Abstract
Intra-abdominal metastasis (IAM) of central nervous system (CNS) tumors through ventriculoperitoneal shunt (VPS) is rare but has been previously reported (e.g., germinomas and medulloblastomas). However, there has been no previous reports in literature involving meningiomas. A case of primary rhabdoid meningioma with widespread intra-abdominal carcinomatosis after placement of a VPS in a 36-year-old man is described. The patient underwent preoperative angioembolization of the tumor, craniotomy, and surgical excision, followed by postoperative gamma knife radiosurgery. Five months later, he underwent a decompressive craniectomy and surgical excision for tumor recurrence causing raised intracranial pressure and communicating hydrocephalus, necessitating placement of a VPS. One month after placement of the VPS, the patient developed abdominal distension and confusion. He was treated for a VPS infection and the shunt was explanted. He continued to deteriorate with high output from the peritoneal drain placed at the time of shunt explantation. An exploratory laparotomy revealed multiple diffuse peritoneal and omental nodules which had the same histopathological and immunohistochemical morphology as the primary tumor. We reviewed the current literature on IAM of primary CNS tumors through VPS, which revealed that patients belonging to the pediatric age group of the male gender and with a primary intracranial germinoma or medulloblastoma have a higher incidence of IAM. The majority of IAM occurred within 2 years of VPS placement, and patients most commonly present with abdominal distension and ascites. Treatment after diagnosis is varied, and the prognosis is poor, with more than half of the patients dying within a year. It is vital for clinicians to maintain a high index of suspicion for similar patients as early intervention could potentially improve patient outcomes and patient expectations managed more effectively.
Keywords: Meningioma, metastasis, ventriculoperitoneal shunt
Introduction
Meningiomas are the second most common symptomatic primary brain tumors in adults and account for one-third of all primary brain and central nervous system (CNS) tumors.[1,2] They are a diverse set of tumors derived from arachnoid cap cells of arachnoid villi in the meninges. Rhabdoid meningioma is an aggressive and unusual variant of meningioma that was first described and introduced by Kepes et al. and Perry et al. in 1998 and then subsequently added into the World Health Organization (WHO) classification of CNS tumors as a Grade III (malignant) tumor in 2000.[3,4,5] The latest 2007 WHO classification identified 15 different types of meningiomas based on morphological criteria and stratified them into three grades.[6] Grade I (benign) tumors form the vast majority, while Grade III (malignant) lesions remain rare.[7] Treatment of Grade III CNS meningiomas usually comprises a combination of surgical excision and radiotherapy.[8]
Any intracranial pathology is a common cause of acquired hydrocephalus. Implantation of a ventriculoperitoneal shunt (VPS) is the most widely used treatment to manage persistent hydrocephalus but can be associated with a number of complications, such as mechanical failures, infections, and dissemination of tumor cells.[9] Intra-abdominal metastasis (IAM) or seeding of CNS tumors through a VPS is rare but has been reported in literature. We describe the first reported case of widespread intra-abdominal carcinomatosis through a VPS in a patient with rhabdoid meningioma and present findings from a literature review of patients with primary CNS tumors and subsequent IAM through VPS.
Case Report
Written consent was obtained from the patient's next of kin for publication. The Institutional Review Board approval was not required as this is the case report.
A 36-year-old man presented with left-sided headaches associated with vertiginous giddiness for 3 months. A magnetic resonance imaging (MRI) scan of the brain revealed a 5.7 cm left temporal mass with a significant left cerebral edema and midline shift [Figure 1a]. The patient underwent preoperative angioembolization of the tumor followed by a left pterional craniotomy and excision of the tumor. Histopathological examination of the resected specimen revealed a meningothelial tumor predominantly consisting of cells with eccentrically placed nuclei, occasional prominent nucleoli, and abundant eosinophilic cytoplasm, consistent with a diagnosis of a meningioma with predominant rhabdoid features [Figure 2a]. Extensive tumor necrosis was also noted [Figure 2b]. Immunohistochemically, the tumor cells showed diffuse strong positivity to vimentin with focal expression of epithelial membrane antigen (EMA) and cytokeratins AE1/3 [Figure 2c]. Placental alkaline phosphatase, activin receptor-like kinase 1, and desmin were not expressed by tumor cells. The patient recovered well postoperatively and received gamma knife radiosurgery 2 months later for treatment of residual tumors.
Figure 1.
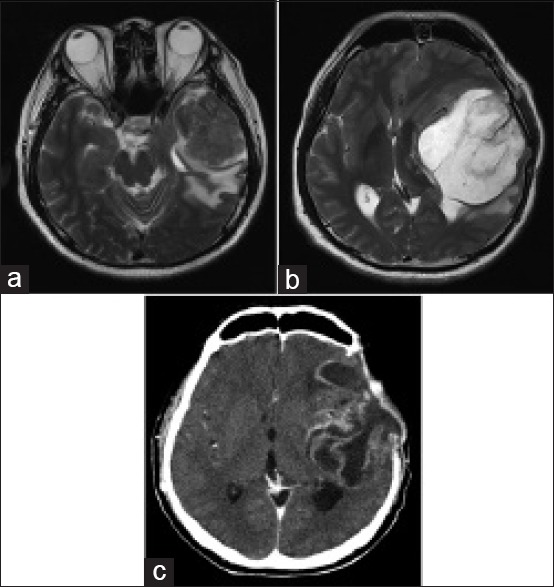
(a) Magnetic resonance imaging scan of the brain showing the initial left temporal mass before surgery. (b) Magnetic resonance imaging of the brain showing tumor recurrence 5 months after initial surgery. (c) Computed tomography of the brain showing tumor recurrence 6 months after diagnosis and after two separate tumor resections
Figure 2.
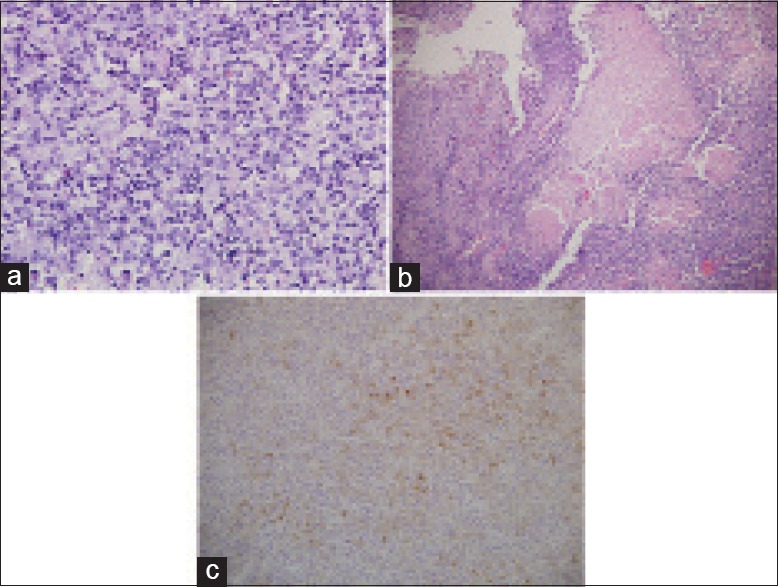
(a) Histology of brain tumor showing meningothelial tumor composed predominantly of cells with abundant eosinophilic cytoplasm and enlarged eccentrically placed nuclei, with occasional prominent nucleoli, consistent with rhabdoid meningioma (H and E, ×400). (b) Extensive geographical tumor necrosis in tumor (H and E, ×100). (c) Positive immunostaining of the tumor cells with epithelial membrane antigen (×200)
Five months following the initial surgery, a routine follow-up MRI scan of the brain showed tumor recurrence in the left middle cranial fossa measuring 7.9 cm with a midline shift, marked sulcal effacement, basal cistern distortion, and left uncal herniation [Figure 1b]. An emergency left decompressive craniectomy and excision of the recurrent tumor were performed. On postoperative day 3, the patient developed wound dehiscence over the lateral aspect of the craniectomy incision with a significant cerebrospinal fluid (CSF) leak. Computed tomography (CT) scan of the brain revealed increasing ventricular dilatation with intracranial herniation and a VPS was implanted. The patient subsequently recovered well.
The patient represented 1 month later with abdominal pain and distension associated with anorexia, lethargy, and confusion. He was disoriented and his abdomen was distended with generalized tenderness. A CT scan of the brain revealed recurrence of a 7.9 cm left frontotemporal tumor with associated perilesional edema [Figure 1c] while a CT scan of the abdomen revealed moderate amounts of ascites with diffuse peritoneal enhancement suggestive of ongoing peritonitis [Figure 3]. The VPS was explanted with placement of separate extraventricular and peritoneal drains in view of a possible VPS infection. The patient remained septic and critically ill even after the removal of the VPS and administration of broad-spectrum antibiotics, requiring increasing inotropic and ventilatory support. CSF and peritoneal fluid cultures were negative. There was 2–3 L of serous fluid drainage from the peritoneal drain daily and multiple samples of peritoneal fluid sent for cytology and culture did not show any malignant cell or infective organisms.
Figure 3.
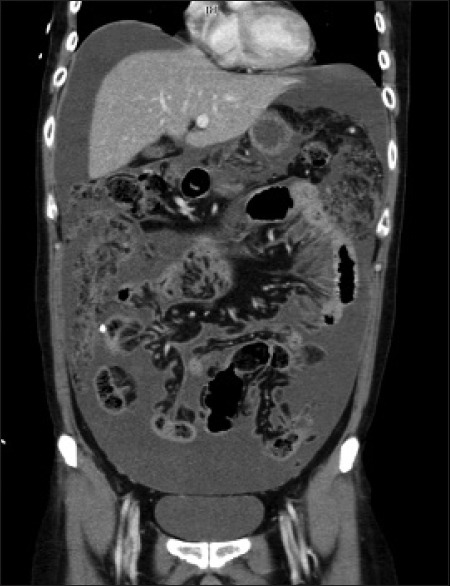
Computed tomography of the abdomen showing ascites with diffuse peritoneal enhancement with diffuse omental thickening and stranding
Diagnostic laparoscopy was attempted to elucidate the cause of persistent sepsis but conversion to a laparotomy was necessary due to dense intra-abdominal adhesions. Intraoperatively, the peritoneal surfaces, greater omentum, serosal surfaces, and mesentery of the small and large bowel were studded with multiple flesh-colored tumor nodules. Histopathological examination of a segment of greater omentum revealed adipose tissue coated and infiltrated by an extensively necrotic tumor which was composed of predominantly epithelioid cells with moderate amount of clear or eosinophilic cytoplasm and marked pleomorphic, irregular, hyperchromatic with prominent nucleoli [Figure 4a]. Some tumor cells had a rhabdoid appearance [Figure 4b], with eccentric nuclei displaced by rounded intracytoplasmic eosinophilic inclusions, and some had a spindled appearance. The tumor cells stained strongly with vimentin and EMA [Figure 4c]. Morphologically and immunohistochemically, the omental biopsy was similar to that of the primary brain tumor which supports the diagnosis of IAM from the primary brain tumor through the VPS. The patient continued to deteriorate and subsequently died 4 days after laparotomy, secondary to massive bilateral pulmonary embolism.
Figure 4.
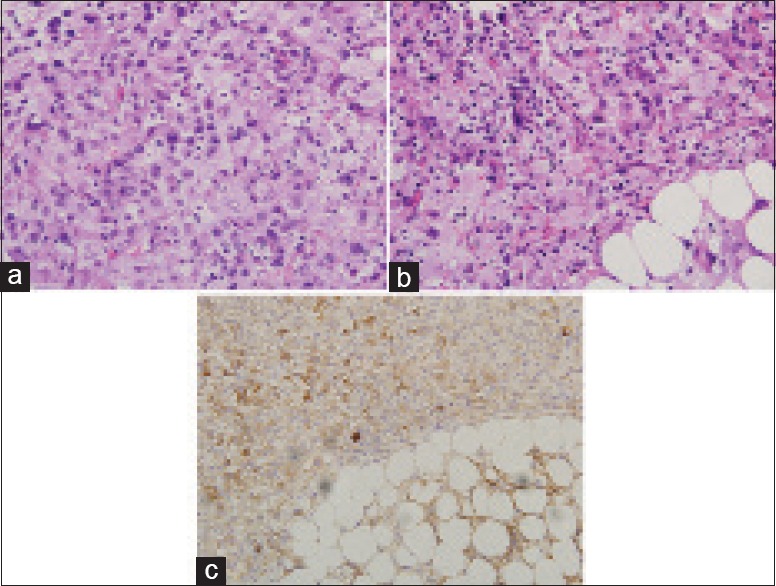
(a) Histology of omental biopsy showing tumor composed of predominantly epithelioid cells with moderate amount of clear or eosinophilic cytoplasm and markedly pleomorphic, irregular, hyperchromatic nuclei with prominent nucleoli, consistent with anaplastic meningioma (H and E, ×400). (b) Cells with rhabdoid appearance characterized by eccentric nuclei displaced by rounded intracytoplasmic eosinophilic inclusions (H and E, ×400). (c) Positive immunostaining of the tumor cells with epithelial membrane antigen (×200)
Discussion
Rhabdoid meningioma is a highly aggressive tumor with a high recurrence rate and poor prognosis. A recent systematic review showed that out of 48 patients with rhabdoid meningioma, almost 50% died within 1 year of surgery and two-thirds had tumor recurrence, with calculated 3- and 5-year recurrence-free survival rates of 41.4% and 27.6%, respectively. It also showed that metastasis most commonly disseminates through the CSF to the spinal cord or other intracranial locations, but extraneural metastasis is rare.[10] Extraneural metastasis to the lung, parotid gland, and liver has been reported and is most likely due to hematogenous seeding.[10,11,12] Tumor dissemination through VPS to the peritoneal cavity causing widespread intra-abdominal carcinomatosis has been reported in CNS tumors of other histological cell types, but to our knowledge, not in rhabdoid meningioma. In our case, we believe that the VPS was the conduit for transcoelomic spread of malignant cells that were present in the CSF into the peritoneal cavity leading to widespread intra-abdominal carcinomatosis. The patient's abdominal symptoms only started 1 month after placement of the VPS, suggesting that IAM developed during this time period. Furthermore, there was no evidence of other synchronous primary malignancies that could have accounted for the intra-abdominal carcinomatosis and the metastatic lesions found in the abdomen had similar histopathological and immunohistochemical morphology as that of the primary rhabdoid meningioma.
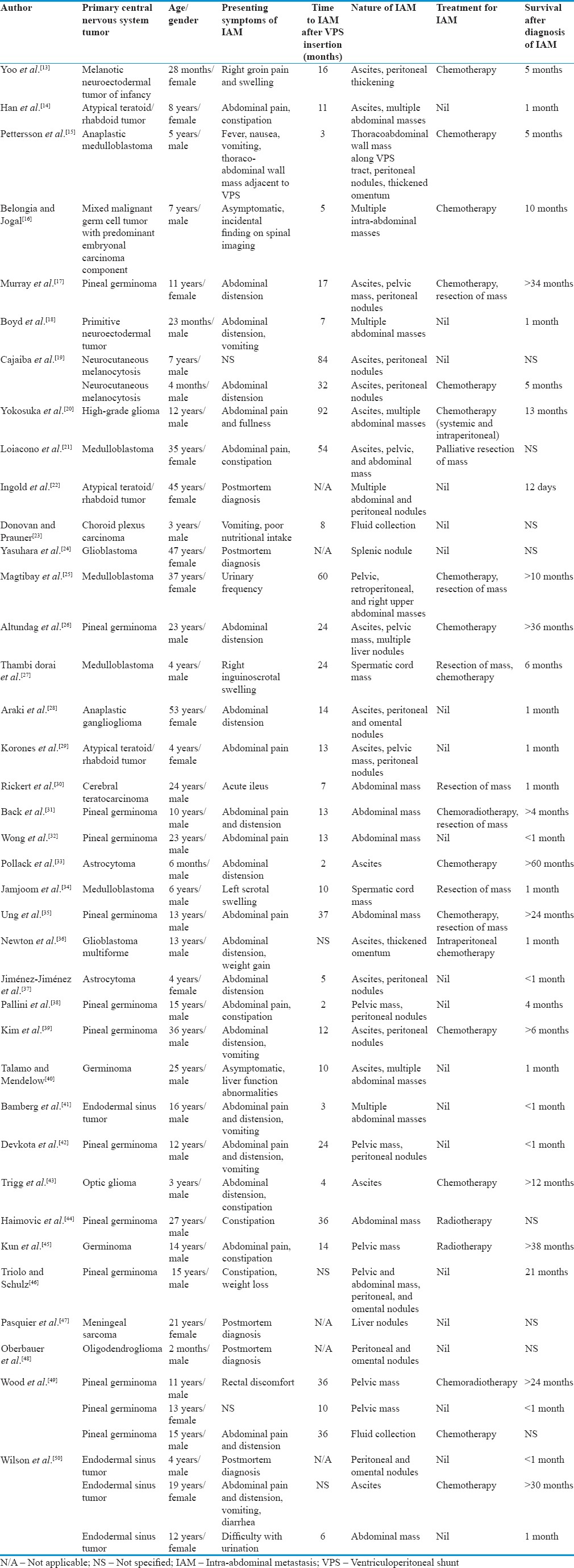
A survey of the literature published from 1979 to 2014 pertaining specifically to patients diagnosed with primary CNS tumors with subsequent IAM from VPS was conducted. Thirty-eight articles with a total of 43 patients were reviewed [Table 1].[13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50] The mean age of patients was 15 years and 3 months, with 69.8% (30 out of 43) of the patients in the pediatric age group (<18 years of age) and 67.4% male (29 out of 43). The overall predominant histological subtypes of primary CNS tumors with VPS-related metastasis were germinomas (11 out of 43, 25.6%) and medulloblastomas (5 out of 43, 11.6%). Other common histological subtypes include endodermal sinus tumors (4 out of 43, 9.3%), glioblastomas (4 out of 43, 9.3%), and atypical teratoid/rhabdoid tumors (3 out of 43, 7.0%). In the adult age group (≥18 years of age), germinomas and medulloblastomas remain the predominant histological subtype at 38.5% (5 out of 13) and 15.4% (2 out of 13) of patients, respectively. The mean time interval from VPS insertion to the occurrence of IAM was 21 months, with 74.3% (26 out of 35) occurring within 2 years of VPS insertion. The most common presenting symptoms of IAM were abdominal distension (41.7%, 15 out of 36), abdominal pain (36.1%, 13 out of 36), constipation (19.4%, 7 out of 36), and vomiting (19.4%, 7 out of 36). The diagnosis of IAM was made after postmortem examination in five patients. Two patients presented with scrotal swelling due to tumor seeding of the spermatic cord through a concurrent hernia defect, and two patients were asymptomatic at the time of diagnosis – one had liver function test abnormalities which led to further imaging and the other was diagnosed incidentally on follow-up spinal imaging. IAM most commonly manifested as ascites (39.5%, 17 out of 43), peritoneal/omental nodules or thickening (34.9%, 15 out of 43), abdominal masses (32.6%, 14 out of 43), and pelvic masses (25.6%, 11 out of 43). Treatment after diagnosis of IAM varied, with 42.1% (16 out of 38) of patients receiving chemotherapy, of which two patients received intraperitoneal chemotherapy. Eight patients (21.1%) underwent surgery to remove the metastatic tumors, of which four of them received prior chemotherapy, radiotherapy, or both. Two patients received combined chemoradiotherapy, two patients received radiotherapy alone, and 15 patients (39.5%) had no further treatment after diagnosis of IAM. Survival outcomes in this particular group of patients were poor, with 45.7% (16 out of 35) of patients dying within 1 month and 57.1% (20 out of 35) dying within 1 year of diagnosis of IAM. There is neither particular histological subtype, nature of IAM, nor treatment modality that rendered a superior survival outcome. However, it is evident that in our review of the literature, a higher incidence of VPS-related IAM is associated with pediatric patients, male gender, and primary intracranial germinomas or medulloblastomas. Our analysis is consistent with an older review by Rickert et al., published in 1998, where patients with VPS-related IAM mainly belonged to the pediatric age group with an overall male predominance and that the most common histological subtypes of primary CNS tumors were germinomas (25.7%) and medulloblastomas (22.9%).[30]
Table 1.
Review of 43 cases of ventriculoperitoneal shunt-related intra-abdominal metastasis reported between 1979 and 2014
With the VPS serving as a conduit for CSF flow from the ventricular cavity into the abdomen, it is a rare but possible means for dissemination of tumor cells, especially in patients with highly aggressive primary tumors that recur or are not completely removed, or tumors that tend to spread through CSF. There have even been cases reported of primary abdominopelvic tumors seeding into the CNS in a retrograde fashion through the VPS.[51,52] In an attempt to prevent the metastasis of tumor cells, a Millipore filter can be placed at the distal peritoneal end of the VPS. However, obstruction due to plugging of the shunt was a commonly reported complication.[21,53] A CSF irradiation filter, which exposes draining CSF to a localized high-intensity radiation field adequately shielded from surrounding tissue, did not produce promising results either.[54] Alternative forms of CSF drainage have also been explored. Placement of only an external ventricular drain can prevent IAM, but patients are at high risk of developing drain-related infections such as ventriculitis or meningitis, which may result in significant morbidity and mortality.[55] Endoscopic third ventriculostomy is a safe and effective method to relieve obstructive hydrocephalus caused by midline tumors and can be a substitute for a VPS in selected cases.[56] Matsumoto et al. developed a percutaneous long-tunneled ventricular drainage (PLTVD) to be used for highly malignant intracranial tumors such as germ cell tumors (GCTs) and medulloblastomas. This involved the cannulation of the frontal horn of the lateral ventricle with a ventricular catheter that was connected to a flow-controlled CSF reservoir. A peritoneal catheter proximally connected to the reservoir was then subcutaneously tunneled, exiting at the upper abdomen, and connected to a drainage system. A study of 13 patients who presented with medulloblastoma or GCT showed zero cases of extraneural metastasis or infections using this method; however, PLTVD had to be converted to a VPS in one case of communicating hydrocephalus due to dissemination and two cases of adhesive aqueductal stenosis not related to the tumor.[57]
Conclusion
We report the index case of a rhabdoid meningioma metastasizing through the VPS into the peritoneal cavity causing widespread intra-abdominal carcinomatosis. As IAM through a VPS is an unusual phenomenon, whether or not the placement of a VPS increases the risk of a patient with a primary intracranial tumor developing IAM remains controversial. The lack of statistical evidence means that the management and treatment of patients who require a VPS should not be changed. Alternative means of CSF drainage or diversion such as PLTVD merit further discussion and risk-benefit assessment. Meanwhile, clinicians should maintain a high index of suspicion in patients with a primary intracranial tumor and VPS presenting with abdominal symptoms for IAM, upon which a low threshold for abdominopelvic imaging should be maintained.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.CBTRUS. Hinsdale, IL: Central Brain Tumour Registry of the United States; 2010. Statistical Report: Primary Brain and Central Nervous System Tumours Diagnosed in the United States in 2004-2006. [Google Scholar]
- 2.Wiemels J, Wrensch M, Claus EB. Epidemiology and etiology of meningioma. J Neurooncol. 2010;99:307–14. doi: 10.1007/s11060-010-0386-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kepes JJ, Moral LA, Wilkinson SB, Abdullah A, Llena JF. Rhabdoid transformation of tumor cells in meningiomas: A histologic indication of increased proliferative activity: Report of four cases. Am J Surg Pathol. 1998;22:231–8. doi: 10.1097/00000478-199802000-00012. [DOI] [PubMed] [Google Scholar]
- 4.Perry A, Scheithauer BW, Stafford SL, Abell-Aleff PC, Meyer FB. “Rhabdoid” meningioma: An aggressive variant. Am J Surg Pathol. 1998;22:1482–90. doi: 10.1097/00000478-199812000-00005. [DOI] [PubMed] [Google Scholar]
- 5.Louis DN, Budka H, von Deimling A, Kepes JJ. World Health Organization classification of tumours pathology and genetics of tumours of the nervous system. In: Kleihues P, Cavenee WK, editors. International Agency for Research on Cancer. Meningiomas. Lyon: IARC Press; 2000. p. 314. [Google Scholar]
- 6.Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114:97–109. doi: 10.1007/s00401-007-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Willis J, Smith C, Ironside JW, Erridge S, Whittle IR, Everington D. The accuracy of meningioma grading: A 10-year retrospective audit. Neuropathol Appl Neurobiol. 2005;31:141–9. doi: 10.1111/j.1365-2990.2004.00621.x. [DOI] [PubMed] [Google Scholar]
- 8.Maclean J, Fersht N, Short S. Controversies in radiotherapy for meningioma. Clin Oncol (R Coll Radiol) 2014;26:51–64. doi: 10.1016/j.clon.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 9.Piatt JH, Jr, Garton HJ. Clinical diagnosis of ventriculoperitoneal shunt failure among children with hydrocephalus. Pediatr Emerg Care. 2008;24:201–10. doi: 10.1097/PEC.0b013e31816a8d43. [DOI] [PubMed] [Google Scholar]
- 10.Zhou Y, Xie Q, Gong Y, Mao Y, Zhong P, Che X, et al. Clinicopathological analysis of rhabdoid meningiomas: Report of 12 cases and a systematic review of the literature. World Neurosurg. 2013;79:724–32. doi: 10.1016/j.wneu.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 11.Parameshwaran Nair R, Vinod, Sarma Y, Nayal B, Kaur Dil S, Tripathi PK. Metastatic rhabdoid meningioma of the parotid – Mimicking primary salivary gland neoplasm. Int J Surg Case Rep. 2015;6C:104–6. doi: 10.1016/j.ijscr.2014.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang Z, Kong M, Li J, Xiao W, Zheng S. Intraspinal rhabdoid meningioma metastasis to the liver. J Clin Neurosci. 2011;18:714–6. doi: 10.1016/j.jocn.2010.07.146. [DOI] [PubMed] [Google Scholar]
- 13.Yoo IH, Yum SK, Oh SJ, Kim KM, Jeong DC. Melanotic neuroectodermal tumor of infancy disseminated by a ventriculoperitoneal shunt and diagnosed from the inguinal sac. J Pediatr Hematol Oncol. 2014;36:e61–4. doi: 10.1097/MPH.0b013e31829dd114. [DOI] [PubMed] [Google Scholar]
- 14.Han YP, Zhao Y, He XG, Ma J. Peritoneal metastasis of third ventricular atypical teratoid/rhabdoid tumor after VP shunt implantation for unexplained hydrocephalus. World J Pediatr. 2012;8:367–70. doi: 10.1007/s12519-012-0384-y. [DOI] [PubMed] [Google Scholar]
- 15.Pettersson D, Schmitz KR, Pollock JM, Hopkins KL. Medulloblastoma: Seeding of VP shunt tract and peritoneum. Clin Pract. 2012;2:e37. doi: 10.4081/cp.2012.e37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Belongia M, Jogal S. Extraneural metastasis of a nongerminomatous germ cell tumor of the central nervous system in a pediatric patient with a ventriculoperitoneal shunt: A case report and review of the literature. J Pediatr Hematol Oncol. 2012;34:e12–6. doi: 10.1097/MPH.0b013e31823dd370. [DOI] [PubMed] [Google Scholar]
- 17.Murray MJ, Metayer LE, Mallucci CL, Hale JP, Nicholson JC, Kirollos RW, et al. Intra-abdominal metastasis of an intracranial germinoma via ventriculo-peritoneal shunt in a 13-year-old female. Br J Neurosurg. 2011;25:747–9. doi: 10.3109/02688697.2011.566383. [DOI] [PubMed] [Google Scholar]
- 18.Boyd DT, Hayeri MR, Vyas PK. Supratentorial primitive neuroectodermal tumor metastasis to the abdomen via a ventriculoperitoneal shunt. Pediatr Radiol. 2010;40(Suppl 1):S123–6. doi: 10.1007/s00247-010-1732-5. [DOI] [PubMed] [Google Scholar]
- 19.Cajaiba MM, Benjamin D, Halaban R, Reyes-Múgica M. Metastatic peritoneal neurocutaneous melanocytosis. Am J Surg Pathol. 2008;32:156–61. doi: 10.1097/PAS.0b013e3181238cd2. [DOI] [PubMed] [Google Scholar]
- 20.Yokosuka K, Ishii R, Suzuki Y, Hirano K, Ishii N, Sekihara Y, et al. Extraneural metastasis of high grade glioma without simultaneous central nervous system recurrence: Case report. Neurol Med Chir (Tokyo) 2007;47:273–7. doi: 10.2176/nmc.47.273. [DOI] [PubMed] [Google Scholar]
- 21.Loiacono F, Morra A, Venturini S, Balestreri L. Abdominal metastases of medulloblastoma related to a ventriculoperitoneal shunt. AJR Am J Roentgenol. 2006;186:1548–50. doi: 10.2214/AJR.04.1718. [DOI] [PubMed] [Google Scholar]
- 22.Ingold B, Moschopulos M, Hutter G, Seeger H, Röthlisberger B, Landolt H, et al. Abdominal seeding of an atypical teratoid/rhabdoid tumor of the pineal gland along a ventriculoperitoneal shunt catheter. Acta Neuropathol. 2006;111:56–9. doi: 10.1007/s00401-005-1112-7. [DOI] [PubMed] [Google Scholar]
- 23.Donovan DJ, Prauner RD. Shunt-related abdominal metastases in a child with choroid plexus carcinoma: Case report. Neurosurgery. 2005;56:E412. doi: 10.1227/01.neu.0000147982.80732.3d. [DOI] [PubMed] [Google Scholar]
- 24.Yasuhara T, Tamiya T, Meguro T, Ichikawa T, Sato Y, Date I, et al. Glioblastoma with metastasis to the spleen – Case report. Neurol Med Chir (Tokyo) 2003;43:452–6. doi: 10.2176/nmc.43.452. [DOI] [PubMed] [Google Scholar]
- 25.Magtibay PM, Friedman JA, Rao RD, Buckner JC, Cliby WA. Unusual presentation of adult metastatic peritoneal medulloblastoma associated with a ventriculoperitoneal shunt: A case study and review of the literature. Neuro Oncol. 2003;5:217–20. doi: 10.1215/S1152-8517-02-00042-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Altundag OO, Celik I, Kars A. Pineal germ cell tumor metastasis via ventriculoperitoneal shunt. Am J Clin Oncol. 2002;25:104–5. doi: 10.1097/00000421-200202000-00025. [DOI] [PubMed] [Google Scholar]
- 27.Thambi dorai CR, Azmi A, Rahman AJ, Subathra S, Hayati AR, Zulfiqar A. Spermatic cord metastasis from a medulloblastoma. Pediatr Surg Int. 2001;17:654–6. doi: 10.1007/s003830100017. [DOI] [PubMed] [Google Scholar]
- 28.Araki M, Fan J, Haraoka S, Moritake T, Yoshii Y, Watanabe T. Extracranial metastasis of anaplastic ganglioglioma through a ventriculoperitoneal shunt: A case report. Pathol Int. 1999;49:258–63. doi: 10.1046/j.1440-1827.1999.00857.x. [DOI] [PubMed] [Google Scholar]
- 29.Korones DN, Meyers SP, Rubio A, Torres C, Constine LS. A 4-year-old girl with a ventriculoperitoneal shunt metastasis of a central nervous system atypical teratoid/rhabdoid tumor. Med Pediatr Oncol. 1999;32:389–91. doi: 10.1002/(sici)1096-911x(199905)32:5<389::aid-mpo16>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 30.Rickert CH, Reznik M, Lenelle J, Rinaldi P. Shunt-related abdominal metastasis of cerebral teratocarcinoma: Report of an unusual case and review of the literature. Neurosurgery. 1998;42:1378–82. doi: 10.1097/00006123-199806000-00118. [DOI] [PubMed] [Google Scholar]
- 31.Back MR, Hu B, Rutgers J, French S, Moore TC. Metastasis of an intracranial germinoma through a ventriculoperitoneal shunt: Recurrence as a yolk-sac tumor. Pediatr Surg Int. 1997;12:24–7. doi: 10.1007/BF01194796. [DOI] [PubMed] [Google Scholar]
- 32.Wong KT, Koh KB, Lee SH, Chee CP. Intracranial germinoma metastasizing via a ventriculo-peritoneal shunt. Singapore Med J. 1996;37:441–2. [PubMed] [Google Scholar]
- 33.Pollack IF, Hurtt M, Pang D, Albright AL. Dissemination of low grade intracranial astrocytomas in children. Cancer. 1994;73:2869–78. doi: 10.1002/1097-0142(19940601)73:11<2869::aid-cncr2820731134>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 34.Jamjoom ZA, Jamjoom AB, Sulaiman AH, Naim-Ur-Rahman, al Rabiaa A. Systemic metastasis of medulloblastoma through ventriculoperitoneal shunt: Report of a case and critical analysis of the literature. Surg Neurol. 1993;40:403–10. doi: 10.1016/0090-3019(93)90221-l. [DOI] [PubMed] [Google Scholar]
- 35.Ung AO, Triscott JA, Leditschke JF, Smith JA. Metastasis of pineal germinoma via ventriculoperitoneal shunt. Aust N Z J Surg. 1993;63:409–12. doi: 10.1111/j.1445-2197.1993.tb00412.x. [DOI] [PubMed] [Google Scholar]
- 36.Newton HB, Rosenblum MK, Walker RW. Extraneural metastases of infratentorial glioblastoma multiforme to the peritoneal cavity. Cancer. 1992;69:2149–53. doi: 10.1002/1097-0142(19920415)69:8<2149::aid-cncr2820690822>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 37.Jiménez-Jiménez FJ, Garzo-Fernández C, De Inovencio-Arocena J, Pérez-Sotelo M, Castro-De Castro P, Salinero-Paniagua E. Extraneural metastases from brainstem astrocytoma through ventriculoperitoneal shunt. J Neurol Neurosurg Psychiatry. 1991;54:281–2. doi: 10.1136/jnnp.54.3.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pallini R, Bozzini V, Scerrati M, Zuppi C, Zappacosta B, Rossi GF. Bone metastasis associated with shunt-related peritoneal deposits from a pineal germinoma. Case report and review of the literature. Acta Neurochir (Wien) 1991;109:78–83. doi: 10.1007/BF01405704. [DOI] [PubMed] [Google Scholar]
- 39.Kim K, Koo BC, Delaflor RR, Shaikh BS. Pineal germinoma with widespread extracranial metastases. Diagn Cytopathol. 1985;1:118–22. doi: 10.1002/dc.2840010207. [DOI] [PubMed] [Google Scholar]
- 40.Talamo TS, Mendelow H. Primary intracranial germinoma with massive ventriculoperitoneal shunt metastases. J Surg Oncol. 1985;28:39–41. doi: 10.1002/jso.2930280111. [DOI] [PubMed] [Google Scholar]
- 41.Bamberg M, Metz K, Alberti W, Heckemann R, Schulz U. Endodermal sinus tumor of the pineal region. Metastases through a ventriculoperitoneal shunt. Cancer. 1984;54:903–6. doi: 10.1002/1097-0142(19840901)54:5<903::aid-cncr2820540525>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 42.Devkota J, Brooks BS, el Gammal T. Ventriculoperitoneal shunt metastasis of a pineal germinoma. Comput Radiol. 1984;8:141–5. doi: 10.1016/0730-4862(84)90051-9. [DOI] [PubMed] [Google Scholar]
- 43.Trigg ME, Swanson JD, Letellier MA. Metastasis of an optic glioma through a ventriculoperitoneal shunt. Cancer. 1983;52:599–601. doi: 10.1002/1097-0142(19830815)52:4<599::aid-cncr2820520403>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 44.Haimovic IC, Sharer L, Hyman RA, Beresford HR. Metastasis of intracranial germinoma through a ventriculoperitoneal shunt. Cancer. 1981;48:1033–6. doi: 10.1002/1097-0142(19810815)48:4<1033::aid-cncr2820480430>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 45.Kun LE, Tang TT, Sty JR, Camitta BM. Primary cerebral germinoma and ventriculoperitoneal shunt metastasis. Cancer. 1981;48:213–6. doi: 10.1002/1097-0142(19810701)48:1<213::aid-cncr2820480134>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 46.Triolo PJ, Schulz EE. Metastatic germinoma (pinealoma) via a ventriculoperitoneal shunt. AJR Am J Roentgenol. 1980;135:854–5. doi: 10.2214/ajr.135.4.854. [DOI] [PubMed] [Google Scholar]
- 47.Pasquier B, Pasquier D, N'Golet A, Panh MH, Couderc P. Extraneural metastases of astrocytomas and glioblastomas: Clinicopathological study of two cases and review of literature. Cancer. 1980;45:112–25. doi: 10.1002/1097-0142(19800101)45:1<112::aid-cncr2820450121>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 48.Oberbauer RW, Tritthart H, Ascher PW, Walter GF, Becker H. Shunt metastases in posterior fossa tumors. Neuropadiatrie. 1979;10:296–300. doi: 10.1055/s-0028-1085332. [DOI] [PubMed] [Google Scholar]
- 49.Wood BP, Haller JO, Berdon WE, Lin SR. Shunt metastases of pineal tumors presenting as a pelvic mass. Pediatr Radiol. 1979;8:108–9. doi: 10.1007/BF00974001. [DOI] [PubMed] [Google Scholar]
- 50.Wilson ER, Takei Y, Bikoff WT, O'Brien MS, Tindall GT, Boehm WM. Abdominal metastases of primary intracranial yolk sac tumors through ventriculoperitoneal shunts: Report of three cases. Neurosurgery. 1979;5:356–64. [PubMed] [Google Scholar]
- 51.Eralp Y, Saip P, Aydin Z, Berkman S, Topuz E. Leptomeningeal dissemination of ovarian carcinoma through a ventriculoperitoneal shunt. Gynecol Oncol. 2008;108:248–50. doi: 10.1016/j.ygyno.2007.09.024. [DOI] [PubMed] [Google Scholar]
- 52.Harada K, Nishizaki T, Kwak T, Fujisawa H, Nishikawa M, Ito H. Intracranial metastasis of Wilms' tumor involving the tectal plate without pulmonary involvement. Case report. Pediatr Neurosurg. 1999;30:331–4. doi: 10.1159/000028819. [DOI] [PubMed] [Google Scholar]
- 53.Reynolds AF, Weinstein PR, Johnson PC. Adenocarcinoma cells trapped on a millipore filter in a patient with meningeal carcinomatosis. Neurosurgery. 1980;7:179–81. doi: 10.1227/00006123-198008000-00013. [DOI] [PubMed] [Google Scholar]
- 54.Halperin EC, Samulski T, Oakes WJ, Friedman HS. Fabrication and testing of a device capable of reducing the incidence of ventricular shunt promoted metastasis. J Neurooncol. 1996;27:39–46. doi: 10.1007/BF00146082. [DOI] [PubMed] [Google Scholar]
- 55.Lwin S, Low SW, Choy DK, Yeo TT, Chou N. External ventricular drain infections: Successful implementation of strategies to reduce infection rate. Singapore Med J. 2012;53:255–9. [PubMed] [Google Scholar]
- 56.O'Brien DF, Hayhurst C, Pizer B, Mallucci CL. Outcomes in patients undergoing single-trajectory endoscopic third ventriculostomy and endoscopic biopsy for midline tumors presenting with obstructive hydrocephalus. J Neurosurg. 2006;105(3 Suppl):219–26. doi: 10.3171/ped.2006.105.3.219. [DOI] [PubMed] [Google Scholar]
- 57.Matsumoto J, Kochi M, Morioka M, Nakamura H, Makino K, Hamada J, et al. A long-term ventricular drainage for patients with germ cell tumors or medulloblastoma. Surg Neurol. 2006;65:74–80. doi: 10.1016/j.surneu.2005.04.036. [DOI] [PubMed] [Google Scholar]


