Abstract
Aims and Objectives:
The aim of this study is to find the outcome of repair and resection of the occipital encephalocele.
Study Design:
Case series.
Materials and Methods:
The clinical data of fifty consecutive occipital encephalocele patients were retrieved from medical records including operative notes, postoperative follow-up visits, and postsurgical complications were noted for analysis from November 2009 to November 2013 at the Department of Neurosurgery, Jinnah Postgraduate Medical Centre, Karachi, Pakistan. All patients were assessed by computed tomography scan, magnetic resonance imaging brain, and ultrasound when needed. Physician's assessment, physical examination, and his/her questions to the family at follow-up were used as a tool to determine if there was a developmental delay rather than quantitative analysis like hydrocephalus questionnaires. Patients who developed complications and delayed milestone were regarded as no improvement and those who did not develop complications and achieved appropriate milestone were regarded as improved at 18 months follow-up.
Results:
Of 50 patients, 17 were males and 33 were females. The average age at presentation was 2.4 months. 16 (32%) patients had increased head circumference and hydrocephalus, 2 (4%) had associated Dandy–Walker cyst, 3 (6%) developed developmental delays, and 8 (15%) had a seizure disorder. None of our patients had neurological deficits. The size of the sac ranged from 2 cm × 3 cm to 27 cm × 15 cm. 9 (18%) patients were admitted with the complication of sac rupture and 2 (4%) patients sac ruptured after admission. Only one patient (2%) had a cerebrospinal fluid leak postoperatively that was repaired primarily without patch graft or dura seal while 4 (8%) developed hydrocephalus after repair of the sac which was treated with placement of ventriculoperitoneal shunt. One (2%) patient did not recover from anesthesia and expired.
Conclusion:
Encephalocele is commonly seen in the practice of neurosurgery in the world as well as in Pakistan. Modern neuroimaging, neurosurgical techniques, and neonatal neurological intensive care have greatly improved morbidity and mortality in the care of encephalocele.
Keywords: Cerebrospinal fluid, encephalocele, hydrocephalus, IQ, ventriculoperitoneal shunt
Introduction
Encephaloceles are congenital group of disorder in which there is a protrusion of brain with or without the protrusion of the meninges through a defect in the skull.[1,2] These congenital lesions are commonly encountered in the practice of neurosurgery in Pakistan and worldwide.[3] The reported epidemiological incidence of the disease is about 0.8–5.6/10,000 live births.[3,4,5] The geographic distribution shows that Western countries have more frequency of occipital encephalocele, whereas in South East Asia frontoethmoidal encephalocele predominates.[6,7,8,9,10] Mesodermal abnormality is thought to be an important factor that causes a defect in calvarium and dura through which protrudes the brain tissue. The exact etiology of the disease is complex, and the associated risk factors have remained obscure. Some studies do show an association between certain risk factors such as hyperthermia, aflatoxin, genetic background, maternal nutritional deficiency, or other environmental factors.[1,7,11,12] The relationship between maternal levels of folate and the incidence of encephalocele is still not clear; although, there is clear evidence about the protective effect of folate in myelomeningocele.[1,7] Children born with large encephalocele sac containing the brain tissue is the single most important risk factor for survival.[7]
In our part of the world, there is no prenatal screening of encephalocele during pregnancy; therefore, the prevalence of encephalocele is higher compared to western world where prenatal screening is routinely done. The women in these countries have the option to terminate the pregnancy in case severe form of encephalocele is detected.[13] Morbidity and mortality rate of occipital encephalocele are quite variable, and was high in the past compared to the present day,[14,15] for example, older studies by Lorber and Schofield[14] reported 57% mortality of occipital encephalocele. Tsuchida et al.[16] reported 41% mortality within 2 years. However, modern high-resolution imaging, good surgical techniques and neonatal postoperative care have tremendously decreased morbidity and mortality of occipital encephaloceles. In a recent study by Bui et al.[15] there were no deaths reported at 9 years of follow-up of encephaloceles. There are very few dedicated studies looking at occipital encephalocele only and these lesions are often studied collectively with other forms of encephalocele. To the best of our knowledge, there is no study that analyzed other parameters such as seizure, hydrocephalus, and developmental delays in a single cohort of occipital encephalocele patients. Therefore the objective of this study was to present surgical treatment and complications of Occipital Encephaloceles such as hydrocephalus, developmental delays, seizure disorder, and to report morbidity and mortality in this modern Era of Microneurosurgery.
Materials and Methods
A large volume of neurosurgical patients are referred from all over Pakistan and adjacent part of Afghanistan and National Institute of Child Health which is affiliated to our Department of Neurosurgery for the pediatric neurosurgery section.
A retrospective study of fifty exclusively occipital encephalocele patients was conducted between November 2009 and November 2013 at the Department of Neurosurgery. The medical records of all operated cases of occipital encephalocele were reviewed, and relevant data such as age, sex, location of encephalocele, the size of the lesion, operative method, seizure, and hydrocephalus along with postoperative complications were recorded for analysis. Patients with follow-up of 18 months were included in the study. These patients were evaluated by computed tomography scan of the brain, magnetic resonance imaging, and ultrasound where appropriate [Figure 1]. Patients with other malformations, large lesions, and a significant amount of cerebral tissue in the sac that could not be repaired without attendant risks, associated syndrome of microcephaly were excluded from this study. Developmental delays and cognition were assessed by senior residents and operating surgeon that were part of the surgical team using examination and interpretation of follow-up questions to the patient's family rather than more quantitative measures such as hydrocephalus outcome questionnaires.[17] Patients who developed complications and delayed milestones were regarded as no improvement and those who did not develop deficits and achieved appropriate milestones were regarded as improved with follow-up examination.
Figure 1.
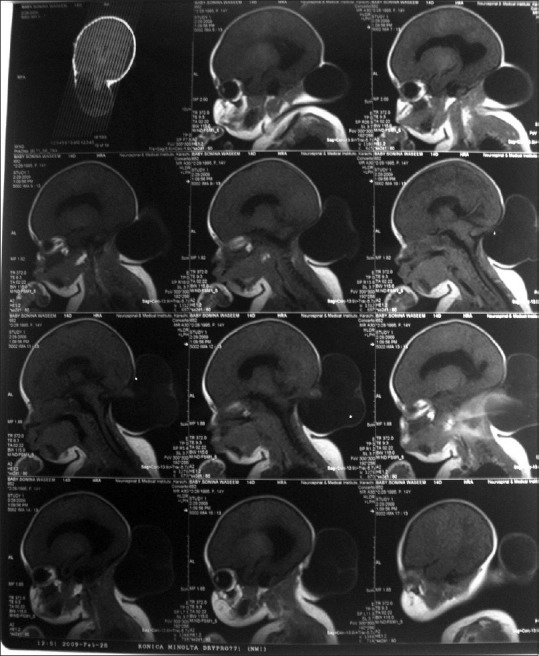
Preoperative T1-weighted magnetic resonance imaging showing occipital encephalocele and its stalk
Direct excision and repair of encephalocele were done and herniated part of the brain which was gliosed and nonviable; safely removed. See Figures 1–3 of the depicted patient. Dural defect closed in a watertight fashion; graft from pericranium used where necessary and fibrin glue was applied to strengthen the graft [Figures 2 and 3]. Ventriculoperitoneal (VP) shunt was placed when hydrocephalus was present. Sacs that ruptured before admission were managed by covering it with normal saline soaked gauze in sterile fashion and were taken to operation theater to repair as soon as possible. We also described postsurgical complications and 18 months follow-up. Data were analyzed using SPSS 14 for windows student version Chicago Illinois, USA software and the relevant descriptive statistic is presented.
Figure 3.
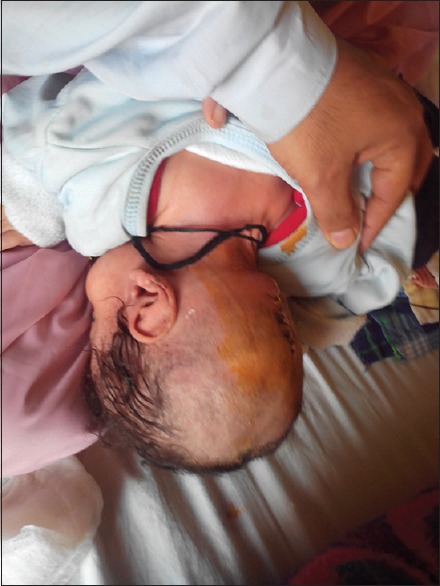
Postoperative picture of the same patient after resection of the encephalocele
Figure 2.
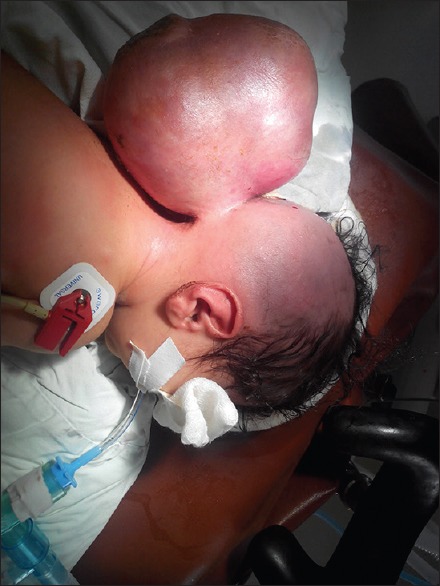
Preoperative picture of the same patient showing massive occipital encephalocele
Results
A total of fifty patients were chosen as per inclusion criteria. Of 50 patients, 17 were males and 33 females. The average age of the patients at the time of presentation was 2.4 months, ranging (4 days to 1.33 years) [Table 1].
Table 1.
Age group and Occipital Encephalocele

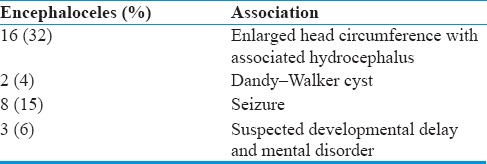
All patients presented with swelling on the head just after birth. A visible mass was situated in either the occipital (supratorcular or infratorcular). Any overlying skin varied from a thick and wrinkled to a thin or shiny covering. Sixteen patients (32%) presented with enlarged head circumference with associated hydrocephalus and two patients (4%) diagnosed with Dandy–Walker cyst. 3 (6%) patients were suspected developmental delay and mental disorders. Eight (15%) patients also had seizure. None of the patients had neurological deficits in our study. Some of the swellings gradually increased in size from birth, while others remained static, or even decreased [Table 2].
Table 2.
Occipital Encephalocele and Associated Features
The size of sac ranged from 2 cm × 3 cm to 27 cm × 15 cm in diameter. It was sometimes difficult to differentiate an encephalocele from other swellings which occur in this region, but the classical cases offered no problem.
Preoperative, postoperative complications, and outcome
9 (18%) patients admitted with the complication of sac rupture with cerebrospinal fluid (CSF) leakage, 2 (4%) patients having rupture of sac after the admission and 1 (2%) patient admitted with the complaint of hemorrhage from the thin and shiny covering skin of the sac. Postoperatively, only 1 (2%) patient had CSF leakage from the repaired wound. 4 (8%) patients developed Hydrocephalus after the repair of protrude sac. 1 (2%) patient did not recover from anesthesia after surgery (repair of encephalocele with the removal of herniated dysplastic brain), he stopped respiration; went on ventilator support and died next day of surgery [Table 3].
Table 3.
Complications of Occipital Encephalocele

Follow-up was done for 18 months from the time of discharge in each patient. Of 50 patients, 45 (90%) came for follow-up. The outcome was assessed according to patient's surgical wound healing, growth, head circumference, symptoms of meningitis, neurological deficits and evidence of recurrence of encephalocele, physical and mental assessment were made by Senior residents and operating surgeon follow-up examination and questions to the family. There was one wound infection that was treated appropriately. No meningitis was observed. None of the recurrences was noted after surgical repair. Four patients (8%) developed hydrocephalus after surgery. Another patient developed postoperative CSF leak and stopped CSF after placement of VP shunt and repair of the leak site. One of the three patients with developmental delays also had a seizure; they did not show improvement at 18 months follow-up regarding developmental milestones and cognition though their seizure was well-controlled on the single epileptic drug. Patients with seizure had good control with one anti-epileptic drug. Mortality was 2% during the 18 months period.
Discussion
In our experience, females were affected predominantly (33%) which correlate with previous reports.[9,11] The average size of the sac was 2 cm × 3 cm to 27 cm × 15 cm in diameter. The contents of the sac varied from dysplastic diverticulum to brain tissue (degenerated) with some amount of CSF always present. The bony defects varied from different sizes, i.e., larger encephalocele had larger bony defects, but this was not always the case because sometimes smaller defects were associated with larger encephaloceles. These larger lesions require urgent surgical intervention to avoid damage to the functioning brain tissues and intracranial vessels that go in and out of the sac to supply the containing brain tissue. The later produce infarction in the brain when this type of tissue is excised.[18] In our series of patients, there was no infarction postoperatively after the repair.
Hydrocephalus is important predictor of developmental delays in patients with encephalocele and develops after surgery in some cases.[2,19,20] VP shunt should be placed before complete repair of encephalocele in these patients.[19,21] Bui et al.[15] reported that occipital encephalocele is commonly associated with hydrocephalus compared to other types of encephalocele. In our series of patients, hydrocephalus was observed in 16 patients (34%) who were treated by placing VP shunt before the repair of the sac. While two patients (4%) developed hydrocephalus after surgery that was again successfully managed by VP shunt as second surgery.
There is always a chance of infection in large encephalocele usually because there is a leakage of CSF.[22,23] In this study, only 3 (6%) patients had the minor infection treated before surgery and one postoperative wound infection treated in the usual manner. All surgeries were elective except nine patients that were admitted with rupture of the sac and CSF leakage which were repaired on emergency basis. Two patients had rupture of sac after the admission and one patient admitted with the complaint of hemorrhage from the thin and shiny covering skin of the sac. All these patients underwent immediate surgery, while remaining 41 patients underwent a presurgery fitness protocol and underwent surgery when they were labeled fit for surgery. Microcephaly is poor prognostic factor which is associated with developmental delays.[14] Gallo[24] used tantalum mesh to manage microcephaly to expand the cranial cavity (expansible cranioplasty) and to place the brain tissue in it that is herniating out.[24] We did not face this problem in our series. We removed all the dysplastic brain tissue which was protruding out of the skull, preoperative imaging was carried out to identify this type of tissue and to prevent postoperative neurological deficits due to any blood vessel and normal brain parenchyma.
A seizure is another important factor to affect the quality of life in these children.[2] Bui et al.[15] reported 17% of seizure in occipital encephalocele. In our series of patients, the seizure was noted in 8 (15%) which is slightly less than other reported studies.[2,15] Seizures in these patients were well-controlled after surgery. Interestingly two of our patients had associated Dandy–Walker cysts along with hydrocephalus. Hydrocephalus should not be treated before treatment of Dandy–Walker cyst due to the risk of upward shifting of posterior fossa contents. We treated hydrocephalus and Dandy–Walker cyst in the same setting by single shunt system connected through Y connector at same setting (locally available in our own assembled system in Pakistan). Mortality was 2% in our series which correlates to recent reports[21] and much less compared to older series.[10,22]
Limitation
Retrospective model of the study with associated bias. Physical assessment, developmental milestones, and cognition were assessed by physician follow-up examinations and interpretation of questions to mothers rather than by hydrocephalus outcome questionnaires used by Kulkarni et al.[17] in patients with hydrocephalus due to any cause. However, only 16 percent of patients had hydrocephalus in our series. Follow-up period is short, and therefore, only short-term surgical and physical outcome were possible.
Conclusion
Encephalocele is commonly seen in the practice of neurosurgery in the world and in Pakistan. It is associated with other congenital anomalies such as hydrocephalus, Dandy–Walker malformation, and microcephaly.
Modern neuroimaging, neurosurgical techniques, and neonatal neurological intensive care have greatly improved morbidity and mortality in the care of encephalocele. It's treatment like excision and repair when done in early age, greatly reduces complications like CSF leak, reduced IQ level of the patients and other effects of associated anomalies are controlled in time. Parents have no difficulty in taking care of their children after repair. Therefore, early repair and excision of occipital encephalocele is recommended.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Agthong S, Wiwanitkit V. Encephalomeningocele cases over 10 years in Thailand: A case series. BMC Neurol. 2002;2:3. doi: 10.1186/1471-2377-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lo BW, Kulkarni AV, Rutka JT, Jea A, Drake JM, Lamberti-Pasculli M, et al. Clinical predictors of developmental outcome in patients with cephaloceles. J Neurosurg Pediatr. 2008;2:254–7. doi: 10.3171/PED.2008.2.10.254. [DOI] [PubMed] [Google Scholar]
- 3.Raja RA, Qureshi AA, Memon AR, Ali H, Dev V. Pattern of encephaloceles: A case series. J Ayub Med Coll Abbottabad. 2008;20:125–8. [PubMed] [Google Scholar]
- 4.Rowland CA, Correa A, Cragan JD, Alverson CJ. Are encephaloceles neural tube defects? Pediatrics. 2006;118:916–23. doi: 10.1542/peds.2005-1739. [DOI] [PubMed] [Google Scholar]
- 5.Thauvin-Robinet C, Callier P, Laurent N, Rousseau T, Masurel-Paulet A, Marle N, et al. Syndromic encephalocele in a fetal case with a 1p35-pter deletion and a 14q32-qter duplication inherited from a maternal balanced translocation. Prenat Diagn. 2007;27:555–9. doi: 10.1002/pd.1724. [DOI] [PubMed] [Google Scholar]
- 6.McDonnell RJ, Johnson Z, Delaney V, Dack P. East Ireland 1980-1994: Epidemiology of neural tube defects. J Epidemiol Community Health. 1999;53:782–8. doi: 10.1136/jech.53.12.782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Siffel C, Wong LY, Olney RS, Correa A. Survival of infants diagnosed with encephalocele in Atlanta, 1979-98. Paediatr Perinat Epidemiol. 2003;17:40–8. doi: 10.1046/j.1365-3016.2003.00471.x. [DOI] [PubMed] [Google Scholar]
- 8.Hoving EW. Nasal encephaloceles. Childs Nerv Syst. 2000;16:702–6. doi: 10.1007/s003810000339. [DOI] [PubMed] [Google Scholar]
- 9.Nath HD, Mahapatra AK, Gunawat P. Case report: A torcular encephalocele with proatlas defect and os-terminale. Asian J Neurosurg. 2012;7:84–6. doi: 10.4103/1793-5482.98652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shilpakar SK, Sharma MR. Surgical management of encephaloceles. J Neurosci. 2004;1:45–8. [Google Scholar]
- 11.Wen S, Ethen M, Langlois PH, Mitchell LE. Prevalence of encephalocele in Texas, 1999-2002. Am J Med Genet A. 2007;143A:2150–5. doi: 10.1002/ajmg.a.31907. [DOI] [PubMed] [Google Scholar]
- 12.Sadewa AH, Sutomo R, Istiadjid M, Nishiyama K, Shirakawa T, Matsuo M, et al. C677T mutation in the MTHFR gene was not found in patients with frontoethmoidal encephalocele in East Java, Indonesia. Pediatr Int. 2004;46:409–14. doi: 10.1111/j.1442-200x.2004.01927.x. [DOI] [PubMed] [Google Scholar]
- 13.Allen WP, Stevenson RE, Thompson SJ, Dean JH. The impact of prenatal diagnosis on NTD surveillance. Prenat Diagn. 1996;16:531–5. doi: 10.1002/(SICI)1097-0223(199606)16:6<531::AID-PD914>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 14.Lorber J, Schofield JK. The prognosis of occipital encephalocele. Dev Med Child Neurol. 1967;13:75–86. doi: 10.1111/j.1469-8749.1967.tb02385.x. [DOI] [PubMed] [Google Scholar]
- 15.Bui CJ, Tubbs RS, Shannon CN, Acakpo-Satchivi L, Wellons JC, Blount JP, et al. Institutional experience with cranial vault encephalocele. J Neurosurg Pediatr. 2008;1:22–5. doi: 10.3171/PED-07/07/022. [DOI] [PubMed] [Google Scholar]
- 16.Tsuchida T, Okada K, Ueki K. The prognosis of encephaloceles (author's transl) No Shinkei Geka. 1981;9:143–50. [PubMed] [Google Scholar]
- 17.Kulkarni AV, Rabin D, Drake JM. An instrument to measure the health status in children with hydrocephalus: The hydrocephalus outcome questionnaire. J Neurosurg. 2004;101(2 Suppl):134–40. doi: 10.3171/ped.2004.101.2.0134. [DOI] [PubMed] [Google Scholar]
- 18.Hoffman H. Comments to the article by Gallo AE. Childs Nerv Syst. 1992;8:229–30. [Google Scholar]
- 19.Gamache FW., Jr Treatment of hydrocephalus in patients with meningomyelocele or encephalocele: A recent series. Childs Nerv Syst. 1995;11:487–8. doi: 10.1007/BF00334972. [DOI] [PubMed] [Google Scholar]
- 20.Docherty JG, Daly JC, Carachi R. Encephaloceles: A review 1971-1990. Eur J Pediatr Surg. 1991;1(Suppl 1):11–3. doi: 10.1055/s-2008-1042528. [DOI] [PubMed] [Google Scholar]
- 21.Mardzuki AI, Abdullah J, Ghazaime G, Ariff AR, Ghazali M. Two-stage management of mega occipito-encephalocele. Med J Malaysia. 2003;58:115–9. [PubMed] [Google Scholar]
- 22.Aslan A, Eser O, Dogru O, Aktepe F, Yurumez Y, Fidan H. Occipital mega encephalocele associated with acute inflammation. Pediatr Neurosurg. 2007;43:65–6. doi: 10.1159/000097530. [DOI] [PubMed] [Google Scholar]
- 23.Martínez-Lage JF, Poza M, Sola J, Soler CL, Montalvo CG, Domingo R, et al. The child with a cephalocele: Etiology, neuroimaging, and outcome. Childs Nerv Syst. 1996;12:540–50. doi: 10.1007/BF00261608. [DOI] [PubMed] [Google Scholar]
- 24.Gallo AE., Jr Repair of giant occipital encephaloceles with microcephaly secondary to massive brain herniation. Childs Nerv Syst. 1992;8:229–30. doi: 10.1007/BF00262854. [DOI] [PubMed] [Google Scholar]


