Abstract
Introduction:
Intracranial meningiomas account for 30% of all primary intracranial tumors. Surgical resection remains the mainstay of the treatment for meningiomas. The magnetic resonance of intracranial meningiomas has been largely discussed in many reports of the radiological and neurosurgical literature. To date, a few studies have been attempted to differentiate the tumor characteristics of meningiomas based on magnetic resonance imaging (MRI) studies.
Objective:
The objective of the study is to evaluate the relationship between MRI signal characteristics of intracranial meningiomas and consistency of tumor using objective measures.
Materials and Methods:
A prospective study included all the patients who were admitted for surgery with an MRI finding suggestive of meningioma. All patients were subjected to routine radiological investigations. Surgical resection was performed for patients eligible for surgery using cavitron ultrasonic aspirator (CUSA). The relationship and correlation between the radiological, intraoperative measurements and the histopathological diagnosis were studied. The tumor consistency was measured using mean CUSA level. Intensity on T2, fluid-attenuated inversion recovery (FLAIR), and diffusion-weighted imaging (DWI) was measured using circular regions of interest (ROI) on the MRI. Multiple ROIs were placed initially on the lesions avoiding the obvious blood vessels, if any, then on the brain cortex to avoid the vasogenic edema. The mean ROI (mROI) results from the lesion were subtracted from the mean ROI from the brain cortex for each lesion to achieve normalized ratio. The results of lesion mROI-cortex mROI were compared to the operative and histopathology results using Pearson's correlation test and linear regression test.
Results:
The total number of patients was seventy. The mean age of the patients was 51 ± 14.8, with 72% of them being females and 28% males. There was a strong statistically significant (P = 0.046) and (P = 0.003) correlation between mean CUSA and FLAIR mROI difference or T2 mROI difference, respectively. On the other hand, there was an inversely proportional relationship between mean CUSA and FLAIR mROI difference and mean CUSA and T2 mROI difference. The value of the regression test (r) shows that there was a slight linear relationship between FLAIR mROI difference or T2 mROI difference and mean CUSA values, in which the mean CUSA value = 50.1 + (−0.088) × FLAIR mROI difference (r = −0.273, P = 0.046) or mean CUSA value = 50.8 + (−0.055) × T2 mROI difference (r = 0.4, P = 0.003). There was no statistical significance in the relation between CUSA values and tumor histological subtypes, DWI values, age, or gender.
Conclusion:
This study presents a new objective method to measure the consistency of intracranial meningiomas based on a simple algorithmic formula. Such information will aid in planning surgery and assessing the resectability of the tumor. To date, this is the first objective measurement of meningioma consistency based on MRI studies and objective intraoperative evaluation.
Keywords: Brain imaging, magnetic resonance imaging, meningioma, neurostimulation, neurosurgery
Introduction
Intracranial meningiomas account for 20–30% of all primary intracranial tumors. They originate from the arachnoid cap cells and occur in middle-aged adults.[1,2] Women are affected twice as often as men.[3,4] Meningiomas are mostly well-differentiated, benign, and encapsulated lesions that indent the brain as they enlarge. Although most meningiomas are benign, they have a surprisingly broad spectrum of clinical characteristics, and histologically distinct subsets are associated with a high risk of recurrence, even after seemingly complete resection. In rare instances, meningiomas are malignant. Most meningiomas are isointense to the brain on T1- and T2-weighted images.[5,6,7,8] A heterogeneous internal texture is found in all but the smallest meningiomas. The mottled pattern is likely due to a combination of flow void from vascularity, focal calcification, small cystic foci, and entrapped cerebrospinal fluid spaces. Hemorrhage is not a common feature.[5,9] Surgical resection remains the mainstay of the treatment for meningiomas; it is usually achieved after devascularizing the tumor from around the capsule then debunking it from inside using various tools including cavitron ultrasound aspirator (CUSA) which is an instrument that combines suction and irrigation, with ultrasound, includes the cavitron ultrasonic surgical aspirator. Difficulties encountered in the surgery for meningiomas are usually due to several factors, such as tumor location, size, vascularity, invasion or encasement of cranial nerves, and important blood vessels. Tumor consistency, although not frequently discussed, seems also to be of importance in determining the respectability and hence, surgical outcome. If the surgeon is aware of the consistency of the tumor preoperatively, the surgery can be planned better. Moreover, the surgical time and the likelihood of adjuvant therapy can be estimated. Different studies have addressed the correlation of the consistency of meningiomas and the preoperative magnetic resonance imaging (MRI) findings and histopathology results.[5,10,11,12,13,14,15,16,17] Controversial results have been obtained while some concluded that MRI could not give definitive answers to the questions of consistency and histological parameters.[11] Others found a correlation between hyperintensity of meningioma in T2-weighted imaging and tissue softness, as well as increased vascularity being found at 1.5T.[13,14,15,17] Hyperintensity in proton attenuation images has also been found to correlate with soft tumors. Meningioma subtypes may affect intensity on T2-weighted images.[12] However, in all studies, statistically, significant correlations have not been shown. It has been found that intensity heterogeneity on T2-weighted imaging correlated with hard tumors at 3T field strength. According to these previous studies, neither signal intensity of meningiomas on unenhanced T1-weighted images correlates with tumor consistency, vascularity, or histologic findings nor the degree of contrast enhancement correlates with these factors.[10,12]
Our purpose in the present study is:
To find out the correlation between surgical tumor consistency, MRI signal intensity, and histopathological fibrous tissue contents in an objective way
To establish operative procedures in reference to the meningioma consistency.
Materials and Methods
All procedures and methods were approved by the King Fahad Medical City Ethics Committee.
Radiology
All patients were subjected to routine radiological investigations including, but not limited to, computed tomography (CT) and MRI.
Magnetic resonance imaging
All patients were studied using a 1.5T. Body MRI equipped with high-performance gradients and a manufacturer-supplied quadrature head coil. The standard imaging protocol consisted of the following sequences: Sagittal T1-weighted (300/14), axial T1-weighted (500/12), fast spin-echo T2-weighted (3000/84), and axial fluid-attenuated inversion recovery (FLAIR) (10,002/162). After the administration of 0.1 mmol/Kg gadopentetate dimeglumine, contrast-enhanced T1-weighted axial (450–500/12) and T1-weighted coronal (600/20) images were obtained. In all sequences, the field of view was 22 or 24 cm and the section thickness was 5 mm, with an interslice gap of 2.5 mm. Before the contrast-enhanced imaging was performed, routine diffusion-weighted MRI was obtained using single-shot multislice spin-echo-planar imaging for all patients. In the diffusion protocol, we used 10,000/99 with b values of 0 and 1000. For all images, a 128 × 128 matrix size 5 mm section thickness, with no gap and field of view of 24 cm × 24 cm, was used. In most studies, thirty images were obtained in an acquisition time of 425 s. The pulse sequence first obtained was T2-weighted images, without a diffusion-sensitizing gradient. Subsequently, a diffusion sensitizing gradient in the direction of the frequency-encoding, phase-encoding, and readout gradients was used to generate three orthogonal diffusion-weighted echo-planar images. The images were transferred to a workstation, and the intensity on T2, FLAIR, and diffusion-weighted imaging (DWI) was measured using circular regions of interest (ROI) on MRI images. Multiple ROIs (three circles) were placed initially on the lesion, avoiding the obvious blood vessels, if any, then on the brain cortex to avoid the vasogenic edema that may be associated with the lesion [Figure 1]. Mean ROI (mROI) results from the lesion were subtracted from the mean ROI from the brain cortex for each lesion. The results of lesion mROI-cortex mROI were compared to the operative and histopathology results.
Figure 1.
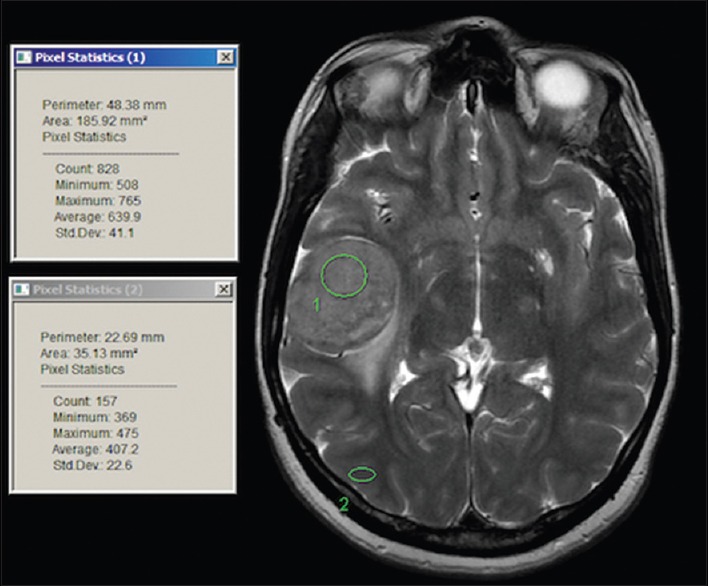
Sample of regions of interest selection on the workstation
Operative procedure
Inclusion criteria: Patients who had a radiological first diagnosis of meningioma where the tumor was deemed partially or completely resectable were included. The patient should have accepted that surgery was the modality of treatment. The patient's general condition should allow a surgical procedure under general or local anesthesia, and the lesion should not have been surgically resected before (not recurrent), but partially resected lesions were included. Patients who met the inclusion criteria were enrolled in this prospective study. Type of anesthesia and surgical approach was dependent on the location and the size of the tumor. As soon as the tumor was exposed, after the initial confirmation of the diagnosis by frozen-section biopsy, three different specimens, measuring at least 3 mm in diameter, were taken from different parts. These pieces were immediately sent for histopathology, labeled as meningioma study specimens. The cavitron ultrasonic surgical aspirator (CUSA) was used in resecting the tumor. The ultrasonic aspirator (CUSA) was applied at 10% amplitude and 10% suction. The CUSA was closely monitored. The amplitude and suction levels and their duration were recorded throughout the procedure.
The CUSA specimen was sent for histopathology as a specimen. All specimens sent to pathology were labeled as “meningioma consistency study;” the remaining were sent for permanent pathology.
Neurosurgeons were always reminded to use the minimum resecting level of the CUSA and the mean value of the amplitude used in the surgery measured. At the end of the procedure, the surgeon's general feeling about the consistency of the tumor was documented (including the vascularity of the lesion and the presence or absence of calcifications, hemorrhage, or necrosis). Cystic components were also described.
Histopathology
Four samples were sent to the neuropathology laboratory: Three fresh specimens and the fourth specimen being the CUSA sample. The fresh samples were processed according to the routine method after taking one section from each centimeter of the maximum dimension of the specimen. Half of them were chosen from the most fibrous looking area. Then, the slides of these sections were stained with hematoxylin and eosin, and the most fibrous sections were stained with Masson trichrome for estimation of the percentage of fibrous tissue. The CUSA sample was centrifuged and a cell block was prepared for the evaluation of the fibrous content. The histopathology diagnosis of both specimens included type of meningioma, grade, size, margins, and percentage of fibrous tissue in the tumor. The percentage of fibrous tissue was estimated per high field power per area in each slide, and the means of the percentages were evaluated. The removed tumor tissue sections were reviewed by a neuropathologist who was blind to the MRI features.
Statistical considerations
The collected data were analyzed using SPSS software version 17.0 (IBM Corporation, USA) computer software. Analysis of variance and correlation/regression tests were performed. The statistical significance was considered at P < 0.05.
Results
A total of seventy patients were included in the study, of whom 16 patients were excluded either for a different diagnosis (five patients) or incomplete data (11 patients). The mean age of the patients was 51 ± 14.8, with 72% of them being females and 28% males. Most of the lesions were WHO Grade I (73%) while 17.8% were Grade II and only 9% were Grade III. Two patients had a necrotic component in their lesions, nine patients (16%) had a hemorrhagic component in their lesion, and only six patients (10%) demonstrated a calcification component in their lesions [Table 1].
Table 1.
A percentages of different variables that was obtained in the study

There was a statistically significant (P = 0.046) and (P = 0.003) correlation between mean CUSA and FLAIR mROI difference or T2 mROI difference, respectively. On the other hand, there was an inversely proportional relationship between mean CUSA and FLAIR difference and mean CUSA and T2 mROI difference. The value of the regression test (r) showed that there was a slight linear relationship between FLAIR mROI difference or T2 mROI difference and mean CUSA values, in which the mean CUSA value = 50.1 + (−0.088 × FLAIR difference) (r = −0.273, P = 0.046) [Figure 2] and mean CUSA value = 50.8 + (−0.055 × T2 difference) (r = 0.4, P = 0.003) [Figure 3]. There was no statistical significance in the relation between CUSA values in one arm and tumor histological subtypes, grade, DWI values, age, presence of calcification, necrosis, or hemorrhage.
Figure 2.
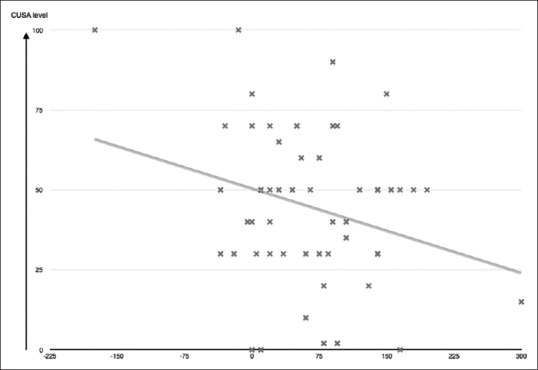
Regression test (r) showed that there is a slight linear relationship between fluid-attenuated inversion recovery mean regions of interest difference mean CUSA values. In which mean CUSA value = 50.1 + (−0.088 × fluid-attenuated inversion recovery mean regions of interest difference) (r = −0.273, P = 0.046)
Figure 3.
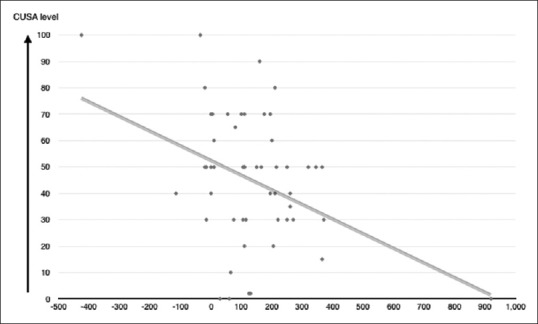
Regression test (r) showed that there is a slight linear relationship between T2 mean regions of interest difference and mean CUSA values. In which mean CUSA value = 50.8 + (−0.055 × T2 mean regions of interest difference) (r = 0.4, P = 0.003)
Discussion
Imaging is an essential tool for neurosurgeons to determine and plan the approach, timing, anticipated risk, and resectability of brain lesions, including meningiomas. Over the years, MRIs have been a helpful tool to achieve these goals, yet there was no objective way to determine the consistency of meningioma, mainly because MRI depends on the hydrogen ions as a tool to reflect the image on the screen. Hydrogen ions are different in number from one individual to another, and also, it is different based on the hydration status of the same individual. As early as 1992, neurosurgeons and radiologists studied the MRI characteristics to determine the meningioma's consistency and its correlation with the histopathological subtypes.[3,5,10,11,12,13,14,15,16,17] Most of the studies showed a significant correlation between T2-weighted images and meningioma consistency, in which hyperintense lesions tended to be soft and hypointense lesions tended to be hard.[5,11,12,13,14,15,16,18] However, the histological subtype was only demonstrated in a study by Bano et al. to determine if DWI could predict that whether the meningioma was benign or atypical, and they found that using DWI at a high b-value could differentiate between benign and malignant meningiomas.[16]
The primary aim of this study was to establish an objective method to predict meningioma consistency based on MRI studies. Our findings were consistent with the previous studies in which hyperintense meningiomas on T2-weighted images were correlated with soft consistency in both the surgeon's opinion and the CUSA mean level; moreover, we were able to develop an algorithmic formula that can moderately predict the tumor's consistency based on T2- weighted images and determine what would be the mean CUSA level on the planned surgery. By this method, we can differentiate between the different hyperintensities of the meningiomas; the regression test showed a weaker correlation between the intensity and FLAIR value, but it was statistically significant. There was no statistical significance in the relationship between DWI value and consistency as measured by CUSA or histological subtypes, which was expected since Bano et al. had a similar finding when using the b-value of 1000. We could not find a statistical significance using our method in measuring the intensity of the lesions with the histological subtypes or the WHO grade, which could be due to the small sample size in the subgroups and the dominancy of WHO Grade I over the other subtypes. Additional studies measuring the tumor elasticity or stiffness with more accurate tools postresection and comparing them with the neurosurgeon's feedback, mean CUSA level, and MRI findings would be of great benefit in determining the accuracy of our method.
Conclusion
This study presents a new objective method to measure the consistency of intracranial meningioma based on a simple algorithmic formula; such information would aid in planning surgery and assessing the resectability of the tumor. To date, this is the first objective measurement of meningioma consistency based on MRI studies and intraoperative objective evaluation.
Financial support and sponsorship
King Fahad Medical City (KFMC) provided financial support in the form of research funding. The sponsor had no role in the design or conduct of this research.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
We thank Mr. Muhammad Salman Bashir for his assistance in the statistical analysis.
References
- 1.Claus EB, Bondy ML, Schildkraut JM, Wiemels JL, Wrensch M, Black PM. Epidemiology of intracranial meningioma. Neurosurgery. 2005;57:1088–95. doi: 10.1227/01.neu.0000188281.91351.b9. [DOI] [PubMed] [Google Scholar]
- 2.Dolecek TA, Propp JM, Stroup NE, Kruchko C. CBTRUS statistical report: Primary brain and central nervous system tumors diagnosed in the United States in 2005-2009. Neuro Oncol. 2012;14(Suppl 5):v1–49. doi: 10.1093/neuonc/nos218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jääskeläinen J, Haltia M, Servo A. Atypical and anaplastic meningiomas: Radiology, surgery, radiotherapy, and outcome. Surg Neurol. 1986;25:233–42. doi: 10.1016/0090-3019(86)90233-8. [DOI] [PubMed] [Google Scholar]
- 4.Central Brain Tumor Registry of the United States. Statistical report: Primary brain tumors in the United States, 1998-2002. Hinsdale, Illinois: Central Brain Tumor Registry of the United States; 2005. [Google Scholar]
- 5.Sheporaitis LA, Osborn AG, Smirniotopoulos JG, Clunie DA, Howieson J, D'Agostino AN. Intracranial meningioma. AJNR Am J Neuroradiol. 1992;13:29–37. [PMC free article] [PubMed] [Google Scholar]
- 6.Julià -Sapé M, Acosta D, Majós C, Moreno-Torres A, Wesseling P, Acebes JJ, et al. Comparison between neuroimaging classifications and histopathological diagnoses using an international multicenter brain tumor magnetic resonance imaging database. J Neurosurg. 2006;105:6–14. doi: 10.3171/jns.2006.105.1.6. [DOI] [PubMed] [Google Scholar]
- 7.Kasuya H, Kubo O, Tanaka M, Amano K, Kato K, Hori T. Clinical and radiological features related to the growth potential of meningioma. Neurosurg Rev. 2006;29:293–6. doi: 10.1007/s10143-006-0039-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yrjänä SK, Tuominen H, Karttunen A, Lähdesluoma N, Heikkinen E, Koivukangas J. Low-field MR imaging of meningiomas including dynamic contrast enhancement study: Evaluation of surgical and histopathologic characteristics. AJNR Am J Neuroradiol. 2006;27:2128–34. [PMC free article] [PubMed] [Google Scholar]
- 9.Tropine A, Dellani PD, Glaser M, Bohl J, Plöner T, Vucurevic G, et al. Differentiation of fibroblastic meningiomas from other benign subtypes using diffusion tensor imaging. J Magn Reson Imaging. 2007;25:703–8. doi: 10.1002/jmri.20887. [DOI] [PubMed] [Google Scholar]
- 10.Chen TC, Zee CS, Miller CA, Weiss MH, Tang G, Chin L, et al. Magnetic resonance imaging and pathological correlates of meningiomas. Neurosurgery. 1992;31:1015–21. doi: 10.1227/00006123-199212000-00005. [DOI] [PubMed] [Google Scholar]
- 11.Carpeggiani P, Crisi G, Trevisan C. MRI of intracranial meningiomas: Correlations with histology and physical consistency. Neuroradiology. 1993;35:532–6. doi: 10.1007/BF00588715. [DOI] [PubMed] [Google Scholar]
- 12.Yamaguchi N, Kawase T, Sagoh M, Ohira T, Shiga H, Toya S. Prediction of consistency of meningiomas with preoperative magnetic resonance imaging. Surg Neurol. 1997;48:579–83. doi: 10.1016/s0090-3019(96)00439-9. [DOI] [PubMed] [Google Scholar]
- 13.Maiuri F, Iaconetta G, de Divitiis O, Cirillo S, Di Salle F, De Caro ML. Intracranial meningiomas: Correlations between MR imaging and histology. Eur J Radiol. 1999;31:69–75. doi: 10.1016/s0720-048x(98)00083-7. [DOI] [PubMed] [Google Scholar]
- 14.Hoover JM, Morris JM, Meyer FB. Use of preoperative magnetic resonance imaging T1 and T2 sequences to determine intraoperative meningioma consistency. Surg Neurol Int. 2011;2:142. doi: 10.4103/2152-7806.85983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sitthinamsuwan B, Khampalikit I, Nunta-aree S, Srirabheebhat P, Witthiwej T, Nitising A. Predictors of meningioma consistency: A study in 243 consecutive cases. Acta Neurochir (Wien) 2012;154:1383–9. doi: 10.1007/s00701-012-1427-9. [DOI] [PubMed] [Google Scholar]
- 16.Bano S, Waraich MM, Khan MA, Buzdar SA, Manzur S. Diagnostic value of apparent diffusion coefficient for the accurate assessment and differentiation of intracranial meningiomas. Acta Radiol Short Rep. 2013;2:2047981613512484. doi: 10.1177/2047981613512484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Romani R, Tang WJ, Mao Y, Wang DJ, Tang HL, Zhu FP, et al. Diffusion tensor magnetic resonance imaging for predicting the consistency of intracranial meningiomas. Acta Neurochir (Wien) 2014;156:1837–45. doi: 10.1007/s00701-014-2149-y. [DOI] [PubMed] [Google Scholar]
- 18.Engelhard HH. Progress in the diagnosis and treatment of patients with meningiomas. Part I: Diagnostic imaging, preoperative embolization. Surg Neurol. 2001;55:89–101. doi: 10.1016/s0090-3019(01)00349-4. [DOI] [PubMed] [Google Scholar]


