Abstract
Objective:
Although posterior communicating artery (PCoA) is a smaller branch of the internal carotid artery, it gives the main contribution in the formation of circle of Willis (CW) by communicating with the internal carotid arterial system and the vertebro-basilar arterial system. The size of PCoA varies frequently. The present work aims to study the PCoA regarding its morphology, morphometry, and symmetry.
Materials and Methods:
This study was conducted on 170 human cadaveric brains. Brains were dissected carefully and delicately to expose all components of CW, especially PCoA. Morphological variations of PCoA were noted along with its morphometry and symmetry.
Results:
Morphological variations of PCoA were aplasia (3.52%), hypoplasia (25.29%), fenestration (0.58%), and persistent fetal pattern (16.47%). In the present study, we found the five different types of terminations of PCoA. Type I termination was the most common type, seen in 92.94% of cases, Type II termination was seen in 1.17%, Type III and Type IV terminations both were seen in 0.58%, and Type V was seen in 1.17%. The mean length of PCoA was 15.9 mm and 15.3 mm on the right and left sides, respectively. The mean diameter of PCoA was 2.1 mm and 1.9 mm on the right and left sides, respectively. Symmetry of PCoA was seen in 65.29% and asymmetric PCoA was seen in 34.70% of cases.
Conclusion:
The present study provides the complete description of PCoA regarding its morphology, symmetry, and morphometry. Awareness of these anatomical variations is important in neurovascular procedures.
Keywords: Brain, circulus arteriosus, morphometry, posterior communicating artery, variation
Introduction
The posterior communicating artery (PCoA) is a smaller branch of the internal carotid artery (ICA). It runs backward to anastomoses with the posterior cerebral artery (PCA) to contribute in the formation of circle of Willis (CW). It forms the main communicating channel between the internal carotid arterial system and the vertebro-basilar arterial system.[1,2] The size of PCoA is usually very small but it varies frequently to form fetal PCoA.[2] The present work aimed to study the PCoA regarding its morphology, morphometry, and symmetry.
Materials and Methods
This study was done in the Department of Anatomy, Rural Medical College, PIMS, Loni, Maharashtra, India. The study was started by undertaking the Institutional Ethical Clearance (PIMS/PhD/RC/2013/28). This study was conducted on 170 pairs of PCoA studied from 170 formalin-preserved human cadaveric brains. The cadaveric bodies from which brains removed were of unknown age and unknown cause of death. The approximate age was 40–60 years. The brains with the gross morphological variations were excluded from the study. Arachnoid mater in the interpeduncular fossa was removed carefully to expose the CW and all its segments including the PCoA. Hence, the PCoA on the either side was carefully dissected. Morphological variations of PCoA were seen, noted, and well photographed. The complete arterial networks of the CW were carefully and delicately separated from brain tissue and pasted on the black plastic sheets for better view. Dimensions of the PCoA were measured with the help of a Vernier caliper. Thus, detailed morphological and morphometrical study of PCoA was performed.
Results
In the present study, in all specimens, we found the origin of PCoA from the ICA. It is a smaller branch arising from the posterolateral aspect of the cranial part of the ICA in the interpeduncular fossa. It arises just before the termination of the ICA into anterior cerebral artery (ACA) and middle cerebral artery (MCA). This point forms the lateral angle of the CW. After the origin, artery runs posteromedially and forms the posterolateral boundaries of CW [Figure 1]. Morphology, symmetry, and morphometry of PCoA were studied.
Figure 1.
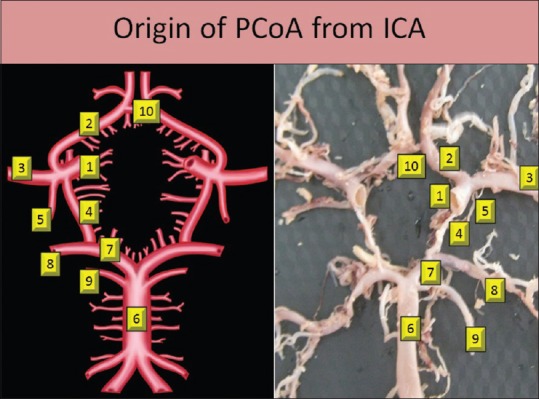
Origin of posterior communicating artery from internal carotid artery
Morphology of posterior communicating artery
Morphological variations of PCoA were seen in 78 specimens (45.88%). Morphological variations noted were aplasia, hypoplasia, fenestration, and fetal pattern of P2 segment.
Aplasia was seen in six specimens (3.52%); two specimens on the right side and four specimens on the left side [Figure 2]. Hypoplasia was seen in 43 specimens (25.29%); 22 specimens on the right side and 21 specimens on the left side. It was bilaterally present in 12 specimens [Figure 3]. Fenestration was seen in one specimen (0.58%) on the right side [Figure 4].
Figure 2.
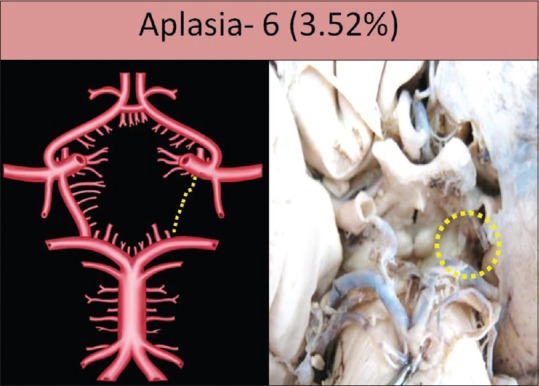
Aplasia of posterior communicating artery shown by dotted circle
Figure 3.
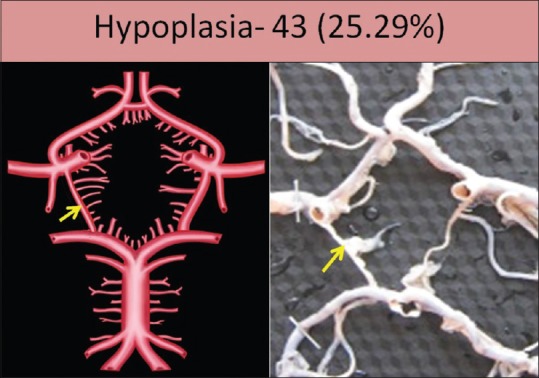
Hypoplasia of posterior communicating artery shown by arrow
Figure 4.
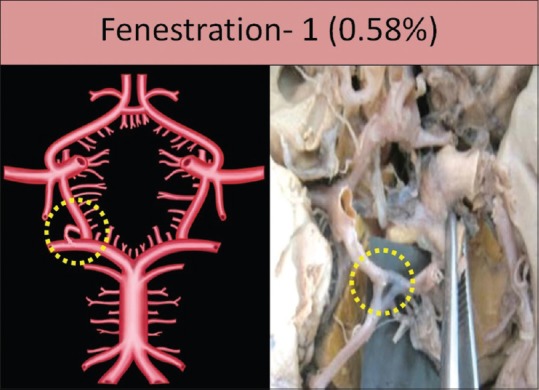
Fenestration of posterior communicating artery shown by dotted circle
Persistent fetal pattern contributing in the formation of P2 segment was seen in 28 specimens (16.47%). This pattern was seen bilaterally in twenty specimens and unilaterally in eight specimens; six on the right side and two on the left side [Figure 5]. Adult type of PCoA artery was seen in 135 specimens (79.41%) [Figure 1]. In the remaining seven specimens, one of the PCoAs was showing aplasticity in six specimens, and in one specimen, fenestrated PCoA was present.
Figure 5.
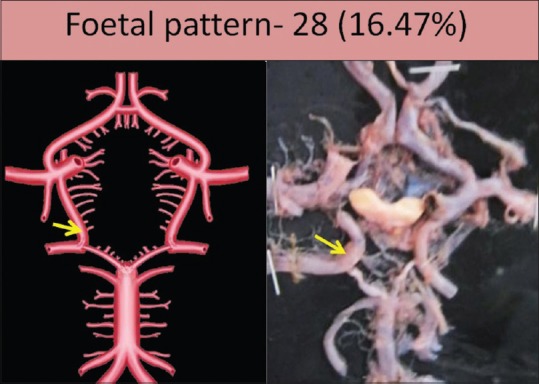
Remnant of fetal pattern of posterior communicating artery shown by arrow
Termination of posterior communicating artery differs as per the morphological variations
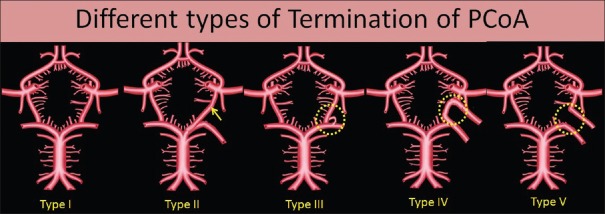
In the present study, termination of PCoA was divided into five different types [Figure 6]. These are as follows:
Figure 6.
Schematic diagram of different types of termination of posterior communicating artery
Type I termination: Typical termination: Artery terminates by anastomoses with the PCA
Type II termination: Artery terminates by anastomosing directly with the basilar artery (BA)
Type III termination: Artery terminates in the fenestration
Type IV termination: Artery anastomoses with an extra loop present in the CW
Type V termination: Artery does not anastomoses with any artery and directly supply the posterior aspect of cerebrum.
Type I termination
This is the typical termination of PCoA in which the artery anastomoses with the PCA to divide it into P1 and P2 segments. This was the most commonly found pattern of termination. It was seen in 158 specimens (92.94%) [Figure 7].
Figure 7.
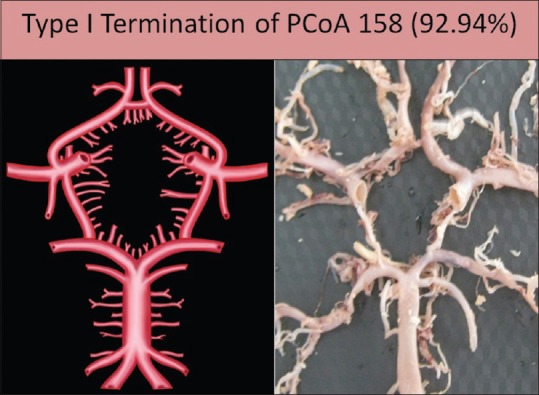
Type I termination of posterior communicating artery
Type II termination
Artery anastomoses directly with the BA at its termination. The terminal end of PCoA instead of anastomosing with PCA joins with the termination of BA. In this type of variation, the PCoA joins the lateral angle of CW with the posterior angle of CW excluding the contribution of PCA from the CW. This was observed in two specimens, both on the left side (1.17%) [Figure 8].
Figure 8.
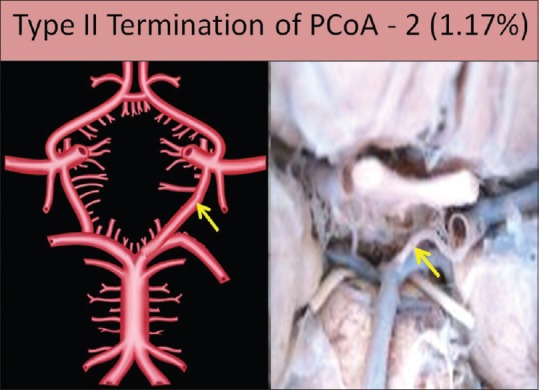
Type II termination of posterior communicating artery
Type III termination
Artery terminates in the fenestration; at the site of anastomosis of PCoA and PCA, there was a large triangular fenestration. It was seen in a single specimen [Figure 9].
Figure 9.
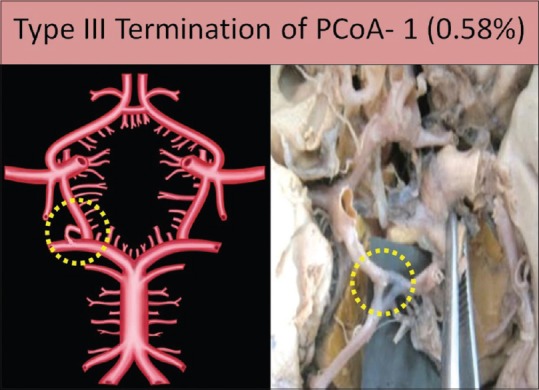
Type III termination of posterior communicating artery
Type IV termination
Artery anastomoses with an extra loop present in the CW: in this type of variation, PCoA runs posteromedially forming the boundary of CW and then it anastomoses with an unnamed extra loop present in the CW. It was forming an extrasegment in the posterior boundary of CW. After anastomoses with an extra loop/segment, PCoA runs posterolaterally to supply the posterior aspect of cerebrum to form an additional P2 segment of the PCA. This type was found in only one specimen on the left side in the present study (0.58%) [Figure 10].
Figure 10.
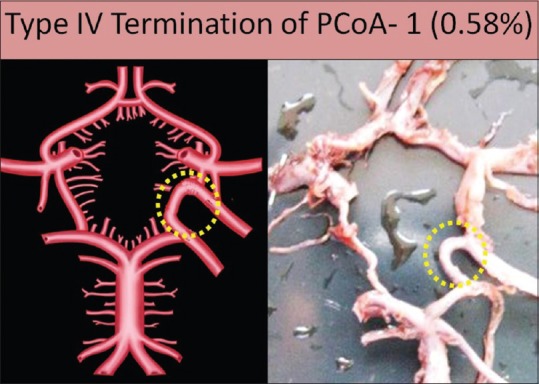
Type IV termination of posterior communicating artery
Type V termination
Artery does not anastomose with any artery and directly supply the posterior aspect of cerebrum: in this type, CW remains incomplete due to failure of communication between PCoA and PCA. PCoA runs posterolaterally to supply the posterior aspect of cerebrum to form an additional P2 segment. This type was seen in two specimens (1.17%), one on the right side and other on the left side [Figure 11].
Figure 11.
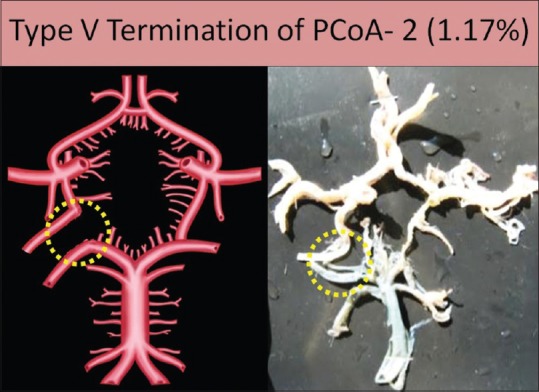
Type V termination of posterior communicating artery
Morphometry of posterior communicating artery
Along with the morphological variations, morphometrical observations of PCoA were done and tabulated [Table 1].
Table 1.
Morphometry of posterior communicating artery (mm)
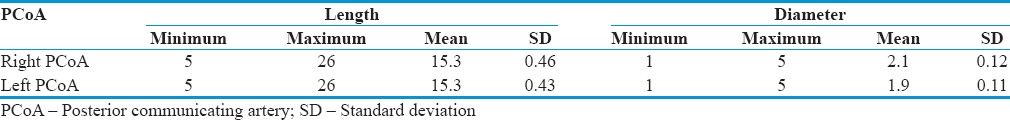
The length of PCoA was taken from its origin to the point of union with the PCA, and diameter was measured at the middle of the artery.
Mean length of PCoA was 15.9 mm and 15.3 mm on the right and left sides, respectively. Mean diameter of PCoA was 2.1 mm and 1.9 mm on the right and left sides, respectively.
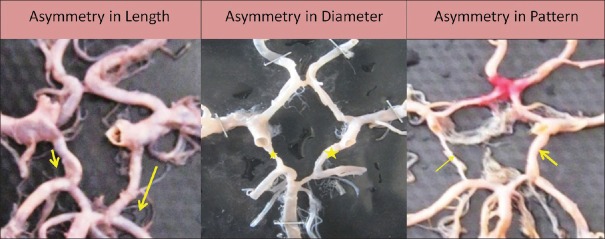
Symmetry of posterior communicating artery
Symmetry of PCoA was seen in 111 specimens (65.29%) [Figure 12], and asymmetry was seen in 59 specimens (34.70%) [Figure 13].
Figure 12.
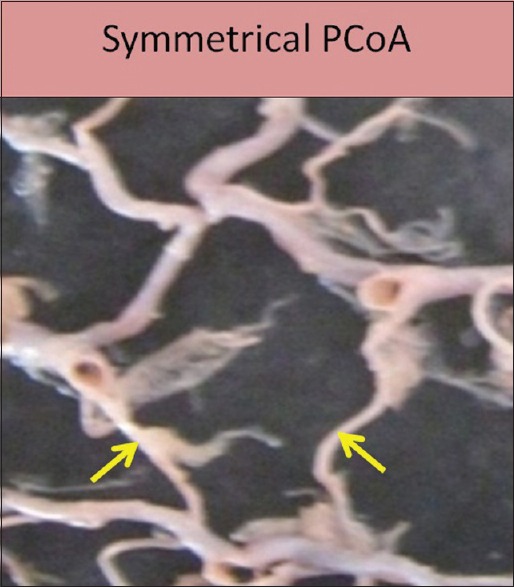
Symmetrical posterior communicating artery
Figure 13.
Asymmetrical posterior communicating artery
Discussion
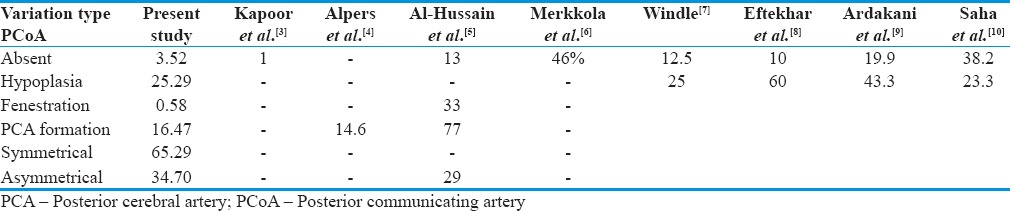
PCoA is the main communicating channel of the CW; it joins ICA and vertebro-basilar arterial system. The present study describes the morphological variations of PCoA in 45.88% of cases. Morphological variations noted were aplasia, hypoplasia, fenestration, and fetal pattern. Morphological variations of PCoA were discussed with the available literature in Table 2.
Table 2.
Comparison of morphological variations of posterior communicating artery
The number of morphological variations associated with the PCoA can be explained on the embryological basis. The blood vessels of the brain develop from a primitive plexus of vascular channels. Certain vascular channels develop and persist and certain channels disappear in accordance with contemporary needs and relations of the areas to be supplied.[11]
In the initial period of development, up to the 14-mm stage in the human embryo, all the cerebral arteries ACA, MCA as well as PCA are primarily derived by the carotid arterial system. Vertebro-basilar system develops later. Thread-like PCoA is due to the “atrophy” of the vestigial connecting branch completing CW during the developmental process.[12]
When the BA develops completely, it begins to supply blood to the posterior part of the brain. Thus, PCoA develops properly and acquires its adult form. If this development does not take place, it shows development of hypoplasia of P1 segment of PCA and an ipsilateral fetal type of PCoA.[13,14,15]
In one of our articles, we have reported various patterns of BA termination. One of the patterns observed was the nonfurcation of BA. In that pattern, BA instead of termination showed its direct continuation into a single PCA, either of the right or left side. This pattern suggests aplasia of one of the PCAs. It was present in 2.94% of cases.[16]
This pattern shows nondevelopment of anastomosis between PCoA and BA through PCA. Thus, PCoA cannot acquire its adult configuration and remains as the fetal type.
Hypoplasia of the PCoA is the most commonly found anomaly in the literature.[1] In the present study, the incidence of the hypoplastic PCoA was 25.29%. Fenestration of the PCoA was the least commonly found variation of the present study in 0.58% of cases.
PCoA can be divided into three different types as follows:
Adult configuration
Transitional configuration and
Fetal or embryonic configuration.
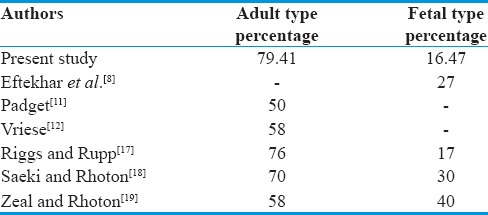
In adult configuration, diameter of PCoA is larger than the P1 diameter. In transitional configuration, diameter of both PCoA and P1 is same and they contribute approximately equal contribution in the formation of P2 segment of PCA. Fetal or embryonic configuration shows the direct continuation of PCoA as P2 segment of PCA. In the present study, we have divided the PCoA into only adult and the fetal type of configuration. Data are compared with the available literature in Table 3.
Table 3.
Comparison of size of posterior communicating artery
For the shunt operations to assess the feasibility, knowledge of the normal size of these vessels is essential. Vessels with 1 mm diameter or less than that are considered abnormal.[3,20]
Dimensions of the PCoA vary greatly. Its hypoplasia was most frequently seen in arteries forming CW. It was mostly seen in PCoA followed by P1 and P2 segments of PCA.[20,21] Morphometric observations of PCoA are compared with the previous studies as shown in Table 4.
Table 4.
Comparison of morphometry of components of posterior communicating artery (mm)

Morphometrical observations could be useful for different stent procedures in treating neurovascular diseases. This knowledge is also useful for microneurosurgeries in revascularization and cerebral bypass surgeries treating aneurysms or occlusion.
In addition to this, the present study also describes the symmetry of PCoA. There is hardly any study describing the symmetry of PCoA.
In normal circumstances, occipital lobes of cerebrum enjoy nutrition both from vertebro-basilar and internal carotid system of arteries. In different morphological variations, the situation changes.
In aplasia of PCoA or P1, blood supply to the occipital pole retains from a single source.
In such cases, collateral circulation lacks. Blood supply may get hampered due to hemodynamic factors affecting vessel wall to change in the development of aneurysm or occlusion.
Complete absence of PCA is never seen, only its origin from BA or ICA changes. Aplasia of P1 seen in the present study is in 2.35% of cases.[26] The present study shows aplasia of PCoA in 3.52% and hypoplasia in 25.29% of cases.
Collateral circulation through CW becomes important in occlusive vascular diseases of the brain. Compensatory circulation will be more effective in the presence of a normal and complete CW.
PCoA plays an important role for effective establishment of collateral circulation through CW. If one of these arteries is thin, hypoplastic or thread like, collateral circulation through the circle may be impaired, partially or completely. For establishment of better collateral circulation, connections between the two sides of the CW as well as the connections between the internal carotid and vertebro-basilar systems are equally important.
Conclusion
These are some interesting facts about the PCoA found in the present study. Hypoplasia of PCoA artery was the most frequently found variation while the fenestration of PCoA is the least found variation. Origin of PCoA shows least morphological variation while the terminal portion of PCoA shows most variety. The present study for the first time described about the different terminations of PCoA.
Morphological variations of PCoA were discussed in detail. Along with this, the present study has described adult and fetal configuration of PCoA in detail. In addition to this, the present study also describes the symmetry of PCoA. The present study adds knowledge by describing the morphological variations of PCoA.
This study provides the complete description of PCoA regarding its morphology, morphometry, and symmetry. Awareness of these anatomical variations is important in neurovascular procedures.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Gunnal SA, Farooqui MS, Wabale RN. Anatomical variations of the circulus arteriosus in cadaveric human brains. Neurol Res Int. 2014;2014:687281. doi: 10.1155/2014/687281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Standring S. Gray's Anatomy. 39th ed. Edinburgh: Elsevier Churchill Livingstone; 2005. p. 300. [Google Scholar]
- 3.Kapoor K, Singh B, Dewan I. Variations in the configuration of the circle of Willis. Anatomical Science International. 2008;83:96–106. doi: 10.1111/j.1447-073X.2007.00216.x. [DOI] [PubMed] [Google Scholar]
- 4.Alpers BJ, Berry RG, Paddison RM. The Anatomical studies of the Circle of Willis in normal brain. Arch Neurol Psychait. 1959;81:40. doi: 10.1001/archneurpsyc.1959.02340160007002. [DOI] [PubMed] [Google Scholar]
- 5.Al-Hussain SM, Shoter AM, Bataina ZM. Circle of willis in adults. Saudi Med J. 2001;22:895–98. [PubMed] [Google Scholar]
- 6.Merkkola P, Tulla H, Ronkainen A, Soppi V, Oksala A, Koivisto T, et al. Incomplete circle of Willis and right axillary artery perfusion. Ann Thorac Surg. 2006;82:74–9. doi: 10.1016/j.athoracsur.2006.02.034. [DOI] [PubMed] [Google Scholar]
- 7.Windle B. On the arteries forming the circle of Willis. J Anat Physiol. 1887;22:289–93. [PMC free article] [PubMed] [Google Scholar]
- 8.Efterkhar B, Dadmehr M, Ansari S, Ghodsi M, Nazparvar B, Ketabchi E. Are the distributions of variations of circle of Willis different in different populations? Results of an anatomical study and review of literature. BMC Neurology. 2006;6:22. doi: 10.1186/1471-2377-6-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ardakani SK, Dadmehr M, Nejat F, Ansari S, Tajik BE, Khashab ME, et al. The cerebral arterial circle (Circulus Arteriosus Cerebri): An anatomical study in fetus and infant samples. Pediatr Neurosurg. 2008;44:388–92. doi: 10.1159/000149906. [DOI] [PubMed] [Google Scholar]
- 10.Saha A, Bhagyalakshmi B, Mandal S, Banopadhyaya M. Variation of posterior communicating artery in human brain: A morphological study. Gomal J Med Sci. 2013;11:42–6. [Google Scholar]
- 11.Padget DH. The circle of Willis: Its embryology and anatomy in Intracranial Arterial Aneurysm. In: Dandy W.E, editor. Ch. 3. New York, NY, USA: Comstock Publishing Company; 1945. [Google Scholar]
- 12.Vriese BD. Sur signification morphologique des art´eries c´er´ebrales. Archives of Oral Biology. 1904;21:357–457. [Google Scholar]
- 13.Gibo H, Lenkey C, Rhoton AL. Microsurgical anatomy of the supraclinoid position of the internal carotid artery. J Neurosurgery. 1981;55:560–74. doi: 10.3171/jns.1981.55.4.0560. [DOI] [PubMed] [Google Scholar]
- 14.Lang J. Stuttgart: Schattauer; 1995. Skull Base and Related Structures: Atlas of clinical anatomy; pp. 25–29. [Google Scholar]
- 15.Yassargil MG. Microsurgery. Vol. 1. Stuttgart and New York: Georgia Thieme; 1984. pp. 60–6. [Google Scholar]
- 16.Gunnal SA, Farooqui MS, Wabale RN. Anatomical variability in termination of basilar artery in human cadaveric brain. Turkish Neurosurgery. 2015 doi: 10.5137/1019-5149.JTN.12812-14.0. DOI: 10.5137/1019-5149.JTN.12812-14.0. [DOI] [PubMed] [Google Scholar]
- 17.Riggs HE, Rupp C. Variations in the form of circle of willis. The relation of the variation to collateral circulation – Anatomic analysis. Arch Neurology. 1963;8:8–14. doi: 10.1001/archneur.1963.00460010024002. [DOI] [PubMed] [Google Scholar]
- 18.Saeki N, Rhoton AL. Microsurgical anatomy of the upper basilar artery and the posterior circle of Willis. Journal of Neurosurgery. 1977;46:563–78. doi: 10.3171/jns.1977.46.5.0563. [DOI] [PubMed] [Google Scholar]
- 19.Zeal AA, Rhoton AL. Microsurgical anatomy of the posterior cerebral artery. Journal of Neurosurgery. 1978;48:534–59. doi: 10.3171/jns.1978.48.4.0534. [DOI] [PubMed] [Google Scholar]
- 20.Fetterman GH, Moran JJ. Anomalies of the circle of Willis in relation to cerebral softening. Archives of Pathology. 1941;32:251–57. [Google Scholar]
- 21.Reddy R, Prabhakar DV, Rao BD. Anatomical study of circle of Willis. Neurology India. 1972;99:8–12. [PubMed] [Google Scholar]
- 22.Pai BS, Varma RG, Kulkarni RN, Nirmala S, Manjunath LC, Rakshith S. Microsurgical anatomy of the posterior circulation. Neurol India. 2007;55:31–41. doi: 10.4103/0028-3886.30424. [DOI] [PubMed] [Google Scholar]
- 23.Aysun Uz, Erbil K. Mine A morphological study of the posterior communicating artery Folia Morphol. 2004;63:397–99. [PubMed] [Google Scholar]
- 24.Krabbe-hartkamp MJ, Van der grond J, De leeuw FE, De groot JC, Algra A, Hillen B, et al. Circle of Willis: morphologic variation on three-dimensional time-of-flight MR angiograms. Radiology. 1998;207:103–11. doi: 10.1148/radiology.207.1.9530305. [DOI] [PubMed] [Google Scholar]
- 25.Moore S, David T, Chase JG, Arnold J, Fink J. 3D models of blood flow in the cerebral vasculature. Journal of Biomechanics. 2006;39:1454–63. doi: 10.1016/j.jbiomech.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 26.Gunnal SA, Farooqui MS, Wabale RN. Study of posterior cerebral artery in human cadaveric brain. Anat Res Int. 2015;2015:681903. doi: 10.1155/2015/681903. [DOI] [PMC free article] [PubMed] [Google Scholar]