Abstract
Introduction:
Ventriculostomy-related infection (VRI) from external ventricular drain (EVD) insertion is a common complication and carries a high mortality rate. Choice of empiric antibiotics depends on the institutions common causative organisms and their susceptibility. We determined risk factors for mortality in patients with VRI, the common organisms causing VRI, and the rate of EVD-related VRI at our institution.
Methods:
Medical records and operative data of patients with cerebrospinal fluid positive cultures with an EVD inserted from 2012 to 2015 were traced. Forty-five patients with EVD-related VRI were included in the study.
Results:
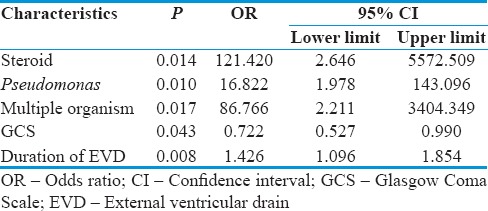
The overall rate of VRI was 6.3%, and the overall mortality rate due to VRI was 48.9%. Acinetobacter baumannii was the most common organism causing VRI (14 patients, 29.2%) with a mortality rate of 64.3%. Only 14.3% of A. baumannii are sensitive to meropenem and imipenem. We found that patients that had a decompressive craniectomy (DC) had a lower mortality rate (P = 0.042) and patients with a longer duration of the EVD being in place before the diagnosis of VRI had poor outcome (P = 0.040). Multivariate logistic regression was performed and we found that the use of steroid (P = 0.014), Pseudomonas aeruginosa infection (P = 0.010), multiple organism infection (P = 0.017), lower Glasgow Coma Scale (P = 0.043), and a longer duration the EVD was in place before the diagnosis of VRI (P = 0.008) were related with higher mortality.
Conclusion:
VRI mortality rate is high with an alarming resistance pattern seen in Acinetobacter VRI. EVDs should be removed as soon as feasible, and DC may be offered to patients with severe ventriculitis or meningitis.
Keywords: Acinetobacter, decompressive craniectomy, fatal outcome, nosocomial meningitis, risk factors, ventriculitis
Introduction
An external ventricular drain (EVD) is a temporary system for drainage of cerebrospinal fluid (CSF) and a conduit for intracranial pressure (ICP) monitoring. Ventriculitis, however, is a common but serious complication associated with ventricular catheters. Reported rates of EVD-related ventriculitis or ventriculostomy-related infection (VRI) range from 0% to 32.2%.[1,2,3] Mortality is as high as 10.3%–40.8%.[4,5,6] A good choice of perioperative prophylactic antibiotics and empiric antibiotics for ventriculitis while waiting for the culture report and sensitivity requires a sound knowledge of the institutions common causative organisms and their susceptibility. There is an alarming increase in resistance to antibiotics among the Gram-negative organisms.[7] Acinetobacter has been increasingly found to be the causative organism, and the greater fear lies in the fact that multidrug-resistant Acinetobacter baumannii (MRAB) infections are getting common.[5,8] Meropenem has been the drug of choice for nosocomial meningitis and ventriculitis to cover Gram-negative bacilli, including A. baumannii. However, polymyxin is frequently the only available therapeutic option with the emergence of carbapenem-resistant A. baumannii. Since the rates of EVD-related ventriculitis can be high, and mortality is not trivial, modifiable factors affecting the outcome of these patients negatively need to be recognized. In this article, we will determine the risk factors for poor prognosis of VRI, outline the common organisms causing VRI with special attention to Acinetobacter VRI, and the rate of EVD-related VRI at our institution.
Methods
This was a retrospective observational study conducted at Penang General Hospital, a neurosurgical referral center for the Northwestern region of Peninsular Malaysia. We obtained approval from the Medical Research and Ethics Committee of Malaysia. CSF positive cultures from the neurosurgical wards were traced from the hospital's microbiology records. Hospital records and operative data of patients with CSF-positive cultures with an EVD inserted from 2012 to 2015 were traced. The total number of EVDs inserted was obtained from the neurosurgical department's records. A total of 45 patients with VRI were included in the study. Patients who had previous central nervous system (CNS) infections were excluded from the study. All patients received prophylactic ceftriaxone or cefuroxime antibiotics before EVD insertion. Most patients suspected to have ventriculitis were started on empiric antibiotics while waiting for the antibiotic susceptibility report. At our center, CSF sampling was done only when there was suspected VRI or before converting an EVD to a ventricular-peritoneal shunt.
There is no clear consensus on the definition of ventriculitis and retrospective analysis of the patient is difficult and maybe biased.[9] Therefore, ventriculitis was defined as follows: (1) any patient with a positive CSF culture from the EVD and was diagnosed and treated for ventriculitis by the neurosurgical or infectious diseases team; (2) any patient with a positive CSF culture from the EVD and increased white cells, elevated protein, and decreased CSF glucose from CSF analysis.
The Glasgow Coma Scale (GCS) is taken to be the last GCS documented before endotracheal intubation or operation. Bacteremia is defined as positive blood culture from the patients yielding the same organism as that in the CSF culture. Patients that still required the EVD even after ventriculitis was diagnosed is defined as a persistent EVD state. Date of the positive CSF culture is used to define the date when ventriculitis is confirmed. Duration of EVD when ventriculitis was first confirmed is the time in days from the date of insertion till the date of confirmation with positive CSF culture. We defined discordant empirical antibiotics as initial antibiotic choice where the cultured organism is resistant to the empiric antibiotic. We categorized the organisms into multi-resistant bacteria if they were resistant to carbapenems or were resistant to methicillin. If a single CSF sample grew more than one organism, the patient is categorized as having multiple organism growths rather than mono-organism growth.
For our statistical analyses, IBM SPSS v23.0.0 (IBM Corp)was used. Chi-square or Fisher's exact test was used for categorical variables and independent Student's t-test for normally distributed continuous variables, and Mann–Whitney test for nonnormally distributed continuous variables. Multivariate logistic regression analysis was also used to identify the risk factors for mortality. A statistically significant result is defined as a test showing a P < 0.05.
Results
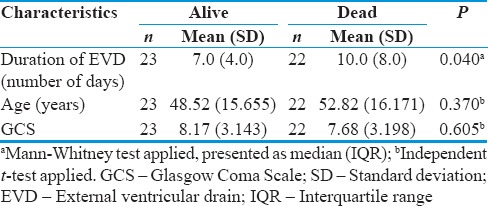
The age ranged from 12 to 75 years (mean, 50.62 years). The main two underlying diagnoses leading to insertion of EVD were spontaneous intracranial bleed 75.6% and traumatic intracranial bleed 15.6%. Slightly more than half of the patients had intraventricular hemorrhage (55.6%). Decompressive craniectomy (DC) was performed on 17 (37.8%) of the patients. Only three patients were on steroids, and 12 (26.7%) patients had diabetes mellitus. Bacteremia with the same organism as that cultured in the CSF was seen in 10 (22.2%) of the patients. Out of the 45 patients, 32 (71.1%) of them still required the EVD even when ventriculitis was diagnosed. In our center, 34 (75.6%) of the patients suffered Gram-negative ventriculitis. Only three (6.7%) patients had endured recurrent infection. Discordant empirical antibiotics were given to 35 (77.8%) patients. The mortality rate from VRI for this study was 48.9%. The time from EVD insertion till confirmation of ventriculitis ranged from 2 to 21 days with a mean of 8.73 days. The mean GCS score before operation or endotracheal intubation was 7.93 with a range of 3–15 [Table 1].
Table 1.
Characteristics of patients with external ventricular drain ventriculitis
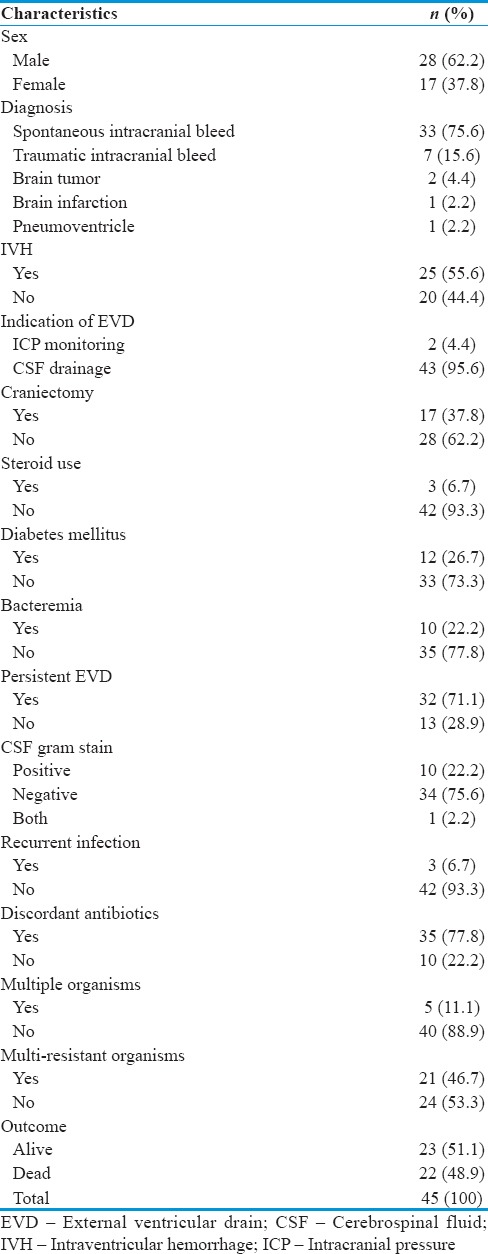
During the 4-year study period of 2012–2015, a total of 796 EVDs were inserted, but 77 of them had preexisting CNS infections. The overall rate over the 4 years was 6.3% [Table 2]. This is in keeping with the published rates of 0%–32.2%.[1,2,3] There is a slow rise in the rate of VRI in our center, and future studies need to be taken to identify the root causes before it escalates further.
Table 2.
Rate of external ventricular drain-related ventriculitis from 2012 to 2015
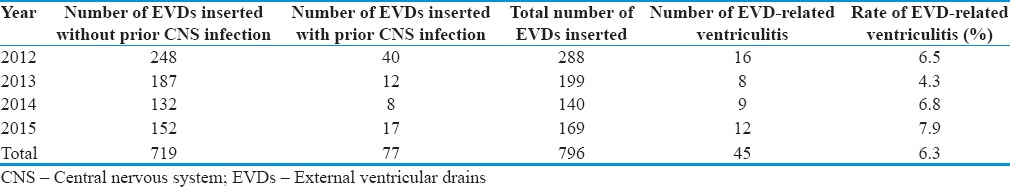
Three patients had recurrent infection, and so the total number of mono-organisms cultured was 48. A. baumannii and Pseudomonas aeruginosa together make up 50% of the causative organisms. Seven (14.6%) patients contracted coagulase negative Staphylococcus (CONS), and only 3 (6.2%) patients were infected with Staphylococcus aureus [Table 3]. Most of the Acinetobacter are resistant to carbapenems and only sensitive to polymyxin [Table 4]. Only 14.3% of Acinetobacter are sensitive to meropenem and imipenem. Ceftriaxone sensitivity is 0%, and resistance is 64.3%. As a whole, a higher proportion of A. baumannii is resistant with very few being sensitive to the common antibiotics. Out of the 14 patients with A. baumannii infection, 12 of them received discordant empiric antibiotics.
Table 3.
Mono-organism cerebrospinal fluid culture results for single episode and recurrent episodes of ventriculitis

Table 4.
Acinetobacter baumannii antibiotic susceptibility from 14 mono-organism cerebrospinal fluid cultures
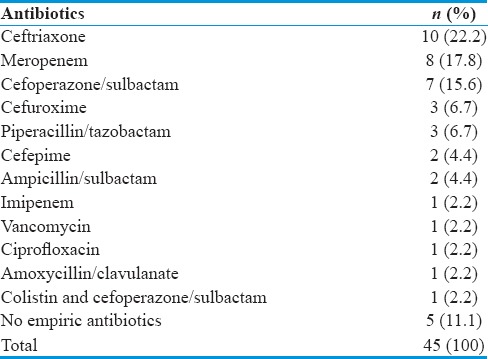
From our group of 45 patients, six of them died before specific antibiotics based on the organism susceptibility reports could be started [Table 5]. Five of the patients did not receive empiric antibiotics presumably due to a missed diagnosis initially, or the patient died before CSF analysis results could be acted on.
Table 5.
Empiric antibiotics
Discussion
Common nosocomial organisms causing infection after neurosurgical procedures include CONS, S. aureus, Enterobacteriaceae, P. aeruginosa, and A. baumannii.[10,11,12,13] Although Gram-positive pathogens were the main organisms isolated in some studies, there is increasing Gram-negative pathogens being responsible for ventriculitis.[1,2,3,8,10,14,15] This is consistent with our study, which showed higher incidence of Gram-negative infections. A. baumannii and P. aeruginosa constituted almost half of the total organisms associated with ventriculitis in our cohort.
The name, Acinetobacter, comes from the Latin word for “motionless” because they lack cilia or flagella with which to move. The respiratory system is the most common site for Acinetobacter infection because of its transient pharyngeal colonization of healthy persons and a high rate of tracheotomy colonization, which is a common occurrence in neurosurgical patients. Most of the A. baumannii isolated in our study was resistant to all cephalosporins and carbapenems, but only sensitive to polymyxin. This observation raises major concern as the occurrence of these multi-resistant Gram-negative bacteria results in a significant reduction of therapeutic options for the treatment of these infections. MRAB is an emerging problem in VRI due to its ability to tolerate desiccation and to accumulate diverse mechanisms of resistance. Polymyxin is frequently the only available therapeutic option for MRAB but has poor CNS penetration. A recent study showed that only the combination of parenteral with intrathecal or intraventricular administration of polymyxin has the potential to achieve therapeutic concentrations and eradicate MRAB from the CNS.[16]
The empirical antibiotics used in our cohort consisted predominantly ceftriaxone, meropenem, and cefoperazone/sulbactam, which covered most of the Gram-negative bacteria but not MRAB Table 5. However, we do not advocate the use of polymyxin as first-line empirical therapy for EVD-related ventriculitis. This is because the indiscriminate use of polymyxin may contribute to the selection of further resistance and may also expose patients to unnecessary renal toxicity. Furthermore, polymyxin has weak activity against P. aeruginosa and other common bacteria. Therefore, careful selection of patients who should receive polymyxin as empirical therapy covering MRAB is essential. Previous colonization with A. baumannii resistant to carbapenems is a variable independently associated with the development of an infection caused by MRAB.[17] New ventriculitis occurring during an outbreak or in a previously colonized patient, and unresolved infections despite treatment with broad-spectrum antibiotics are the most compelling reasons for MRAB empirical coverage with polymyxin. It is important to note that A. baumannii transmission in neurosurgical patients is mainly due to interactions between health-care workers, patients, and contaminated fomites in the ward environment, equipment, and EVD. Infection control interventions, cohort isolation, improved hand hygiene compliance, enhanced cleaning, and environmental disinfection have been successful at reducing nosocomial infection rates and controlling outbreaks due to A. baumannii.[18] Ventriculitis caused by Acinetobacter carries a mortality rate of 29.6%–52.9%.[5,6,8,19,20] In our cohort, it is shocking that out of 14 patients with Acinetobacter meningitis, 9 (64.3%) of them died.
We believe our findings will create awareness among other hospitals about the rising trend of MRAB ventriculitis, which was also highlighted in other reports.[5,6,8,19,20] The level of discordance in empiric antibiotics at our center is very high (77.8%), but was statistically insignificant in terms of mortality with our small sample size (P = 0.170, odds ratio = 2.77). By knowing our center's organisms, it is our hope that there will be less wastage of antibiotics, prolongation of hospital stay, or even morbidity due to discordant antibiotics. Since there is a high rate of nosocomial MRAB ventriculitis, we will have to consider giving our patients polymyxin when they are not responding to a course of empirical antibiotics. Based on our experience, we strongly encourage every neurosurgical center to conduct a similar study to identify common causative organisms in their own ventriculitis cohort.
Various regimes of surgical prophylactic antibiotics have been tested, including cefepime, cephalothin, ampicillin/sulbactam, aztreonam, ceftaroline, trimethoprim-sulfamethoxazole, and cefuroxime.[21,22,23,24,25] Cefuroxime is the main surgical prophylactic antibiotic used at our center. Although there is evidence that antibiotic prophylaxis may reduce VRI, the data available are still of suboptimal quality.[26] It is postulated that the use of cephalosporins, especially aminothiazolyl cephalosporins such as cefuroxime and ceftriaxone are associated with promoting resistance due to their broad spectrum cover.[27] In view of these, it may be reasonable to use single-dose cefazolin for patients undergoing clean neurosurgical procedures, CSF-shunting procedures, or intrathecal pump placement to prevent the increase in number of resistant organisms at our center as recommended by the American Society of Health-System Pharmacists report in 2013.[28] A dosage of 1 g of cefazolin just before skin incision translates to a CSF concentration above the minimal inhibitory concentration level for approximately 5 h.[29]
There are four main postulated mechanisms of VRI by Mounier et al.: (1) during insertion, (2) during disconnection or manipulation of the EVD system, (3) colonization of the drain at the insertion site, and (4) hematogenous seeding. The study suggests that VRI is chiefly caused by pathogens colonizing the drain insertion site and hence, the higher number of CONS VRI.[30] Looking at the trend of organisms at our center, it is highly likely that Gram-negative infections did not occur during the insertion of EVDs. Colonization of the drain or hematogenous seeding may be a possibility since 22.2% of our patients had the same organism both in blood cultures and CSF cultures, but it could also be hematogenous dissemination of the VRI instead.
Our analysis showed that the duration of the EVD being in place before the diagnosis of VRI was made is statistically significant in determining the outcome of patients with VRI [Table 6]. The results indicated that there is a significant association between the duration of EVD being in place before the diagnosis of VRI was made and the outcome of patients with VRI (P = 0.040). It has been shown that EVD colonization was strongly associated with the development of VRI.[31] It is possible that colonization or VRI precedes detectable signs and symptoms, and therefore, the longer the EVD is within the ventricles, the higher the bacteria burden is and hence more difficult to cure. On top of that, prolonged indwelling catheters may promote the selection of resistant strains. Although a persistent EVD state was not associated with a higher mortality rate in our study, it was significant in another study.[5] Yet, another paper recommends the removal of infected EVDs to improve cure rate.[32] All these information supports the early removal of EVDs if possible even if clinical VRI is not present yet. Not only does prolonged duration of EVD increase the risk of contracting VRI but it also increases the mortality rate if VRI does occur.[33]
Table 6.
Statistical analysis of univariate continuous variables in association with outcome
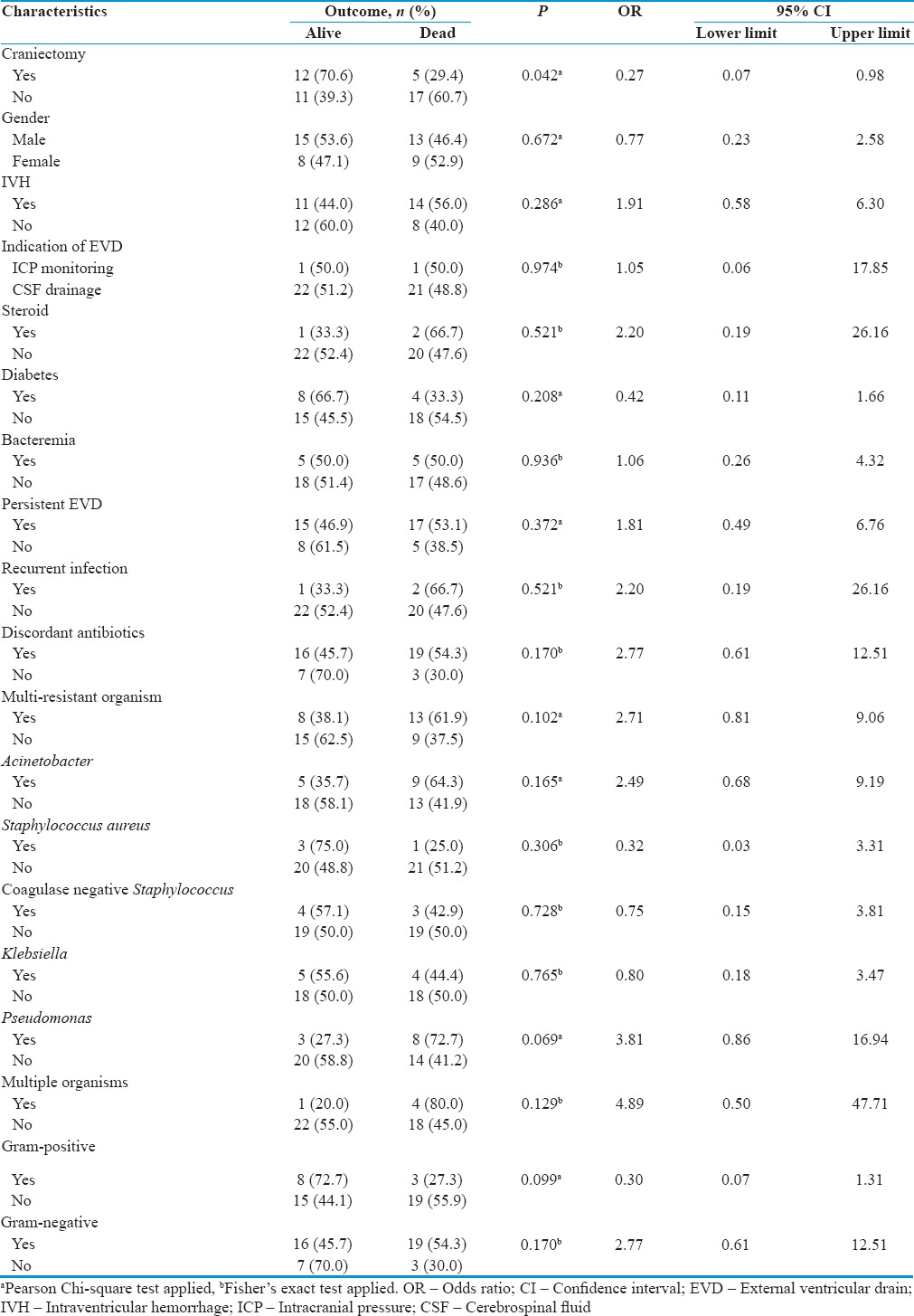
Those who underwent a DC in our cohort of patients had a lower proportion of mortality (P = 0.042) [Table 7]. The mortality rate among those that had a DC was 29.4% as compared to 60.7% in those that did not have a DC. Our findings support the anecdotal evidence that DC may be beneficial in patients with bacterial meningitis with high refractory ICP.[34,35] It must be clarified that all patients in this study had the DC done for other reasons such as intracranial bleeds and not for VRI and therefore the DC was done before VRI was diagnosed. Although just a postulation, brain edema causing raised, ICP may be a cause of reduced cerebral perfusion and thus reduced antibiotic delivery and impaired transport of immune cells and factors to combat the infection. This is further supported by the fact that ICP-targeted treatment has also been recommended for bacterial meningitis.[36] In neurosurgical patients with reduced GCS and ventriculitis, it would be ideal for these patients to have their ICP monitored so that DC may be carried out if other treatment measures fail to reduce the ICP.
Table 7.
Statistical analysis of univariate categorical variables in association with outcome
Multivariate logistic regression was performed, and we found that the use of steroid, P. aeruginosa infection, multiple organism infection, lower GCS, and a longer duration the EVD was in place before the diagnosis of VRI were related with higher mortality [Table 8]. There are various other factors that have been identified to be risk factors for poor outcome in ventricultis. In one study, mortality was strongly related to age, white cell counts, and removal of EVD.[32] Another study showed that shock, C-reactive protein ≥10 mg/dL, and persistent EVD state was associated with higher mortality rates.[5] Gram-negative infection, CSF glucose <30 mg/dL, CSF protein >200 mg/dL, concurrent nosocomial infection, and GCS score <10 were associated with higher mortality in another study.[6]
Table 8.
Multivariate logistic regression test (using enter method)
This study has its limitations. The sample size is small even with 4 years of data, and this is a retrospective study where there maybe information bias and missing data. The study was done in a single center, and generalization of its findings to other centers is limited. Performing a prospective study and expanding the scope to include other hospitals is a suitable aim.
Conclusion
The rate of EVD-related ventriculitis in our center remains relatively low but increasing nonetheless. Worrying susceptibility patterns, especially those of Acinetobacter VRI needs special attention before it is too late. EVDs should be removed as soon as feasible to avoid VRI and higher mortality rates. In patients with ventriculitis/meningitis, DC maybe considered as a life-saving measure.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
We would like to acknowledge and thank the Director General of Health Malaysia for allowing us to perform and publish this research. Our gratitude goes to the Clinical Research Center of Penang General Hospital for guiding us during this study.
References
- 1.Lwin S, Low SW, Choy DK, Yeo TT, Chou N. External ventricular drain infections: Successful implementation of strategies to reduce infection rate. Singapore Med J. 2012;53:255–9. [PubMed] [Google Scholar]
- 2.Schade RP, Schinkel J, Visser LG, Van Dijk JM, Voormolen JH, Kuijper EJ. Bacterial meningitis caused by the use of ventricular or lumbar cerebrospinal fluid catheters. J Neurosurg. 2005;102:229–34. doi: 10.3171/jns.2005.102.2.0229. [DOI] [PubMed] [Google Scholar]
- 3.Omar MA, Mohd Haspani MS. The risk factors of external ventricular drainage-related infection at hospital Kuala Lumpur: An observational study. Malays J Med Sci. 2010;17:48–54. [PMC free article] [PubMed] [Google Scholar]
- 4.Do BH, Kim SW, Oh JT, Son JW, Ha SW, Lee EK, et al. The external ventricular drain-related ventriculitis: Organisms and appropriateness of empiric antibiotic therapy. Infect Chemother. 2005;37:92–8. [Google Scholar]
- 5.Kim HI, Kim SW, Park GY, Kwon EG, Kim HH, Jeong JY, et al. The causes and treatment outcomes of 91 patients with adult nosocomial meningitis. Korean J Intern Med. 2012;27:171–9. doi: 10.3904/kjim.2012.27.2.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Erdem I, Hakan T, Ceran N, Metin F, Akcay SS, Kucukercan M, et al. Clinical features, laboratory data, management and the risk factors that affect the mortality in patients with postoperative meningitis. Neurol India. 2008;56:433–7. doi: 10.4103/0028-3886.44629. [DOI] [PubMed] [Google Scholar]
- 7.O'Neill E, Humphreys H, Phillips J, Smyth EG. Third-generation cephalosporin resistance among Gram-negative bacilli causing meningitis in neurosurgical patients: Significant challenges in ensuring effective antibiotic therapy. J Antimicrob Chemother. 2006;57:356–9. doi: 10.1093/jac/dki462. [DOI] [PubMed] [Google Scholar]
- 8.Yadegarynia D, Gachkar L, Fatemi A, Zali A, Nobari N, Asoodeh M, et al. Changing pattern of infectious agents in postneurosurgical meningitis. Caspian J Intern Med. 2014;5:170–5. [PMC free article] [PubMed] [Google Scholar]
- 9.Ramanan M, Lipman J, Shorr A, Shankar A. A meta-analysis of ventriculostomy-associated cerebrospinal fluid infections. BMC Infect Dis. 2015;15:3. doi: 10.1186/s12879-014-0712-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Logigan C, Mihalache D, Turcu T. Clinical study of 57 cases of nosocomial meningitis. J Prev Med. 2008;16:59–68. [Google Scholar]
- 11.Streharova A, Benca J, Holeckova K, Balik J, Sula I, Lesnakova A, et al. Comparison of postsurgical and community acquired bacterial meningitis – Analysis of 372 cases within a nationwide survey. Neuro Endocrinol Lett. 2007;28(Suppl 3):7–9. [PubMed] [Google Scholar]
- 12.Weisfelt M, van de Beek D, Spanjaard L, de Gans J. Nosocomial bacterial meningitis in adults: A prospective series of 50 cases. J Hosp Infect. 2007;66:71–8. doi: 10.1016/j.jhin.2007.02.017. [DOI] [PubMed] [Google Scholar]
- 13.Wang KW, Chang WN, Huang CR, Tsai NW, Tsui HW, Wang HC, et al. Post-neurosurgical nosocomial bacterial meningitis in adults: Microbiology, clinical features, and outcomes. J Clin Neurosci. 2005;12:647–50. doi: 10.1016/j.jocn.2004.09.017. [DOI] [PubMed] [Google Scholar]
- 14.van de Beek D, Drake JM, Tunkel AR. Nosocomial bacterial meningitis. N Engl J Med. 2010;362:146–54. doi: 10.1056/NEJMra0804573. [DOI] [PubMed] [Google Scholar]
- 15.McClelland S, 3rd, Hall WA. Postoperative central nervous system infection: Incidence and associated factors in 2111 neurosurgical procedures. Clin Infect Dis. 2007;45:55–9. doi: 10.1086/518580. [DOI] [PubMed] [Google Scholar]
- 16.Ziaka M, Markantonis SL, Fousteri M, Zygoulis P, Panidis D, Karvouniaris M, et al. Combined intravenous and intraventricular administration of colistin methanesulfonate in critically ill patients with central nervous system infection. Antimicrob Agents Chemother. 2013;57:1938–40. doi: 10.1128/AAC.01461-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Corbella X, Montero A, Pujol M, Domínguez MA, Ayats J, Argerich MJ, et al. Emergence and rapid spread of carbapenem resistance during a large and sustained hospital outbreak of multiresistant Acinetobacter baumannii. J Clin Microbiol. 2000;38:4086–95. doi: 10.1128/jcm.38.11.4086-4095.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fierobe L, Lucet JC, Decré D, Muller-Serieys C, Deleuze A, Joly-Guillou ML, et al. An outbreak of imipenem-resistant Acinetobacter baumannii in critically ill surgical patients. Infect Control Hosp Epidemiol. 2001;22:35–40. doi: 10.1086/501822. [DOI] [PubMed] [Google Scholar]
- 19.Rodríguez Guardado A, Blanco A, Asensi V, Pérez F, Rial JC, Pintado V, et al. Multidrug-resistant Acinetobacter meningitis in neurosurgical patients with intraventricular catheters: Assessment of different treatments. J Antimicrob Chemother. 2008;61:908–13. doi: 10.1093/jac/dkn018. [DOI] [PubMed] [Google Scholar]
- 20.Khan FY, Abukhattab M, Baager K. Nosocomial postneurosurgical Acinetobacter baumannii meningitis: A retrospective study of six cases admitted to Hamad General Hospital, Qatar. J Hosp Infect. 2012;80:176–9. doi: 10.1016/j.jhin.2011.08.021. [DOI] [PubMed] [Google Scholar]
- 21.Stein GE, Yasin F, Smith C, Scharmen A, Havlichek D, Bill C. A pharmacokinetic/pharmacodynamic analysis of ceftaroline prophylaxis in patients with external ventricular drains. Surg Infect (Larchmt) 2015;16:169–73. doi: 10.1089/sur.2014.098. [DOI] [PubMed] [Google Scholar]
- 22.Wong GK, Poon WS, Lyon D, Wai S. Cefepime vs. ampicillin/sulbactam and aztreonam as antibiotic prophylaxis in neurosurgical patients with external ventricular drain: Result of a prospective randomized controlled clinical trial. J Clin Pharm Ther. 2006;31:231–5. doi: 10.1111/j.1365-2710.2006.00729.x. [DOI] [PubMed] [Google Scholar]
- 23.Blomstedt GC. Results of trimethoprim-sulfamethoxazole prophylaxis in ventriculostomy and shunting procedures. A double-blind randomized trial. J Neurosurg. 1985;62:694–7. doi: 10.3171/jns.1985.62.5.0694. [DOI] [PubMed] [Google Scholar]
- 24.Lucey MA, Myburgh JA. Antibiotic prophylaxis for external ventricular drains in neurosurgical patients: An audit of compliance with a clinical management protocol. Crit Care Resusc. 2003;5:182–5. [PubMed] [Google Scholar]
- 25.Alleyne CH, Jr, Hassan M, Zabramski JM. The efficacy and cost of prophylactic and perioprocedural antibiotics in patients with external ventricular drains. Neurosurgery. 2000;47:1124–7. doi: 10.1097/00006123-200011000-00020. [DOI] [PubMed] [Google Scholar]
- 26.Sonabend AM, Korenfeld Y, Crisman C, Badjatia N, Mayer SA, Connolly ES., Jr Prevention of ventriculostomy-related infections with prophylactic antibiotics and antibiotic-coated external ventricular drains: A systematic review. Neurosurgery. 2011;68:996–1005. doi: 10.1227/NEU.0b013e3182096d84. [DOI] [PubMed] [Google Scholar]
- 27.Dancer SJ. The problem with cephalosporins. J Antimicrob Chemother. 2001;48:463–78. doi: 10.1093/jac/48.4.463. [DOI] [PubMed] [Google Scholar]
- 28.Bratzler DW, Dellinger EP, Olsen KM, Perl TM, Auwaerter PG, Bolon MK, et al. Clinical practice guidelines for antimicrobial prophylaxis in surgery. Surg Infect (Larchmt) 2013;14:73–156. doi: 10.1089/sur.2013.9999. [DOI] [PubMed] [Google Scholar]
- 29.Klekner A, Ga'spa'r A, Kardos S, Szabó J, Cse'csei G. Cefazolin prophylaxis in neurosurgery monitored by capillary electrophoresis. J Neurosurg Anesthesiol. 2003;15:249–54. doi: 10.1097/00008506-200307000-00013. [DOI] [PubMed] [Google Scholar]
- 30.Mounier R, Lobo D, Cook F, Martin M, Attias A, Aït-Mamar B, et al. From the skin to the brain: Pathophysiology of colonization and infection of external ventricular drain, a prospective observational study. PLoS One. 2015;10:e0142320. doi: 10.1371/journal.pone.0142320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hetem DJ, Woerdeman PA, Bonten MJ, Ekkelenkamp MB. Relationship between bacterial colonization of external cerebrospinal fluid drains and secondary meningitis: A retrospective analysis of an 8-year period. J Neurosurg. 2010;113:1309–13. doi: 10.3171/2010.6.JNS10258. [DOI] [PubMed] [Google Scholar]
- 32.Kim BN, Peleg AY, Lodise TP, Lipman J, Li J, Nation R, et al. Management of meningitis due to antibiotic-resistant Acinetobacter species. Lancet Infect Dis. 2009;9:245–55. doi: 10.1016/S1473-3099(09)70055-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Holloway KL, Barnes T, Choi S, Bullock R, Marshall LF, Eisenberg HM, et al. Ventriculostomy infections: The effect of monitoring duration and catheter exchange in 584 patients. J Neurosurg. 1996;85:419–24. doi: 10.3171/jns.1996.85.3.0419. [DOI] [PubMed] [Google Scholar]
- 34.Hoehne J, Friedrich M, Brawanski A, Melter M, Schebesch KM. Decompressive craniectomy and early cranioplasty in a 15-year-old boy with N. meningitidis meningitis. Surg Neurol Int. 2015;6:58. doi: 10.4103/2152-7806.154776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Di Rienzo A, Iacoangeli M, Rychlicki F, Veccia S, Scerrati M. Decompressive craniectomy for medically refractory intracranial hypertension due to meningoencephalitis: Report of three patients. Acta Neurochir (Wien) 2008;150:1057–65. doi: 10.1007/s00701-008-0019-1. [DOI] [PubMed] [Google Scholar]
- 36.Glimåker M, Johansson B, Halldorsdottir H, Wanecek M, Elmi-Terander A, Ghatan PH, et al. Neuro-intensive treatment targeting intracranial hypertension improves outcome in severe bacterial meningitis: An intervention-control study. PLoS One. 2014;9:e91976. doi: 10.1371/journal.pone.0091976. [DOI] [PMC free article] [PubMed] [Google Scholar]


