Abstract
The past few years have seen increasing support for gross total resection in the management of low-grade gliomas (LGGs), with a greater extent of resection correlated with better overall survival, progression-free survival, and time to malignant transformation. There is consistent evidence in literature supporting extent of safe resection as a good prognostic indicator as well as positively affecting seizure control, symptomatic relief in pressure symptoms, and longer progression-free and total survival. The operative goal in most LGG cases is to maximize the extent of resection for these benefits while avoiding postoperative neurologic deficits. Several advanced invasive and noninvasive surgical techniques such as intraoperative magnetic resonance imaging (MRI), fluorescence-guided surgery, intraoperative functional pathway mapping, and neuronavigation have been developed in an attempt to better achieve maximal safe resection. We present a case of LGG in a young patient with a 5-year history of refractory seizures and gradual onset walking difficulty. Serial MRI brain scans revealed a progressive increase in right frontal tumor size with substantial edema and parafalcine herniation. Noninvasive brain mapping by functional MRI (fMRI) and sleep-awake-sleep type of anesthesia with endotracheal tube insertion was utilized during an awake craniotomy. Histopathology confirmed a Grade II oligodendroglioma, and genetic analysis revealed no codeletion at 1p/19q. Neurological improvement was remarkable in terms of immediate motor improvement, and the patient remained completely seizure free on a single antiepileptic drug. There is no radiologic or clinical evidence of recurrence 6 months postoperatively. This is the first published report of an awake craniotomy for LGG in Pakistan. The contemporary concept of supratotal resection in LGGs advocates generous functional resection even beyond MRI findings rather than mere excision of oncological boundaries. This relatively aggressive approach is only possible with an awake craniotomy, which ensures preservation of functional status and thus less postoperative morbidity and better outcomes. Noninvasive mapping for intracranial space-occupying lesions, including fMRI and blood-oxygen-level dependent (BOLD) imaging modality, is an essential tool in a resource-limited setting such as Pakistan.
Keywords: Awake craniotomy, brain mapping, low-grade glioma, oligodendroglioma, Pakistan
Introduction
Low-grade gliomas (LGG), defined by the World Health Organization as Grade I and Grade II glial origin brain tumors, include ependymomas, pilocytic astrocytomas, pleomorphic xanthoastrocytomas, diffuse astrocytomas, oligodendrogliomas, and mixed gliomas. Although not uncommon in clinical neurosurgical practice, they remain challenging in terms of management strategies.[1,2] Currently available treatment options include conservative therapy, fractionated radiotherapy, surgical resection, and chemotherapy. There is a growing consensus and increasing support that “gross total resection” (GTR) plays a vital role in the outcome of LGG. Outcome parameters such as improvement in survival time, functional recovery, delay in tumor recurrence rate, and time to malignant transformation are important factors in the consideration of treatment strategy, and there is consistent evidence in literature supporting extent of safe resection as a good prognostic indicator as well as positively affecting seizure control, symptomatic relief in pressure symptoms, and longer progression-free and total survival.[1,2,3,4]
Conversion to high-grade glioma, residual or recurrent tumor, and the need of adjuvant therapy remain the main concerns during management.[5,6] The subcortical location preferred by the tumor and extension along the cortico-subcortical plane is the usual source of recurrence and the reason for intraoperative tumor identification failure. There is growing evidence in support of GTR leading to better survival and seizure control[1] by eliminating tumor burden and mass effect. However, achieving GTR without compromising neurological function is not always realizable, and skepticism remains that maximizing the extent of resection may potentially lead to more frequent adverse events. Recent advancements in neurodiagnostic and microneurosurgical techniques have fortunately seen a significant improvement in surgical results.
Awake craniotomy is a technique, which augments the probability of better resection with preservation of functional status and thus better outcomes. It reduces the possibility of postoperative morbidity, facilitates early discharge from the hospital, and helps in achieving maximum possible resection of tumor in carefully selected patients.[7] Results from a recent study suggest that preoperative functional magnetic resonance imaging (fMRI) is a suitable clinical tool for patients diagnosed with LGG and emphasizes the strength of fMRI as a tool for the preoperative noninvasive mapping in LGG cases.[8] The blood-oxygen-level dependent (BOLD) imaging modality in addition to fMRI helps in improved outcome of patients presenting with intracranial space-occupying lesions.[9]
We present a case of LGG in a young patient with a 5-year history of refractory seizures and gradual onset walking difficulty. Serial magnetic resonance imaging (MRI) brain scans revealed a progressive increase in right frontal tumor size with substantial edema and parafalcine herniation.
Case Report
A 43-year-old right-handed gentleman, doctor by profession, with no known comorbid, was presented elsewhere initially with a 4-year history of generalized tonic–clonic seizures and a right frontal lesion and was diagnosed to have an LGG. Family history was not significant for tumors or epilepsy, and the patient opted for conservative management with anticonvulsants at this time. Despite his known lesion and tumor-related seizures, he volunteered to work in West Africa, where his seizures progressively became refractory and recurrent in nature, and he developed bilateral leg and left upper extremity weakness. He was started on high-dose steroids in Ghana (West Africa) and presented to us soon after with a fumbling gait, slurred speech, disturbed behavior, poor memory, and waning conscious levels. Preoperative MRI brain scan was promptly performed, which showed a significant increase in the size of the right frontal lesion with minimal enhancement, significantly increased edema, and subfalcine herniation [Figure 1].
Figure 1.
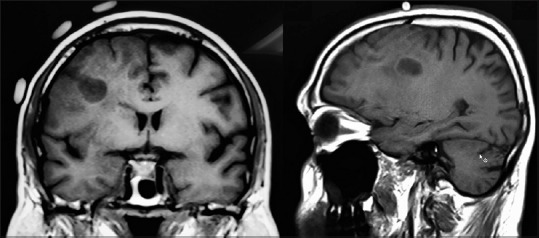
Preoperative magnetic resonance imaging with markers
Subsequently, fMRI was performed preoperatively for noninvasive brain mapping, revealing an 8 mm interface of the tumor's posterior margin to the motor area [Figure 2].
Figure 2.
Sagittal and coronal sections of functional magnetic resonance imaging brain
MRI-based scalp markers were utilized in the planning of the craniotomy incision. Sleep-awake-sleep pattern of anesthesia was chosen for right frontal craniotomy. Microscopic middle frontal gyrus and inferior frontal gyrus excision without jeopardizing cortical venous channels under microscopic vision were performed with the help of awake motor movement confirmation during the critical tumor resection phase. Frozen section confirmed LGG [Figure 3].
Figure 3.
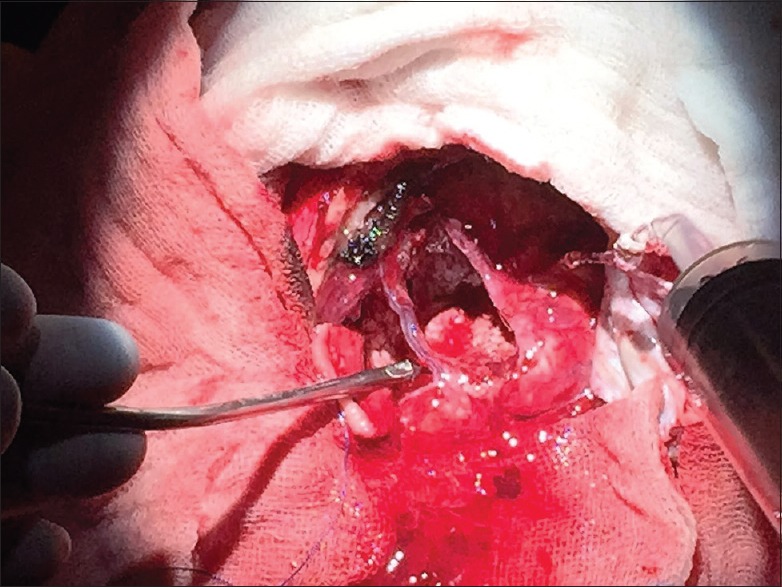
Resection cavity showing intact superior frontal sulcal and inferior frontal sulcal veins
Operative course was uneventful with no seizures; hemostasis in the resection cavity was achieved without any difficulty; durotomy and cranial flap replaced, and extubation was successful with an optimal postoperative course in neurointensive care unit (ICU) [Figure 4].
Figure 4.

Immediate postoperative computed tomography brain scan
Later, histopathology confirmed a Grade II oligodendroglioma, and genetic analysis revealed no codeletion at 1p/19q. A 3-Tesla MRI (3T MRI) brain scan with contrast within 36 h postoperatively showed no residual tumor and resolved edema. Clinically, leg and arm weakness resolved completely rendering the patient New York Heart Association functional class I, who went back to work as a general physician in Muzaffarabad, Kashmir. A second 3T MRI brain scan reaffirmed earlier status of tumor-free resection cavity at about 6 months postoperatively. One year down the line, the patient has not experienced a single seizure postoperatively. The oncologist recommended a “wait and watch” policy for adjuvant treatment and linked this decision to any clinical/radiological evidence of tumor recurrence [Figure 5].
Figure 5.
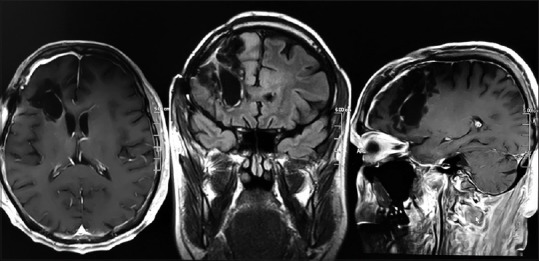
Magnetic resonance imaging brain 6 months postoperatively
Discussion
Invasive cortical mapping both surrounding the lesion deep in subfascial subcortical planes and at the surface is an informative and reliable technique. Unfortunately, in developing countries, these invasive facilities are not widely available and thus rarely practiced. The major challenges facing neurosurgeons in Pakistan are the lack of resources, unavailable technical expertise, and suboptimal invasive monitoring tools.
In our case, we utilized noninvasive brain mapping by fMRI and MRI markers as part of the preoperative workup. Sleep-awake-sleep type of anesthesia with endotracheal tube insertion enabled the patient to actively respond during the most vulnerable part of brain tumor resection. A frozen section was also done intraoperatively to verify the histopathological diagnosis.
The timing of postoperative MRA brain and its magnetic strength can influence the results and hence the claim of the extent of resection. We made sure that a 3T MRI brain with contrast was performed within 36 h of surgery, and this, revealing no residual tumor, forms the basis of our claim of total resection. Radiologic or clinical recurrence of tumor was not present 6 months postoperatively. We further plan to do annual MRI brain with contrast, provided that the patient remains symptom free. This follow-up strategy is thought to aid in diagnosing possible late-onset recurrences[10] and the need of adjuvant treatment in such a scenario, as advised by the oncology team.
Uneventful perioperative and postoperative course with <4 h postoperative ventilation and <16 h total ICU stay were important parameters noted in the management course. Neurological improvement was remarkable in terms of immediate motor improvement, and the patient remained completely seizure free on a single antiepileptic drug. Histopathology confirmed the diagnosis of oligodendroglioma Grade II; genetic analysis revealed no codeletion at 1p/19q, which is unfavorable in terms of prognosis. However, based on GTR, the oncologist proposed to wait and watch with adjuvant therapy (chemotherapy and/or radiation) reserved for any probable recurrence in the future.
We subscribe to Duffau's[11] concept of supratotal resection in LGG, which advocates that generous functional resection even beyond MRI findings rather than excision of oncological boundaries is the future of neuro-oncology practice. We achieved excellent tumor resection, despite the constraints and limitations faced in our current practice, thereby improving the quality of life. A seizure-free status, functional improvement, and the ability to resume baseline activities in our patients are encouraging and promising.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Aghi MK, Nahed BV, Sloan AE, Ryken TC, Kalkanis SN, Olson JJ. The role of surgery in the management of patients with diffuse low grade glioma: A systematic review and evidence-based clinical practice guideline. J Neurooncol. 2015;125:503–30. doi: 10.1007/s11060-015-1867-1. [DOI] [PubMed] [Google Scholar]
- 2.Turkoglu E, Gurer B, Sanli AM, Dolgun H, Gurses L, Oral NA, et al. Clinical outcome of surgically treated low-grade gliomas: A retrospective analysis of a single institute. Clin Neurol Neurosurg. 2013;115:2508–13. doi: 10.1016/j.clineuro.2013.10.010. [DOI] [PubMed] [Google Scholar]
- 3.Youland RS, Schomas DA, Brown PD, Nwachukwu C, Buckner JC, Giannini C, et al. Changes in presentation, treatment, and outcomes of adult low-grade gliomas over the past fifty years. Neuro Oncol. 2013;15:1102–10. doi: 10.1093/neuonc/not080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Youland RS, Khwaja SS, Schomas DA, Keating GF, Wetjen NM, Laack NN. Prognostic factors and survival patterns in pediatric low-grade gliomas over 4 decades. J Pediatr Hematol Oncol. 2013;35:197–205. doi: 10.1097/MPH.0b013e3182678bf8. [DOI] [PubMed] [Google Scholar]
- 5.Cochereau J, Herbet G, Duffau H. Patients with incidental WHO grade II glioma frequently suffer from neuropsychological disturbances. Acta Neurochir (Wien) 2016;158:305–12. doi: 10.1007/s00701-015-2674-3. [DOI] [PubMed] [Google Scholar]
- 6.Cochereau J, Herbet G, Rigau V, Duffau H. Acute progression of untreated incidental WHO Grade II glioma to glioblastoma in an asymptomatic patient. J Neurosurg. 2016;124:141–5. doi: 10.3171/2014.12.JNS141851. [DOI] [PubMed] [Google Scholar]
- 7.Duffau H. Awake surgery for brain gliomas: Plea for an increased involvement of anesthesiologists. Ann Fr Anesth Reanim. 2012;31:e81–6. doi: 10.1016/j.annfar.2012.04.009. [DOI] [PubMed] [Google Scholar]
- 8.Morrison MA, Churchill NW, Cusimano MD, Schweizer TA, Das S, Graham SJ. Reliability of task-based fMRI for preoperative planning: A test-retest study in brain tumor patients and healthy controls. PLoS One. 2016;11:e0149547. doi: 10.1371/journal.pone.0149547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ahsan H, Akbar M, Bhatti AU. Application and advantage of functional magnetic resonance imaging and blood oxygen level dependant (BOLD) imaging modality. J Pak Med Assoc. 2009;59:794–6. [PubMed] [Google Scholar]
- 10.Rudà R, Bello L, Duffau H, Soffietti R. Seizures in low-grade gliomas: Natural history, pathogenesis, and outcome after treatments. Neuro Oncol. 2012;14(Suppl 4):iv55–64. doi: 10.1093/neuonc/nos199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duffau H. A new concept of diffuse (low-grade) glioma surgery. Adv Tech Stand Neurosurg. 2012;38:3–27. doi: 10.1007/978-3-7091-0676-1_1. [DOI] [PubMed] [Google Scholar]



