Abstract
The etiology of up to 95% of cerebral aneurysms may be accounted for by hemodynamically-induced factors that create vascular injury. The purpose of this review is to describe key physical properties that stents have and how they affect cerebral aneurysms. We performed a two-step screening process. First, a structured search was performed using the PubMed database. The following search terms and keywords were used: “Hemodynamics,” “wall shear stress (WSS),” “velocity,” “viscosity,” “cerebral aneurysm,” “intracranial aneurysm,” “stent,” “flow diverter,” “stent porosity,” “stent geometry,” “stent configuration,” and “stent design.” Reports were considered if they included original data, discussed hemodynamic changes after stent-based treatment of cerebral aneurysms, examined the hemodynamic effects of stent deployment, and/or described the geometric characteristics of both stents and the aneurysms they were used to treat. The search strategy yielded a total of 122 articles, 61 were excluded after screening the titles and abstracts. Additional articles were then identified by cross-checking reference lists. The final collection of 97 articles demonstrates that the geometric characteristics and configurations of deployed stents influenced hemodynamic parameters such as aneurysmal WSS, inflow, and pressure. The geometric characteristics of the aneurysm and its position also had significant influences on intra-aneurysmal hemodynamics after treatment. In conclusion, changes in specific aneurysmal hemodynamic parameters that result from stenting relate to a number of factors including the geometric properties and configurations of deployed stents, the geometric properties of the aneurysm, and the pretreatment hemodynamics.
Keywords: Cerebral aneurysms, cerebrovascular, hemodynamics, stents
Introduction
Cerebral aneurysms are present in an estimated 6% of the global population.[1,2] Moreover, often directly dependent on their size, cerebral aneurysms rupture occurs in 1% of those patients ultimately leading to subarachnoid hemorrhage.[3]
The etiology of up to 95% of all cerebral aneurysms is accounted for by hemodynamic factors that create vascular trauma.[4] The specific hemodynamic factors leading to cerebral aneurysms, however, remain unclear. The general role of hemodynamics is recognized and is supported by the higher incidence of intracranial aneurysms in patients with aortic coarctation, the development of aneurysms contralaterally following cervical carotid occlusion, and flow-related aneurysms related to arteriovenous malformations. As such, endovascular treatments attempt to modify hemodynamic properties within the aneurysm as a method of preventing eventual rupture.
Intravascular treatment techniques include embolization with liquid embolic agents such as Onyx HD 500 (eV3-Irvine CA, USA) and platinum coils. While coiling has proven effective in treating cerebral aneurysms, this technique can fail to achieve durable occlusion, especially in large and wide-neck aneurysms. Coiling is recognized for its considerable recanalization potential specifically, in wide-necked aneurysms and ineffective in aneurysmal recurrence.[5] Moreover, the stent prevents coils from herniating into the parent vessel and facilitating endothelialization of the aneurysmal orifice.[6]
Recently, a new paradigm in the treatment of large and wide-neck aneurysms, endoluminal therapy, has been gaining recognition. Endoluminal therapies include treatment with stand-alone stents known as flow diverters (FDs).[7] Specifically, FDs reduce wall shear stress (WSS) at the aneurysmal sac, diverting flow away from the aneurysm.[8,9,10,11,12,13,14,15,16] FDs also prevent recanalization by promoting endothelial growth within the stent struts, repairing the vessel and permanently excluding the aneurysm. In addition, FDs mitigate pulsatility within the aneurysm, protecting the aneurysm from the vigorous nature of the arterial flow.[17]
Interestingly, Kadirvel et al.[18] suggest with in vivo animal models that endothelialization of the FD is actually more significant than thrombus formation in achieving complete occlusion of the aneurysm. Specifically, their in vivo model histologically demonstrated that complete aneurysmal occlusion occurs secondary to healing at the neck of the aneurysm with endothelial cells overlying the smooth muscle.[18] This recent study suggests, that newly developing FDs should concentrate on accelerating endothelialization at the aneurysmal neck over the device struts, in hopes to ultimately stimulate complete occlusion of the aneurysm.[18]
FDs lead to changes in aneurysmal hemodynamics that promote thrombosis and remodeling processes, which eventually exclude the aneurysm from circulation.[7] These remodeling processes take time and, therefore, immediate aneurysmal thrombosis does not occur.
To date, no single hemodynamic parameter has been identified as the major factor responsible for aneurysmal thrombosis. The goal of this review is to discuss the physical parameters of aneurysms and how they affect the stents facilitating aneurysm occlusion.
Materials and Methods
A structured search was performed using the PubMed database. Title, abstract, keywords, and full text were searched using combinations of the following terms and keywords: “Hemodynamics” or “WSS” or “velocity” or “viscosity;” “cerebral aneurysm” or “intracranial aneurysm;” and “stent” or “FD” or “stent porosity” or “stent geometry” or “stent configuration” or “stent design.”
The database search results were screened for potentially relevant articles based on title/abstract and full-text using predefined inclusion criteria. A cross-check of reference lists from identified articles were performed to identify additional articles for inclusion.
Initial screen of articles for eligibility
Abstracts were identified from the electronic literature search and were independently evaluated by two authors (K. A. and L. F. G.). Eligible articles were selected for full-text review. During the initial screening, abstracts were considered if they reported original data and if the corresponding articles made specific mention of the search terms. The search was limited to articles written in the English language.
Second screen of articles for eligibility issues (inclusion and exclusion criteria)
K. A. and L. F. G. independently reviewed the full-text articles and selected those that included: Original data, detailed hemodynamic changes in the cerebral aneurysm, effects of stent deployment, a description of stent, and aneurysm properties. Studies were excluded if they discussed the effects of coils rather than stents on aneurysmal hemodynamics, had limited or no hemodynamic data or did not discuss cerebral aneurysms. Editorials, letters, review articles, case reports, and animal experimental studies were excluded.
Selection and data extraction
Data were extracted based on the following three categories: Hemodynamic changes in WSS, velocity, viscosity, turnover time, vortex formation, and other parameters, aneurysm (geometric) properties including type, size, location, and relation to the parent artery and stent (geometric) properties including design, configuration, porosity, filament diameter, and cell shape.
Results and Discussion
Our literature search yielded 927 papers. Of these, 801 were duplicates and four were not written in English. Of the 122 papers remaining, 61 were excluded by title and abstract screening. Of these 61 remaining, 29 lacked a baseline hemodynamic discussion. Another 11 papers were excluded because they discussed coils rather that stents, and six discussed noncerebral aneurysms. Additional six papers did not discuss stent effects; nine additional lacked relevance or were not full text. Full texts were accessed for the remaining 61 papers. Additional papers were obtained by cross-checking of reference lists from identified articles. A total of 97 papers were included.
Aneurysmal flow
Aneurysm flow is studied at three different points. First is the inflow entering the aneurysm, occurring at the distal aspect of the neck. Second is the outflow exiting the aneurysm, occurring at the proximal neck. Finally, the central slow flow vortex forms at the entrance to the aneurysm during peak systole.
The concept behind a stent as an FD relies on the creation of aneurysmal flow stagnation; facilitating thrombosis. Stagnation occurs when the outflow of the aneurysm slows, decreasing exchange between the parent vessel and the aneurysm. Flow stagnation is readily achieved in the setting of side-wall aneurysms which exhibit a shear driven flow. Augsburger et al. state, the velocity and vorticity in inertia driven flow are up to 4 times higher than in shear driven flow.[8] This observation has been consistent, with and without stenting. Meng et al.[19] propose in high curvature models, stasis index, inflow momentum reduction, and impact flow reduction decrease. They report that inflow is 103–104 times greater in inertia driven flow than in shear driven flow.[19] The findings of Kim et al.[20] also support this. With increased artery curve, the flow diverting efficacy of a stent decreases secondary to increased aneurysmal inflow. Kim et al. found that the use of the Tristar™ stent (Guidant, St. Paul, MN, USA), a laser cut, open-cell design, was associated with improved efficacy in strongly curved vessels. Conversely, the Wallstent® (Boston Scientific/Target, San Leandro, CA, USA), a woven mesh design, was more effective in the treatment of less angulated vessels.[20]
Struffert et al.[21] measured time-density curves in elastase-induced aneurysms following placement of Neuroform (Stryker Neurovascular, Freemont, CA, USA) and Pipeline Embolization Device (PED) (eV3, Irvine, CA, USA). They observed a considerable increase in time to peak the PED group, and less of an increase in the Neuroform group. The average inflow and outflow were prolonged in the PED group; indicating that PED has greater efficacy in intra-aneurysmal stagnation.[21]
The pulsatility of the blood flow may contribute to hemodynamic changes within cerebral aneurysms. Augsburger et al.[8] reported that flow reduction is similar regardless if the flow was pulsatile or not in the devices tested. The authors also found that, in the case of steady flow, reduction in flow was inversely proportional to flow rate. In conditions of pulsatile flow, FDs create a more homogenous flow by reducing the gap between the high- and low-flow magnitude during the cardiac cycle.[8]
Flow pattern may have predictive value for aneurysmal rupture. In a study of 210 cerebral aneurysm models, 83% of aneurysmal ruptures were associated with complex or unstable flow patterns, while unruptured aneurysms were associated with simple flow patterns.[22] An example of simple and complex flow patterns is presented in Figure 1. The simple flow pattern in Figure 1a exhibits one aneurysmal inflow jet and a single vortex structure while the complex flow pattern in Figure 1b shows several inflow jets and several vortex structures within the aneurysm. Complex flow patterns have been associated with increased inflammatory cell infiltration, a finding not unusual in the walls of ruptured aneurysms.[23] Flow complexity can be dampened by a single stent and further reductions in complexity occur with sequential telescopic constructs.[24] A diffuse flow is less likely to cause aneurysm rupture than with a concentrated jet flow.[25] Such diffuse flow can be achieved by employing multiple stents in a telescoping manner. Telescoping stents reduce the risk of developing jet-like inflow, decreasing the potential of aneurysmal rupture.[22] Overlapping multiple stents decreases porosity, diverting jet flow away from the aneurysm.[22,26,27] This technique is not without limitations. The use of multiple stents may alter the flow to downstream perforating branches, resulting in subsequent ischemia or infarction.[28,29] A single stent carries the risk of compromising flow to downstream branches and multiple stents increase this risk. Darsaut et al.[30] found that FDs were more likely to occlude the aneurysm itself than the branch and are less effective in bifurcation aneurysms.[30]
Figure 1.
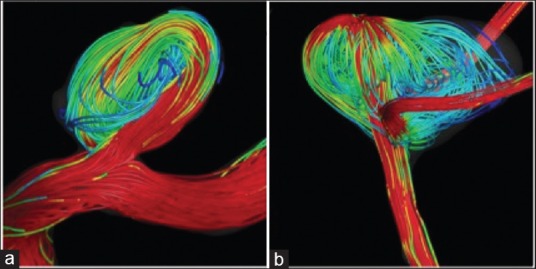
Simple (a) and complex (b) flow patterns within the aneurysm
Using computational hemodynamics analysis, in an eight patient series, four failed and four successful (defined as complete angiographic exclusion of the aneurysm from the circulation within 6 months treatment) Chong et al. demonstrated noticeable obliteration of jet flow in successful aneurysm occlusion.[31] Specifically, the researchers demonstrated that a successful case showed a favorable posttreatment flow pattern identified as a (reduction of jet flow speed, central diversion, and peripheral stasis). Clinically, the researches elude that confirming patient-specific computational FDs (CFDs) with pre- and post-treatment angiograms could aid in predicting patient treatment outcomes and the need for further early intervention before rupture such as (double FD technique) or closer interval imaging follow-up.[31]
Wall shear stress
Aneurysmal WSS is the tangential drag force produced by blood moving across the endothelial surface.[20] WSS is elevated when its value becomes greater than that of the parent vessel.[22,32] There is an unequal distribution of WSS along the aneurysm, with the fundus being exposed to low-values of WSS during the cardiac cycle.[11] Increased WSS values have been associated with aneurysmal growth.[33] The high-impact WSS zone is considered the active site of aneurysmal initiation and progression.[34] Aneurysmal remodeling is effected by tangential force acting on the endothelial surface.[12] Elevated WSSs causes outward remodeling of the artery for the ultimate purpose of ameliorating WSS. Several groups have reported that a prolonged elevation is associated with internal elastic lamina fragmentation; facilitating aneurysmal dilatation secondary to destructive remodeling.[12,32,35,36]
Both high- and low-WSS are associated with aneurysmal rupture.[12,27,34,35] Shojima et al.[35] and Jou et al.[37] report low-WSS for the majority of ruptures in middle cerebral artery (MCA) and internal carotid artery (ICA) aneurysms. Endothelial cells have mechanoreceptors such as surface adhesion molecules and ion channels; promoting vascular adaptation with equilibrium occurring at 1.5–2 Pa. This has substantiated the hypothesis that low-WSS induces inflammatory and atherosclerotic processes potentiating wall weakening.[12,27,34,35] Malek et al.[38] demonstrate that excessively low-WSS (<0.4 Pa) within the aneurysm leads to atherosclerotic infiltration.[38] Similar effects are seen within the carotid bifurcation. Recently, Meng et al.[39] propose a bimodal theory identifying factors of low- and high- WSS contributing to aneurysm growth and rupture. They propose two biologic pathways: An inflammatory-cell-mediated pathway that is induced by low-WSS and a high-oscillatory shear index; and a mural and cell-mediated pathway-induced by high-WSS.[39]
Stent placement across the aneurysmal neck reduces WSS to favor thrombus formation.[40,41] Figure 2 demonstrates reductions in WSS magnitudes at the aneurysm and parent vessel after FD deployment. It should be noted that higher WSS increases prostacyclin 2 and nitric oxide production, inhibiting thrombus formation.[42,43]
Figure 2.
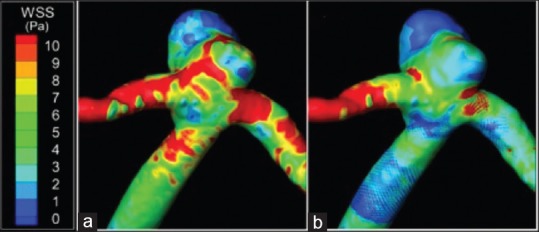
Wall shear stress in a computational aneurysm model before treatment (a) and after treatment with a flow diverter (b)
Shear stress distributes unevenly over spatial locations within aneurysms. At the distal wall, WSS may reach up to 10 times that of the proximal wall.[11] The direction of WSS is reversed at the distal wall.[10] Aenis et al.[44] report maximal systolic WSS values (approximately 50 dyn/cm2) were found at the distal aspect of the neck. These values are not elevated enough to induce endothelial damage in a normal artery,[45,46] although they are substantial enough to induce endothelial damage in a preexisting aneurysm.[45] It is known intact artery endothelium will not be damaged under forces below 300 dyn/cm2.[45] Stents have been found to decrease the exposure of the distal aneurysm neck to high-WSS, protecting the aneurysm from further expansion.[19,47]
Stent placement and arterial curvature are associated with changes to the impact zone.[48,49] The changes were encountered under physiologic high-flow conditions (Re = 490) and only minimally in low-flow conditions (Re = 128).[19] The impact zone is negligible for untreated side-wall aneurysms but increases markedly as parent artery curvature increases. The reduction of the impact zone was 61%, 96%, 98%, and 56% for curvatures of 0/mm, 0.03/mm, 0.07/mm, and 0.11/mm, respectively, after treatment using the Wallstent® (Boston Scientific/Target, San Leandro, CA, USA).[19] These results mirror those by Kim et al.[20] They describe larger impact zones are associated with higher curvature as a result of the parent vessel flow gaining a more direct path toward the distal wall. In a study comparing Tristar™ (Guidant, St. Paul, MN, USA) stent and Wallstent® (Boston Scientific/Target, San Leandro, CA, USA), were found to ameliorate the impact zone magnitude; although the reduction was greater with the Wallstent at the tested curvature.[20] This is explained by the design of the Wallstent disperses flow at the aneurysmal neck as a result of its double helical woven wire pattern and less elongated cells.[20] Kim et al. conclude that above certain limits of curvature (i.e., above 0.07/mm), stenting becomes ineffective.[20] This is explained by the dominance of inertia-driven flow in the context of substantial curvatures.[19,20] In addition, the gradual transition from a split inflow to whole jet inflow is associated with increasing curvature.[20]
Aneurysmal location to the parent artery plays an important role in the determination of WSS values. Arterial bifurcations and bends are known to be points of high-shear stress that favor aneurysmal formation.[50] When comparing side-wall aneurysms to fusiform aneurysms, the magnitude and pulsatility of the WSS is decreased in side-wall aneurysms following stent placement. Rhee et al.[40] found that differences in the height-to-neck ratio in fusiform and lateral aneurysm models may explain this finding. The ratio of dome height-to-neck width was approximately 2-times larger in the side-wall model in their study. Further studies will be helpful to characterize this finding. Saccular and blister-like aneurysm models exhibited different WSS values following the placement of Enterprise™ VRD stents (Codman and Shurtleff, Inc., Raynham, Massachusetts, USA).[51] Wide and narrow aneurysmal necks exhibit different hemodynamics following stent deployment.[52] In a CFD simulation of wide-necked and narrow-necked cerebral aneurysms, FDs were found to be more effective in narrow neck aneurysms.[52] This highlights the importance of hemodynamic variability on architectural and geometric aneurysmal variety.
In a long-term clinical study designed by Briganti et al., from November 2008 to January 2012, 35 patients with a total of 39 intracranial aneurysms were treated by FD and followed for an average of 41 months (94). In short, the 35 patient series demonstrated that the rate and time to complete aneurysmal occlusion did not statistically correlate with the aneurysm size. Moreover, the data suggested that the aneurysm neck and neck/sac ratio is more predictive of occlusion rates: Neck/sac ratio (ranging from 0.5 to 1.0) demonstrated rather early occlusion within 3 months, in contrast 71% of aneurysms with larger neck (neck/sac ratio 0.9 or 1.0) demonstrated late occlusion (6 months or more) or partial occlusion (94). Finally, although limited by patient sample size, the location of the aneurysm appears to correlate with occlusion rate: Aneurysms arise from the ICA and circle of Willis demonstrate higher occlusion rates than those of arising from the MCA (94).
WSS exhibited remarkable changes following the placement of multiple telescoping stents in a study by Kim et al.,[53] comparing Neuroform 2 (Stryker Neurovascular Freemont, CA, USA), Wingspan (Stryker Neurovascular Freemont, CA, USA) and Vision (Guidant Corp., Santa Clara, CA, USA) stents. They found that the low-WSS area in the dome of the aneurysm increased following the placement of multiple stents. This group also reported that a single-stent showed an insignificant (<10%) reduction in the elevated WSS area while double-stented models demonstrate a 45% reduction. WSS reduction reached maximum value with triple stent placement regardless of stent type.[53] Tremmel et al.[24] found that placement of a single Vision (Abbott Vascular, Tremecula, CA, USA), or Enterprise (Codman Neurovascular, Miami, FL, USA) stent reduces WSS by 85% when compared to no treatment. The triple Enterprise stent model showed the largest reduction of average WSS (54.7%).
Larrabide et al.[54] demonstrated that an inverse relationship exists between WSS and aneurysm morphology variables such as (aneurysm depth, aneurysm dome area, aneurysm volume, aneurysm neck maximum width, and aneurysm neck area). Specifically, WSS decreases with the aforementioned increasing aneurysmal morphology variables. This study again confirmed that FD reduces WSS within aneurysm. Furthermore, they noted that aneurysms with relatively high-WSS before treatment had a larger reduction in WSS after FD treatment. In short, their study suggests that intra-aneurysmal hemodynamics before and after FD treatment is most strongly influenced by aneurysmal morphology and to a lesser extent the aneurysmal position and orientation with respect to its parent vessel.[54]
Turnover time
Turnover time is the time it takes for blood to circulate within the aneurysm and return to the parent vessel. It is calculated by dividing the aneurysmal volume by the aneurysmal inflow rate.[20] Turnover time is an indicator of stasis and is effected by curvature, with a smaller curvature correlating with a greater turnover time.[20,55] Prolonged times are associated with aneurysmal stasis and, therefore, thrombosis.
The effect of stenting and especially sequential stenting on the turnover time is well-reported.[20,24,53] Kim et al.[20] compared two different stents and found that the Wallstent is more effective at increasing the turnover time than Tristar. This is explained by Tristar's higher hydraulic resistance. Longer turnover times were difficult to achieve at greater curvature, with both stents proving ineffective at curvatures > 0.07/mm.[20]
Following a comparison between Neuroform 2 (Stryker vascular Freemont, CA, USA), Wingspan (Stryker vascular Freemont, CA, USA) and Vision (Abbott Vascular, Tremecula, CA, USA), Kim et al.[53] did not report a considerable difference in turnover time in single-stented and nonstented models (1.2–1.3 times longer in stented-than nonstented models). An appreciable increase in turnover time (up to 1.8 times longer) was obtained by double stenting, decreasing porosity by construct overlapping. Triple stenting was associated with substantial increases in turnover time, up to 2.4 times.[53] In the Enterprise stent, turnover times increased by 117%, 128%, and 141% when compared to the nonstented model after deployment of a single-stent, double-stent, and triple-stent, respectively.[24]
Velocity
The flow velocity within the aneurysm can reach up to 70% of the parent vessel during systolic deceleration. This was observed in lateral aneurysmal models by Cantón et al.,[11] indicating that aneurysmal flow velocity is an important parameter to study. Maximal velocity is concentrated in the distal aneurysmal neck contributing to enlargement of the aneurysm.[11]
Both devices reduce flow velocities within the aneurysm. Figure 3 shows flow velocity magnitudes in a bifurcation aneurysm model that was virtually treated with the PED (PED; Covidien Vascular Therapies, Mansfield, Massachusetts, USA). The PED reduced the average flow velocity magnitude within the aneurysmal sac by 25.5%. Cantón et al.[10] and Babiker et al.[9] used open-cell crossing-Y stents and observed 11–30% and 22.0–42.9% reductions in velocity, respectively. Another group suggested deploying Y-configuration stents would provide an advantage of second stent focal narrowing, improving its flow diverting properties.[56] As the second stent passes through the interstices of the first, a waist occurs on the second stent at the cell where it pierces the first stent.[57] When such an outcome is desired, Y-configuration stents with closed-cell designs may be superior to the open-cell design.[56,58,59] Enterprise stents, have a closed-cell design, reducing velocity among various stent configurations: A single stent (19%) < nonoverlapping-Y (29%) < virtual-Y (32%) < horizontal (39%) < kissing-Y (48%) < crossing-Y (54%). This is especially true when open-cell stents are used, where a “fish mouth” effect is exhibited opening toward the nonstented side. This may lead to increased WSS, increasing the risk of aneurysmal rupture and bleeding.[9] The previous findings regarding full-Y configurations correlated with clinical outcomes found by Roszelle et al.[60] Evidence of thrombosis within the aneurysmal sac after treatment with stents alone was observed before coil embolization was performed.[60]
Figure 3.
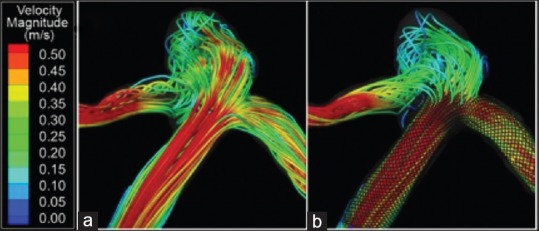
Flow velocity streamtraces in a computational aneurysm model before treatment (a) and after treatment with a flow diverter (b)
Dorn et al.[61] evaluated three stents: Solitaire (eV3, Irvine, CA, USA), Silk (Balt, Montmorency, France) and Phenox FD (Phenox, Bochum, Germany). The solitaire stent reduced in flow velocity within a side-wall aneurysm model in the following zones: 67.59% (inflow zone), 9.65% (dome), and 37.94% (outflow zone). The Silk stent reduced the flow by 58.15% (inflow zone), 89.06% (dome), and 90.06% (outflow zone). A reduction in velocity was noted in a study by Kulcsár et al.,[7] showing a 44% velocity reduction by silk-like stents. The Phenox FD had the greatest velocity reduction of 96.76% (inflow zone), 90% (dome) and 90.91% (outflow zone).
Tortuous vessels have an untoward effect the function of high-porosity Neuroform and Wingspan (Stryker Neurovascular Freemont, CA, USA) stents. This is explained by the stents bend at an acute angle leading to a “fish mouth” shape. This is common to open-cell stent designs and has the potential to form a jet flow into the aneurysm. In contrast to the high-porosity stent, PED showed improved flexibility and apposition to vessel walls when deployed. Generally speaking, FDs have proven superiority over other stents in reducing aneurysmal flow velocities, correlating with favorable clinical outcomes.[30,31,62,63,64]
In the case of side-wall aneurysm models, the effects of multiple stents on the intra-aneurysmal flow velocity are substantial. Cantón et al.[11] evaluated sequential use of Neuroform stents in side-wall aneurysm models. Following deployment of the initial stent, a 40% reduction in the maximum flow velocity was observed at peak systole.[11] The reduction continued to increase with the deployment of subsequent stents, reaching 60% upon placement of the third. Further reduction was observed in the diastolic phase, reaching 80% after the deployment of the third stent.[11] Using Enterprise stents in a wide-necked saccular basilar trunk aneurysm model, Tremmel et al.[24] observed considerable velocity reduction following the first stent and reached up to 38% reduction following the third stent placement. As the number of stents increased, the high-velocity regions move away from the aneurysmal cavity (especially the distal neck) and into the stent lumen.
Vorticity
Aneurysmal flows exhibit an unsteadiness and three-dimensionality including phenomena including vortex formation and shear stresses previously discussed.[65] Flow changes are associated with changes in the strength and vortex propagation.[44,66] During the various phases of the pulsatile cycle, the vortex center moves away from the parent vessel and towards the distal neck. The vortex is formed near the proximal neck in early systole and propagates briskly toward the distal wall during diastole. At the conclusion of diastole, the vortex dissipates.[65]
The vortex core at the distal wall creates the largest gradient of WSS.[11] Higher WSS's are associated with aneurysm growth and rupture. Thus, an analysis of the effect of stenting on flow vortices is clinically relevant. Cantón et al.[11] observed changes in the vortex location after placement of two or three sequential telescoping Neuroform stents. Single stent placement did not alter the vortex location.[11]
Using a Wallstent, Meng et al.[19] found stent placement disrupted vortex flow patterns in a side-wall aneurysm models, although not in curved aneurysm models. In addition, the authors found that in straight vessels the counter clockwise vortex was disrupted. Conversely, an inertia-driven inflow vortex in curved models becomes weaker but maintains counter clockwise rotation.[19] This is attributed to the fact that in the lateral wall aneurysm the shear driven flow is sluggish, allowing for a less arduous stent mission. Curved vessels exhibited stronger inflow jet rendering the vortex more challenging to disrupt.[19]
In the setting of vortices, stents strongly disarrange the main vortex, yielding multiple smaller vortices with counter clockwise rotation.[46,67] This observation was made using a saccular aneurysm model. Tateshima et al.[67] had similar finding with deploying a Neuroform stent in side-wall and bifurcation aneurysm models.
Intra-aneurysmal pressure
FDs have recently gained favor despite a very low-incidence (<2%) of aneurysm rupture following treatment. Alterations in intra-aneurysmal pressure after treatment have been postulated as a contributor to late rupture.
The pressure reduction within the aneurysm following stenting is insignificant when compared to the physiologic blood pressure.[24,68,69,70,71] In a model of an ICA-ophthalmic artery aneurysm to study the effect of FDs on intra-aneurysmal pressure found that treatments with Neuroform EZ (Boston Scientific, Fremont, CA, USA) and PED led to reductions in intra-aneurysmal pressure of only 4 mm Hg and 8 mm Hg, respectively, despite major reductions in flow velocity. They concluded, “… intra-aneurysmal pressure remained essentially unchanged regardless of the level of reduction of the intra-aneurysmal flow velocity.”[72]
Cebral et al.[17] performed a CFD analysis including treatment data from three giant aneurysms that ruptured after treatment and four successfully treated aneurysms. All aneurysms were located in the ICA and were treated with PEDs. In each posttreatment rupture case, the FD led to a pressure increase within the aneurysm. Schneiders et al.[73] used silk FDs in vivo and concluded, “flow-diverting stent is not a pressure-diverting stent.” In a more recent study, pressure at the aneurysmal ostium before FD placement correlated with the pressure change inside the aneurysm after treatment.[74] It therefore be of benefit to evaluate an aneurysm prior to FD placement to decide if additional devices (e.g. coils) would optimize the posttreatment hemodynamic environment.
Aneurysms with proximal stenosis in the parent artery are at risk of increased intra-aneurysmal pressure caused by opening of the stenosis.[17] Aneurysms that have most flow from the parent artery due to its tortuosity, may have greater potential for intra-aneurysmal pressure increase following FD placement.[17] This resistance triggers auto-regulatory mechanisms to maintain perfusion by decreasing vascular resistance distal to the aneurysm. Therefore, flow rate within the aneurysm is increased, augmenting intra-aneurysmal pressure. To summarize, a combination of factors contribute to aneurysmal rupture.[17]
In an idealized basilar bifurcation model by Roszelle et al.,[71] the effect of telescoping high-porosity stents (i.e. Enterprise) and PED on intra-aneurysmal pressure was evaluated. Significant pressure changes within the aneurysm following stenting were not demonstrated. The greatest pressure difference was observed in the PED-treated model, which was nearly double that observed for a three-stent high-porosity stent deployment.[71]
Stent properties
Porosity
Porosity and metal coverage are not interchangeable terms. Metal coverage is the ratio of surface area covered by metal to the total surface area of the stent.[40,75] Porosity is the ratio of metal-free surface area to total surface area of the stent. Examples of high- and low-porosity stents are shown in Figure 4. Porosity is an important parameter when considering a stent in redirecting flow.[8,40,66,76,77,78,79] Increased stagnation and thrombosis can be achieved with less porous stents. Darsaut et al.[78] found a significant correlation between aneurysm occlusion rates and metallic porosity. However, a study by Sadasivan et al.[80] found that the pore density may also be a critical factor in device efficacy. For a woven stent, such as the PED, the maximum coverage is obtained when the device is deployed into a vessel that matches its diameter. Aneurysms in curved vessels occur at the outer curve more frequently than inner and stent porosity is greater at the outer edge of the curve.
Figure 4.
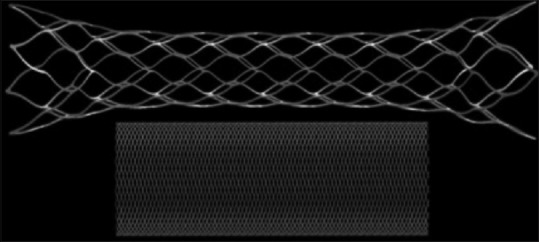
Computational models of the Enterprise™ high porosity stent (above) and the low porosity Pipeline Embolization Device (below)
The lower the stent porosity, the more effective the stent is in diverting flow. It is important to consider an exceedingly low-porosity (<65%) has the potential to occlude perforating vessels. Covered or nonporous stents lack the flexibility needed for navigation through the intracranial circulation. Higher metal coverage of the vessel wall was found to trigger stent stenosis secondary to intimal hyperplasia.[72]
In basilar tip aneurysms, kissing-Y and crossing-Y stent configurations result in the smallest pore size. In the kissing-Y configuration, stents are deployed in parallel contribute to the uniform narrowing of both stents. On the other hand, deployment of a second stent through the first stent (Y-configuration) leads to focal narrowing. The narrowed elements in both configurations contributed to decreased porosity and, thus, improved efficacy over horizontally deployed stents.[58]
Augsburger et al.[8] compared the effects of porosity reductions following stent telescoping. The authors observed reduction in intra-aneurysmal flow proportional to porosity reduction. Porosities tested were 87%, 74%, 63%, and 45% for Neuroform II, stent-in-stent combinations of Neuroform II, and low-porosity devices. The lowest-porosity reduced the flow velocity to 14–19% of the untreated control under shear-driven flows and to 5–31% of the untreated control under inertia-driven flows.[8]
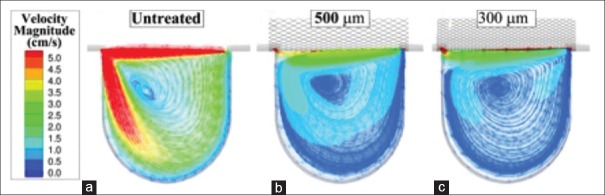
Pore density refers to the number of cells per unit area and has demonstrated an important role in flow diversion. The effect of pore density on aneurysmal hemodynamics is explained by the results in Figure 5. Sadasivan et al.[80] found that a device with a porosity of 70% and a pore density of 18 pores/mm2 performed better than devices with 65% porosity, 14 pores/mm2 and 70% porosity, 12 pores/mm2, respectively.
Figure 5.
Flow velocity reductions in a computational aneurysm model with increasing pore density. Specifically, results are presented for the untreated case (a) and after treatment with 8 pores/mm2 (b) and 21 pores/mm2 (c) stents
Several studies have indicated that a less porous stent with greater pore density may still have a considerable effect on blood flow. Another flow-disrupting strategy is to employ multiple high-porosity, low-pore density stents in a telescoping fashion. With each sequentially deployed stent, there is an increase in the amount of metal coverage in the aneurysmal ostium.[24,66,81]
Design
Stent geometry is a critical factor in modifying flow. Kim et al.[20] found differences in stent efficacy as a result of geometry. It is suggested that a double-helical woven mesh has greater impact on WSS, while a rectangular strut cross-section produced higher resistance, thereby reducing inflow rate and increasing turnover time.[20] Fu et al.[82] agreed that rectangular stent strut cross-sections have the maximum effect on flow rate. They found that the average wall shear rate in the majority of stented aneurysm was <100/s.[83]
A study by Wang et al.[84] suggested that occlusion correlated with local metal coverage provided by the stent at the aneurysm neck. The authors found that FDs with 35% metal coverage achieved more than 95% angiographic occlusion.
Stent filament diameter, at a constant porosity, also has proven to affect stent performance. The reduction in filament diameter is associated with flow reduction.[46] Lieber et al.[46] studied three helical stents with 76% porosity with different filament sizes of 178, 153, and 127 μm. A 30% reduction in the mean circulation was obtained by decreasing the filament diameter but equivalently increasing the number of stent filaments.[46] Alterations in strut orientation with respect to the parent vessel axis were found to affect hemodynamics differently; perpendicular struts diminished flow velocity more than parallel.[85]
Oversizing is sometimes required to achieve desirable apposition against the parent artery wall and avoid device migration. However, the efficacy of FDs in altering aneurysmal flow can be significantly reduced by oversizing the devices due to changes in stent-cell angles and dimensions.[86]
Conclusion
Flow alterations in cerebral aneurysms that occur following stent deployment are influenced by a number of factors. These include the stent itself, the aneurysmal geometry, and the pretreatment hemodynamics. Interactions among these factors have been discussed widely in the literature. To date, there is no a single factor that can be modified in order to obtain an aneurysm occlusion. Rather, aneurysmal occlusion is achieved by multitude interrelated factors. Further studies are necessary to elucidate the relationship between stenting and aneurysmal hemodynamics. Will see soon a patient specific treatment that offers the best occlusion rate for a particular anatomy and geometry.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Wiebers DO, Whisnant JP, Huston J, 3rd, Meissner I, Brown RD, Jr, Piepgras DG, et al. Unruptured intracranial aneurysms: Natural history, clinical outcome, and risks of surgical and endovascular treatment. Lancet. 2003;362:103–10. doi: 10.1016/s0140-6736(03)13860-3. [DOI] [PubMed] [Google Scholar]
- 2.Winn HR, Britz GW. Unruptured aneurysms. J Neurosurg. 2006;104:179–80. doi: 10.3171/jns.2006.104.2.179. [DOI] [PubMed] [Google Scholar]
- 3.Broderick JP, Brott TG, Duldner JE, Tomsick T, Huster G. Volume of intracerebral hemorrhage. A powerful and easy-to-use predictor of 30-day mortality. Stroke. 1993;24:987–93. doi: 10.1161/01.str.24.7.987. [DOI] [PubMed] [Google Scholar]
- 4.Osborn AG, Tong KA. 2nd ed. St Louis, MO, USA: Mosby; 1996. Handbook of Neuroradiology: Brain and Skull. [Google Scholar]
- 5.Hayakawa M, Murayama Y, Duckwiler GR, Gobin YP, Guglielmi G, Viñuela F. Natural history of the neck remnant of a cerebral aneurysm treated with the Guglielmi detachable coil system. J Neurosurg. 2000;93:561–8. doi: 10.3171/jns.2000.93.4.0561. [DOI] [PubMed] [Google Scholar]
- 6.Lylyk P, Cohen JE, Ceratto R, Ferrario A, Miranda C. Endovascular reconstruction of intracranial arteries by stent placement and combined techniques. J Neurosurg. 2002;97:1306–13. doi: 10.3171/jns.2002.97.6.1306. [DOI] [PubMed] [Google Scholar]
- 7.Kulcsár Z, Augsburger L, Reymond P, Pereira VM, Hirsch S, Mallik AS, et al. Flow diversion treatment: Intra-aneurismal blood flow velocity and WSS reduction are parameters to predict aneurysm thrombosis. Acta Neurochir (Wien) 2012;154:1827–34. doi: 10.1007/s00701-012-1482-2. [DOI] [PubMed] [Google Scholar]
- 8.Augsburger L, Farhat M, Reymond P, Fonck E, Kulcsar Z, Stergiopulos N, et al. Effect of flow diverter porosity on intraaneurysmal blood flow. Clin Neuroradiol. 2009;19:204–14. doi: 10.1007/s00062-009-9005-0. [DOI] [PubMed] [Google Scholar]
- 9.Babiker MH, Gonzalez LF, Ryan J, Albuquerque F, Collins D, Elvikis A, et al. Influence of stent configuration on cerebral aneurysm fluid dynamics. J Biomech. 2012;45:440–7. doi: 10.1016/j.jbiomech.2011.12.016. [DOI] [PubMed] [Google Scholar]
- 10.Cantón G, Levy DI, Lasheras JC. Hemodynamic changes due to stent placement in bifurcating intracranial aneurysms. J Neurosurg. 2005;103:146–55. doi: 10.3171/jns.2005.103.1.0146. [DOI] [PubMed] [Google Scholar]
- 11.Cantón G, Levy DI, Lasheras JC, Nelson PK. Flow changes caused by the sequential placement of stents across the neck of sidewall cerebral aneurysms. J Neurosurg. 2005;103:891–902. doi: 10.3171/jns.2005.103.5.0891. [DOI] [PubMed] [Google Scholar]
- 12.Hashimoto T, Meng H, Young WL. Intracranial aneurysms: Links among inflammation, hemodynamics and vascular remodeling. Neurol Res. 2006;28:372–80. doi: 10.1179/016164106X14973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Levitt MR, McGah PM, Aliseda A, Mourad PD, Nerva JD, Vaidya SS, et al. Cerebral aneurysms treated with flow-diverting stents: Computational models with intravascular blood flow measurements. AJNR Am J Neuroradiol. 2014;35:143–8. doi: 10.3174/ajnr.A3624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang Q, Xu J, Cheng J, Wang S, Wang K, Liu JM. Hemodynamic changes by flow diverters in rabbit aneurysm models: A computational fluid dynamic study based on micro-computed tomography reconstruction. Stroke. 2013;44:1936–41. doi: 10.1161/STROKEAHA.113.001202. [DOI] [PubMed] [Google Scholar]
- 15.Mut F, Scrivano E, Bleise C, Lylyk P, Cebral J. Hemodynamics in two tandem aneurysms treated with flow diverters. Int J Numer Method Biomed Eng. 2014;30:517–24. doi: 10.1002/cnm.2614. [DOI] [PubMed] [Google Scholar]
- 16.Seong J, Wakhloo AK, Lieber BB. In vitro evaluation of flow divertors in an elastase-induced saccular aneurysm model in rabbit. J Biomech Eng. 2007;129:863–72. doi: 10.1115/1.2800787. [DOI] [PubMed] [Google Scholar]
- 17.Cebral JR, Mut F, Raschi M, Scrivano E, Ceratto R, Lylyk P, et al. Aneurysm rupture following treatment with flow-diverting stents: Computational hemodynamics analysis of treatment. AJNR Am J Neuroradiol. 2011;32:27–33. doi: 10.3174/ajnr.A2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kadirvel R, Ding YH, Dai D, Rezek I, Lewis DA, Kallmes DF. Cellular mechanisms of aneurysm occlusion after treatment with a flow diverter. Radiology. 2014;270:394–9. doi: 10.1148/radiol.13130796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meng H, Wang Z, Kim M, Ecker RD, Hopkins LN. Saccular aneurysms on straight and curved vessels are subject to different hemodynamics: Implications of intravascular stenting. AJNR Am J Neuroradiol. 2006;27:1861–5. [PMC free article] [PubMed] [Google Scholar]
- 20.Kim M, Taulbee DB, Tremmel M, Meng H. Comparison of two stents in modifying cerebral aneurysm hemodynamics. Ann Biomed Eng. 2008;36:726–41. doi: 10.1007/s10439-008-9449-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Struffert T, Ott S, Kowarschik M, Bender F, Adamek E, Engelhorn T, et al. Measurement of quantifiable parameters by time-density curves in the elastase-induced aneurysm model: First results in the comparison of a flow diverter and a conventional aneurysm stent. Eur Radiol. 2013;23:521–7. doi: 10.1007/s00330-012-2611-2. [DOI] [PubMed] [Google Scholar]
- 22.Cebral JR, Castro MA, Burgess JE, Pergolizzi RS, Sheridan MJ, Putman CM. Characterization of cerebral aneurysms for assessing risk of rupture by using patient-specific computational hemodynamics models. AJNR Am J Neuroradiol. 2005;26:2550–9. [PMC free article] [PubMed] [Google Scholar]
- 23.Chiu JJ, Chen CN, Lee PL, Yang CT, Chuang HS, Chien S, et al. Analysis of the effect of disturbed flow on monocytic adhesion to endothelial cells. J Biomech. 2003;36:1883–95. doi: 10.1016/s0021-9290(03)00210-0. [DOI] [PubMed] [Google Scholar]
- 24.Tremmel M, Xiang J, Natarajan SK, Hopkins LN, Siddiqui AH, Levy EI, et al. Alteration of intra-aneurysmal hemodynamics for flow diversion using enterprise and vision stents. World Neurosurg. 2010;74:306–15. doi: 10.1016/j.wneu.2010.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cebral JR, Mut F, Weir J, Putman CM. Association of hemodynamic characteristics and cerebral aneurysm rupture. AJNR Am J Neuroradiol. 2011;32:264–70. doi: 10.3174/ajnr.A2274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Benndorf G, Herbon U, Sollmann WP, Campi A. Treatment of a ruptured dissecting vertebral artery aneurysm with double stent placement: Case report. AJNR Am J Neuroradiol. 2001;22:1844–8. [PMC free article] [PubMed] [Google Scholar]
- 27.Doerfler A, Wanke I, Egelhof T, Stolke D, Forsting M. Double-stent method: Therapeutic alternative for small wide-necked aneurysms. Technical note. J Neurosurg. 2004;100:150–4. doi: 10.3171/jns.2004.100.1.0150. [DOI] [PubMed] [Google Scholar]
- 28.Kallmes DF, Ding YH, Dai D, Kadirvel R, Lewis DA, Cloft HJ. A new endoluminal, flow-disrupting device for treatment of saccular aneurysms. Stroke. 2007;38:2346–52. doi: 10.1161/STROKEAHA.106.479576. [DOI] [PubMed] [Google Scholar]
- 29.Lopes DK, Ringer AJ, Boulos AS, Qureshi AI, Lieber BB, Guterman LR, et al. Fate of branch arteries after intracranial stenting. Neurosurgery. 2003;52:1275–8. doi: 10.1227/01.neu.0000064567.15270.27. [DOI] [PubMed] [Google Scholar]
- 30.Darsaut TE, Bing F, Salazkin I, Gevry G, Raymond J. Flow diverters can occlude aneurysms and preserve arterial branches: A new experimental model. AJNR Am J Neuroradiol. 2012;33:2004–9. doi: 10.3174/ajnr.A3075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chong W, Zhang Y, Qian Y, Lai L, Parker G, Mitchell K. Computational hemodynamics analysis of intracranial aneurysms treated with flow diverters: Correlation with clinical outcomes. AJNR Am J Neuroradiol. 2014;35:136–42. doi: 10.3174/ajnr.A3790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cebral JR, Castro MA, Appanaboyina S, Putman CM, Millan D, Frangi AF. Efficient pipeline for image-based patient-specific analysis of cerebral aneurysm hemodynamics: Technique and sensitivity. IEEE Trans Med Imaging. 2005;24:457–67. doi: 10.1109/tmi.2005.844159. [DOI] [PubMed] [Google Scholar]
- 33.Tateshima S, Murayama Y, Villablanca JP, Morino T, Takahashi H, Yamauchi T, et al. Intraaneurysmal flow dynamics study featuring an acrylic aneurysm model manufactured using a computerized tomography angiogram as a mold. J Neurosurg. 2001;95:1020–7. doi: 10.3171/jns.2001.95.6.1020. [DOI] [PubMed] [Google Scholar]
- 34.Hoi Y, Meng H, Woodward SH, Bendok BR, Hanel RA, Guterman LR, et al. Effects of arterial geometry on aneurysm growth: Three-dimensional computational fluid dynamics study. J Neurosurg. 2004;101:676–81. doi: 10.3171/jns.2004.101.4.0676. [DOI] [PubMed] [Google Scholar]
- 35.Shojima M, Oshima M, Takagi K, Torii R, Hayakawa M, Katada K, et al. Magnitude and role of wall shear stress on cerebral aneurysm: Computational fluid dynamic study of 20 middle cerebral artery aneurysms. Stroke. 2004;35:2500–5. doi: 10.1161/01.STR.0000144648.89172.0f. [DOI] [PubMed] [Google Scholar]
- 36.Masuda H, Zhuang YJ, Singh TM, Kawamura K, Murakami M, Zarins CK, et al. Adaptive remodeling of internal elastic lamina and endothelial lining during flow-induced arterial enlargement. Arterioscler Thromb Vasc Biol. 1999;19:2298–307. doi: 10.1161/01.atv.19.10.2298. [DOI] [PubMed] [Google Scholar]
- 37.Jou LD, Lee DH, Morsi H, Mawad ME. Wall shear stress on ruptured and unruptured intracranial aneurysms at the internal carotid artery. AJNR Am J Neuroradiol. 2008;29:1761–7. doi: 10.3174/ajnr.A1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Malek AM, Alper SL, Izumo S. Hemodynamic shear stress and its role in atherosclerosis. JAMA. 1999;282:2035–42. doi: 10.1001/jama.282.21.2035. [DOI] [PubMed] [Google Scholar]
- 39.Meng H, Tutino VM, Xiang J, Siddiqui A. High WSS or low WSS? Complex interactions of hemodynamics with intracranial aneurysm initiation, growth, and rupture: Toward a unifying hypothesis. AJNR Am J Neuroradiol. 2014;35:1254–62. doi: 10.3174/ajnr.A3558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rhee K, Han MH, Cha SH. Changes of flow characteristics by stenting in aneurysm models: Influence of aneurysm geometry and stent porosity. Ann Biomed Eng. 2002;30:894–904. doi: 10.1114/1.1500406. [DOI] [PubMed] [Google Scholar]
- 41.Hashimoto S, Manabe S, Matsumoto Y, Ikegami K, Tsuji H, Nakamura T, et al. The Effect of Pulsatile Shear Flow on Thrombus Formation and Hemolysis. Paper Presented at: Engineering in Medicine and Biology Society, 2000. Proceedings of the nd Annual International Conference of the IEEE. 2000 [Google Scholar]
- 42.Diamond SL, Sharefkin JB, Dieffenbach C, Frasier-Scott K, McIntire LV, Eskin SG. Tissue plasminogen activator messenger RNA levels increase in cultured human endothelial cells exposed to laminar shear stress. J Cell Physiol. 1990;143:364–71. doi: 10.1002/jcp.1041430222. [DOI] [PubMed] [Google Scholar]
- 43.Frangos JA, Eskin SG, McIntire LV, Ives CL. Flow effects on prostacyclin production by cultured human endothelial cells. Science. 1985;227:1477–9. doi: 10.1126/science.3883488. [DOI] [PubMed] [Google Scholar]
- 44.Aenis M, Stancampiano AP, Wakhloo AK, Lieber BB. Modeling of flow in a straight stented and nonstented side wall aneurysm model. J Biomech Eng. 1997;119:206–12. doi: 10.1115/1.2796081. [DOI] [PubMed] [Google Scholar]
- 45.Steiger HJ, Poll A, Liepsch D, Reulen HJ. Haemodynamic stress in lateral saccular aneurysms. An experimental study. Acta Neurochir (Wien) 1987;86:98–105. doi: 10.1007/BF01402292. [DOI] [PubMed] [Google Scholar]
- 46.Lieber BB, Livescu V, Hopkins LN, Wakhloo AK. Particle image velocimetry assessment of stent design influence on intra-aneurysmal flow. Ann Biomed Eng. 2002;30:768–77. doi: 10.1114/1.1495867. [DOI] [PubMed] [Google Scholar]
- 47.Baráth K, Cassot F, Rüfenacht DA, Fasel JH. Anatomically shaped internal carotid artery aneurysm in vitro model for flow analysis to evaluate stent effect. AJNR Am J Neuroradiol. 2004;25:1750–9. [PMC free article] [PubMed] [Google Scholar]
- 48.Liou TM, Li YC, Wang TC. Hemodynamics altered by placing helix stents in an aneurysm at a 45 degrees angle to the curved vessel. Phys Med Biol. 2008;53:3763–76. doi: 10.1088/0031-9155/53/14/004. [DOI] [PubMed] [Google Scholar]
- 49.Zhang YS, Yang XJ, Wang SZ, Qiao AK, Chen JL, Zhang KY, et al. Hemodynamic effects of stenting on wide-necked intracranial aneurysms. Chin Med J (Engl) 2010;123:1999–2003. [PubMed] [Google Scholar]
- 50.Cervós-Navarro J, Pätzold C, Tokuriki Y, Takebe Y, Hori K. In vivo study of flow pattern at human carotid bifurcation with regard to aneurysm development. Acta Neurochir (Wien) 1992;115:112–7. doi: 10.1007/BF01406368. [DOI] [PubMed] [Google Scholar]
- 51.Tanemura H, Ishida F, Miura Y, Umeda Y, Fukazawa K, Suzuki H, et al. Changes in hemodynamics after placing intracranial stents. Neurol Med Chir (Tokyo) 2013;53:171–8. doi: 10.2176/nmc.53.171. [DOI] [PubMed] [Google Scholar]
- 52.Wu YF, Yang PF, Shen J, Huang QH, Zhang X, Qian Y, et al. A comparison of the hemodynamic effects of flow diverters on wide-necked and narrow-necked cerebral aneurysms. J Clin Neurosci. 2012;19:1520–4. doi: 10.1016/j.jocn.2011.10.015. [DOI] [PubMed] [Google Scholar]
- 53.Kim M, Levy EI, Meng H, Hopkins LN. Quantification of hemodynamic changes induced by virtual placement of multiple stents across a wide-necked basilar trunk aneurysm. Neurosurgery. 2007;61:1305–12. doi: 10.1227/01.NEU.0000280168.25968.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Larrabide I, Geers AJ, Morales HG, Aguilar ML, Rüfenacht DA. Effect of aneurysm and ICA morphology on hemodynamics before and after flow diverter treatment. J Neurointerv Surg. 2015;7:272–80. doi: 10.1136/neurintsurg-2014-011171. [DOI] [PubMed] [Google Scholar]
- 55.Liepsch DW. Flow in tubes and arteries – A comparison. Biorheology. 1986;23:395–433. doi: 10.3233/bir-1986-23408. [DOI] [PubMed] [Google Scholar]
- 56.Kono K, Terada T. Hemodynamics of 8 different configurations of stenting for bifurcation aneurysms. AJNR Am J Neuroradiol. 2013;34:1980–6. doi: 10.3174/ajnr.A3479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rohde S, Bendszus M, Hartmann M, Hähnel S. Treatment of a wide-necked aneurysm of the anterior cerebral artery using two Enterprise stents in “Y”-configuration stenting technique and coil embolization: A technical note. Neuroradiology. 2010;52:231–5. doi: 10.1007/s00234-009-0603-y. [DOI] [PubMed] [Google Scholar]
- 58.Lozen A, Manjila S, Rhiew R, Fessler R. Y-stent-assisted coil embolization for the management of unruptured cerebral aneurysms: Report of six cases. Acta Neurochir (Wien) 2009;151:1663–72. doi: 10.1007/s00701-009-0436-9. [DOI] [PubMed] [Google Scholar]
- 59.Cekirge HS, Yavuz K, Geyik S, Saatci I. A novel “Y” stent flow diversion technique for the endovascular treatment of bifurcation aneurysms without endosaccular coiling. AJNR Am J Neuroradiol. 2011;32:1262–8. doi: 10.3174/ajnr.A2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Roszelle BN, Nair P, Gonzalez LF, Haithem Babiker M, Ryan J, Frakes D. Comparison among different high porosity stent configurations: Hemodynamic effects of treatment in a large cerebral aneurysm. J Biomech Eng. 2014;136:021013. doi: 10.1115/1.4026257. [DOI] [PubMed] [Google Scholar]
- 61.Dorn F, Niedermeyer F, Balasso A, Liepsch D, Liebig T. The effect of stents on intra-aneurysmal hemodynamics: In vitro evaluation of a pulsatile sidewall aneurysm using laser Doppler anemometry. Neuroradiology. 2011;53:267–72. doi: 10.1007/s00234-010-0723-4. [DOI] [PubMed] [Google Scholar]
- 62.Janiga G, Rössl C, Skalej M, Thévenin D. Realistic virtual intracranial stenting and computational fluid dynamics for treatment analysis. J Biomech. 2013;46:7–12. doi: 10.1016/j.jbiomech.2012.08.047. [DOI] [PubMed] [Google Scholar]
- 63.Zhang Y, Chong W, Qian Y. Investigation of intracranial aneurysm hemodynamics following flow diverter stent treatment. Med Eng Phys. 2013;35:608–15. doi: 10.1016/j.medengphy.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 64.Kojima M, Irie K, Fukuda T, Arai F, Hirose Y, Negoro M. The study of flow diversion effects on aneurysm using multiple enterprise stents and two flow diverters. Asian J Neurosurg. 2012;7:159–65. doi: 10.4103/1793-5482.106643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Le TB, Troolin DR, Amatya D, Longmire EK, Sotiropoulos F. Vortex phenomena in sidewall aneurysm hemodynamics: Experiment and numerical simulation. Ann Biomed Eng. 2013;41:2157–70. doi: 10.1007/s10439-013-0811-9. [DOI] [PubMed] [Google Scholar]
- 66.Lieber BB, Stancampiano AP, Wakhloo AK. Alteration of hemodynamics in aneurysm models by stenting: Influence of stent porosity. Ann Biomed Eng. 1997;25:460–9. doi: 10.1007/BF02684187. [DOI] [PubMed] [Google Scholar]
- 67.Tateshima S, Tanishita K, Hakata Y, Tanoue SY, Viñuela F. Alteration of intraaneurysmal hemodynamics by placement of a self-expandable stent. Laboratory investigation. J Neurosurg. 2009;111:22–7. doi: 10.3171/2009.2.JNS081324. [DOI] [PubMed] [Google Scholar]
- 68.Shobayashi Y, Tateshima S, Kakizaki R, Sudo R, Tanishita K, Viñuela F. Intra-aneurysmal hemodynamic alterations by a self-expandable intracranial stent and flow diversion stent: High intra-aneurysmal pressure remains regardless of flow velocity reduction. J Neurointerv Surg. 2013;5(Suppl 3):iii38–42. doi: 10.1136/neurintsurg-2012-010488. [DOI] [PubMed] [Google Scholar]
- 69.Lieber BB, Gounis MJ. The physics of endoluminal stenting in the treatment of cerebrovascular aneurysms. Neurol Res. 2002;24(Suppl 1):S33–42. doi: 10.1179/016164102101200014. [DOI] [PubMed] [Google Scholar]
- 70.Roszelle BN, Babiker MH, Hafner W, Gonzalez LF, Albuquerque FC, Frakes DH. In vitro and in silico study of intracranial stent treatments for cerebral aneurysms: Effects on perforating vessel flows. J Neurointerv Surg. 2013;5:354–60. doi: 10.1136/neurintsurg-2012-010322. [DOI] [PubMed] [Google Scholar]
- 71.Roszelle BN, Gonzalez LF, Babiker MH, Ryan J, Albuquerque FC, Frakes DH. Flow diverter effect on cerebral aneurysm hemodynamics: An in vitro comparison of telescoping stents and the Pipeline. Neuroradiology. 2013;55:751–8. doi: 10.1007/s00234-013-1169-2. [DOI] [PubMed] [Google Scholar]
- 72.Tominaga R, Harasaki H, Sutton C, Emoto H, Kambic H, Hollman J. Effects of stent design and serum cholesterol level on the restenosis rate in atherosclerotic rabbits. Am Heart J. 1993;126:1049–58. doi: 10.1016/0002-8703(93)90654-r. [DOI] [PubMed] [Google Scholar]
- 73.Schneiders JJ, VanBavel E, Majoie CB, Ferns SP, van den Berg R. A flow-diverting stent is not a pressure-diverting stent. AJNR Am J Neuroradiol. 2013;34:E1–4. doi: 10.3174/ajnr.A2613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Karmonik C, Chintalapani G, Redel T, Zhang YJ, Diaz O, Klucznik R, et al. Hemodynamics at the ostium of cerebral aneurysms with relation to post-treatment changes by a virtual flow diverter: A computational fluid dynamics study. Conf Proc IEEE Eng Med Biol Soc. 2013;2013:1895–8. doi: 10.1109/EMBC.2013.6609895. [DOI] [PubMed] [Google Scholar]
- 75.Ionita CN, Paciorek AM, Hoffmann KR, Bednarek DR, Yamamoto J, Kolega J, et al. Asymmetric vascular stent: Feasibility study of a new low-porosity patch-containing stent. Stroke. 2008;39:2105–13. doi: 10.1161/STROKEAHA.107.503862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Liou TM, Li YC. Effects of stent porosity on hemodynamics in a sidewall aneurysm model. J Biomech. 2008;41:1174–83. doi: 10.1016/j.jbiomech.2008.01.025. [DOI] [PubMed] [Google Scholar]
- 77.Trager AL, Sadasivan C, Lieber BB. Comparison of the in vitro hemodynamic performance of new flow diverters for bypass of brain aneurysms. J Biomech Eng. 2012;134:084505. doi: 10.1115/1.4006454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Darsaut TE, Bing F, Salazkin I, Gevry G, Raymond J. Flow diverters failing to occlude experimental bifurcation or curved sidewall aneurysms: An in vivo study in canines. J Neurosurg. 2012;117:37–44. doi: 10.3171/2012.4.JNS111916. [DOI] [PubMed] [Google Scholar]
- 79.Seshadhri S, Janiga G, Beuing O, Skalej M, Thévenin D. Impact of stents and flow diverters on hemodynamics in idealized aneurysm models. J Biomech Eng. 2011;133:071005. doi: 10.1115/1.4004410. [DOI] [PubMed] [Google Scholar]
- 80.Sadasivan C, Cesar L, Seong J, Wakhloo AK, Lieber BB. Treatment of rabbit elastase-induced aneurysm models by flow diverters: Development of quantifiable indexes of device performance using digital subtraction angiography. IEEE Trans Med Imaging. 2009;28:1117–25. doi: 10.1109/TMI.2008.2012162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kallmes DF, Ding YH, Dai D, Kadirvel R, Lewis DA, Cloft HJ. A second-generation, endoluminal, flow-disrupting device for treatment of saccular aneurysms. AJNR Am J Neuroradiol. 2009;30:1153–8. doi: 10.3174/ajnr.A1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fu W, Gu Z, Meng X, Chu B, Qiao A. Numerical simulation of hemodynamics in stented internal carotid aneurysm based on patient-specific model. J Biomech. 2010;43:1337–42. doi: 10.1016/j.jbiomech.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 83.Bando K, Berger S. Research on fluid-dynamic design criterion of stent used for treatment of aneurysms by means of computational simulation. Comut Fluid Dyn J. 2003;11:527–31. [Google Scholar]
- 84.Wang K, Huang Q, Hong B, Li Z, Fang X, Liu J. Correlation of aneurysm occlusion with actual metal coverage at neck after implantation of flow-diverting stent in rabbit models. Neuroradiology. 2012;54:607–13. doi: 10.1007/s00234-011-0922-7. [DOI] [PubMed] [Google Scholar]
- 85.Ohta M, Hirabayashi M, Wetzel S, Lylyk P, Wata H, Tsutsumi S, et al. Impact of stent design on intra-aneurysmal flow. A computer simulation study. Interv Neuroradiol. 2004;10(Suppl 2):85–94. doi: 10.1177/15910199040100S216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mut F, Cebral JR. Effects of flow-diverting device oversizing on hemodynamics alteration in cerebral aneurysms. AJNR Am J Neuroradiol. 2012;33:2010–6. doi: 10.3174/ajnr.A3080. [DOI] [PMC free article] [PubMed] [Google Scholar]