Abstract
Background:
Geissoschizine methyl ether (GM) is one of the indole alkaloids in Uncaria hook, and an active ingredient of yokukansan (YKS) that improves behavioral and psychological symp-toms of dementia (BPSD) in patients with several types of dementia. The pharmacological action of GM has been related to various serotonin (5-HT) receptor subtypes.
Objective:
The aim of this article is to review the binding characteristics of GM to the 5-HT receptor sub-types in the brains using our own data and previous findings.
Methods:
Competitive receptor-binding and agonist/antagonist activity assays for several 5-HT receptor subtypes were performed. Moreover, the articles describing pharmacokinetics and brain distribution of GM were searched in PubMed.
Results:
GM bound the following 5-HT receptor subtypes: 5-HT1A, 5-HT1B, 5-HT2A, 5-HT2B, 5-HT2C, 5-HT4, 5-HT5A, 5-HT6, and 5-HT7. Among these receptors, GM had partial agonistic activity for 5-HT1A receptors and antagonistic activity for 5-HT2A, 5-HT2B, 5-HT2C, and 5-HT7 receptors. Also, GM was me-tabolized by various CYP isoforms, mainly CYP3A4. Parent/unchanged GM was detected in both the blood and brain of rats after oral administration of YKS. In the brains, GM was presumed to bind to 5-HT1A, 5-HT2A, 5-HT2B, 5-HT2C, and 5-HT7 receptors on neuron-like large cells mainly in the frontal cor-tex.
Conclusion:
These results suggest that GM is a pharmacologically important alkaloid that regulates vari-ous serotonergic activities or functions by binding to multiple 5-HT receptor subtypes. Thus, this review provides recent 5-HT receptor-related evidence that GM is partly responsible for pharmacological effects of YKS.
Keywords: Geissoschizine methyl ether, 5-HT receptor, pharmacokinetics, pharmacological aspect, yokukansan, BPSD, dementia
1. INTRODUCTION
Yokukansan (YKS) is one of the traditional Japanese medicines called Kampo medicines in Japan, and has been approved by the Japanese Ministry of Health, Labour, and Welfare as a remedy for neurosis, insomnia, and irritability and night crying in children. YKS reportedly improves behavioral and psychological symptoms of dementia (BPSD) such as hallucinations, agitation, and aggressiveness in patients with different types of dementia, including Alzheimer’s disease [1-4], dementia with Lewy bodies [5], vascular dementia [6], and frontotemporal dementia [7], without severe adverse effects.
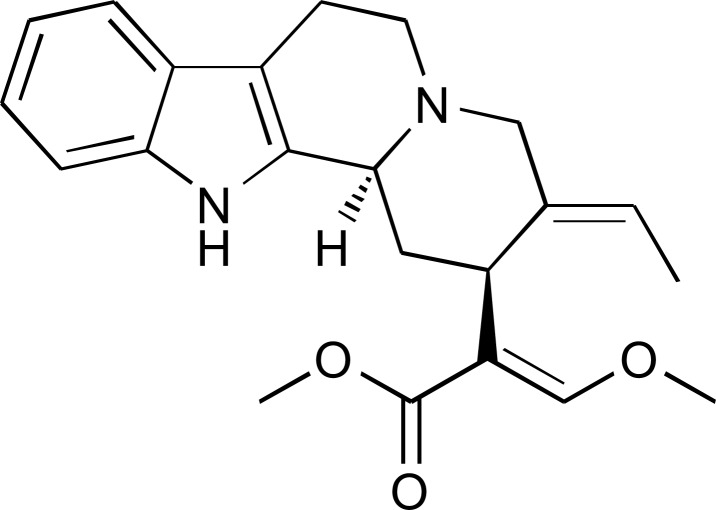
Accumulated basic research has demonstrated that the serotonergic system in the central nervous system (CNS) plays an important role in the psychotropic effects of YKS [8-10]. An in vitro binding study showed that YKS bound to a serotonin 1A (5-HT1A) receptor as a partial agonist [9]. A subsequent study clarified that only Uncaria hook, among seven constituent medicinal herbs of YKS, had the partial agonistic activity to 5-HT1A receptor [9]. This finding was also verified by the evidence that the partial agonistic binding of YKS disappeared after removing Uncaria hook from YKS. These results imply that the active ingredients showing 5-HT1A receptor partial agonistic activity are contained in Uncaria hook. Further in vitro receptor-binding assay identified geissoschizine methyl ether (GM) as the active ingredient, which is an indole alkaloid in Uncaria plants [11,12] (Fig. 1).
Fig. (1).
Chemical structure of geissoschizine methyl ether (GM).
These in vitro findings were supported by in vivo studies using rodents, which have demonstrated that oral YKS (1.0 g/kg) ameliorated BPSD-like aggressive and social behaviors and that these ameliorative effects were counteracted by a 5-HT1A receptor antagonist [8,10]. This finding was also verified by the study that the ameliorative effect of YKS on isolation stress-induced aggressive behavior was completely abolished by the removal of Uncaria hook, suggesting that the effect of YKS is mainly attributed to Uncaria hook [10]. Moreover, Uncaria hook alone (150 mg/kg, the approximate amount of Uncaria hook contained in 1.0 g/kg of YKS) or GM alone (150 μg/kg, the approximate amount of GM contained in 1.0 g/kg of YKS) also ameliorated isolation stress-induced aggressive behavior, which had similar efficacy to YKS [10]. Pharmacokinetic study demonstrated that GM was detected in the plasma and brain of rats after oral administration of YKS [13,14]. These results suggest that GM is a potent 5-HT1A receptor agonist and a candidate ingredient for the psychopharmacological effect of YKS.
GM has an indole structure similar to that of the neurotransmitter 5-HT. 5-HT receptors that are instrumental in various physiological functions are known to have at least 14 subtypes from seven distinct families (5-HT1–5-HT7) [15]. Therefore, GM might mediate multiple serotonergic physiological functions via several 5-HT receptor subtypes. Indeed, to date, GM has demonstrated binding ability to several subtypes of 5-HT receptor [10,16-21]. In this review, we describe our data indicating the binding profile and agonist/antagonist activity of GM for various 5-HT receptor subtypes, with reference to previous findings. The pharmacokinetics and pharmacological aspects of GM and YKS are also described. These findings provide druggable information of a natural compound GM, and would be useful in understanding the contribution of GM to the pharmacological effects of YKS.
2. ISOLATION AND IDENTIFICATION OF GM
We isolated GM from Uncaria hook, i.e. the hook of Uncaria rhynchophilla Miquel, Rubiaceae [22]. In brief, 319.4 g of a dried crude drug of Uncaria hook dissolved in 2.5 L of distilled water was refluxed at 120°C for 2 h. The extracted solution was passed through a 100 mesh-size stainless steel filter and then lyophilized to give a dried powder (38.2 g). The extract was chromatographed on a Diaion HP-20 (Mitsubishi, Tokyo, Japan), eluted with 2 L of water, 2 L of aqueous methanol (50% v/v), and 1 L of methanol, successively. The methanol eluate was evaporated to remove the solvent and then lyophilized to afford the dried methanol–eluate powder (0.529 g). The indole alkaloids were further isolated from the methanol extract by eluting with 0.05 M ammonium acetate buffer (pH 3.6)–acetonitrile (1:1) on a separation column (ODS, 5 cm i.d. × 30 cm, Inertsil, GL Science, Tokyo, Japan), yielding 10 mg of GM. In direct comparison with an authentic standard substance, the isolated GM was confirmed to be a single peak by high performance liquid chromatography, and was identified by analyses of the 1H and 13C nuclear magnetic resonance spectra and mass spectrum.
3. RECEPTOR BINDING
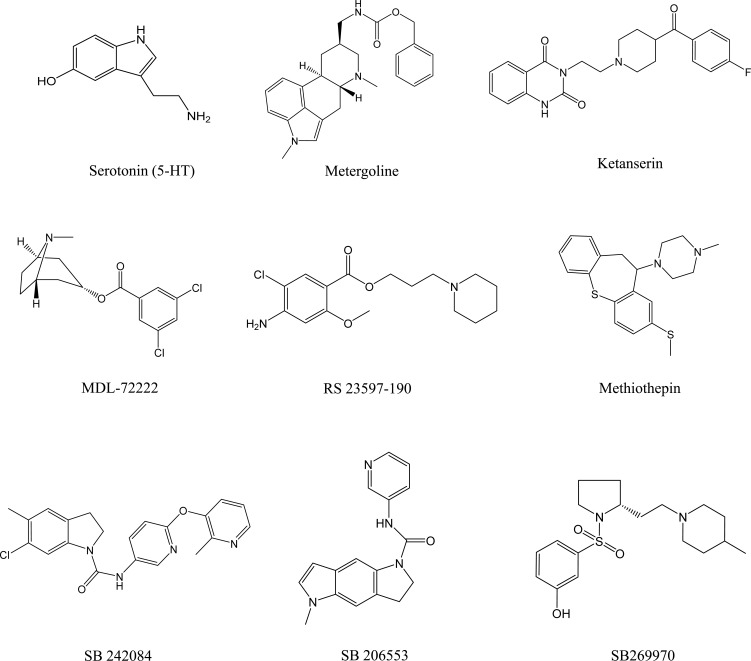
This section introduces the foundational data regarding the binding of GM on 5-HT receptor subtypes. Competitive binding assays for 5-HT1A [23, 24], 5-HT1B [25, 26], 5-HT2A [27, 28], 5-HT2B [27], 5-HT2C [29], 5-HT3 [30, 31], 5-HT4 [32], 5-HT5A [33], 5-HT6 [34], and 5-HT7 [35, 36] were performed according to the previously reported procedures. The membrane preparations of Chinese hamster ovary (CHO) cells stably expressing human recombinant 5-HT1A and 5-HT7, CHO-K1 cells stably expressing human recombinant 5-HT2A, 5-HT2B, 5-HT2C, and 5-HT5A, human embryonic kidney (HEK)-293 cells stably expressing human recombinant 5-HT3, and Hela cells stably expressing human recombinant 5-HT6, were used for the respective corresponding binding assays. Membrane preparations of rat cerebral cortex and guinea pig striatum were used for the binding assays of 5-HT1B and 5-HT4 receptors. Radioligands used for each receptor assay were [3H]8-hydroxy-2-(di-n-propylamino)tetralin (8-OH-DPAT) for 5-HT1A; [125I]cyanopindiolol for 5-HT1B; [3H]ketanserin for 5-HT2A; [3H]lysergic acid diethylamide for 5-HT2B, 5-HT5A, 5-HT6, and 5-HT7; [3H]mesulergine for 5-HT2C; [3H]GR-65630 for 5-HT3; and [3H]GR-113808 for 5-HT4. Metergoline (5-HT1A), serotonin (5-HT1B, 5-HT2B, 5-HT4, 5-HT5A, 5-HT6, and 5-HT7), mianserin (5-HT2A and 5-HT2C), and MDL 72222 (5-HT3) were used to determine the nonspecific binding for each receptor. The binding specificities of these binding assay procedures were approximately 75%–95%. In these assays, metergoline, 5-HT, ketanserin, SB242084, MDL 72222, RS-23595-190, and methiothepin were used as the reference compounds (Fig. 2).
Fig. (2).
Chemical structures of the reference compounds used in the binding and agonist/antagonist assays to 5-HT receptor subtypes.
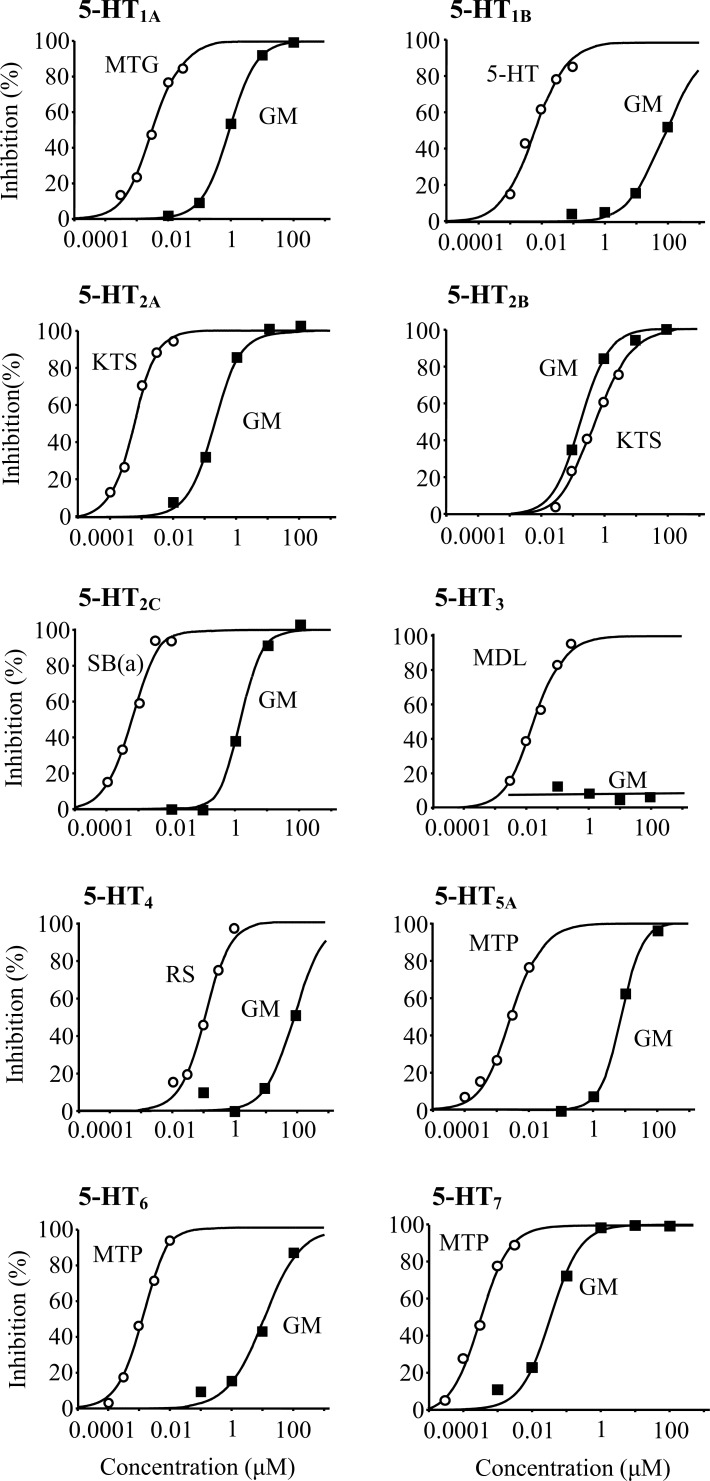
Figure (3) shows the concentration-response curves to determine the half maximal inhibitory concentration (IC50) values of GM to each 5-HT receptor subtype in the competitive binding assays. The sigmoidal curve for each reference compound indicated that the binding assays used in this study were appropriated to evaluate the binding of test substances. GM strongly inhibited the radioligand bindings to 5-HT1A (IC50 = 0.904 μM), 5-HT2A (IC50 = 0.197 μM), 5-HT2B (IC50 = 0.191 μM), 5-HT2C (IC50 = 1.480 μM), and 5-HT7 (IC50 = 0.034 μM) receptors rather than other subtypes of 5-HT1B (IC50 = 88.3 μM), 5-HT3 (non-binding), 5-HT4 (IC50 = 94.5 μM), 5-HT5A (IC50 = 6.84 μM), and 5-HT6 (IC50 = 12.2 μM). The results suggest that GM bound to 5-HT1A, 5-HT2A, 5-HT2B, 5-HT2C, and 5-HT7 receptor subtypes.
Fig. (3).
The concentration–response curves of GM and reference compounds to 5-HT receptor subtypes in the competitive binding assays. Each data represents the mean of duplicate determinations. MTG, metergoline; KTS, ketanserin; SB(a), SB242084; MDL, MDL 72222; RS, RS-23597-190; MTP, methiothepin.
Kanatani et al. [16] reported that GM inhibited specific [3H]5-HT binding to rat brain membrane; however, they did not determine the target receptor subtype. Thereafter, Pengsuparp et al. [18] demonstrated that GM inhibited the specific binding of [3H]radioligands for 5-HT1A, 5-HT2A, and 5-HT2C receptors to mouse brain membrane. Our competitive binding assays using radioligands demonstrated that GM shows more potently binds to not only 5-HT1A, 5-HT2A and 5-HT2C but also 5-HT2B and 5-HT7 receptors in various cells expressing each human recombinant 5-HT receptor subtype.
4. AGONIST AND ANTAGONIST ACTIVITIES
Subsequently, we examined whether GM shows agonistic or antagonistic activity to five 5-HT receptor subtypes (5-HT1A, 5-HT2A, 5-HT2B, 5-HT2c, and 5-HT7) that GM showed potent binding in the competitive receptor-binding assays. The agonistic effects of GM were evaluated by measuring [35S]GTPγS binding for 5-HT1A [37] and 5-HT2C [37, 38] receptors, inositol monophosphate (IP1) for 5-HT2A [39, 40] and 5-HT2B [41,42] receptors, or cAMP for 5-HT7 receptors [43] in the cells expressing each receptor subtype. The antagonistic effects of GM on these receptors were assessed by examining the inhibition of 5-HT-induced increases in [35S]GTPγS binding, IP1 production, or cAMP pro-duction. In these assays, metergoline, 5-HT, ketanserin, SB242084, MDL 72222, RS-23595-190, and methiothepin were used as the reference compounds (Fig. 2).
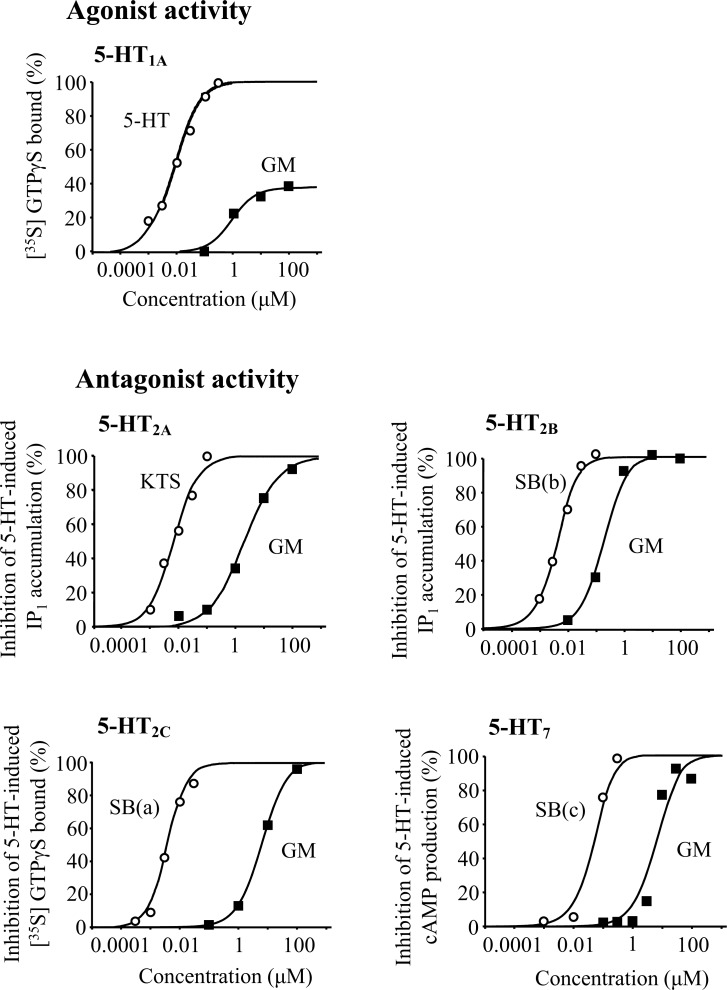
Figure (4) shows the concentration-response curves of GM and reference compounds to each receptor. Agonistic activity was found in 5-HT1A receptors: the [35S]GTPγS binding was increased by GM or 5-HT, a full agonist, in a concentration-dependent manner. However, the binding rate of GM reached a plateau at approximately 40% of that of 5-HT, suggesting that GM is a partial agonist for 5-HT1A receptor [10]. Regarding the four other receptors, GM showed antagonistic activity with IC50 values of 2.31 μM (5-HT2A), 0.182 μM (5-HT2B), 6.19 μM (5-HT2C), and 6.00 μM (5-HT7).
Fig. (4).
Agonist and antagonist activities of GM. GM showed partial agonistic activity for 5-HT1A receptor and antagonistic activity for 5-HT2A, 5-HT2B, 5-HT2C, and 5-HT7 receptors. Each data represents the mean of duplicate determinations. KTS, ketanserin, SB(a), SB242084; SB(b), SB206553; SB(c), SB269970.
GM was initially found by Kanatani et al. [16] to have partial agonistic activity for 5-HT receptors in a combination of [3H]5-HT-binding assay of rat brain membrane and bioassay using guinea-pig ileum, but they did not determine the receptor subtypes. Meanwhile, Zhu et al. [17] reported that Uncaria hook exhibited strong binding to 5-HT1A and 5-HT2 receptors, but they did not determine the active ingredient. In 2001, Pengsuparp et al. [18] reported that GM possessed mixed 5-HT1A receptor agonist/5-HT2A/2C receptor antagonist activities by using various bioassays such as hypothermic response, head-twitch response, and head-weaving response. Recently, Ueda et al. [19] verified them in another analytical approach, i.e., a single-cell-based calcium imaging assay using HEK-293T cells expressing each human recombinant 5-HT receptor subtype. From these findings and our results (Figs. 3 and 4), it is no doubt that GM possesses 5-HT1A receptor partial agonist and 5-HT2A/2C receptor antagonist activities.
We also found that GM possessed antagonistic activity to the 5-HT2B receptor. GM contains tetrahydro-β-carboline (THBC) in its structure, which has been reported to show selective antagonist activity on the 5-HT2B receptor [44, 45]. Rauwolscine is also an indole alkaloid containing the THBC structure, and is reported to behave as a 5-HT1A receptor partial agonist, a 5-HT2A/2B receptor antagonist [46-48], as well as an α2-adrenergic receptor antagonist [49, 50]. It is suggested that the presence of the D-ring and the substituents of THBC [18], in other words, the C1-substituted optically activity of THBC [51], increases the affinity for 5-HT receptor subtypes. Since GM is also a C1-substituted THBC, GM is thought to have 5-HT2B antagonistic activity.
Regarding the 5-HT7 receptor, Ueda et al. [19] first demonstrated that GM behaved as the antagonist in addition to 5-HT1A partial agonist, 5-HT2A/2C antagonist, and a D2L receptor partial agonist/antagonist in a single-cell-based calcium imaging assay. The 5-HT7 receptor is a G protein-coupled receptor linked to Gαs that activates adenylate cyclase, and increases second messenger cAMP [52]. Because this receptor does not link to an intracellular calcium mobilization ([Ca2+]i) system, it is different from Gαq-linked G protein-coupled receptor-like 5-HT2 receptors, which activate inositol trisphosphate, and then induce [Ca2+]i mobilization [53]. Thus, although it is generally impossible to evaluate the intrinsic activity of 5-HT7 receptors by changes in [Ca2+]i mobilization, the calcium imaging assay newly developed by Ueda et al. [54] enabled it by transfection of Gα15 (Gα15 integrates into the downstream calcium flux) in HEK-293T cells expressing human recombinant 5-HT7 receptors. However, the receptor binding rate of the test substance is not clear in this method, and measurement of the cAMP level is the most appropriate for the G protein-coupled receptors linked to Gαs and Gαi for direct and absolute evaluation. Our present data support these issues by clarifying the antagonistic effect of GM on 5-HT7 receptor using competitive binding assay (Fig. 3, 5-HT7) and direct measurement of intracellular cAMP levels (Fig. 4, 5-HT7). In our 5-HT7 receptor assay [20], only GM, among the seven alkaloids in Uncaria hook (indole alkaloids: GM, hirsuteine, and hirsutine; oxindole alkaloids: rhynchophylline, isorhynchophylline, corynoxeine, and isocorynoxeine), showed 5-HT7 receptor antagonistic activity. Structural comparison of these ingredients inferred that the binding to 5-HT7 receptor also depends on the difference of optical isomer at the C1-substituent in the THBC structure [51], as described above.
Several chemical compounds with 5-HT7 receptor antagonistic activity reportedly also have 5-HT1 agonist and 5-HT2 receptor antagonist activities [55-57]. As already described, GM has an agonistic effect on 5HT1A receptors, and an antagonistic effect on 5-HT2A receptors. These findings also support that GM has a high affinity for 5-HT7 receptors.
5. PHARMACOKINETICS
In vitro studies using rat and human liver microsomes reported that GM was metabolized into at least 13 metabolites including hydroxylated, dehydrogenated, hydroxylated + dehydrogenated, demethylated, and hydration forms by several CYP isoforms, and CYP3A4 was found to mainly contribute to GM metabolism [58, 59]. Parent/unchanged GM was detected in both plasma and brain of rats after orally administered YKS, and demonstrated that GM was able to cross the blood-brain barrier (BBB) in an in vitro BBB assay [13,14]. Recently, Kitagawa et al. [60] verified GM to be detected in the plasma after oral administration of YKS in humans. These in vivo and in vitro results suggest that GM in orally administered YKS is absorbed into the blood, and then reaches the brain through the BBB. The GM that entered the brain was presumed to bind to dopamine D2, adrenergic α2A, and μ-opioid receptors and L-type Ca2+ channels, as well as 5-HT1A, 5-HT2A, 5-HT2B, 5-HT2C, and 5-HT7 receptors on neuron-like large cells mainly in the frontal cortex, which were evaluated by autoradiography using [3H]GM in rat brain slices [21]. This result agrees with those in previous in vitro binding assays [10,18-20].
Pharmacokinetics of GM metabolites identified in the in vitro study has not yet been verified in vivo study. However, we confirmed in a preliminary study that 23-O-demethylated GM was also detected in the brain of YKS-treated rats, and in vitro receptor binding assay showed that this metabolite did not bind to the 5-HT1A receptors (unpublished observations). These results suggest that GM but not the metabolite is the active form.
6. PHARMACOLOGICAL ASPECT
In humans, 90% of the 5-HT in the body exists in the gastrointestinal tract, 8%–10% in platelets, and 1%–2% in the CNS. In the peripheral nervous system, 5-HT is involved in smooth muscle contraction, gastrointestinal function, and platelet aggregation. In the CNS as a neurotransmitter, it is related to physiological functions such as mood and emotional regulation, sleep-wake cycle, thermoregulation, sexual behavior, algesia, cognition/memory formation, and biorhythm. Dysfunction of the serotonergic system is involved in various mental disorders such as anxiety, aggressiveness, duress, mood disorders, schizophrenia, autism, and drug dependence [61]. Complex natural alkaloids that contain the THBC structure such as yohimbine or reserpine have a wide range of pharmacological activities. These types of molecule are known to have 5-HT receptor antagonist and α-adrenergic receptor antagonist activity, and have a broad spectrum of pharmacological properties including central action related to hallucination, vasodilation, and analgesic actions, as well as antimicrobial activities [44, 51, 62]. YKS containing GM, one of the THBCs, has various pharmacological effects that act to improve symptoms that are similar to BPSDs, like aggressiveness, hallucinations, anxiety, and sleep disturbance, as well as symptoms like tardive dyskinesia, neuropathic pain, morphine tolerance/physical dependency, allergy/atopic dermatitis, and cognitive deficits [63]. These multiple potential actions include serotonergic, glutamatergic, cholinergic, dopaminergic, adrenergic, and GABAnergic neurotransmissions as well as neuroprotection, anti-stress effect, promotion of neuroplasticity, and anti-inflammatory effect [63]. Among these neuropsychopharmacological effects, YKS, Uncaria hook, or GM has been demonstrated to enhance 5-HT1A receptor agonist-induced decrease in rearing behavior, concomitant with up-regulation of prefrontal 5-HT1A receptors in mice [64], or to ameliorate aggressiveness and decreased sociability [10] in isolation-stressed mice, anxiety in fear-conditioned rats [65, 66] through their agonistic effect to 5-HT1A receptors [10], 5-hydroxy-L-tryptophan-induced head-twitch response which are related to 5-HT2A and 5-HT2C receptor antagonisms [18], and 5-HT2A receptor agonist-induced head-twitch response by down-regulating 5-HT2A receptors in the prefrontal cortex [67, 68]. In addition, these substances act on other neurotransmitter systems to improve symptoms, e.g., adrenergic/dopaminergic agonist-induced decrease in locomotion [18, 69], morphine-induced tolerance/physical dependency in mice by blocking α2A-adrenoceptors [70], norepinephrine-induced contraction of rat aorta [22], and glutamate-induced neuronal death [71].
Although the physiological functions of 5-HT7 receptor are not fully understood, several studies suggest an involvement in vascular relaxation [36, 72] and circadian rhythm control [73, 74]. Hedlund and Sutcliffe [75] also suggest important functional roles for the 5-HT7 receptor in thermoregulation, circadian rhythm, learning and memory, hippocampal signaling, and sleep. In addition, because atypical antipsychotics, such as clozapine and risperidone, and some antidepressants display high affinity for the 5-HT7 receptor as antagonists, blocking effects of this receptor by these drugs are involved in antipsychotic or antidepressant action [57, 76, 77]. Ueda et al. [19] suggest that the pharmacological profiles of GM at dopamine and serotonin receptors are similar to those of aripiprazole, a third-generation antipsychotic. As described above, GM having 5-HT7 receptor antagonist activity (Figs. 3 and 4) was actually demonstrated to have anti-aggressive and vasorelaxant effects. YKS also has an ameliorative effect on rapid eye movement sleep behavior disorder in humans [78], which is related to circadian rhythm control. Thus, 5-HT7 receptor antagonism is thought to relate to the psychotropic and vasorelaxant effects of GM and YKS.
Recently, Deng et al. [79] reported that several ingredients in Angelica sinensis exhibited affinity toward 5-HT7 receptors in the in vitro competitive binding assay. Ofir et al. [80] reported that several isoflavans isolated from the roots of Glycyrrhiza glabra inhibited in vitro serotonin re-uptake. Although these herbal medicines differ from those included in YKS in the botanical origin; Angelica acutiloba and Glycyrrhiza uralensis are used in YKS, they are also informative for drug discovery and development for serotonin receptors in future.
CONCLUSION
This review provided 5-HT receptor-related evidence of GM responsible for pharmacological effects of YKS. GM is thought to be a pharmacologically important alkaloid in regulating various serotonergic activities or functions by binding multiple 5-HT receptor subtypes. We hope this review forms the foundation for assessing the usefulness of natural compounds on neurotransmitter systems in the CNS.
ACKNOWLEDGEMENTS
For studies conducted at Tsumura Research Laboratories, the authors thank the following researchers and managers for planning the studies, performing the experiments, analyzing the data, writing the papers, and stimulating discussion: Toshiyuki Ueki, Zenji Kawakami, Masahiro Tabuchi, Sachiko Imamura, Akinori Nishi, Hitomi Kanno, Takuji Yamaguchi, Hiroaki Ooizumi, Nae Kuriyama, Takashi Matsumoto, Hirotaka Kushida, Junko Watanabe, Tomohisa Hattori, and Yoshio Kase.
The authors would like to thank Enago (www.enago.jp) for the English language review.
LIST OF ABBRIBIATIONS
- 5-HT
5-Hydroxytriptamine (serotonin)
- 8-OH-DPAT
8-Hydroxy-2-(di-n-propylamino)tetralin
- BBB
Blood-brain barrier
- BPSD
Behavioral and psychological symptoms of dementia
- cAMP
Cyclic adenosine 3',5'-monophosphate
- CHO
Chinese hamster ovary
- CNS
Central nervous system
- CYP
Cytochrome P450
- GABA
Gamma-aminobutyric acid
- GM
Geissoschizine methyl ether
- GTPγS
Guanosine 5'-O-(3-thiotriphosphate)
- HEK
Human embryonic kidney
- IC50
Half maximal inhibitory concentration
- KTS
Ketanserin
- MDL
MDL 72222
- MTG
Metergoline
- MTP
Methiothepin
- RS
RS-23597-190
- SB(a)
SB206553
- SB(b)
SB242084
- SB(c)
SB269970.
- THBC
Tetrahydro-β-carboline
- YKS
Yokukansan
Consent for Publication
Not applicable.
CONFLICT OF INTEREST
The authors are employees of Tsumura & Co. The authors declare that except for income received from the employer, no financial support or compensation has been received from any individual or corporate entity and no conflict of interest exists.
REFERENCES
- 1.Iwasaki K., Satoh-Nakagawa T., Maruyama M., Monma Y., Nemoto M., Tomita N., Tanji H., Fujiwara H., Seki T., Fujii M., Arai H., Sasaki H. A randomized, observer-blind, controlled trial of the traditional Chinese medicine Yi-Gan San for improvement of behavioral and psychological symptoms and activities of daily living in dementia patients. J. Clin. Psychiatry. 2005;66(2):248–252. doi: 10.4088/jcp.v66n0214. [DOI] [PubMed] [Google Scholar]
- 2.Mizukami K., Asada T., Kinoshita T., Tanaka K., Sonohara K., Nakai R., Yamaguchi K., Hanyu H., Kanaya K., Takao T., Okada M., Kudo S., Kotoku H., Iwakiri M., Kurita H., Miyamura T., Kawasaki Y., Omori K., Shiozaki K., Odawara T., Suzuki T., Yamada S., Nakamura Y., Toba K. A randomized cross-over study of a traditional Japanese medicine (kampo), yokukansan, in the treatment of the behavioural and psychological symptoms of dementia. Int. J. Neuropsychopharmacol. 2009;12(2):191–199. doi: 10.1017/S146114570800970X. [DOI] [PubMed] [Google Scholar]
- 3.Monji A., Takita M., Samejima T., Takaishi T., Hashimoto K., Matsunaga H., Oda M., Sumida Y., Mizoguchi Y., Kato T., Horikawa H., Kanba S. Effect of yokukansan on the behavioral and psychological symptoms of dementia in elderly patients with Alzheimer’s disease. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2009;33(2):308–311. doi: 10.1016/j.pnpbp.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 4.Matsuda Y., Kishi T., Shibayama H., Iwata N. Yokukansan in the treatment of behavioral and psychological symptoms of dementia: A systematic review and meta-analysis of randomized controlled trials. Hum. Psychopharmacol. 2013;28(1):80–86. doi: 10.1002/hup.2286. [DOI] [PubMed] [Google Scholar]
- 5.Iwasaki K., Kosaka K., Mori H., Okitsu R., Furukawa K., Manabe Y., Yoshita M., Kanamori A., Ito N., Wada K., Kitayama M., Horiguchi J., Yamaguchi S., Takayama S., Fukuhara R., Ouma S., Nakano S., Hashimoto M., Kinoshita T. Improvement in delusions and hallucinations in patients with dementia with Lewy bodies upon administration of yokukansan, a traditional Japanese medicine. Psychogeriatrics. 2012;12(4):235–241. doi: 10.1111/j.1479-8301.2012.00413.x. [DOI] [PubMed] [Google Scholar]
- 6.Nagata K., Yokoyama E., Yamazaki T., Takano D., Maeda T., Takahashi S., Terayama Y. Effects of yokukansan on behavioral and psychological symptoms of vascular dementia: An open-label trial. Phytomedicine. 2012;19(6):524–528. doi: 10.1016/j.phymed.2012.02.008. [DOI] [PubMed] [Google Scholar]
- 7.Kimura T., Hayashida H., Furukawa H., Takamatsu J. Pilot study of pharmacological treatment for frontotemporal dementia: Effect of Yokukansan on behavioral symptoms. Psychiatry Clin. Neurosci. 2010;64(2):207–210. doi: 10.1111/j.1440-1819.2010.02072.x. [DOI] [PubMed] [Google Scholar]
- 8.Kanno H., Sekiguchi K., Yamaguchi T., Terawaki K., Yuzurihara M., Kase Y., Ikarashi Y. Effect of yokukansan, a traditional Japanese medicine, on social and aggressive behaviour of para-chloroamphetamine-injected rats. J. Pharm. Pharmacol. 2009;61(9):1249–1256. doi: 10.1211/jpp/61.09.0016. [DOI] [PubMed] [Google Scholar]
- 9.Terawaki K., Ikarashi Y., Sekiguchi K., Nakai Y., Kase Y. Partial agonistic effect of yokukansan on human recombinant serotonin 1A receptors expressed in the membranes of Chinese hamster ovary cells. J. Ethnopharmacol. 2010;127(2):306–312. doi: 10.1016/j.jep.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 10.Nishi A., Yamaguchi T., Sekiguchi K., Imamura S., Tabuchi M., Kanno H., Nakai Y., Hashimoto K., Ikarashi Y., Kase Y. Geissoschizine methyl ether, an alkaloid in Uncaria hook, is a potent serotonin 1A receptor agonist and candidate for amelioration of aggressiveness and sociality by yokukansan. Neuroscience. 2012;207:124–136. doi: 10.1016/j.neuroscience.2012.01.037. [DOI] [PubMed] [Google Scholar]
- 11.Haginiwa J., Sakai S., Aimi N., Yamanaka E., Shinma N. Studies of plants containing indole alkaloids. 2. On the alkaloids of Uncaria rhynchophylla Miq. Yakugaku Zasshi. 1973;93(4):448–452. doi: 10.1248/yakushi1947.93.4_448. [DOI] [PubMed] [Google Scholar]
- 12.Mimaki Y., Toshimizu N., Yamada K., Sashida Y. Yakugaku Zasshi. 1997;117(12):1011–1021. doi: 10.1248/yakushi1947.117.12_1011. [Anti-convulsion effects of choto-san and chotoko (Uncariae Uncis cam Ramlus) in mice, and identification of the active principles]. [DOI] [PubMed] [Google Scholar]
- 13.Imamura S., Tabuchi M., Kushida H., Nishi A., Kanno H., Yamaguchi T., Sekiguchi K., Ikarashi Y., Kase Y. The blood-brain barrier permeability of geissoschizine methyl ether in Uncaria hook, a galenical constituent of the traditional Japanese medicine yokukansan. Cell. Mol. Neurobiol. 2011;31(5):787–793. doi: 10.1007/s10571-011-9676-3. [DOI] [PubMed] [Google Scholar]
- 14.Kushida H., Fukutake M., Tabuchi M., Katsuhara T., Nishimura H., Ikarashi Y., Kanitani M., Kase Y. Simultaneous quantitative analyses of indole and oxindole alkaloids of Uncaria Hook in rat plasma and brain after oral administration of the traditional Japanese medicine Yokukansan using high-performance liquid chromatography with tandem mass spectrometry. Biomed. Chromatogr. 2013;27(12):1647–1656. doi: 10.1002/bmc.2974. [DOI] [PubMed] [Google Scholar]
- 15.Hoyer D., Engel G., Kalkman H.O. Molecular pharmacology of 5-HT1 and 5-HT2 recognition sites in rat and pig brain membranes: radioligand binding studies with [3H]5-HT, [3H]8-OH-DPAT, (-)[125I]iodocyanopindolol, [3H]mes-ulergine and [3H]ketanserin. Eur. J. Pharmacol. 1985;118(1-2):13–23. doi: 10.1016/0014-2999(85)90658-2. [DOI] [PubMed] [Google Scholar]
- 16.Kanatani H., Kohda H., Yamasaki K., Hotta I., Nakata Y., Segawa T., Yamanaka E., Aimi N., Sakai S. The active principles of the branchlet and hook of Uncaria sinensis Oliv. examined with a 5-hydroxytryptamine receptor binding assay. J. Pharm. Pharmacol. 1985;37(6):401–404. doi: 10.1111/j.2042-7158.1985.tb03023.x. [DOI] [PubMed] [Google Scholar]
- 17.Zhu M., Bowery N.G., Greengrass P.M., Phillipson J.D. Application of radioligand receptor binding assays in the search for CNS active principles from Chinese medicinal plants. J. Ethnopharmacol. 1996;54(2-3):153–164. doi: 10.1016/s0378-8741(96)01454-7. [DOI] [PubMed] [Google Scholar]
- 18.Pengsuparp T., Indra B., Nakagawasai O., Tadano T., Mimaki Y., Sashida Y., Ohizumi Y., Kisara K. Pharmacological studies of geissoschizine methyl ether, isolated from Uncaria sinensis Olivera., in the central nervous system. Eur. J. Pharmacol. 2001;425(3):211–218. doi: 10.1016/s0014-2999(01)01195-5. [DOI] [PubMed] [Google Scholar]
- 19.Ueda T., Ugawa S., Ishida Y., Shimada S. Geissoschizine methyl ether has third-generation antipsychotic-like actions at the dopamine and serotonin receptors. Eur. J. Pharmacol. 2011;671(1-3):79–86. doi: 10.1016/j.ejphar.2011.09.007. [DOI] [PubMed] [Google Scholar]
- 20.Ueki T., Nishi A., Imamura S., Kanno H., Mizoguchi K., Sekiguchi K., Ikarashi Y., Kase Y. Effects of geissoschizine methyl ether, an indole alkaloid in Uncaria hook, a constituent of yokukansan, on human recombinant serotonin 7 receptor. Cell. Mol. Neurobiol. 2013;33(1):129–135. doi: 10.1007/s10571-012-9878-3. [DOI] [PubMed] [Google Scholar]
- 21.Mizoguchi K., Kushida H., Kanno H., Igarashi Y., Nishimura H., Ikarashi Y., Kase Y. Specific binding and characteristics of geissoschizine methyl ether, an indole alkaloid of Uncaria Hook, in the rat brain. 2014. [DOI] [PubMed]
- 22.Yuzurihara M., Ikarashi Y., Goto K., Sakakibara I., Hayakawa T., Sasaki H. Geissoschizine methyl ether, an indole alkaloid extracted from Uncariae Ramulus et Uncus, is a potent vasorelaxant of isolated rat aorta. Eur. J. Pharmacol. 2002;444(3):183–189. doi: 10.1016/s0014-2999(02)01623-0. [DOI] [PubMed] [Google Scholar]
- 23.Martin G.R., Humphrey P.P. Receptors for 5-hydroxytryptamine: Current perspectives on classification and nomenclature. Neuropharmacology. 1994;33(3-4):261–273. doi: 10.1016/0028-3908(94)90058-2. [DOI] [PubMed] [Google Scholar]
- 24.May J.A., McLaughlin M.A., Sharif N.A., Hellberg M.R., Dean T.R. Evaluation of the ocular hypotensive response of serotonin 5-HT1A and 5-HT2 receptor ligands in conscious ocular hypertensive cynomolgus monkeys. J. Pharmacol. Exp. Ther. 2003;306(1):301–309. doi: 10.1124/jpet.103.049528. [DOI] [PubMed] [Google Scholar]
- 25.Hoyer D., Clarke D.E., Fozard J.R., Hartig P.R., Martin G.R., Mylecharane E.J., Saxena P.R., Humphrey P.P. International Union of Pharmacology classification of receptors for 5-hydroxytryptamine (Serotonin). Pharmacol. Rev. 1994;46(2):157–203. [PubMed] [Google Scholar]
- 26.Pazos A., Hoyer D., Palacios J.M. Mesulergine, a selective serotonin-2 ligand in the rat cortex, does not label these receptors in porcine and human cortex: evidence for species differences in brain serotonin-2 receptors. Eur. J. Pharmacol. 1984;106(3):531–538. doi: 10.1016/0014-2999(84)90056-6. [DOI] [PubMed] [Google Scholar]
- 27.Bonhaus D.W., Bach C., DeSouza A., Salazar F.H., Matsuoka B.D., Zuppan P., Chan H.W., Eglen R.M. The pharmacology and distribution of human 5-hydroxytryptamine2B (5-HT2B) receptor gene products: comparison with 5-HT2A and 5-HT2C receptors. Br. J. Pharmacol. 1995;115(4):622–628. doi: 10.1111/j.1476-5381.1995.tb14977.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saucier C., Albert P.R. Identification of an endogenous 5-hydroxytryptamine2A receptor in NIH-3T3 cells: agonist-induced down-regulation involves decreases in receptor RNA and number. J. Neurochem. 1997;68(5):1998–2011. doi: 10.1046/j.1471-4159.1997.68051998.x. [DOI] [PubMed] [Google Scholar]
- 29.Wolf W.A., Schutz L.J. The serotonin 5-HT2C receptor is a prominent serotonin receptor in basal ganglia: evidence from functional studies on serotonin-mediated phosphoinositide hydrolysis. J. Neurochem. 1997;69(4):1449–1458. doi: 10.1046/j.1471-4159.1997.69041449.x. [DOI] [PubMed] [Google Scholar]
- 30.Miller K., Weisberg E., Fletcher P.W., Teitler M. Membrane-bound and solubilized brain 5HT3 receptors: improved radioligand binding assays using bovine area postrema or rat cortex and the radioligands 3H-GR65630, 3H-BRL43694, and 3H-LY278584. Synapse. 1992;11(1):58–66. doi: 10.1002/syn.890110108. [DOI] [PubMed] [Google Scholar]
- 31.Boess F.G., Steward L.J., Steele J.A., Liu D., Reid J., Glencorse T.A., Martin I.L. Analysis of the ligand binding site of the 5-HT3 receptor using site directed mutagenesis: importance of glutamate 106. Neuropharmacology. 1997;36(4-5):637–647. doi: 10.1016/s0028-3908(97)00044-0. [DOI] [PubMed] [Google Scholar]
- 32.Grossman C.J., Kilpatrick G.J., Bunce K.T. Development of a radioligand binding assay for 5-HT4 receptors in guinea-pig and rat brain. Br. J. Pharmacol. 1993;109(3):618–624. doi: 10.1111/j.1476-5381.1993.tb13617.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rees S., den Daas I., Foord S., Goodson S., Bull D., Kilpatrick G., Lee M. Cloning and characterisation of the human 5-HT5A serotonin receptor. FEBS Lett. 1994;355(3):242–246. doi: 10.1016/0014-5793(94)01209-1. [DOI] [PubMed] [Google Scholar]
- 34.Monsma F.J., Jr, Shen Y., Ward R.P., Hamblin M.W., Sibley D.R. Cloning and expression of a novel serotonin receptor with high affinity for tricyclic psychotropic drugs. Mol. Pharmacol. 1993;43(3):320–327. [PubMed] [Google Scholar]
- 35.Roth B.L., Craigo S.C., Choudhary M.S., Uluer A., Monsma F.J., Jr, Shen Y., Meltzer H.Y., Sibley D.R. Binding of typical and atypical antipsychotic agents to 5-hydroxytryptamine-6 and 5-hydroxytryptamine-7 receptors. J. Pharmacol. Exp. Ther. 1994;268(3):1403–1410. [PubMed] [Google Scholar]
- 36.Shen Y., Monsma F.J., Jr, Metcalf M.A., Jose P.A., Hamblin M.W., Sibley D.R. Molecular cloning and expression of a 5-hydroxytryptamine7 serotonin receptor subtype. J. Biol. Chem. 1993;268(24):18200–18204. [PubMed] [Google Scholar]
- 37.Adlersberg M., Arango V., Hsiung S., Mann J.J., Underwood M.D., Liu K., Kassir S.A., Ruggiero D.A., Tamir H. In vitro autoradiography of serotonin 5-HT(2A/2C) receptor-activated G protein: guanosine-5′-(gamma-[(35)S]thio)triphosphate binding in rat brain. J. Neurosci. Res. 2000;61(6):674–685. doi: 10.1002/1097-4547(20000915)61:6<674::AID-JNR11>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 38.Cussac D., Newman-Tancredi A., Duqueyroix D., Pasteau V., Millan M.J. Differential activation of Gq/11 and Gi(3) proteins at 5-hydroxytryptamine(2C) receptors revealed by antibody capture assays: Influence of receptor reserve and relationship to agonist-directed trafficking. Mol. Pharmacol. 2002;62(3):578–589. doi: 10.1124/mol.62.3.578. [DOI] [PubMed] [Google Scholar]
- 39.Brea J., Castro M., Giraldo J., López-Giménez J.F., Padín J.F., Quintián F., Cadavid M.I., Vilaró M.T., Mengod G., Berg K.A., Clarke W.P., Vilardaga J.P., Milligan G., Loza M.I. Evidence for distinct antagonist-revealed functional states of 5-hydroxytryptamine(2A) receptor homodimers. Mol. Pharmacol. 2009;75(6):1380–1391. doi: 10.1124/mol.108.054395. [DOI] [PubMed] [Google Scholar]
- 40.Sharif N.A., Kelly C.R., McLaughlin M. Human trabecular meshwork cells express functional serotonin-2A (5HT2A) receptors: Role in IOP reduction. Invest. Ophthalmol. Vis. Sci. 2006;47(9):4001–4010. doi: 10.1167/iovs.06-0062. [DOI] [PubMed] [Google Scholar]
- 41.Fitzgerald L.W., Burn T.C., Brown B.S., Patterson J.P., Corjay M.H., Valentine P.A., Sun J.H., Link J.R., Abbaszade I., Hollis J.M., Largent B.L., Hartig P.R., Hollis G.F., Meunier P.C., Robichaud A.J., Robertson D.W. Possible role of valvular serotonin 5-HT(2B) receptors in the cardiopathy associated with fenfluramine. Mol. Pharmacol. 2000;57(1):75–81. [PubMed] [Google Scholar]
- 42.Porter R.H., Benwell K.R., Lamb H., Malcolm C.S., Allen N.H., Revell D.F., Adams D.R., Sheardown M.J. Functional characterization of agonists at recombinant human 5-HT2A, 5-HT2B and 5-HT2C receptors in CHO-K1 cells. Br. J. Pharmacol. 1999;128(1):13–20. doi: 10.1038/sj.bjp.0702751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shultz S., Worzella T., Gallagher A., Shieh J., Goueli S., Hsiao K., Vidugiriene J. Miniaturized GPCR signaling studies in 1536-well format. J. Biomol. Tech. 2008;19(4):267–274. [PMC free article] [PubMed] [Google Scholar]
- 44.Audia J.E., Evrard D.A., Murdoch G.R., Droste J.J., Nissen J.S., Schenck K.W., Fludzinski P., Lucaites V.L., Nelson D.L., Cohen M.L. Potent, selective tetrahydro-beta-carboline antagonists of the serotonin 2B (5HT2B) contractile receptor in the rat stomach fundus. J. Med. Chem. 1996;39(14):2773–2780. doi: 10.1021/jm960062t. [DOI] [PubMed] [Google Scholar]
- 45.Singh P., Kumar R. Quantitative structure-activity relationship study on tetrahydro-beta-carboline antagonists of the serotonin 2B (5HT2B) contractile receptor in the rat stomach fundus. J. Enzyme Inhib. 2001;16(6):491–497. doi: 10.1080/14756360127570. [DOI] [PubMed] [Google Scholar]
- 46.Arthur J.M., Casañas S.J., Raymond J.R. Partial agonist properties of rauwolscine and yohimbine for the inhibition of adenylyl cyclase by recombinant human 5-HT1A receptors. Biochem. Pharmacol. 1993;45(11):2337–2341. doi: 10.1016/0006-2952(93)90208-e. [DOI] [PubMed] [Google Scholar]
- 47.Kaumann A.J. Yohimbine and rauwolscine inhibit 5-hydroxytryptamine-induced contraction of large coronary arteries of calf through blockade of 5 HT2 receptors. Naunyn Schmiedebergs Arch. Pharmacol. 1983;323(2):149–154. doi: 10.1007/BF00634263. [DOI] [PubMed] [Google Scholar]
- 48.Wainscott D.B., Sasso D.A., Kursar J.D., Baez M., Lucaites V.L., Nelson D.L. [3H]Rauwolscine: an antagonist radioligand for the cloned human 5-hydroxytryptamine2b (5-HT2B) receptor. Naunyn Schmiedebergs Arch. Pharmacol. 1998;357(1):17–24. doi: 10.1007/pl00005133. [DOI] [PubMed] [Google Scholar]
- 49.Perry B.D. U’Prichard, D.C. [3H]rauwolscine (alpha-yohimbine): A specific antagonist radioligand for brain alpha 2-adrenergic receptors. Eur. J. Pharmacol. 1981;76(4):461–464. doi: 10.1016/0014-2999(81)90123-0. [DOI] [PubMed] [Google Scholar]
- 50.Qin K., Sethi P.R., Lambert N.A. Abundance and stability of complexes containing inactive G protein-coupled receptors and G proteins. FASEB J. 2008;22(8):2920–2927. doi: 10.1096/fj.08-105775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Laine A.E., Lood C., Koskinen A.M. Pharmacological importance of optically active tetrahydro-β-carbolines and synthetic approaches to create the C1 stereocenter. Molecules. 2014;19(2):1544–1567. doi: 10.3390/molecules19021544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sunahara R.K., Dessauer C.W., Gilman A.G. Complexity and diversity of mammalian adenylyl cyclases. Annu. Rev. Pharmacol. Toxicol. 1996;36:461–480. doi: 10.1146/annurev.pa.36.040196.002333. [DOI] [PubMed] [Google Scholar]
- 53.Exton J.H. Regulation of phosphoinositide phospholipases by hormones, neurotransmitters, and other agonists linked to G proteins. Annu. Rev. Pharmacol. Toxicol. 1996;36:481–509. doi: 10.1146/annurev.pa.36.040196.002405. [DOI] [PubMed] [Google Scholar]
- 54.Ueda T., Ugawa S., Ishida Y., Hondoh A., Shimada S. Development of generic calcium imaging assay for monitoring Gi-coupled receptors and G-protein interaction. J. Biomol. Screen. 2009;14(7):781–788. doi: 10.1177/1087057109335258. [DOI] [PubMed] [Google Scholar]
- 55.Heidmann D.E., Metcalf M.A., Kohen R., Hamblin M.W. Four 5-hydroxytryptamine7 (5-HT7) receptor isoforms in human and rat produced by alternative splicing: Species differences due to altered intron-exon organization. J. Neurochem. 1997;68(4):1372–1381. doi: 10.1046/j.1471-4159.1997.68041372.x. [DOI] [PubMed] [Google Scholar]
- 56.Krobert K.A., Bach T., Syversveen T., Kvingedal A.M., Levy F.O. The cloned human 5-HT7 receptor splice variants: A comparative characterization of their pharmacology, function and distribution. Naunyn Schmiedebergs Arch. Pharmacol. 2001;363(6):620–632. doi: 10.1007/s002100000369. [DOI] [PubMed] [Google Scholar]
- 57.Bonaventure P., Nepomuceno D., Kwok A., Chai W., Langlois X., Hen R., Stark K., Carruthers N., Lovenberg T.W. Reconsideration of 5-hydroxytryptamine (5-HT)(7) receptor distribution using [(3)H]5-carboxamidotryptamine and [(3)H]8-hydroxy-2-(di-n-propylamino)tetraline: analysis in brain of 5-HT(1A) knockout and 5-HT(1A/1B) double-knockout mice. J. Pharmacol. Exp. Ther. 2002;302(1):240–248. doi: 10.1124/jpet.302.1.240. [DOI] [PubMed] [Google Scholar]
- 58.Kushida H., Matsumoto T., Igarashi Y., Nishimura H., Watanabe J., Maemura K., Kase Y. Metabolic profiling of the Uncaria hook alkaloid geissoschizine methyl ether in rat and human liver microsomes using high-performance liquid chromatography with tandem mass spectrometry. Molecules. 2015;20(2):2100–2114. doi: 10.3390/molecules20022100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Matsumoto T., Kushida H., Maruyama T., Nishimura H., Watanabe J., Maemura K., Kase Y. In vitro identification of human cytochrome P450 isoforms involved in the metabolism of Geissoschizine methyl ether, an active component of the traditional Japanese medicine Yokukansan. Xenobiotica. 2016;46(4):325–334. doi: 10.3109/00498254.2015.1076585. [DOI] [PubMed] [Google Scholar]
- 60.Kitagawa H., Munekage M., Ichikawa K., Fukudome I., Munekage E., Takezaki Y., Matsumoto T., Igarashi Y., Hanyu H., Hanazaki K., Fukudome I., Munekage E., Takezaki Y., Matsumoto T., Igarashi Y., Hanyu H., Hanazaki K. Pharmacokinetics of active components of yokukansan, a traditional Japanese herbal medicine after a single oral administration to healthy Japanese volunteers: A cross-over, randomized study. PLoS One. 2015;10(7):e0131165. doi: 10.1371/journal.pone.0131165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jacobs B.L., Azmitia E.C. Structure and function of the brain serotonin system. Physiol. Rev. 1992;72(1):165–229. doi: 10.1152/physrev.1992.72.1.165. [DOI] [PubMed] [Google Scholar]
- 62.Cao R., Peng W., Wang Z., Xu A. beta-Carboline alkaloids: Biochemical and pharmacological functions. Curr. Med. Chem. 2007;14(4):479–500. doi: 10.2174/092986707779940998. [DOI] [PubMed] [Google Scholar]
- 63.Ikarashi Y., Mizoguchi K. Neuropharmacological efficacy of the traditional Japanese Kampo medicine yokukansan and its active ingredients. Pharmacol. Ther. 2016;166:84–95. doi: 10.1016/j.pharmthera.2016.06.018. [DOI] [PubMed] [Google Scholar]
- 64.Ueki T., Mizoguchi K., Yamaguchi T., Nishi A., Ikarashi Y., Hattori T., Kase Y. Yokukansan increases 5-HT1A receptors in the prefrontal cortex and enhances 5-HT1A receptor agonist-induced behavioral responses in socially isolated mice. Evid. Based Complement. Alternat. Med. 2015;2015:726471. doi: 10.1155/2015/726471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jung J.W., Ahn N.Y., Oh H.R., Lee B.K., Lee K.J., Kim S.Y., Cheong J.H., Ryu J.H. Anxiolytic effects of the aqueous extract of Uncaria rhynchophylla. J. Ethnopharmacol. 2006;108(2):193–197. doi: 10.1016/j.jep.2006.05.019. [DOI] [PubMed] [Google Scholar]
- 66.Yamaguchi T., Tsujimatsu A., Kumamoto H., Izumi T., Ohmura Y., Yoshida T., Yoshioka M. Anxiolytic effects of yokukansan, a traditional Japanese medicine, via serotonin 5-HT1A receptors on anxiety-related behaviors in rats experienced aversive stress. J. Ethnopharmacol. 2012;143(2):533–539. doi: 10.1016/j.jep.2012.07.007. [DOI] [PubMed] [Google Scholar]
- 67.Egashira N., Iwasaki K., Ishibashi A., Hayakawa K., Okuno R., Abe M., Uchida N., Mishima K., Takasaki K., Nishimura R., Oishi R., Fujiwara M. Repeated administration of Yokukansan inhibits DOI-induced head-twitch response and decreases expression of 5-hydroxytryptamine (5-HT)2A receptors in the prefrontal cortex. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2008;32(6):1516–1520. doi: 10.1016/j.pnpbp.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 68.Ueki T., Mizoguchi K., Yamaguchi T., Nishi A., Sekiguchi K., Ikarashi Y., Kase Y. Yokukansan, a traditional Japanese medicine, decreases head-twitch behaviors and serotonin 2A receptors in the prefrontal cortex of isolation-stressed mice. J. Ethnopharmacol. 2015;166:23–30. doi: 10.1016/j.jep.2015.02.046. [DOI] [PubMed] [Google Scholar]
- 69.Sakakibara I., Terabayashi S., Kubo M., Higuchi M., Komatsu Y., Okada M., Taki K., Kamei J. Effect on locomotion of indole alkaloids from the hooks of uncaria plants. Phytomedicine. 1999;6(3):163–168. doi: 10.1016/S0944-7113(99)80004-X. [DOI] [PubMed] [Google Scholar]
- 70.Nakagawa T., Nagayasu K., Nishitani N., Shirakawa H., Sekiguchi K., Ikarashi Y., Kase Y., Kaneko S. Yokukansan inhibits morphine tolerance and physical dependence in mice: the role of α2A-adrenoceptor. Neuroscience. 2012;227:336–349. doi: 10.1016/j.neuroscience.2012.09.079. [DOI] [PubMed] [Google Scholar]
- 71.Kawakami Z., Ikarashi Y., Kase Y. Isoliquiritigenin is a novel NMDA receptor antagonist in kampo medicine yokukansan. Cell. Mol. Neurobiol. 2011;31(8):1203–1212. doi: 10.1007/s10571-011-9722-1. [DOI] [PubMed] [Google Scholar]
- 72.Bard J.A., Zgombick J., Adham N., Vaysse P., Branchek T.A., Weinshank R.L. Cloning of a novel human serotonin receptor (5-HT7) positively linked to adenylate cyclase. J. Biol. Chem. 1993;268(31):23422–23426. [PubMed] [Google Scholar]
- 73.Lovenberg T.W., Baron B.M., de Lecea L., Miller J.D., Prosser R.A., Rea M.A., Foye P.E., Racke M., Slone A.L., Siegel B.W. A novel adenylyl cyclase-activating serotonin receptor (5-HT7) implicated in the regulation of mammalian circadian rhythms. Neuron. 1993;11(3):449–458. doi: 10.1016/0896-6273(93)90149-l. [DOI] [PubMed] [Google Scholar]
- 74.Gannon R.L. 5HT7 receptors in the rodent suprachiasmatic nucleus. J. Biol. Rhythms. 2001;16(1):19–24. doi: 10.1177/074873040101600103. [DOI] [PubMed] [Google Scholar]
- 75.Hedlund P.B., Sutcliffe J.G. Functional, molecular and pharmacological advances in 5-HT7 receptor research. Trends Pharmacol. Sci. 2004;25(9):481–486. doi: 10.1016/j.tips.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 76.Eglen R.M., Jasper J.R., Chang D.J., Martin G.R. The 5-HT7 receptor: Orphan found. Trends Pharmacol. Sci. 1997;18(4):104–107. doi: 10.1016/s0165-6147(97)01043-2. [DOI] [PubMed] [Google Scholar]
- 77.Vanhoenacker P., Haegeman G., Leysen J.E. 5-HT7 receptors: Current knowledge and future prospects. Trends Pharmacol. Sci. 2000;21(2):70–77. doi: 10.1016/s0165-6147(99)01432-7. [DOI] [PubMed] [Google Scholar]
- 78.Shinno H., Kamei M., Nakamura Y., Inami Y., Horiguchi J. Successful treatment with Yi-Gan San for rapid eye movement sleep behavior disorder. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2008;32(7):1749–1751. doi: 10.1016/j.pnpbp.2008.06.015. [DOI] [PubMed] [Google Scholar]
- 79.Deng S., Chen S.N., Yao P., Nikolic D., van Breemen R.B., Bolton J.L., Fong H.H., Farnsworth N.R., Pauli G.F. Serotonergic activity-guided phytochemical investigation of the roots of Angelica sinensis. J. Nat. Prod. 2006;69(4):536–541. doi: 10.1021/np050301s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ofir R., Tamir S., Khatib S., Vaya J. Inhibition of serotonin re-uptake by licorice constituents. J. Mol. Neurosci. 2003;20(2):135–140. doi: 10.1385/JMN:20:2:135. [DOI] [PubMed] [Google Scholar]