Abstract
The role of language in communication plays a crucial role in human development and function. In patients who have a surgical lesion at the functional language areas, surgery should be intricately planned to avoid incurring further morbidity. This normally requires extensive functional and anatomical mappings of the brain to identify regions that are involved in language processing and production. In our case report, regions of the brain that are important for language functions were studied before surgery by employing (a) extraoperative methods such as functional magnetic resonance imaging, transmagnetic stimulation, and magnetoencephalography; (b) during the surgery by utilizing intraoperative awake surgical methods such as an intraoperative electrical stimulation; and (c) a two-stage surgery, in which electrical stimulation and first mapping are made thoroughly in the ward before second remapping during surgery. The extraoperative methods before surgery can guide the neurosurgeon to localize the functional language regions and tracts preoperatively. This will be confirmed using single-stage intraoperative electrical brain stimulation during surgery or a two-stage electrical brain stimulation before and during surgery. Here, we describe two cases in whom one has a superficial lesion and another a deep-seated lesion at language-related regions, in which language mapping was done to preserve its function. Additional review on the neuroanatomy of language regions, language network, and its impairment was also described.
Keywords: Awake surgery, brain mapping, brain network, language mapping
Introduction
Language is an important aspect of human cognition. It signifies how complex human beings are and this is reflected by our capabilities in expressing ideas and emotions, solving mathematical problems, retrieving memories, and even in hiding the truth. These functions are sufficient to make us aware that language has vast connections with various parts of the brain (cortical and subcortical regions), particularly auditory areas, visual areas, areas concerned with the hands and mouth, memory, limbic system, insular region, corpus striatum, cerebellum, and several other areas in the frontal and parietal lobes. The left hemisphere is thought of as the dominant hemisphere for language in the majority of patients, but lately, the contralateral right hemisphere has been shown to have some language functions.[1,2,3] The afferents for language system mainly arise from ears and eyes, and in some people from the hands or feet; subsequently, it is processed further in many parts of the brain as mentioned above. The main efferents for language originate mainly from the frontal lobes: mouth and face, hand or leg motor skill areas. Based on these complex connections, one should study not only the anatomical and functional aspects of the brain for language but also try to gain some understanding in language processing and language evolution in animals. Anatomical language areas were initially described by Pierre Paul Broca in 1861 involving areas at the posterior part of the inferior frontal gyrus (Brodmann's 44 and 45) which was later validated by further studies and which expanded on the initial knowledge on language expression.[4,5] A few years later, in 1876, Karl Wernicke described another type of language disorder, involving a failure to comprehend language rather than a failure to speak. Location of the lesion in Wernicke's patient was at the junction of the temporal, parietal, and occipital lobes, which is now called Wernicke–Geschwind area - the cortical areas surrounding the distal end of the Sylvian fissure (supramarginal gyrus and part of inferior parietal lobule) and the area surrounding the superior temporal sulcus (angular gyrus).[5,6]
Current studies on language utilize functional magnetic resonance imaging (MRI), transmagnetic stimulation, magnetoencephalography (MEG), and electrical brain stimulation in awake patients for language mapping, whereas tractography and diffusion tensor imaging give additional anatomical knowledge related to white matter tracts for language processing.[7,8,9,10,11,12,13,14] Currently, seven tracts are thought to be important in language processing and function: arcuate fasciculus (AF), superior longitudinal fasciculus (SLF branches II and III), inferior fronto-occipital fasciculus (IFOF), uncinate fasciculus (UF), middle longitudinal fasciculus (MLF), and inferior longitudinal fasciculus (ILF). Surgery in areas and tracts involved in language connectivity can cause further morbidity to the patient. Therefore, surgery in these areas should aim to preserve the language-related anatomy and function and hence reduce further morbidity. Here, we describe our preoperative and intraoperative surgical techniques to preserve language function in two patients who harbored a surgical lesion in dominant language areas: one superficially located and the other, a deep-seated one. Further literature review on language was made to have a better understanding of its network, development, and impairment.
Case Reports
Case 1
A 54-year-old right-handed male was noted by his wife to have changes in his behavior for the past 1 month, in which he became less talkative, responding to questions only with words, not in sentences. The cognitive and clinical assessment revealed marked expressive aphasia with nonfluent speech, impairment in naming and repetition, and mild facial weakness affecting the lower part of the right side of the face and ipsilateral mild limb weakness. The MRI of the brain disclosed a tumoral lesion at the left frontal lobe [Figure 1a and b]. The planned procedure was explained to the patient and consent was taken.
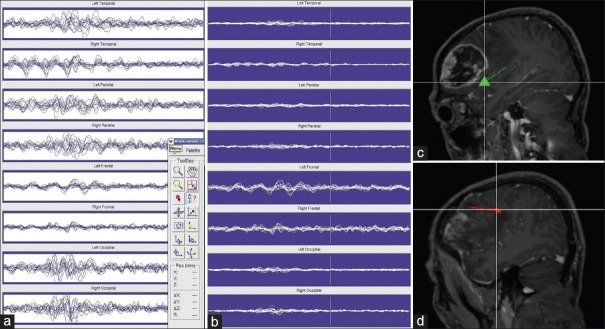
Figure 1.
(a and b) Magnetic resonance imaging of the brain disclosed a tumoral lesion at the left frontal lobe. Image fusion between magnetic resonance imaging and 71-region (c) and Brodmann cortical brain atlas (d) using MATLAB anatomical software. The lesion seems to affect the areas concerned with speech: Brodmann areas 44 and 45, and largely the middle and inferior frontal gyrus or operculum
Extraoperative language-related mapping
The MRI images were then fused with the 71-region and Brodmann cortical brain atlas using an in-house Matlab-based MEG-pipeline programme was used to analyse the MEG data. This was accomplished with statistical parametric mapping (SPM)-based Matlab 7.4–R2008a (MathWorks Inc., Natick, MA, USA). The lesion seemed to affect the areas concerned with speech: Brodmann areas 44 and 45 and largely the middle and inferior frontal gyrus or operculum [Figure 1c and d]. Fractional anisotropy maps showed marked alteration in white matter tracts connecting the left temporoparietal with the ipsilateral frontal lobe [Figure 1b]. The obvious tracts involved were the AF, IFOF, and SLF branch II and III [Figure 2]. MEG was used to localize the areas concerned with speech using equivalent current dipole technique analyzing the brainwaves' oscillations between 200 and 400 ms in the frontal lobe (after suppressing the oscillations in other lobes) [Figure 3a and b]. The areas concerned with silent picture naming [Figure 3c] and spoken language were also identified [Figure 3d].
Figure 2.
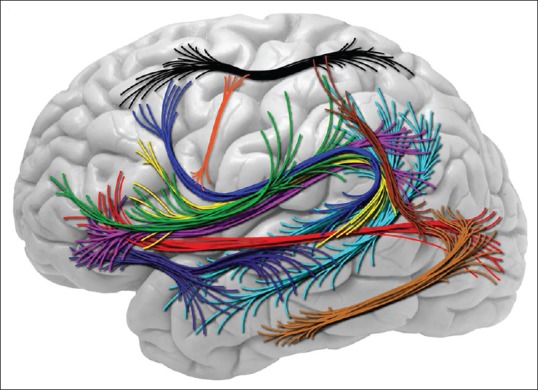
White matter tracts involved in language are arcuate fasciculus (dark blue, yellow), inferior fronto-occipital fasciculus (red), superior longitudinal fasciculus branch I (black); II (magenta/pink) and III (green), uncinate fasciculus (purple), middle (cyan/light blue) and inferior longitudinal fasciculus (light brown). Additional fascicles are also shown: Occipitotemporal motion areas to the intraparietal sulcus (dark brown) and frontal aslant tract is the newly described tract that traverses along the motor gyrus connecting facial laterally to limb-genital region medially (orange)
Figure 3.
(a and b) Magnetoencephalography localization for silent picture naming of speech using equivalent current dipole technique analyzing the brainwaves oscillations between 200 and 400 ms in the frontal lobe. The cortical areas for silent picture naming (c) and spoken language (d) were identified
Intraoperative language-related mapping and surgical strategy
The extraoperative language-related mapping data were then transferred to the intraoperative navigation system (Medtronics S7). The patient was initially operated under general anesthesia, and partial removal of visualized tumor was done for histopathological examination which later disclosed a glioblastoma multiforme (GBM) [Figure 4a]. Grid electrodes were put onto the surface, which were identified as language areas in our initial extraoperative mapping. The patient was then transferred to neurointensive care, and on the following day, detailed mapping of the expressive language areas was made using the grid-stimulatory method (grid electrodes as an anode and a cathode). We successfully identified and confirmed the expressive language area by asking the patient to count and name objects. Patient stopped or was unable to do those tasks at stimulation parameters of 10–20 trains of 2–3 mA, 50 Hz, and 1000 us pulse width involving electrodes no 9, 10, 11, 17, 18, 19, 25, 26, 27, and 28 [Figure 4a]. Of note, these areas seemed to correspond with the extraoperative MEG language-related mapping. With all these data, the neurosurgeon then proceeded with further removal of the GBM under an awake state but sparing the language-related areas [Figure 4b]. After more aggressive surgery, the tumoral bed was irradiated with intraoperative radiation therapy, and later, he received further adjuvant chemotherapy [Figure 4c]. At 1-month postsurgery, his speech improved from words to full sentences and weakness of the face and limbs disappeared gradually. The postoperative image at 4 months is provided [Figure 4d].
Figure 4.
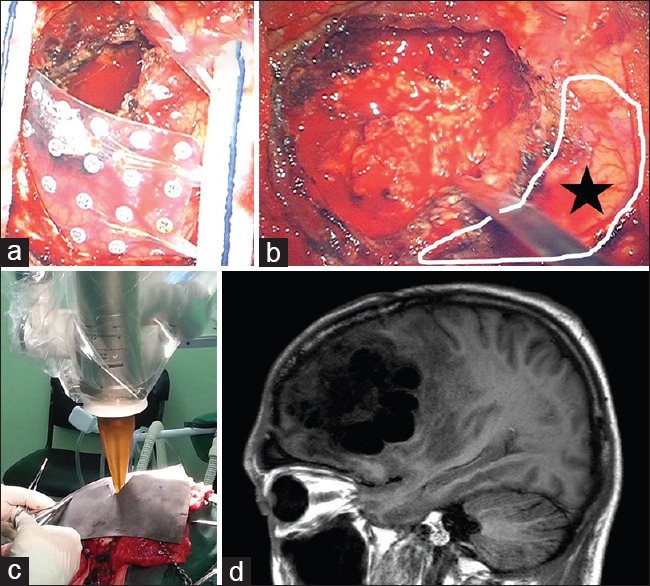
(a) Patient was initially operated under general anesthesia, and partial removal of seen tumor was removed for histopathological examination. Grid electrodes were laid onto the surface which was identified as language area on extraoperative neuronavigated data. (b) Further removal of the tumor was made under awake state and spared the language-related areas. (c) After aggressive surgery, the tumoral bed was irradiated with intraoperative radiation therapy. (d) The postoperative magnetic resonance imaging at four months
Case 2
A 19-year-old girl was admitted following several sudden episodes of headache and associated with right limb weakness. She was able to communicate in full sentences but did have slight slurring of speech and admitted to having difficulty in finding the right words to speak. The MRI of the brain showed a popcorn-cavernoma-like lesion medial and posterior to the left insular lobe adjacent to the descending corticospinal tract [Figure 5a]. The planned procedure was explained to the patient and consent was taken.
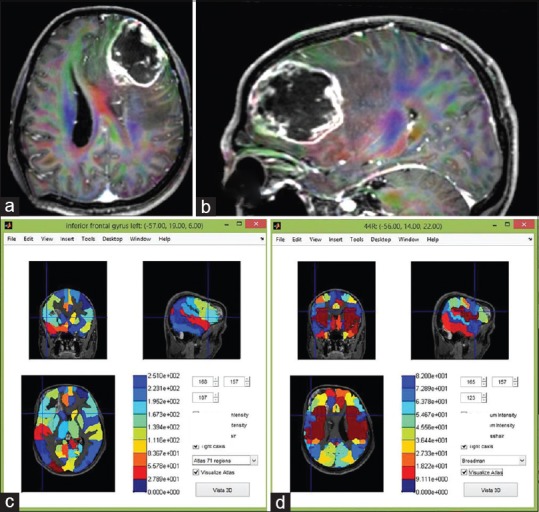
Figure 5.
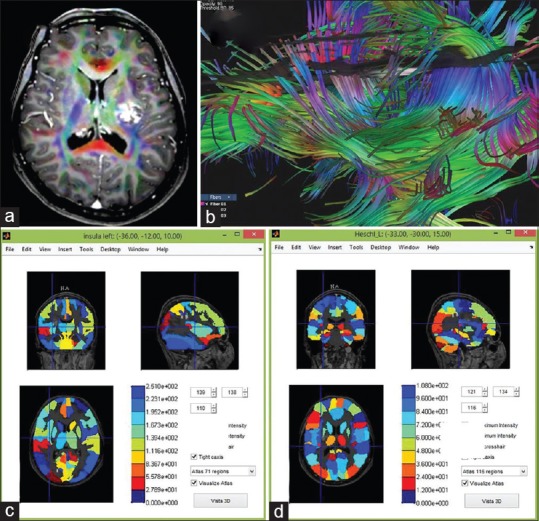
(a) Magnetic resonance imaging of the brain showed a popcorn-cavernoma like feature at medial and posterior to the left insular lobe adjacent to the descending corticospinal tract. (b) The cranial tractography revealed the cavernoma is surrounded by the corticospinal tract, AF, SLF II and III, middle longitudinal fasciculus, and inferior fronto-occipital fasciculus. Magnetic resonance imaging fusion with 71- (c) and 116-regions cortical brain atlas (d) using MATLAB anatomical software. The cavernoma is situated closed to the left insula and Heschl gyrus
Extraoperative language-related mapping
The cranial tractography revealed a cavernoma surrounded by the corticospinal tract, AF, SLF II and III, MLF, and IFOF [Figure 5b]. MRI images were then fused with 71 and 116-regions cortical brain atlas using MATLAB anatomical software. The cavernoma was situated close to the left insula and Heschl's gyrus [Figure 5c and d]. The MEG was used to localize the eloquent areas in the left cerebral hemisphere (sensorimotor and hearing areas).
Intraoperative language-related mapping and surgical strategy
Under awake surgery [Figure 6a] and based on the extraoperative data, further mapping was made using bipolar Ojemann stimulator to localize the important tracts for speech and areas representing the right lower face. To configure the anatomy of the tract, evoked responses were noted in some grid electrodes whenever the tract was stimulated proximal or distal to the grid at low ampere to give evoked responses taking care to avoid causing persistent discharges or clinical seizures [Figure 6b and c]. The face area was identified using bipolar stimulator and facial electromyography (EMG). The approach to remove the cavernoma was made through left distal transsylvian approach [Figure 6d and e]. The histology confirmed the cavernoma [Figure 6f]. Her speech and limb weakness gradually recovered a month after surgery.
Figure 6.
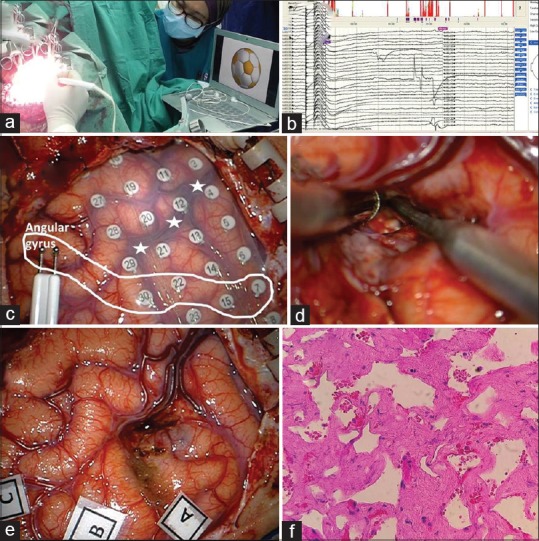
(a) Awake surgery in lateral position with language testing. (b and c) Based on extraoperative data, further mapping was made using bipolar Ojemann stimulator to localize the important tracts for speech and area representing the right lower face. To configure the anatomy of the tract, evoked responses were noted in some grid electrodes whenever the tract was stimulated proximal or distal to the grid at low ampere to give enough evoked responses. (d and e) The distal transsylviann approach to remove the cavernoma. (f) Histology confirmed the cavernoma
Discussion
A review on language network: Anatomy, development, and impairment
Two distinct lobes of the dominant (commonly left) cerebral hemisphere, the temporal and frontal lobes are related to language. There is a network connecting the temporal with the frontal language areas. This network involves several cortical gray matter areas and white matter tracts with two major streams (similar to the two visual streams): (a) the dorsal stream for language involving AF (the most important dorsal stream tract), SLF (branches II and III), and MLF; (b) the ventral stream involving IFOF (the most important ventral stream tract), UF, and ILF.[15] Definite gray matter areas involved in language are debated and are also postulated to involve both hemispheres.[1,2] Nonetheless, Brodmann's area 44, 45, supramarginal gyrus (the gyrus that caps the distal end of exposed Sylvian fissure), angular gyrus (the gyrus that caps the distal end of superior temporal sulcus), and dominant mesial temporal lobe are known to participate in some forms of language production and thought to be vital in language processing.[4,5,6] Therefore, this manuscript emphasizes on the discussion involving the regions and tracts mentioned above.
Child language development tells us a lot about the function of these two lobes. Progressive language development in a child starts mainly with facial expression, sounds, and later on, words. This initial part of language development is known as “lexical-semantic language” (lexical for sound, auditory, word; and semantic for meaning) and has its roots in animal communication systems. By having this type of communication system, humans can, therefore, be viewed as evolutionary. However, at the subsequent part of language development which begins around the age of 12 months onward, the child starts to acquire words and also has the ability to repeat words as a result of the maturation of the arcuate fasciculus. Later when a larger vocabulary is acquired and stored in mesial structures, the child can start to combine them, initially with two words, then three and more words with grammatical function.[16] Consequently, there is a steady progression in language development. This subsequent language development is known as “grammatical language” and is not seen in animals. Interestingly, the emergence of grammatical language seems to correspond with the emergence of so-called “metacognitive executive functions” such as problem-solving, concept formation, strategy development, controlling attention, working memory, planning, and judgment.[17,18] Therefore, grammatical language development runs parallel with the development and maturation of the frontal lobe. Remarkably, humans differ from animals not only in this language development but also in the microgravity position of the body; animals tend to be in horizontal or in curved positions which could have an evolutionary ancestor deep in the ocean (the largest microgravity or buoyant space on earth), whereas humans are mainly in gravity or vertical position, except at early development of the nervous system; embryo, early gestational age (microgravity due to buoyancy), and later at old age, suggesting humans origin could also be coming from a microgravity environment but not from the ocean because of the two main differences: (a) language-related frontal lobe development and (b) body position.[19]
Language and memory are closely related. Memory ensures the development and production of language occurs normally, and this is obviously noted in patients who have had mesial temporal lobe epilepsy surgery, in which language and memory dominancy must be ascertained beforehand. Deleted memories can affect language functions. Therefore, one must have some knowledge in memory concepts to understand language function optimally. There are two memory systems associated with language: (a) declarative/explicit (learning) memory and (b) procedural/implicit memory (acquired incidentally).[20,21,22] Lexical-semantic aspect of the language is “explicitly learned” and represents a type of knowledge we are aware of (declarative memory). It depends on retrorolandic cortical structures (including temporal lobe) and the hippocampus. Grammar (set of structural rules governing the composition of sentences: syntax-words-morphology) is “acquired incidentally.” Procedural or implicit memory is for grammar. Procedural grammatical learning (skilled articulatory acts and grammar) is related to the execution of sequences of elements used for speaking. Procedural memory is related with frontal-subcortical circuitries or lobes.
Based on these observations, one can generally say that the lexical or semantic language is dealt with mainly by the temporal lobe, whereas the grammatical language is mostly dealt with by the frontal lobe. Hence, these two lobes must be connected to ensure well-developed language development in a child: from sound, single words (lexical-semantic) to many words which are interconnected (grammatical). Thus, the previously mentioned seven white matter tracts (AF, SLF II and III, IFOF, UF, MLF, and ILF) are seen to be involved. In short, two main disturbances in language can happen based on these two language systems: (a) impairment in grammatical system and (b) impairment in lexical-semantic system.[16,23] Injury to the pars opercularis (Brodmann area 44)/triangularis (Brodmann area 45) tends to cause “content without grammar” type of grammatical system language impairment (before was viewed as Broca's aphasia). Patients found difficulty in making sentences or connecting the two words, manifested as impairment in finding the right words or poor expression. It means the frontal lobe or grammatical lobe is affected (higher cognitive functions). This feature is noted to happen in case one whereby the patient was able only to communicate in isolated words but later improved to full sentences after the surgery. On the other hand, injury to the area surrounding the supramarginal and/or angular gyrus, leads to “grammar without content” type of lexical-semantic system language impairment (before was viewed as Wernicke's aphasia). In this type of language impairment, the temporal lobe connectivity is affected; therefore, the lexical or semantic (sounds-words/meaning) components are affected. The lexical repertoire tends to decrease, and language understanding difficulties are evident. Patients manifest as having difficulty to understand but is seemingly able to converse in full sentences (intact grammatical language/system) which can sometimes be overused or grammar (sentences of two words or more are connected) is intact but lacking in their meaning (semantic) and words (lexical), difficulty in recalling the words (memory of the words), and poor association and poor acoustic discrimination in speech. Pure injury to AF (main tract in dorsal stream) can cause impairment in word-retrieving memory: manifested as a reduction in lexical-semantic repertoire or difficulty in finding the right words, poor repetition, and naming (as partly noted in case 2). With injury to UF or ILF (ventral stream), a compensatory phenomenon occurs with function normally taken over by the IFOF (the most important and longest associative bundle for language tract in ventral stream); but once injury to the IFOF occurs, there is no compensation. Thus, preserving the IFOF during surgery is thought to be important and commonly best executed under awake brain surgery.[24] In summary, neuroplasticity is applied commonly for gray matter but not for the white matter; and the white matter tracts have limited compensatory mechanism, therefore avoiding injury to the white matter is important during surgery.
Language mapping and surgical strategy
We describe two different approaches in managing a superficial lesion and a deep-seated lesion near the eloquent language-related areas. For a superficial lesion as in case one, the surgery was done in two stages: (1) first, patient was put under general anesthesia, the “visualized tumor” was debulked partially and sent for histopathological confirmatory diagnosis. Neuronavigation loaded with extraoperative mapping data was used to localize the eloquent areas. Grid electrodes were laid onto the areas concerned with language, identified initially by the extraoperative mapping data. Language tests were then done at the ward rather than in the operating theater. We preferred this method of language testing because of the calm and relaxed environment and without sedative effects from the anesthetic medications. Our extraoperative data for language seemed to have a good correlation with grid electrodes' tests done in the ward. (2) We proceeded with a second surgery after taking into consideration the results obtained in the ward (first through language mapping) and with further intraoperative language mapping (remapping) under awake state; therefore more aggressive surgery could be done. Using this two-stage approach, we had successfully preserved the patient's language areas.
For a deep-seated lesion in language areas, as in case two, detailed anatomical mapping including tracts mapping and functional sensorimotor gray matter mapping were obviously important. The surgical approach for a deep-seated lesion is to avoid additional damage to the gray and white matters of the normal brain by the selected approach. The transsylvian approach was chosen to achieve this goal. From an anatomical perspective, the lesion situated at the left retrorolandic area is closely related to the eloquent cortices and commonly surrounded by several major white matter tracts. The transsylvian is probably the best approach but should be done carefully and gently because splitting of the distal half of the Sylvian fissure is not as easy as splitting the proximal half. Neuronavigation is again important to track the areas of interest, and under awake state, bipolar or grid stimulation can further map the eloquent areas and anatomical configuration for the white matter tracts. These two described approaches are currently our preferred approach to managing lesions in the dominant hemisphere for language.
Conclusions
Language mapping should be done for surgical lesions in the dominant (commonly left) hemisphere. The extraoperative language mapping seems as important because it can help the neurosurgeon to localize early the areas of interest and therefore further intraoperative mapping can be done quickly and optimally. By studying the language-related anatomy and functional data obtained from the patient, one can have a better understanding in language functions, and with further current literature review, one can also have a better understanding of language development and its systems.
Financial support and sponsorship
This study was supported by short-term grants (Ref: 304/PPSP/61312142) from Universiti Sains Malaysia (USM) and approved by Human Research Ethics Committee USM, School of Medical Sciences, Kubang Kerian, Kelantan, Malaysia.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Cogan GB, Thesen T, Carlson C, Doyle W, Devinsky O, Pesaran B. Sensory-motor transformations for speech occur bilaterally. Nature. 2014;507:94–8. doi: 10.1038/nature12935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gandour J, Tong Y, Wong D, Talavage T, Dzemidzic M, Xu Y, et al. Hemispheric roles in the perception of speech prosody. Neuroimage. 2004;23:344–57. doi: 10.1016/j.neuroimage.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 3.Tzourio N, Crivello F, Mellet E, Nkanga-Ngila B, Mazoyer B. Functional anatomy of dominance for speech comprehension in left handers vs. right handers. Neuroimage. 1998;8:1–16. doi: 10.1006/nimg.1998.0343. [DOI] [PubMed] [Google Scholar]
- 4.Dronkers NF, Plaisant O, Iba-Zizen MT, Cabanis EA. Paul Broca's historic cases: High resolution MR imaging of the brains of Leborgne and Lelong. Brain. 2007;130(Pt 5):1432–41. doi: 10.1093/brain/awm042. [DOI] [PubMed] [Google Scholar]
- 5.1st ed. San Diego, California, USA: Academic Press Elsevier; 2014. Historical background. In: Neuroanatomy of Language Regions of the Human Brain; pp. 1–17. [Google Scholar]
- 6.Nieuwenhuys R, Voogd C, Van Huijzen C. 4th ed. Wurzburg, Germany: Springer-Verlag Berlin Heidelberg; 2008. Telencephalon: Neocortex in greater limbic system; pp. 491–649. [Google Scholar]
- 7.Bizzi A, Nava S, Ferrè F, Castelli G, Aquino D, Ciaraffa F, et al. Aphasia induced by gliomas growing in the ventrolateral frontal region: Assessment with diffusion MR tractography, functional MR imaging and neuropsychology. Cortex. 2012;48:255–72. doi: 10.1016/j.cortex.2011.11.015. [DOI] [PubMed] [Google Scholar]
- 8.de Bruin A, Roelofs A, Dijkstra T, Fitzpatrick I. Domain-general inhibition areas of the brain are involved in language switching: FMRI evidence from trilingual speakers. Neuroimage. 2014;90:348–59. doi: 10.1016/j.neuroimage.2013.12.049. [DOI] [PubMed] [Google Scholar]
- 9.Kim SH, Lee DG, You H, Son SM, Cho YW, Chang MC, et al. The clinical application of the arcuate fasciculus for stroke patients with aphasia: A diffusion tensor tractography study. NeuroRehabilitation. 2011;29:305–10. doi: 10.3233/NRE-2011-0706. [DOI] [PubMed] [Google Scholar]
- 10.Krieg SM, Tarapore PE, Picht T, Tanigawa N, Houde J, Sollmann N, et al. Optimal timing of pulse onset for language mapping with navigated repetitive transcranial magnetic stimulation. Neuroimage. 2014;100:219–36. doi: 10.1016/j.neuroimage.2014.06.016. [DOI] [PubMed] [Google Scholar]
- 11.Lei M, Akama H, Murphy B. Neural basis of language switching in the brain: FMRI evidence from Korean-Chinese early bilinguals. Brain Lang. 2014;138:12–8. doi: 10.1016/j.bandl.2014.08.009. [DOI] [PubMed] [Google Scholar]
- 12.Matsuda R, Coello AF, De Benedictis A, Martinoni M, Duffau H. Awake mapping for resection of cavernous angioma and surrounding gliosis in the left dominant hemisphere: Surgical technique and functional results: Clinical article. J Neurosurg. 2012;117:1076–81. doi: 10.3171/2012.9.JNS12662. [DOI] [PubMed] [Google Scholar]
- 13.Pylkkänen L, Bemis DK, Blanco Elorrieta E. Building phrases in language production: An MEG study of simple composition. Cognition. 2014;133:371–84. doi: 10.1016/j.cognition.2014.07.001. [DOI] [PubMed] [Google Scholar]
- 14.Song X, Dornbos D, 3rd, Lai Z, Zhang Y, Li T, Chen H, et al. Diffusion tensor imaging and diffusion tensor imaging-fibre tractograph depict the mechanisms of Broca-like and Wernicke-like conduction aphasia. Neurol Res. 2011;33:529–35. doi: 10.1179/016164111X13007856084322. [DOI] [PubMed] [Google Scholar]
- 15.Hickok G, Poeppel D. Dorsal and ventral streams: A framework for understanding aspects of the functional anatomy of language. Cognition. 2004;92:67–99. doi: 10.1016/j.cognition.2003.10.011. [DOI] [PubMed] [Google Scholar]
- 16.Ardila A. There are two different language systems in the brain. Journal of Behavioral and Brain Science. 2011;1:23–36. [Google Scholar]
- 17.Ardila A. On the evolutionary origins of executive functions. Brain Cogn. 2008;68:92–9. doi: 10.1016/j.bandc.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 18.Ardila A. Development of metacognitive and emotional executive functions in children. Appl Neuropsychol Child. 2013;2:82–7. doi: 10.1080/21622965.2013.748388. [DOI] [PubMed] [Google Scholar]
- 19.Idris Z, Muzaimi M, Ghani RI, Idris B. Principles, anatomical origin and applications of brainwaves: A review, our experience and hypothesis related to microgravity and the question on soul. J Biomedical Science and Engineering, 2014;7:435–445. [Google Scholar]
- 20.Fabbro F. The bilingual brain: Cerebral representation of languages. Brain Lang. 2001;79:211–22. doi: 10.1006/brln.2001.2481. [DOI] [PubMed] [Google Scholar]
- 21.Ullman MT. The declarative/procedural model of lexicon and grammar. J Psycholinguist Res. 2001;30:37–69. doi: 10.1023/a:1005204207369. [DOI] [PubMed] [Google Scholar]
- 22.Ullman MT. Contributions of memory circuits to language: The declarative/procedural model. Cognition. 2004;92:231–70. doi: 10.1016/j.cognition.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 23.Ullman MT, Pierpont EI. Specific language impairment is not specific to language: The procedural deficit hypothesis. Cortex. 2005;41:399–433. doi: 10.1016/s0010-9452(08)70276-4. [DOI] [PubMed] [Google Scholar]
- 24.Caverzasi E, Papinutto N, Amirbekian B, Berger MS, Henry RG. Q-ball of inferior fronto-occipital fasciculus and beyond. PLoS One. 2014;9:e100274. doi: 10.1371/journal.pone.0100274. [DOI] [PMC free article] [PubMed] [Google Scholar]