Abstract
Introduction:
Solitary fibrous tumor (SFT) is rarely diagnosed in clinical practice. Since its initial descriptions in the central nervous system (CNS) and the orbits, very few case reports and small case series have expanded their clinical and pathological characterization. We sought to describe a cases series of SFT from a single laboratory of neuropathology belonging to a tertiary university hospital.
Methods:
Retrospective clinical and histopathological description of eight cases of CNS and orbital SFT diagnosed over a 21-year period of time.
Results:
Median age was 47.3 years and four were males. Clinical presentation was related to local mass effect in all. Tumors occurred in the orbits (5/62.5%), intracranial dura attached (2), and the spinal medulla (1). The neuropathology showed the presence of hemangiopericytoma type (2), classic type (3), and mixed type (3). Histological anaplasia was present in two cases. Widespread/total immunoreactivity for vimentin, CD34, and Bcl-2 was present in all. Gross total removal was conducted in the majority (6/75%) and subtotal removal in 2 (25%). Three patients were submitted to adjuvant treatment (radiosurgery and radiotherapy). Recurrence occurred in four patients, 13–120 months after surgical intervention. Anaplasia was present in one case of recurrence.
Conclusion:
Our case series confirms the clinical and neuropathological diversity of CNS and orbital SFTs. Studies with longer follow-up periods are necessary to better understand the clinical behavior and prognosis of the SFT in the CNS and orbits.
Keywords: Central nervous system, neuropathology, orbit, solitary fibrous tumor
Introduction
Solitary fibrous tumor (SFT) is an uncommon tumor of mesenchymal origin initially described in the pleura under the term “localized fibrous mesothelioma.”[1] Its first description in the orbit and the central nervous system (CNS) was in 1994[2] and 1996,[3] respectively. The incidence of CNS SFTs is unknown, but it may represent 0.09% of all “dural-adherent” tumors.[4] One recent review reported 80 cases of orbital SFTs in the literature.[5] The 2007 World Health Organization (WHO) classification of tumors of the CNS includes SFTs among the mesenchymal nonmeningothelial tumors.[6] Meningeal involvement occurs in the majority of CNS SFTs demanding differential diagnosis with other dura-based lesions, most frequently the meningiomas.[7,8] Furthermore, intracranial extension of orbital SFT can occur,[9] and misdiagnosis of both CNS and orbital SFT by other tumors, such as meningiomas and angiofibromas is not rare.[10,11,12,13,14]
Contrary to peripheral soft tissue, where SFT and hemangiopericytoma (HPC) have a common clinicopathological profile and are included in the spectrum of the same tumor,[15] in the CNS and orbits, SFT and HPC have been described as distinct entities, often showing disparate postsurgery outcomes.[5,16]
Clinicopathological characterization of CNS[3,15,17] and orbital SFTs[5,18] has been guided by retrospective case reports, institutional case series, and systematic reviews.[4,7,8,19,20] We sought to describe a consecutive case series of CNS and orbital SFTs from a single neuropathology laboratory of a tertiary university hospital addressing the controversy issue of the origin of SFTs and HPC with these particular locations.
Methods
We perform a retrospective review of all cases of SFTs diagnosed at the laboratory of neuropathology of our tertiary hospital in the last 21 years. All patients submitted to surgery and followed at the neurosurgery department were included in this study.
Demographic data, clinical presentation, imagiological features, extension of surgical resection, histopathological features, adjuvant treatment, and outcome were reviewed. For the neuropathological study, representative formalin-fixed, paraffin-embedded tissues were stained with hematoxylin and eosin and immunohistochemical techniques, with the following primary antibodies: vimentin (monoclonal, 1/100, Leica Biosystems, UK), epithelial membrane antigen (EMA, monoclonal, 1/100, Dako, Denmark), S-100 (polyclonal, 1/200, Leica Biosystems, UK), CD34 (monoclonal, 1/100, Leica Biosystems, UK), smooth muscle actin (monoclonal, 1/100, Leica Biosystems, UK), Bcl-2 (monoclonal, 1/100, Leica Biosystems, UK), and Ki-67 (clone MM1, monoclonal, 1/100, Leica Biosystems, UK). As a secondary antibody, polymer Dako Envision (Dako, Denmark) was used. The immunostaining was graded as follows: −/+ in the absence or in the presence of few reactive elements, + for focal staining, ++ for broad staining but not widespread, and +++ for widespread or total staining.
Results
Among the 1716 tumors with meningeal implantation and the 90 orbital tumors diagnosed during the period of time considered, 3 (0.2%) and 5 (5.6%), respectively, were SFTs. Among the three meningeal SFTs, two were intracranial and one was spinal. The mean age at diagnosis for all cases was 47.3 years (range 19–68 years). Table 1 summarizes the clinical, treatment, and outcome data of all patients. Symptoms and signs at presentation were related to tumor location. On magnetic resonance we verify contrast enhancement, hypointense or isointense on T1-weighted, and hyperintense or hypointense/hyperintense on T2-weighted imaging in all cases [Figure 1]. Gross total resection was performed in all orbital (Cases 1, 2, 3, 6, and 7) and in one CNS (Case 8) SFT; in other two CNS SFT (Cases 4 and 5), a subtotal resection was made. Three patients were submitted to adjuvant treatment: stereotactic radiosurgery in two CNS (Cases 4 and 5) and radiotherapy in one orbital SFT (Case 2). Median follow-up time after surgery was 92.6 months (range 21–252 months). Recurrences occurred after 60 and 13 months in orbital SFTs (Case 1 and 2, respectively) and after 120 and 36 months in two CNS SFT (Case 4 and 5, respectively). In two patients (Case 1 and 4), a previous misdiagnosis of malignant fibrous histiocytoma and HPC was performed.
Table 1.
Demographic and clinical data of seven patients with solitary fibrous tumors of the orbits and the central nervous system
Figure 1.
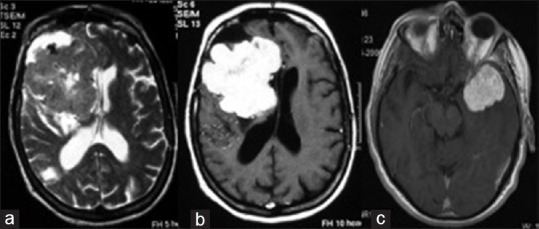
(a) T2- and (b) T1-weighted magnetic resonance imaging after gadolinium injection showed a right sphenoid wing solitary fibrous tumor; (c) T1-weighted magnetic resonance imaging after gadolinium injection showed left temporal lobe
Regarding histopathology [Figure 2], two orbitals' tumor (Case 1 and 2) presented mainly round to fusiform cells tightly arranged around a ramifying, variable in caliber, vascular network. Extensive zones of hyalinization could hardly be seen. This pattern was considered to be “predominantly of the hemangiopericytoma type” of SFT. Three tumors, two orbitals and one of the CNS (Cases 3, 6, and 5, respectively), were composed mainly of spindle cells presenting several arrangements along with extensive areas of hyalinization where cells were arranged singly or in small clusters. The vascular component was much less impressive. This pattern was considered to be “predominantly of the classic SFT type.” Finally, another three tumors, one orbital and two of the CNS (Cases 4, 7, and 8, respectively), presented features of both types and were considered to be of the “mixed type.” Two orbital tumors (Case 1 and 3) disclosed histological anaplasia with high cellularity, nuclei pleomorphism, and a mitotic activity of about 3–5 mitosis/10 high-power fields and were considered malignant. Immunohistochemical study [Table 2] disclosed widespread/total or broad immunoreactivity for vimentin, CD34, and Bcl-2 (all cases). All the other used antibodies showed immunonegativity/only few immunoreactive elements or only very focal immunoreactivity. Cases 1 and 3 (both orbital SFT) showed a high proliferative index (Ki-67), with recurrence of one of them (Case 1).
Figure 2.
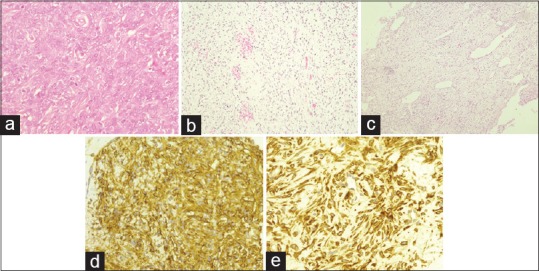
(a) Solitary fibrous tumor of the hemangiopericytoma type (H and E, ×40); (b) solitary fibrous tumor of the classic type (H and E, ×20); (c) solitary fibrous tumor of the mixed type (H and E, ×10); (d) neoplastic elements extensively CD34 immunoreactivity (×40); (e) neoplastic elements extensively Bcl-2 immunoreactivity (×40)
Table 2.
Histopathology and immunohistochemistry of seven patients with solitary fibrous tumors of the orbits and the central nervous system
Discussion
The diagnosis of SFT in our laboratory is rare. Roughly, most cases arouse from the orbits, followed by intracranial dural-attached masses. Spinal location was presented in one case.[21] In a review of 189 published cases of CNS SFT over a 14-year period,[8] three-quarters were intracranial. In our hospital, orbital tumors are usually operated by neurosurgeons, partially explaining the finding of high (62.5%) orbital SFTs in our series. The percentage of orbital SFTs in the two available case series of CNS and orbital SFT was lower compared to ours, 11.2% and 8.3%, respectively.[19,22]
As in other reports of CNS and orbital SFT, the majority of patients were middle-age males, and the clinical manifestations were mainly related to local mechanic growing effect.[7,8,11,23]
The 2007 WHO classification of tumors of the CNS defines typical SFT as “partternless architecture characterized by a combination of alternating hypocellular and hypercellular areas separated from each other by thick bands of hyalinized somewhat keloidal, collagen and branching hemangiopericytoma-like vessels.”[24] We identified cases in which the majority of the morphology was, either very similar to the one of the classic HPCs or harbored mixed morphological types of SFTs and HPCs. The presence of focal HPC patterns in SFT[11] may explain the frequent misdiagnosis with that neoplasm in a good proportion of SFT.[11,25] As in previous studies,[7,11] these histological variants of SFTs did not seem to play any role in the postsurgery outcome.
Histological criteria of “aggressive” extrapleural SFTs are hypercellularity, cytological atypias, necrosis, and more than 4 mitoses/10 high-power fields and/or an infiltrative margin.[7,8,26] These features are thought to be associated with a tendency to develop local recurrence and/or distant metastases.[27] In our series, four tumors recurred, but only one presented previous anaplastic features. Paradoxically, in one patient, besides the presence of aggressive histological characteristics, the tumor did not recur after a long, 9-year, follow-up period. This unpredictable biological behavior seems to differentiate CNS and orbital SFTs from their peripheral soft tissues counterparts[7,8,18] and suggests that all patients, including those with the so-called benign tumors, need a postsurgical regular follow-up.
As mentioned before, in opposition to the peripheral soft tissue tumors, in which SFT and HPC are regarded as a continuum of the same entity,[24,28] the possibility of misdiagnosis with HPC should be considered in both orbital and CNS SFT. This consideration may be important due to high risk of local recurrence and distant late metastases in those tumors displaying exclusively classical HPC features.[7] However, recent data described an overlapping of pathological and prognosis features in both CNS SFT and classical HPC.[15] Indeed, CD34 immunoreactivity, which is considered a marker of SFT, is present in 33%–100% of cases of classical HPC[29] and can be negative in up to 10% of cases of SFT.[15] The proportion of collagen in comparison to cellularity is also used to differentiate SFT from HPC. However, this distinction is arbitrary, reflecting, once again, the difficulty in the histological differentiation of these tumors.[15] Furthermore, there are few cases of CNS HPC that recurred with histological features of SFT, a finding supporting a common spectrum between the two entities.[13,30]
Cytogenetic studies demonstrated the existence of shared markers between the two tumors (NAB2-STAT6 fusion) giving further evidence that CNS SFTs and HPCs are variants of a single tumor.[17] In agreement with the literature,[7] our cases showed, in general, strong and diffuse immunohistochemical positivity for CD34, vimentin and Bcl-2, and negativity for EMA and desmin, rendering needless any cytogenetic study.
In cases of orbital SFT, it has been proposed that fibrous histiocytomas, HPCs, and giant cell angiofibromas should be reclassified as SFTs because all lesions showed considerable microscopic similarity.[11]
In our series, one patient (Case 1) had had a previous diagnosis of an orbital malignant fibrous histiocytoma, harbored a total resection, and the recurrence showed a SFT with signs of anaplasia. However, in our Case 4 that had had a previously diagnosed of HPC, the tumor recurred and unpredictably metastasized outside the CNS. Systemic metastases of SFT are rare and more often associated with anaplasia features.[25] Still, in this last case, the tumor did not display any anaplastic histological feature suggesting an aggressive postsurgery behavior.
Surgical extension is determinant for orbital and CNS SFTs prognosis.[7,8] A large systematic review of 189 cases of CNS SFTs found 58.1% of recurrences in cases of subtotal resection compared with 14.0% with gross total resection.[8] A review described recurrences in 26% of orbital SFT, most in incomplete excised cases.[5] In our series, 50% (4/8) of the patients had tumor recurrence, two after gross total (both orbital SFT) and two after subtotal resections (two CNS SFT). The relatively long-term follow-up after surgery, median of 92.6 months, in comparison to other series (median of 36.0 and 51.5 months),[19,30] and the subtotal resection in two cases, might explain the higher tumor recurrence.
Conclusion
Our series confirms the clinical and neuropathological diversity of CNS and orbital SFTs. Prospective studies, with longer follow-up periods, are required to better understand the clinical behavior and prognosis of these tumors. Cytogenetic studies are also needed to better comprehend the overlapping clinical and neuropathological features that SFTs and classical HPCs may disclose.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
The authors would like to thank Dr. Herculano Carvalho and Dr. José Lavrador for providing clinical and surgical data about the patients.
References
- 1.Klemperer P, Rabin CB. Primary neoplasms of the pleura. Arch Pathol. 1931;11:385–412. [Google Scholar]
- 2.Westra WH, Gerald WL, Rosai J. Solitary fibrous tumor. Consistent CD34 immunoreactivity and occurrence in the orbit. Am J Surg Pathol. 1994;18:992–8. doi: 10.1097/00000478-199410000-00003. [DOI] [PubMed] [Google Scholar]
- 3.Carneiro SS, Scheithauer BW, Nascimento AG, Hirose T, Davis DH. Solitary fibrous tumor of the meninges: A lesion distinct from fibrous meningioma. A clinicopathologic and immunohistochemical study. Am J Clin Pathol. 1996;106:217–24. doi: 10.1093/ajcp/106.2.217. [DOI] [PubMed] [Google Scholar]
- 4.Brunori A, Cerasoli S, Donati R, Giangaspero F, Chiappetta F. Solitary fibrous tumor of the meninges: Two new cases and review of the literature. Surg Neurol. 1999;51:636–40. doi: 10.1016/s0090-3019(98)00115-3. [DOI] [PubMed] [Google Scholar]
- 5.Le CP, Jones S, Valenzuela AA. Orbital solitary fibrous tumor: A case series with review of the literature. Orbit. 2014;33:145–51. doi: 10.3109/01676830.2013.853806. [DOI] [PubMed] [Google Scholar]
- 6.Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114:97–109. doi: 10.1007/s00401-007-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bisceglia M, Galliani C, Giannatempo G, Lauriola W, Bianco M, D'angelo V, et al. Solitary fibrous tumor of the central nervous system: A 15-year literature survey of 220 cases (August 1996-July 2011) Adv Anat Pathol. 2011;18:356–92. doi: 10.1097/PAP.0b013e318229c004. [DOI] [PubMed] [Google Scholar]
- 8.Fargen KM, Opalach KJ, Wakefield D, Jacob RP, Yachnis AT, Lister JR. The central nervous system solitary fibrous tumor: A review of clinical, imaging and pathologic findings among all reported cases from 1996 to 2010. Clin Neurol Neurosurg. 2011;113:703–10. doi: 10.1016/j.clineuro.2011.07.024. [DOI] [PubMed] [Google Scholar]
- 9.Young TK, Hardy TG. Solitary fibrous tumor of the orbit with intracranial involvement. Ophthal Plast Reconstr Surg. 2011;27:e74–6. doi: 10.1097/IOP.0b013e3181ed3590. [DOI] [PubMed] [Google Scholar]
- 10.Bernardini FP, de Conciliis C, Schneider S, Kersten RC, Kulwin DR. Solitary fibrous tumor of the orbit: Is it rare? Report of a case series and review of the literature. Ophthalmology. 2003;110:1442–8. doi: 10.1016/S0161-6420(03)00459-7. [DOI] [PubMed] [Google Scholar]
- 11.Furusato E, Valenzuela IA, Fanburg-Smith JC, Auerbach A, Furusato B, Cameron JD, et al. Orbital solitary fibrous tumor: Encompassing terminology for hemangiopericytoma, giant cell angiofibroma, and fibrous histiocytoma of the orbit: Reappraisal of 41 cases. Hum Pathol. 2011;42:120–8. doi: 10.1016/j.humpath.2010.05.021. [DOI] [PubMed] [Google Scholar]
- 12.Goldsmith JD, van de Rijn M, Syed N. Orbital hemangiopericytoma and solitary fibrous tumor: A morphologic continuum. Int J Surg Pathol. 2001;9:295–302. doi: 10.1177/106689690100900406. [DOI] [PubMed] [Google Scholar]
- 13.Hori E, Kurimoto M, Fukuda O, Takahashi C, Nagai S, Oya T, et al. Recurrent intracranial solitary fibrous tumor initially diagnosed as hemangiopericytoma. Brain Tumor Pathol. 2007;24:31–4. doi: 10.1007/s10014-006-0212-y. [DOI] [PubMed] [Google Scholar]
- 14.Tam ES, Chen EC, Nijhawan N, Harvey JT, Howarth D, Oestreicher JH. Solitary fibrous tumor of the orbit: A case series. Orbit. 2008;27:426–31. doi: 10.1080/01676830802344508. [DOI] [PubMed] [Google Scholar]
- 15.Bouvier C, Métellus P, de Paula AM, Vasiljevic A, Jouvet A, Guyotat J, et al. Solitary fibrous tumors and hemangiopericytomas of the meninges: Overlapping pathological features and common prognostic factors suggest the same spectrum of tumors. Brain Pathol. 2012;22:511–21. doi: 10.1111/j.1750-3639.2011.00552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gengler C, Guillou L. Solitary fibrous tumour and haemangiopericytoma: Evolution of a concept. Histopathology. 2006;48:63–74. doi: 10.1111/j.1365-2559.2005.02290.x. [DOI] [PubMed] [Google Scholar]
- 17.Schweizer L, Koelsche C, Sahm F, Piro RM, Capper D, Reuss DE, et al. Meningeal hemangiopericytoma and solitary fibrous tumors carry the NAB2-STAT6 fusion and can be diagnosed by nuclear expression of STAT6 protein. Acta Neuropathol. 2013;125:651–8. doi: 10.1007/s00401-013-1117-6. [DOI] [PubMed] [Google Scholar]
- 18.Ali MJ, Honavar SG, Naik MN, Vemuganti GK. Orbital solitary fibrous tumor: A rare clinicopathologic correlation and review of literature. J Res Med Sci. 2013;18:529–31. [PMC free article] [PubMed] [Google Scholar]
- 19.Chen H, Zeng XW, Wu JS, Dou YF, Wang Y, Zhong P, et al. Solitary fibrous tumor of the central nervous system: A clinicopathologic study of 24 cases. Acta Neurochir (Wien) 2012;154:237–48. doi: 10.1007/s00701-011-1160-9. [DOI] [PubMed] [Google Scholar]
- 20.Harmouch A, Chefchaouni MC, Maher M, Sefiani S. Solitary fibrous tumor of the orbit: Report of two cases and review of literature. J Fr Ophtalmol. 2011;34:133–7. doi: 10.1016/j.jfo.2010.11.006. [DOI] [PubMed] [Google Scholar]
- 21.Lavrador JP, Oliveira E, Neto L, Pimentel J, Francisco AF, Livraghi S. Dumbbell-shaped spinal solitary fibrous tumor: Combined approach and a review of the literature. Neurochirurgie. 2015;61:287–91. doi: 10.1016/j.neuchi.2015.03.006. [DOI] [PubMed] [Google Scholar]
- 22.Metellus P, Bouvier C, Guyotat J, Fuentes S, Jouvet A, Vasiljevic A, et al. Solitary fibrous tumors of the central nervous system: Clinicopathological and therapeutic considerations of 18 cases. Neurosurgery. 2007;60:715–22. doi: 10.1227/01.NEU.0000255418.93678.AD. [DOI] [PubMed] [Google Scholar]
- 23.Krishnakumar S, Subramanian N, Mohan ER, Mahesh L, Biswas J, Rao NA. Solitary fibrous tumor of the orbit: A clinicopathologic study of six cases with review of the literature. Surv Ophthalmol. 2003;48:544–54. doi: 10.1016/s0039-6257(03)00087-0. [DOI] [PubMed] [Google Scholar]
- 24.Fletcher CD, Unni KK, Mertens F. WHO Classification of Tumours, Pathology and Genetics of Tumours of Soft Tissue and Bone. Lyon: IARC Press; 2002. [Google Scholar]
- 25.Gessi M, Gielen GH, Roeder-Geyer ED, Sommer C, Vieth M, Braun V, et al. Extracranial metastasizing solitary fibrous tumors (SFT) of meninges: Histopathological features of a case with long-term follow-up. Neuropathology. 2013;33:68–74. doi: 10.1111/j.1440-1789.2012.01319.x. [DOI] [PubMed] [Google Scholar]
- 26.Vallat-Decouvelaere AV, Dry SM, Fletcher CD. Atypical and malignant solitary fibrous tumors in extrathoracic locations: Evidence of their comparability to intra-thoracic tumors. Am J Surg Pathol. 1998;22:1501–11. doi: 10.1097/00000478-199812000-00007. [DOI] [PubMed] [Google Scholar]
- 27.Moran CA, Suster S, Koss MN. The spectrum of histologic growth patterns in benign and malignant fibrous tumors of the pleura. Semin Diagn Pathol. 1992;9:169–80. [PubMed] [Google Scholar]
- 28.Ambrosini-Spaltro A, Eusebi V. Meningeal hemangiopericytomas and hemangiopericytoma/solitary fibrous tumors of extracranial soft tissues: A comparison. Virchows Arch. 2010;456:343–54. doi: 10.1007/s00428-010-0888-6. [DOI] [PubMed] [Google Scholar]
- 29.Perry A, Scheithauer BW, Nascimento AG. The immunophenotypic spectrum of meningeal hemangiopericytoma: A comparison with fibrous meningioma and solitary fibrous tumor of meninges. Am J Surg Pathol. 1997;21:1354–60. doi: 10.1097/00000478-199711000-00010. [DOI] [PubMed] [Google Scholar]
- 30.Vassal F, Manet R, Forest F, Camdessanche JP, Péoc'h M, Nuti C. Solitary fibrous tumors of the central nervous system: Report of five cases with unusual clinicopathological and outcome patterns. Acta Neurochir (Wien) 2011;153:377–84. doi: 10.1007/s00701-010-0866-4. [DOI] [PubMed] [Google Scholar]


