Abstract
Objectives:
Diffuse axonal injury is one of the major causes of unconsciousness, profound neurologic deficits and persistent vegetative state after head trauma. In recent years, MR imaging has been gaining popularity as an adjunctive imaging method in patients with DAI. Our study aims to assess the relative diagnostic and prognostic capability of various MRI sequences.
Patients and Methods:
Retrospective observational study done in 1 year duration on 30 DAI patients. Clinical assessment done with GCS at admission and GOS at 6 month. MRI Brain FLAIR, DWI, T2*GRE AND SWI sequences taken. DAI grade were evaluated for different MRI sequences. Prognosis was correlated to total number of lesion/locations and DAI grade of patients. Statistical analysis was done using SPSS Statistical software (ver.20.0.0) and XL-Stat and ANOVA one way test, post hoc test (Turkey test) and Chi square test.
Result:
We studied 30 male patients, mean age 32.57±8.72 ranges. The commonest mode of injury is RTA-80%, fall-16% followed by assault-3.33%. Out of 30 patients, 17 patients (56.67%) had GCS <=8, 13 patients (43.33%) had GCS between 9 and 12 and no patient had a GCS score between 13 and 15. The mean GCS score was 8.47±1.50. At a 6 month follow up, out of a total of 30 patients, 2 patients (6.66%) expired (GOS-1), 3 patients (10%) remained in persistent vegetative state (GOS-2), 11 patients (36.67%) and 10 patients (33.33%) were found to be severely (GOS-3) and moderately (GOS-4) disabled respectively and 4 patients (13.33%) showed good recovery (GOS-5). Mean GOS is 3.37+/-1.06. Newer imaging -SWI able to detects lesion better (diagnosis of DAI) as compared to other older sequences like FLAIR,DWI,T2*GRE. But no statistically significant found between total number of lesion/locations to the outcome and also newer imaging do not change the grade of DAI patients.
Conclusion:
Although advanced imaging in head injury, SWI helps in diagnosing the diffuse axonal injury more efficiently than other imaging sequences, but it is the grade of patients at admission that predicts the outcome best.
Keywords: Diffuse axonal injury prognosis, newer magnetic resonance imaging sequence, susceptibility-weighted imaging
Introduction
Diffuse axonal injury (DAI) is one of the major causes of unconsciousness and persistent vegetative state after head trauma.[1] It results from accelerative declarative/rotational forces associated with high-energy head trauma (Gentry, 2001; Hammoud and Wasserman, 2002), leading to extensive tissue damage with the involvement of multiple brain areas and profound neurologic deficits.[2,3,4,5,6,7,8]
In recent years, magnetic resonance imaging (MRI) is gaining popularity as an adjunctive imaging method in patients with DAI because it permits more precise identification and localization of various hemorrhagic and nonhemorrhagic lesions (NHLs). It also provides useful information regarding mechanisms of injury and potential clinical outcome.[9]
Recommended MRI sequences include T1- and T2-weighted, fluid attenuation inversion recovery (FLAIR), and diffusion-weighted images (DWIs).[10]
Recently, gradient recalled echo (GRE) and susceptibility-weighted imaging (SWI) have developed into a powerful diagnostic tool to provide additional clinically useful information that is often complementary to conventional MRI sequences.
Our study aims to assess the relative diagnostic and prognostic capability of various MRI sequences.
Materials and Methods
Patient selection
We retrospectively reviewed patient's records who presented as a closed head injury from February 2014 to January 2015. They underwent MRI as part of their clinical care. We included patients aged above 18 years who sustained head injury due to any mode. Review of the medical records by one of the authors showed that the mechanism of trauma (acceleration, deceleration, or rotatory force) was consistent with DAI of the brain in all patients.
We excluded those patients from the study, who were reaching hospital late (after 24 h) of injury, having associated intracranial lesion like acute subdural hematoma, intracerebral hemorrhage requiring emergency surgery, diagnosed case of seizure disorder, having any other intracranial pathology like tuberculoma, brain tumor, have been previously operated for any intracranial pathology, history of alcohol or drug intake, or having any past history of medical, neurological, or psychiatric illness and MRI brain all sequences either missing or distorted.
Furthermore, those patients with DAI who expired during hospital stay due to respiratory tract infection, severe dyselectrolytemia, or any other cause were also excluded from the study. Hence, ultimately, only thirty patients were included in the study. We followed the patients for 6 months for the assessment of their outcomes.
Clinical scoring
We correlated the number and location of the lesions to the patients' clinical status after admission (24 h) and the same to the outcome of the patients for 6 months after sustaining head injury. Patient's clinical status was evaluated by Glasgow coma scale (GCS) score after the admission and by Glasgow outcome scale (GOS) 6 months after the injury. We considered the GCS score after 24 h to rule out the confliction effect of hyperacute injury with the GCS score. Patient's levels of consciousness may not truly reflect the GCS level of patients during an acute setting.
Imaging
All patients underwent computed tomography (CT) head plain, as early as possible after admission to the hospital to rule out any intracranial lesions requiring urgent surgical interventions. Patients suspected of having DAI, after initial management, underwent MRI brain plain with our recommended sequences which included FLAIR, DWI, GRE, and SWI sequences, within 72 h of injury.
MRI was done with the Phillips Ingenia 3T machine. The technical details of each sequence are given in Table 1.
Table 1.
Technical details of various MRI sequences
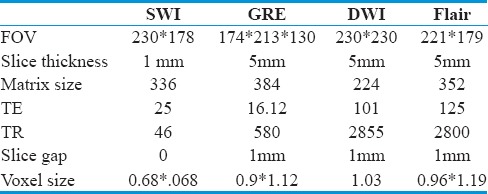
Image assessment
Image assessment was done with a radiologist having experience in traumatic brain injury (TBI) imaging. The lesions (1–15 mm) were defined as circumscribed foci of signal-intensity abnormality, including hyperintense, hypointense, and mixed hyperintense and hypointense lesions. Lesions at the base and surface and perilesional edema associated with lesion signal void due to blood vessels or calcification are not included.
FLAIR images show hyperintense signals to the lesion areas. DWIs were evaluated in conjunction with the corresponding apparent diffusion coefficient (ADC) maps to differentiate between lesions with decreased, increased, or unchanged diffusion rates. Since susceptibility effects from blood products can interfere with reliable ADC measurements, hypointense (hemorrhagic) lesions depicted on DWIs were not evaluated for their ADC change. Gradient-echo sequences and SWI sequences are both particularly useful in revealing the paramagnetic effects of petechial hemorrhages and produce a hypointense signal in areas of acute and early subacute hemorrhage. The GRE sequence is found to be very sensitive in identifying small contusions and minute older hemorrhages. It detects the lesions which were not visualized on previous sequences.
The total number of lesions was determined for each sequence separately, as well as for the combination of all sequences.
The sensitivity was assessed by comparing the ability to detect the number of lesions and their distribution at various locations. The locations were selected in five districts; for example, cortico-medullary junction, corpus callosum, deep cerebral nuclei, postfossa, and brain stem, and locations (n = 20) were included as follows: bilateral frontal lobe, bilateral temporal lobe, bilateral parietal lobe, bilateral occipital lobe, corpus callosum-body, splenium, bilateral basal ganglia, bilateral thalamus, bilateral cerebellar hemisphere, vermis, midbrain, pons, and medulla.
The prognostic role was assessed by comparing the final GOS to the total number as well as the location of lesions detected by different sequences.
Grades of DAI were analyzed as per different imaging sequences and compared to the final GOS score.
Statistical analysis
All the data were entered into Excel sheet Microsoft Office Excel-2007 and analyzed statistically using SPSS Statistical Software (version 20.0.0: IBM Corp: Armonk, NY) and XL-STAT (Addinsoft, NY, USA).
All the outcome variables, i.e., quantitative data, were summarized in the form of mean ± standard deviation. The difference between the mean value of the groups was analyzed using the ANOVA one-way test and was further analyzed using a post hoc test (Turkey test) and all the qualitative data were summarized in the form of proportions. The differences between proportions were analyzed using the Chi-square test.
Observations/Results
We studied thirty male patients with a mean age of 32.57 ± 8.72 years ranging from 18 to 50 years. The most common mode of injury is road traffic accident (RTA) - 80%, followed by fall - 16% and assault - 3.33%.
Out of thirty patients, 17 patients (56.67%) had GCS ≤8, 13 patients (43.33%) had GCS between 9 and 12, and no patient had GCS score between 13 and 15. The mean GCS score was 8.47 ± 1.50.
At a 6-month follow-up, out of thirty patients, 2 patients (6.66%) expired (GOS-1) due to very low neurological and general condition, 3 patients (10%) remained in persistent vegetative state (GOS-2), 11 patients (36.67%) and 10 patients (33.33%) were found to be severely (GOS-3) and moderately (GOS-4) disabled, respectively, and 4 patients (13.33%) showed good recovery (GOS-5). Mean GOS is 3.37 ± 1.06 [Tables 2, 3 and Figure 1].
Table 2.
Clinico-demographic profile of patients
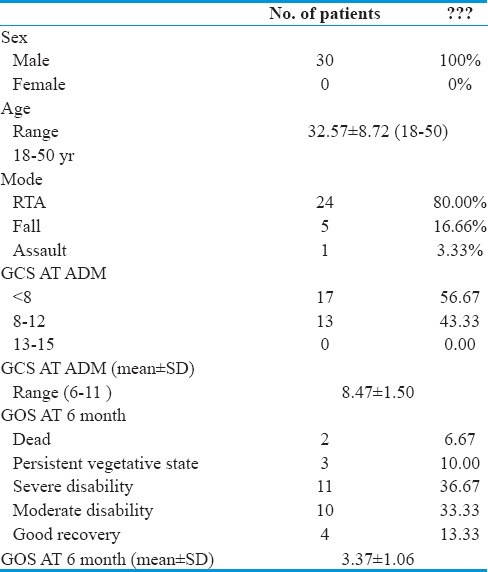
Table 3.
Distribution of lesions in dai patients
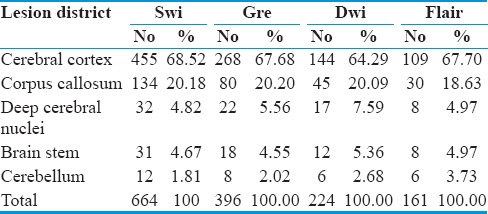
Figure 1.
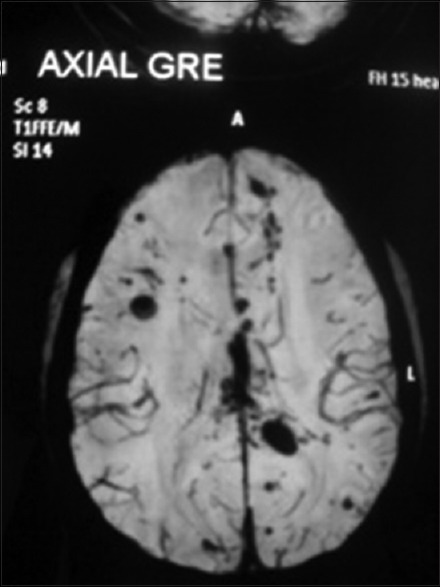
Axial T2* gradient recalled echo image showing bilateral cerebral cortex multiple contusion
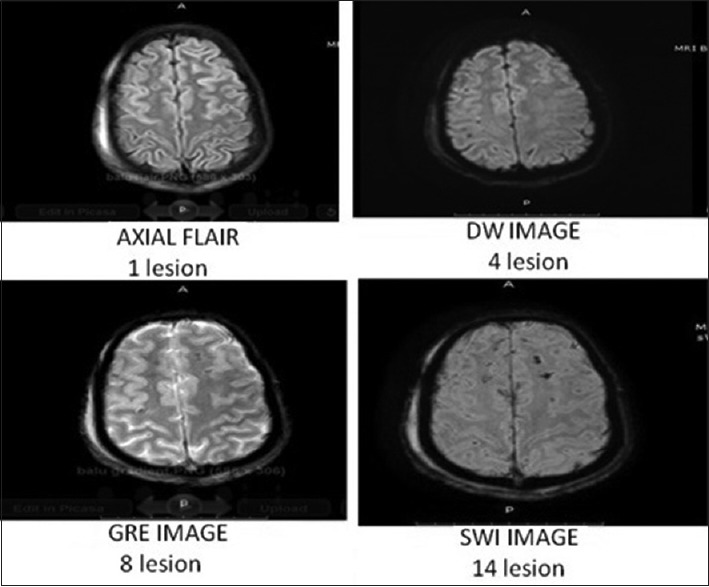
On assessing the various images individually and together, the most affected region (district) was cerebral cortex (cortico-medullary junction). It is mainly the frontal and temporal regions that are injured more as compared to the parietal and occipital regions. This is followed by the corpus callosum, in which the posterior part of body is found to be more affected then the anterior.
After this, deep cerebral nuclei, brainstem, mainly midbrain and cerebellum (superior medullary velum), were found to be affected [Table 4].
Table 4.
Relative senstivity of various imaging sequences in detecting the number and locatons of lesions
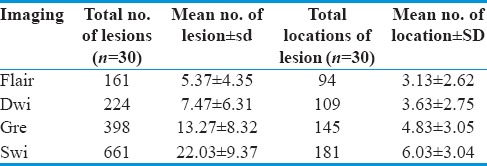
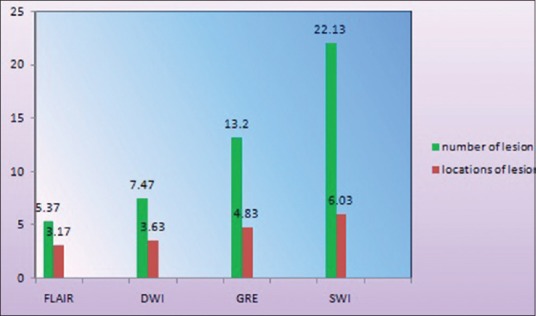
In our study of thirty patients, among MRI sequences, FLAIR images detect 161 lesions, with a mean of 5.37 ± 4.35 and a total of 95 locations with a mean of 3.13 ± 2.62. DWI detects 224 lesions, with a mean of 7.47 ± 6.31 and a total of 109 locations with a mean of 3 ± 2.75. GRE sequences pick up 396 lesions, with a mean 13.27 ± 8.32 and a total of 145 locations with mean of 4.83 ± 3.05, whereas SWI detects 664 lesions, with a mean of 22.03 ± 9.37 at a total of 181 locations with a mean of 6.03 ± 3.04.
To test the relative sensitivity of various sequences, we applied the HOC test which shows that SWI can detect a significantly higher number of lesions at the highest number of locations as compared to other sequences with a P < 0.005 [Figures 2, 3 and Table 5].
Figure 2.
Comparison of various sequences
Figure 3.
Role of magnetic resonance imaging sequences in detecting lesions
Table 5.
Association of outcome of patients with gcs at admission
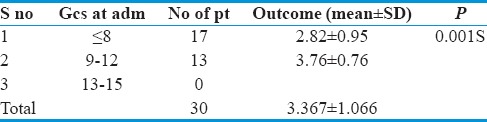
Among thirty patients, those having GCS ≤8 (17 patients) had an average GOS of 2.82 ± 0.95 and those having GCS between 9 and 12 (13 patients) had an average GOS score of 3.76 ± 0.76.
On analyzing statistically, a significant association (P = 0.001) was found between GCS and outcome. Those patients having poorer GCS at admission had a poorer outcome and vice versa [Table 6].
Table 6.
Association between the outcome and the number of lesions detected by various sequences
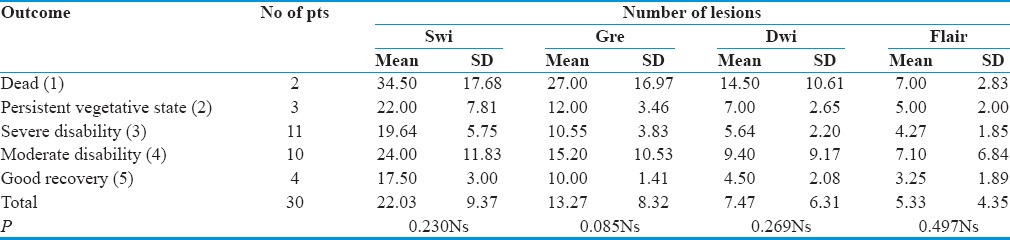
It appears that in every sequence, patients with a greater number lesion had a poorer outcome. However, on analyzing the outcome to the number of lesions in every sequence, we found that there was no statistical association present between the numbers of lesions to the outcome (P > 0.005) [Figures 4, 5 and Table 7].
Figure 4.
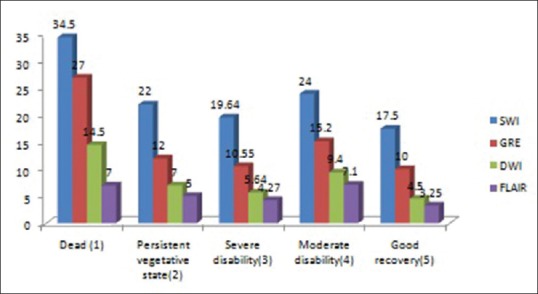
Number of lesions detected by different sequences in each Glasgow outcome scale group
Figure 5.
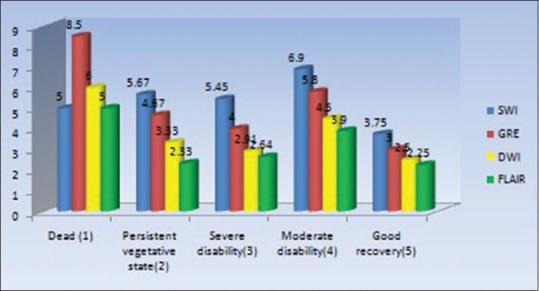
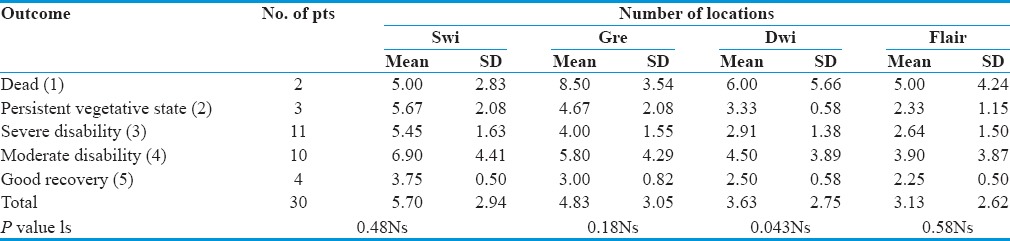
Association between patients outcome to the location of lesions detected by various sequences
Table 7.
Association between patients’ outcome to the location of lesions detected by various sequences
Furthermore, there was no significant association.
No statistical significant difference was observed between locations of lesion and outcome (P > 0.05) [Table 8].
Table 8.
Correlation of outcome to the grade of diffuse axonal injury patients as per different imaging sequences
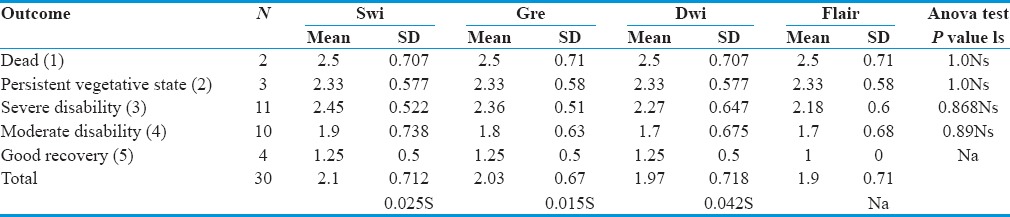
When association was tested between the outcome to the grade of DAI patients, based on various imaging sequences, it was shown that those patients (n = 2) who expired (GOS-1) during the follow-up period had an average grade of 2.5 according to all imaging sequences. Those patients (n = 3) with GOS 2 had Grade 3 as per SWI and 2.66 as other sequences. Severely disabled (GOS-3) patients[12] had an average grade of 2.33 on SWI sequences, 2.16 as per GRE and DWI, and 2 as per FLAIR image. Moderately disabled (GOS-4) patients[9] had an average grade 1.77 as per SWI, 1.66 as per GRE, and 1.55 as per both DWI and FLAIR images. Patients[4] who recovered well (GOS-5) had a 1.25 grade as per SWI, GRE, and DWI and 1 as per FLAIR image.
However, no significant difference was observed in the outcome to the grade of DAI patients based on various imaging sequences (P > 0.005).
Discussion
In our study, RTA is the most common cause head injury, constituting 80.00% of total cases, followed by fall (16.6%) and assault (3.33%). The lobes of the brain most likely to be injured are the frontal and temporal lobes.[11] Centroaxial white matter regions, including the corpus callosum, basal ganglia, thalamus, and deep hemispheric nuclei internal capsule, the superior cerebral peduncles,[23] (Gentry et al, 1988a; Cotran et al, 1999, Adam JH 1982, 1989). These areas may be more easily damaged because of the difference in density between them and the rest of the brain.[22] and fornix due to their rigid attachment to relatively more mobile cerebral hemispheres.[30] These areas may be more easily damaged because of the difference in density between them and the rest of the brain.[14]
In the present study, among all districts we categorized in the brain parenchyma, it is the cerebral cortex (455 lesions out of 664-68.52%), which was affected most, followed by the corpus callosum (134/664-20.18%), deep cerebral nuclei (32/664-4.81%), brainstem (31/664-4.66%), and cerebellum (12/664-1.80%) in descending order of prevalence. Chung et al. in 2012 also reported the lesions in almost the same frequency order as in our study. Rainer Scheid in 2002 reported involvement of the corpus callosum (21.2%) which is almost as same as our study (20.18%).
Several studies highlighted the role of GCS in predicting the outcome.[15,16] Wilberger et al. found a correlation between the admission GCS score and the eventual outcome of patients with DAI. In severe head injury, 42% of the patients died or were severely disabled or vegetative at discharge. Mild and moderate head injury did well. In patients with brainstem or corpus callosum tissue tear hemorrhage, the outcome is extremely poor.[17]
Horn et al.[18] suggested best prognosticators for TBI: patient's initial GCS score, presence of abnormalities on CT, and pupillary responses. He inversely correlated the outcome to GCS. Our study too reflects the correlation of GCS at admission to the outcome. Our patients (17/30) who sustained severe head injury with GCS score ≤8 had poor outcome. Two of them died (11%) and the rest of them remained in persistent vegetative state or were severely disabled. Those patients (13/30) who had moderate head injury (GCS-9–12) were moderately disabled.
Some studies reported that the GCS itself is not a sufficient predictor of outcome.[19,20] They reported that cognitive dysfunction seems to be more responsible for overall disability after TBI than do motor deficits.[21] More detailed neuropsychological testing is needed for clarification.
The classification was first proposed by Adams et al. in 1989[7] and divided DAI into three grades. According to the anatomic distribution of injury:
-
Grade I involves gray-white matter interfaces
- Most commonly: parasagittal regions of frontal lobes, periventricular temporal lobes
- Less commonly: parietal and occipital lobes, internal and external capsules, and cerebellum
- Often inapparent on conventional imaging, it may show changes on MRS.[26]
-
Grade II involves corpus callosum in addition to Stage I locations
- Observed in approximately 20% of patients
- Most commonly: posterior body and splenium, however, do advance anteriorly with increasing severity of injury
- Most frequently, unilateral may be seen on SWI.[26]
-
Grade III involves brainstem in addition to Stage I and II locations.
- Most commonly: rostral midbrain, superior cerebellar peduncles, medial lemnisci and corticospinal tracts [Figure 6].
Figure 6.
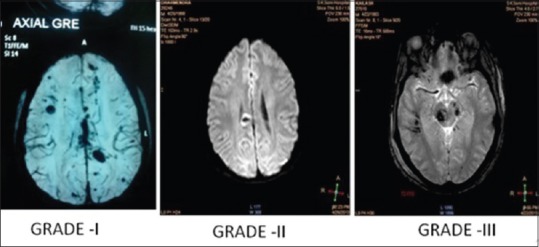
Grades of diffuse axonal injury
Controversy in diffuse axonal injury-hemorrhagic versus nonhemorrhagic
There has been great controversy regarding the nature of the lesions present in DAI patients. Previous studies reported that the lesions are NHL.[4,23,24]
Gentry et al.[4] define these lesions as mainly T2-hyperintense, diffuse, small (5–15 mm), focal abnormalities limited to white matter tracts not seen on CT. Furthermore, in one MRI study, only 10%–30% of DAIs were hemorrhagic,[5] but our study reveals that it is the hemorrhagic lesions that are found mainly in DAI patients. Periventricular hyperintensities are reported to be present in 74% of young normal persons and in 89% of elderly normal persons. The frequency of subcortical lesions in these two groups is reported to be 6% and 39%, respectively.[4] It is therefore questionable whether all the reported hyperintensities represent sites of axonal shear injury. A study was done by Chung et al., MD, in 2012 in DAI patients, correlating NHLs to the outcome. He mentioned that the distribution, evolution, and developmental mechanism of NHL are neither well known nor detailed studies. They also emphasizes that most NHLs (about 50%) resolve completely; they are probably less significant to prognosis than hemorrhagic lesions. Gentry et al.[4] said that acute callosal lesions were commonly nonhemorrhagic in nature on MRI features because 21 of 31 cases (67.7%) showed a nonhemorrhagic nature. However, these cases seemed to include the edema surrounding the hemorrhagic lesion mentioned as “usually, large nonhemorrhagic zones of injury surrounded the foci of blood.” The same authors reported brain stem injuries as well, but these were just mentioned as NHLs being more detectable on T2-weighted MRI than T1-weighted MRI or CT.[25]
Yanagawa et al. noted that possible asymptomatic cerebral infarctions may comprise one cause of T2-hyperintensities, especially if elderly patients are included in a study. During the acute stage of TBI, focal edema might be another cause of T2-hyperintense signal intensity changes. Furthermore, Gentry reported a high incidence of intraventricular hemorrhage due to high vasularity of the corpus callosum. This finds it unlikely that incidence of hemorrhagic lesions are less in corpus callosum (Mittl et al.).[23]
Hyperintense lesions have been reported in patients of assault which seems unlikely. Recently, a study performed by Jun Liu in 2014 states that DAI that is more common and associated with microbleeding is called hemorrhagic DAI. DAI without microbleeding or with isolated microbleeding has also been reported (Chen et al., 2013b). Intraparenchymal bleeding, including white matter microbleeding, is also a major feature of TBI. There are two manifestations of DAI. The first one is small petechial hemorrhages throughout the brain that result from the rupture of small blood vessels. The second is linear hemorrhages at subcortical white matter regions, particularly in the posterior frontal and parietal lobes (Ju et al., 2013). These small petechial hemorrhages scattered throughout the white matter, particularly in parasagittal white matter, are typically called diffuse vascular injury (DVI) (Costello et al., 2013; Zhu et al., 2013a). All cases of DVI show severe DAI and fall in a spectrum of similar pathological conditions (Chen et al., 2013a; Costello et al., 2013). Thus, appreciating the properties of DVI, as understood through microbleeding petechial hemorrhages, helps give diagnostic insight into potential DAI. In our study, SWI and GRE sequences detect a greater number of lesions as compared to DWI and FLAIR images. Hence, it can be appreciated that, contrary to popular belief, DAI is mainly hemorrhagic, but not nonhemorrhagic, and prognosis can be better correlated with hemorrhagic lesions as compared to NHLs.
Diagnostic role
Due to its rapid availability, low cost, rapidity,[26] short examination time, compatibility with life-support devices, and the convenience in planning for any surgical intervention, a noncontrast CT scan of the head is a first method of choice to evaluate seriously injured patients (Tchekmedyian et al., 2013). However, in DAI patients, it can be grossly normal or shows multiple scattered tiny contusions in the brain parenchyma. MRI is recommended in these situations (Gentry et al., 1988b; Ogawa et al., 1992).
Anatomical imaging with MRI is very sensitive and accurate in diagnosing cerebral pathology in DAI patients.[27]
Routine sequences (T1, T2, FLAIR, or DWI) might miss the actual burden of pathology. A sequence (such as gradient echo) that accentuates the susceptibility artifact arising from blood products must be performed to recognize small petechial hemorrhages. In the present study, only four sequences are taken into consideration.
FLAIR imaging shows lesions as white matter hyperintensities. Carlos Marquez de la Plata et al. performed quantitative assessment of white matter lesions in DAI patients in 2007 using FLAIR images. However, FLAIR images are sensitive to NHLs and those are found far below the hemorrhagic lesions, so diagnostic capability is not reliable. In our study, FLAIR imaging can identify a 5.33 mean number of lesions (24%) and a 3.13 mean number of locations. Pamela W. Schaefer in her study in 2003 reported 64% lesions, but it takes into consideration only NHLs so seems to be less reliable.
Conventionally, DWI has been used for the diagnosis of acute stroke given its high sensitivity for acute ischemia; however, DWI has been shown to be sensitive for other cerebral disease processes, including DAI. However, DWI is very sensitive only to NHLs and not very good at detecting hemorrhagic lesions on DWI. DWI can detect a number of lesions with a mean 7.47 and with a number of locations with mean 3.63. Toshibumi Kinoshita performed a study in 2006 comparing DWI and FLAIR imaging and found that the sensitivity of DWI to lesional conspicuity in DAI lesions was almost equal to that of FLAIR. Our study too found that the detection rate of DWI was comparable with FLAIR imaging.
Gradient-echo axial MRIs demonstrate numerous small foci of diminished signal consistent with the paramagnetic effect of the hemoglobin content of many acute hemorrhages. The abnormal signal on gradient-echo images can persist for many years after the injury helped to detect chronic injury.
Our study detects a 13.27 mean number of lesions with a 4.83 mean number of locations. We found the lesions are hypointense on T2* images. Lesions detected on a T2* image are far more then routine sequences. Previous studies (Ezaki Yin 2006) also emphasize the role of T2* GRE images in detecting the lesions in DAI patients over DWI and FLAIR images. Although a study performed by Pamela W. Schaefer et al. in 2003 said that DWI sequencing can detect lesions more than T2* images, they consider that DAI mainly contain NHLs.
SWI is particularly helpful in the evaluation of DAI, often associated with punctate hemorrhages in the deep subcortical white matter, which are not routinely visible on CT or conventional MRI sequences. SWI exploits the magnetic susceptibility differences between tissues, resulting in phase differences between regions containing paramagnetic deoxygenated blood products (deoxyhemoglobin, intracellular methemoglobin, and hemosiderin) and surrounding tissue. Not many studies have been done comparing diagnostic capability between GRE and SWI images. Studies by Tong et al.[28,29] and Babikian et al.[30] have shown that SWI is 3–6 times more sensitive than conventional T2*-weighted gradient-echo sequences in detecting the size, number, volume, and distribution of hemorrhagic lesions in DAI. Jun Liu in 2014 emphasized the role of SWI in detecting lesions to be better than conventional MRI sequences and also helpful in predicting outcome.
In our study, SWI imaging is able to detect the highest number of lesions with 22.13 lesions per person. It can detect a rate of 6.03 lesions per person.
Hence, SWI is definitely better than GRE imaging and other routine sequences for the detection of lesions.
Prognostic role
Paterakis et al. in 2000 had reported the outcome in patients with DAI and highlighted the prognostic role of MRI.[31]
DAIs can be visualized indirectly through shear hemorrhages (traumatic microbleeds) caused by inertial injuries on blood vessels (Tong et al., 2004; Scheid et al., 2003, 2006) or more directly by analyzing white matter hyperintensities on FLAIR MRI (Takaoka et al., 2002; Pierallini et al., 2000).
In our study, we tried to correlate the outcome to the number/location of the lesions. We counted the number/location of lesions shown by each sequence separately for each outcome group, but none of the sequences, including SWI, could show any correlation to the outcome. Although patients having a greater number of lesions seem to have a poorer outcome as compared to those having a lesser number, there was no statistical significance between the number/location of lesions to the outcome.
Carlos Marquez de la Plata in 2007 performed a quantification study of white matter volume and correlated the outcome to the outcome of patients. He said that volumetric assessment of DAI lesions in adult patients with TBI during the acute phase of the illness is reliable, is reproducible, and is associated, albeit modestly, with functional outcome, but there is no correlation between outcome and number of hemorrhagic lesions in DAI patients. Pamela W. Schaefer showed good correlation to outcome with the number of lesion using DWI images, but only very weak correlation to the number of hemorrhagic lesions (T2* images). In a recent study, Jun Liu in 2014 emphasized the role of SWI in detecting lesions showing that they were better than conventional MRI sequences and also more helpful in predicting the outcome.
A discrepancy has been reported between findings using MRI sequences and final outcomes. Studies have shown that although extensive white matter injury was consistently associated with a poor prognosis, the presence of focal signal-intensity abnormalities indicating shearing injury was seen in patients with both poor and good clinical outcomes.[32]
When we could not find any significant correlation to the outcome, we categorized the patients in different grades of DAI as per the Adam's classification according to different sequences. Surprisingly, we could not find significant difference in grade depicted by FLAIR, DWI, GRE, and SWI. This means that the lesion deciding the grade of DAI patients can be picked up by conventional sequences with almost equal accuracy. Therefore, the grade of DAI patients will not vary according to different sequences. However, with increasing grade in either sequence, a patient's prognosis becomes poorer. As per author's best knowledge, and PubMed/Google Search, no prior study had been done over this issue.
Our study shows that although the SWI and GRE are better than DWI and FLAIR sequences in diagnosing the DAI by picking up a greater number of lesions, the number/location of lesions does not correlate with the outcome. It is the grade at admission that predicts the outcome. In patients found to have sustained injury at deep-seated brain parenchyma, near basal cisterns, the brainstem had a comparatively poor prognosis. The involvement of the brainstem in TBI is a very important predictor of long-term outcome.[33]
Conclusion
Although advanced imaging in head injury, SWI helps in diagnosing the DAI more efficiently than other imaging sequences, but it is the grade of patients at admission that predicts the outcome best.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
We gratefully thank Dr. Rashmi Gupta (MD), Assistant Professor, Department of Social and Preventive Medicine, SMS, Jaipur, Rajasthan, India, for doing excellent job of statistical analysis of the data. We are also thankful to all involved radiological technicians for their excellent help perform all magnetic resonance examinations.
References
- 1.Wasserman J, Koenigsberg RA. Diffuse Axonal Injury. 2007. [Last retrieved on 2008 Jan 26]. Available from: http://www.Emedicine.com .
- 2.Adams JH, Graham DI, Murray LS, Scott G. Diffuse axonal injury due to nonmissile head injury in humans: An analysis of 45 cases. Ann Neurol. 1982;12:557–63. doi: 10.1002/ana.410120610. [DOI] [PubMed] [Google Scholar]
- 3.Gennarelli TA, Thibault LE, Adams JH, Graham DI, Thompson CJ, Marcincin RP. Diffuse axonal injury and traumatic coma in the primate. Ann Neurol. 1982;12:564–74. doi: 10.1002/ana.410120611. [DOI] [PubMed] [Google Scholar]
- 4.Gentry LR, Godersky JC, Thompson B. MR imaging of head trauma: Review of the distribution and radiopathologic features of traumatic lesions. AJR Am J Roentgenol. 1988;150:663–72. doi: 10.2214/ajr.150.3.663. [DOI] [PubMed] [Google Scholar]
- 5.Gentry LR. Imaging of closed head injury. Radiology. 1994;191:1–17. doi: 10.1148/radiology.191.1.8134551. [DOI] [PubMed] [Google Scholar]
- 6.Gentry LR, Thompson B, Godersky JC. Trauma to the corpus callosum: MR features. AJNR Am J Neuroradiol. 1988;9:1129–38. [PMC free article] [PubMed] [Google Scholar]
- 7.Adams JH, Doyle D, Ford I, Gennarelli TA, Graham DI, McLellan DR. Diffuse axonal injury in head injury: Definition, diagnosis and grading. Histopathology. 1989;15:49–59. doi: 10.1111/j.1365-2559.1989.tb03040.x. [DOI] [PubMed] [Google Scholar]
- 8.Strich SJ. Diffuse degeneration of the cerebral white matter in severe dementia following head injury. J Neurochem. 1956;19:163–85. doi: 10.1136/jnnp.19.3.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mittal S, Wu Z, Neelavalli J, Haacke EM. Susceptibility-weighted imaging: Technical aspects and clinical applications, part 2. AJNR Am J Neuroradiol. 2009;30:232–52. doi: 10.3174/ajnr.A1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schrader H, Mickeviciene D, Gleizniene R, Jakstiene S, Surkiene D, Stovner LJ, et al. Magnetic resonance imaging after most common form of concussion. BMC Med Imaging. 2009;9:11. doi: 10.1186/1471-2342-9-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boon R, de Montfor GJ. Brain Injury. Learning Discoveries Psychological Services. 2002. [Last retrieved on 2008 Jan 17]. Available from: www.angelfire.com/journal/ldps/BrainInjury.htm .
- 12.Smith D, Greenwald B. Management and Staging of Traumatic Brain Injury. [Last retrieved on 2008 Jan 17]. Available from: http://www.Emedicine.com .
- 13.Vik A, Kvistad KA, Skandsen T, Ingebrigtsen T. Diffuse axonal injury in traumatic brain injury. Tidsskr Nor Laegeforen. 2006;126:2940–4. [PubMed] [Google Scholar]
- 14.Singh J, Stock A. Head Trauma. 2006. Sep 25, [Last retrieved on 2008 Jan 17]. Available from: http://www.Emedicine.com .
- 15.Braakman R, Gelpke GJ, Habbema JD, Maas AI, Minderhoud JM. Systematic selection of prognostic features in patients with severe head injury. Neurosurgery. 1980;6:362–70. [PubMed] [Google Scholar]
- 16.Fearnside MR, Cook RJ, McDougall P, McNeil RJ. The Westmead Head Injury Project outcome in severe head injury. A comparative analysis of pre-hospital, clinical and CT variables. Br J Neurosurg. 1993;7:267–79. doi: 10.3109/02688699309023809. [DOI] [PubMed] [Google Scholar]
- 17.Wilberger JE, Jr, Rothfus WE, Tabas J, Goldberg AL, Deeb ZL. Acute tissue tear hemorrhages of the brain: Computed tomography and clinicopathological correlations. Neurosurgery. 1990;27:208–13. doi: 10.1097/00006123-199008000-00006. [DOI] [PubMed] [Google Scholar]
- 18.Horn EM, Feiz-Erfan I, Harrington TR. Prognostic utility of magnetic resonance imaging in traumatic brain injury. Barrow Quarterly. 2003:19. [Google Scholar]
- 19.Katz DI, Alexander MP. Traumatic brain injury.Predicting course of recovery and outcome for patients admitted to rehabilitation. Arch Neurol. 1994;51:661–70. doi: 10.1001/archneur.1994.00540190041013. [DOI] [PubMed] [Google Scholar]
- 20.Wilson JT, Hadley DM, Wiedmann KD, Teasdale GM. Neuropsychological consequences of two patterns of brain damage shown by MRI in survivors of severe head injury. J Neurol Neurosurg Psychiatry. 1995;59:328–31. doi: 10.1136/jnnp.59.3.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jennett B, Snoek J, Bond MR, Brooks N. Disability after severe head injury: Observations on the use of the Glasgow Outcome Scale. J Neurol Neurosurg Psychiatry. 1981;44:285–93. doi: 10.1136/jnnp.44.4.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Teasdale GM. Head injury. J Neurol Neurosurg Psychiatry. 1995;58:526–39. doi: 10.1136/jnnp.58.5.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mittl RL, Grossman RI, Hiehle JF, Hurst RW, Kauder DR, Gennarelli TA, et al. Prevalence of MR evidence of diffuse axonal injury in patients with mild head injury and normal head CT findings. AJNR Am J Neuroradiol. 1994;15:1583–9. [PMC free article] [PubMed] [Google Scholar]
- 24.Gentry LR, Godersky JC, Thompson B, Dunn VD. Prospective comparative study of intermediate-field MR and CT in the evaluation of closed head trauma. AJR Am J Roentgenol. 1988;150:673–82. doi: 10.2214/ajr.150.3.673. [DOI] [PubMed] [Google Scholar]
- 25.Chung SW, Park YS, Nam TK, Kwon JT, Min BK, Hwang SN. Locations and clinical significance of non-hemorrhagic brain lesions in diffuse axonal injuries. J Korean Neurosurg Soc. 2012;52:377–83. doi: 10.3340/jkns.2012.52.4.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thomas M, Dufour L. Challenges of diffuse axonal injury diagnosis. Rehabil Nurs. 2009;34:179–80. doi: 10.1002/j.2048-7940.2009.tb00276.x. [DOI] [PubMed] [Google Scholar]
- 27.Lee B, Newberg A. Neuroimaging in traumatic brain imaging. NeuroRx. 2005;2:372–83. doi: 10.1602/neurorx.2.2.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tong KA, Ashwal S, Holshouser BA, Shutter LA, Herigault G, Haacke EM, et al. Hemorrhagic shearing lesions in children and adolescents with posttraumatic diffuse axonal injury: Improved detection and initial results. Radiology. 2003;227:332–9. doi: 10.1148/radiol.2272020176. [DOI] [PubMed] [Google Scholar]
- 29.Tong KA, Ashwal S, Holshouser BA, Nickerson JP, Wall CJ, Shutter LA, et al. Diffuse axonal injury in children: Clinical correlation with hemorrhagic lesions. Ann Neurol. 2004;56:36–50. doi: 10.1002/ana.20123. [DOI] [PubMed] [Google Scholar]
- 30.Babikian T, Freier MC, Tong KA, Nickerson JP, Wall CJ, Holshouser BA, et al. Susceptibility weighted imaging: Neuropsychologic outcome and pediatric head injury. Pediatr Neurol. 2005;33:184–94. doi: 10.1016/j.pediatrneurol.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 31.Paterakis K, Karantanas AH, Komnos A, Volikas Z. Outcome of patients with diffuse axonal injury: The significance and prognostic value of MRI in the acute phase. J Trauma. 2000;49:1071–5. doi: 10.1097/00005373-200012000-00016. [DOI] [PubMed] [Google Scholar]
- 32.Kelly AB, Zimmerman RD, Snow RB, Gandy SE, Heier LA, Deck MD. Head trauma: Comparison of MR and CT – Experience in 100 patients. AJNR Am J Neuroradiol. 1988;9:699–708. [PMC free article] [PubMed] [Google Scholar]
- 33.Mannion RJ, Cross J, Bradley P, Coles JP, Chatfield D, Carpenter A, et al. Mechanism-based MRI classification of traumatic brainstem injury and its relationship to outcome. J Neurotrauma. 2007;24:128–35. doi: 10.1089/neu.2006.0127. [DOI] [PubMed] [Google Scholar]


