoo@skku.edu.
Keon Hee Yoo, Email: hema2170@skku.edu.
Appendix A. Supplementary Data
Supplementary material
References
- Ankrum J.A., Ong J.F., Karp J.M. Mesenchymal stem cells: immune evasive, not immune privileged. Nat. Biotechnol. 2014;32:252–260. doi: 10.1038/nbt.2816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asavaroengchai W., Wang H., Wang S., Wang L., Bronson R., Sykes M., Yang Y.G. An essential role for IFN-gamma in regulation of alloreactive CD8 T cells following allogeneic hematopoietic cell transplantation. Biol. Blood Marrow Transplant. 2007;13:46–55. doi: 10.1016/j.bbmt.2006.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augello A., Tasso R., Negrini S.M., Amateis A., Indiveri F., Cancedda R., Pennesi G. Bone marrow mesenchymal progenitor cells inhibit lymphocyte proliferation by activation of the programmed death 1 pathway. Eur. J. Immunol. 2005;35:1482–1490. doi: 10.1002/eji.200425405. [DOI] [PubMed] [Google Scholar]
- Baksh D., Song L., Tuan R.S. Adult mesenchymal stem cells: characterization, differentiation, and application in cell and gene therapy. J. Cell. Mol. Med. 2004;8:301–316. doi: 10.1111/j.1582-4934.2004.tb00320.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry F.P., Murphy J.M. Mesenchymal stem cells: clinical applications and biological characterization. Int. J. Biochem. Cell Biol. 2004;36:568–584. doi: 10.1016/j.biocel.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Bucher C.M., Passweg J.R. Towards rational graft-versus-host disease prophylaxis. Haematologica. 2012;97:1779–1780. doi: 10.3324/haematol.2012.080226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bürgler D., Medinger M., Passweg J., Fischmann A., Bucher C. Intra-arterial catheter guided steroid administration for the treatment of steroid-refractory intestinal GvHD. Leuk. Res. 2014;38:184–187. doi: 10.1016/j.leukres.2013.10.024. [DOI] [PubMed] [Google Scholar]
- Caplan A.I. Mesenchymal stem cells. J. Orthop. Res. 1991;9:641–650. doi: 10.1002/jor.1100090504. [DOI] [PubMed] [Google Scholar]
- Chan J.L., Tang K.C., Patel A.P., Bonilla L.M., Pierobon N., Ponzio N.M., Rameshwar P. Antigen-presenting property of mesenchymal stem cells occurs during a narrow window at low levels of interferon-gamma. Blood. 2006;107:4817–4824. doi: 10.1182/blood-2006-01-0057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X., Armstrong M.A., Li G. Mesenchymal stem cells in immunoregulation. Immunol. Cell Biol. 2006;84:413–421. doi: 10.1111/j.1440-1711.2006.01458.x. [DOI] [PubMed] [Google Scholar]
- Dazzi F., Krampera M. Mesenchymal stem cells and autoimmune diseases. Best Pract. Res. Clin. Haematol. 2011;24:49–57. doi: 10.1016/j.beha.2011.01.002. [DOI] [PubMed] [Google Scholar]
- English K., French A., Wood K.J. Mesenchymal stromal cells: facilitators of successful transplantation? Cell Stem Cell. 2010;7:431–442. doi: 10.1016/j.stem.2010.09.009. [DOI] [PubMed] [Google Scholar]
- Fallarino F., Grohmann U. Using an ancient tool for igniting and propagating immune tolerance: IDO as an inducer and amplifier of regulatory T cell functions. Curr. Med. Chem. 2011;18:2215–2221. doi: 10.2174/092986711795656027. [DOI] [PubMed] [Google Scholar]
- Koç O.N., Gerson S.L., Cooper B.W., Dyhouse S.M., Haynesworth S.E., Caplan A.I., Lazarus H.M. Rapid hematopoietic recovery after coinfusion of autologous-blood stem cells and culture-expanded marrow mesenchymal stem cells in advanced breast cancer patients receiving high-dose chemotherapy. J. Clin. Oncol. 2000;18:307–316. doi: 10.1200/JCO.2000.18.2.307. [DOI] [PubMed] [Google Scholar]
- Krampera M. Mesenchymal stromal cells: more than inhibitory cells. Leukemia. 2011;25:565–566. doi: 10.1038/leu.2011.8. [DOI] [PubMed] [Google Scholar]
- Le Blanc K., Tammik C., Rosendahl K., Zetterberg E., Ringdén O. HLA expression and immunologic properties of differentiated and undifferentiated mesenchymal stem cells. Exp. Hematol. 2003;31:890–896. doi: 10.1016/s0301-472x(03)00110-3. [DOI] [PubMed] [Google Scholar]
- Lee M.W., Choi J., Yang M.S., Moon Y.J., Park J.S., Kim H.C., Kim Y.J. Mesenchymal stem cells from cryopreserved human umbilical cord blood. Biochem. Biophys. Res. Commun. 2004;320:273–278. doi: 10.1016/j.bbrc.2004.04.206. [DOI] [PubMed] [Google Scholar]
- Medinger M., Tichelli A., Bucher C., Halter J., Dirnhofer S., Rovo A., Passweg J., Tzankov A. GVHD after allogeneic haematopoietic SCT for AML: angiogenesis, vascular endothelial growth factor and VEGF receptor expression in the BM. Bone Marrow Transplant. 2013;48:715–721. doi: 10.1038/bmt.2012.200. [DOI] [PubMed] [Google Scholar]
- Meirelles Lda S., Fontes A.M., Covas D.T., Caplan A.I. Mechanisms involved in the therapeutic properties of mesenchymal stem cells. Cytokine Growth Factor Rev. 2009;20:419–427. doi: 10.1016/j.cytogfr.2009.10.002. [DOI] [PubMed] [Google Scholar]
- Meisel R., Zibert A., Laryea M., Göbel U., Däubener W., Dilloo D. Human bone marrow stromal cells inhibit allogeneic T-cell responses by indoleamine 2,3-dioxygenase-mediated tryptophan degradation. Blood. 2004;103:4619–4621. doi: 10.1182/blood-2003-11-3909. [DOI] [PubMed] [Google Scholar]
- Mitchell K.E., Weiss M.L., Mitchell B.M., Martin P., Davis D., Morales L., Helwig B., Beerenstrauch M., Abou-Easa K., Hildreth T., Troyer D., Medicetty S. Matrix cells from Wharton's jelly form neurons and glia. Stem Cells. 2003;21:50–60. doi: 10.1634/stemcells.21-1-50. [DOI] [PubMed] [Google Scholar]
- Müller M., Carter S., Hofer M.J., Campbell I.L. The chemokine receptor CXCR3 and its ligands CXCL9, CXCL10 and CXCL11 in neuroimmunity - a tale of conflict and conundrum. Neuropathol. Appl. Neurobiol. 2010;36:368–387. doi: 10.1111/j.1365-2990.2010.01089.x. [DOI] [PubMed] [Google Scholar]
- Munn D.H., Sharma M.D., Mellor A.L. Ligation of B7-1/B7-2 by human CD4 + T cells triggers indoleamine 2,3-dioxygenase activity in dendritic cells. J. Immunol. 2004;172:4100–4110. doi: 10.4049/jimmunol.172.7.4100. [DOI] [PubMed] [Google Scholar]
- Nauta A.J., Fibbe W.E. Immunomodulatory properties of mesenchymal stromal cells. Blood. 2007;110:3499–3506. doi: 10.1182/blood-2007-02-069716. [DOI] [PubMed] [Google Scholar]
- Opitz C.A., Litzenburger U.M., Lutz C., Lanz T.V., Tritschler I., Köppel A., Tolosa E., Hoberg M., Anderl J., Aicher W.K., Weller M., Wick W., Platten M. Toll-like receptor engagement enhances the immunosuppressive properties of human bone marrow-derived mesenchymal stem cells by inducing indoleamine-2,3-dioxygenase-1 via interferon-beta and protein kinase R. Stem Cells. 2009;27:909–919. doi: 10.1002/stem.7. [DOI] [PubMed] [Google Scholar]
- Phinney D.G. Biochemical heterogeneity of mesenchymal stem cell populations: clues to their therapeutic efficacy. Cell Cycle. 2007;6:2884–2889. doi: 10.4161/cc.6.23.5095. [DOI] [PubMed] [Google Scholar]
- Phinney D.G. Functional heterogeneity of mesenchymal stem cells: implications for cell therapy. J. Cell. Biochem. 2012;113:2806–2812. doi: 10.1002/jcb.24166. [DOI] [PubMed] [Google Scholar]
- Pittenger M.F., Mackay A.M., Beck S.C., Jaiswal R.K., Douglas R., Mosca J.D., Moorman M.A., Simonetti D.W., Craig S., Marshak D.R. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- Polchert D., Sobinsky J., Douglas G., Kidd M., Moadsiri A., Reina E., Genrich K., Mehrotra S., Setty S., Smith B., Bartholomew A. IFN-gamma activation of mesenchymal stem cells for treatment and prevention of graft versus host disease. Eur. J. Immunol. 2008;38:1745–1755. doi: 10.1002/eji.200738129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prockop D.J. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science. 1997;276:71–74. doi: 10.1126/science.276.5309.71. [DOI] [PubMed] [Google Scholar]
- Qian L., Wu Z., Shen J. Advances in the treatment of acute graft-versus-host disease. J. Cell. Mol. Med. 2013;17:966–975. doi: 10.1111/jcmm.12093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren G., Zhang L., Zhao X., Xu G., Zhang Y., Roberts A.I., Zhao R.C., Shi Y. Mesenchymal stem cell-mediated immunosuppression occurs via concerted action of chemokines and nitric oxide. Cell Stem Cell. 2008;2:141–150. doi: 10.1016/j.stem.2007.11.014. [DOI] [PubMed] [Google Scholar]
- Reyes M., Lund T., Lenvik T., Aguiar D., Koodie L., Verfaillie C.M. Purification and ex vivo expansion of postnatal human marrow mesodermal progenitor cells. Blood. 2001;98:2615–2625. doi: 10.1182/blood.v98.9.2615. [DOI] [PubMed] [Google Scholar]
- Ringdén O., Remberger M., Svahn B.M., Barkholt L., Mattsson J., Aschan J., Le Blanc K., Gustafsson B., Hassan Z., Omazic B., Svenberg P., Solders G., von Döbeln U., Winiarski J., Ljungman P., Malm G. Allogeneic hematopoietic stem cell transplantation for inherited disorders: experience in a single center. Transplantation. 2006;81:718–725. doi: 10.1097/01.tp.0000181457.43146.36. [DOI] [PubMed] [Google Scholar]
- Ringdén O., Uzunel M., Rasmusson I., Remberger M., Sundberg B., Lönnies H., Marschall H.U., Dlugosz A., Szakos A., Hassan Z., Omazic B., Aschan J., Barkholt L., Le Blanc K. Mesenchymal stem cells for treatment of therapy-resistant graft-versus-host disease. Transplantation. 2006;81:1390–1397. doi: 10.1097/01.tp.0000214462.63943.14. [DOI] [PubMed] [Google Scholar]
- Ruutu T., van Biezen A., Hertenstein B., Henseler A., Garderet L., Passweg J., Mohty M., Sureda A., Niederwieser D., Gratwohl A., de Witte T. Prophylaxis and treatment of GVHD after allogeneic haematopoietic SCT: a survey of centre strategies by the European Group for Blood and Marrow Transplantation. Bone Marrow Transplant. 2012;47:1459–1464. doi: 10.1038/bmt.2012.45. [DOI] [PubMed] [Google Scholar]
- Ryan J.M., Barry F., Murphy J.M., Mahon B.P. Interferon-gamma does not break, but promotes the immunosuppressive capacity of adult human mesenchymal stem cells. Clin. Exp. Immunol. 2007;149:353–363. doi: 10.1111/j.1365-2249.2007.03422.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato K., Ozaki K., Oh I., Meguro A., Hatanaka K., Nagai T., Muroi K., Ozawa K. Nitric oxide plays a critical role in suppression of T-cell proliferation by mesenchymal stem cells. Blood. 2007;109:228–234. doi: 10.1182/blood-2006-02-002246. [DOI] [PubMed] [Google Scholar]
- Sekiya I., Larson B.L., Smith J.R., Pochampally R., Cui J.G., Prockop D.J. Expansion of human adult stem cells from bone marrow stroma: conditions that maximize the yields of early progenitors and evaluate their quality. Stem Cells. 2002;20:530–541. doi: 10.1634/stemcells.20-6-530. [DOI] [PubMed] [Google Scholar]
- Soleymaninejadian E., Pramanik K., Samadian E. Immunomodulatory properties of mesenchymal stem cells: cytokines and factors. Am. J. Reprod. Immunol. 2012;67:1–8. doi: 10.1111/j.1600-0897.2011.01069.x. [DOI] [PubMed] [Google Scholar]
- Sotiropoulou P.A., Perez S.A., Salagianni M., Baxevanis C.N., Papamichail M. Characterization of the optimal culture conditions for clinical scale production of human mesenchymal stem cells. Stem Cells. 2006;24:462–471. doi: 10.1634/stemcells.2004-0331. [DOI] [PubMed] [Google Scholar]
- Spaggiari G.M., Capobianco A., Abdelrazik H., Becchetti F., Mingari M.C., Moretta L. Mesenchymal stem cells inhibit natural killer-cell proliferation, cytotoxicity, and cytokine production: role of indoleamine 2,3-dioxygenase and prostaglandin E2. Blood. 2008;111:1327–1333. doi: 10.1182/blood-2007-02-074997. [DOI] [PubMed] [Google Scholar]
- Stec M., Baran J., Baj-Krzyworzeka M., Weglarczyk K., Gozdzik J., Siedlar M., Zembala M. Chemokine receptors and chemokine production by CD34 + stem cell-derived monocytes in response to cancer cells. Anticancer Res. 2012;32:4749–4753. [PubMed] [Google Scholar]
- Tan P.H., Bharath A.K. Manipulation of indoleamine 2,3 dioxygenase; a novel therapeutic target for treatment of diseases. Expert Opin. Ther. Targets. 2009;13:987–1012. doi: 10.1517/14728220903018940. [DOI] [PubMed] [Google Scholar]
- Vogel W., Grünebach F., Messam C.A., Kanz L., Brugger W., Bühring H.J. Heterogeneity among human bone marrow-derived mesenchymal stem cells and neural progenitor cells. Haematologica. 2003;88:126–133. [PubMed] [Google Scholar]
- Wang H., Asavaroengchai W., Yeap B.Y., Wang M.G., Wang S., Sykes M., Yang Y.G. Paradoxical effects of IFN-gamma in graft-versus-host disease reflect promotion of lymphohematopoietic graft-versus-host reactions and inhibition of epithelial tissue injury. Blood. 2009;113:3612–3619. doi: 10.1182/blood-2008-07-168419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Chen X., Cao W., Shi Y. Plasticity of mesenchymal stem cells in immunomodulation: pathological and therapeutic implications. Nat. Immunol. 2014;15:1009–1016. doi: 10.1038/ni.3002. [DOI] [PubMed] [Google Scholar]
- Waterman R.S., Tomchuck S.L., Henkle S.L., Betancourt A.M. A new mesenchymal stem cell (MSC) paradigm: polarization into a pro-inflammatory MSC1 or an immunosuppressive MSC2 phenotype. PLoS One. 2010;5 doi: 10.1371/journal.pone.0010088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterman R.S., Henkle S.L., Betancourt A.M. Mesenchymal stem cell 1 (MSC1)-based therapy attenuates tumor growth whereas MSC2-treatment promotes tumor growth and metastasis. PLoS One. 2012;7 doi: 10.1371/journal.pone.0045590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto M., Ota A., Hori T., Imai S., Sohma H., Suzuki N., Hatakeyama N., Inazawa N., Ito Y.M., Kimura H., Tsutsumi H., Kokai Y. Early expression of plasma CCL8 closely correlates with survival rate of acute graft-vs-host disease in mice. Exp. Hematol. 2011;39:1101–1112. doi: 10.1016/j.exphem.2011.07.006. [DOI] [PubMed] [Google Scholar]
- Zuk P.A., Zhu M., Mizuno H., Huang J., Futrell J.W., Katz A.J., Benhaim P., Lorenz H.P., Hedrick M.H. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7:211–228. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material