Abstract
Hemoglobin subunit alpha (HBA) expression in endothelial cells (ECs) has recently been shown to control vascular tone and function. We sought to elucidate the transcriptional regulation of HBA expression in the EC. Gain of KLF2 or KLF4 function studies led to significant induction of HBA in ECs. An opposite effect was observed in ECs isolated from animals with endothelial-specific ablation of Klf2, Klf4 or both. Promoter reporter assays demonstrated that KLF2/KLF4 transactivated the hemoglobin alpha promoter, an effect that was abrogated following mutation of all four putative KLF-binding sites. Fine promoter mutational studies localized three out of four KLF-binding sites (sites 2, 3, and 4) as critical for the transactivation of the HBA promoter by KLF2/KLF4. Chromatin immunoprecipitation studies showed that KLF4 bound to the HBA promoter in ECs. Thus, KLF2 and KLF4 serve as important regulators that promote HBA expression in the endothelium.
Keywords: endothelial cells, hemoglobin subunit alpha, Kruppel-like factors, myoendothelial junction
Introduction
Hemoglobin is expressed in both erythroid cells and a variety of non-erythroid cells, such as endothelial cells (ECs), macrophages, and epithelial cells.1 In erythroid cells, two alpha-globin chains assemble with two beta-globin chains to form adult hemoglobin heterotetramers with four heme prosthetic groups, which carry oxygen to tissues and transport carbon dioxide away from tissues to the lungs.2 Transcription factors such as KLF1, GATA1, and NFE2 play a role in the regulation of hemoglobin genes in erythroid cells.3–6 However, the mechanisms of hemoglobin regulation in non-erythroid cells remain unknown.
ECs are critical constituents of the blood vessel wall7, and their dysfunction is a proximate event in the development of numerous cardiovascular diseases.8–10 Previous studies have documented that hemoglobin subunit alpha (HBA) is expressed in ECs and enriched in the myoendothelial junction (MEJ) – a portion of ECs that protrudes through the internal elastic lamina and juxtaposes vascular smooth muscle cells (SMCs).11–13 These specialized structures serve as a conduit for communication between these two cell types, such as the passage of nitric oxide (NO) from ECs to SMCs, a process critical for the regulation of vascular tone and function.11,14,15 The MEJ is typically observed in smaller diameter vessels (e.g. small arteries, veins, or arterioles) as opposed to larger ones. Endothelial HBA functions to fine-tune NO diffusion to SMCs.11 The efficiency of NO scavenging by HBA requires its coupling to endothelial nitric oxide synthase (eNOS) and active heme iron redox cycling by cytochrome B5 reductase 3 (CYB5R3), which reduces the HBA heme iron from the Fe3+, non-reactive form, to the Fe2+ state, which is capable of blocking NO diffusion.11–13 While such post-translational regulation of HBA in ECs is well described, the transcriptional regulation of endothelial HBA remains unknown. Hence, elucidating the molecular mechanisms of how endothelial HBA is regulated may provide a deeper understanding of vascular homeostasis and how alterations in this process lead to cardiovascular disease.
Studies over the past decade from our group and others have identified Kruppel-like factors (KLFs), termed KLF2 and KLF4, as essential regulators of endothelial homeostasis, endothelial identity, and vascular integrity.16–18 KLFs are members of the zinc-finger family of DNA-binding transcription factors, of which a total of 18 mammalian family members have been identified to date.19 In ECs, KLF2 and KLF4 are induced by laminar flow and regulate the expression of many flow-dependent endothelial gene products (e.g. eNOS) that are critical for endothelial function.20–23 Furthermore, both factors have been shown to inhibit EC pro-inflammatory activation by virtue of their ability to inhibit key inflammatory mediators such as nuclear factor kappa-B (NFκB). Here we demonstrate that KLF2 and KLF4 serve as potent regulators of HBA expression in ECs.
Methods
Detailed methods are available as online supplemental materials. These include adenoviral infection and plasmid DNA transfection, EC isolation, RNA isolation, and real-time polymerase chain reaction (PCR), western blot, protein digestion, mass spectrometry, luciferase assay, site-directed mutagenesis, and chromatin immunoprecipitation. Real-time PCR and site-directed mutagenic oligonucleotide primers are listed in Table S1 and Table S2, respectively.
Animals
All animals used were the C57BL6/J mouse strain. Endothelial-specific Klf2 and/or Klf4 knockout mice were generated by breeding floxed mice (Klf2/Klf4) with tamoxifen-inducible Cdh5(PAC)-CreERT2 mice (CRE; originally from R Adams, University of Münster, Münster, Germany). Cdh5(PAC)-CreERT2 mice were used as controls. Male mice aged 8–10 weeks old were intraperitoneally injected with tamoxifen (2 mg/25 g) to trigger endothelial-specific gene deletion. All animal protocols carried out in this study were approved by the Case Western Reserve University Institutional Animal Care and Use Committee.
Cell culture and a vascular cell co-culture model
Human umbilical vein endothelial cells (HUVECs), human coronary artery endothelial cells (HCAECs), and human coronary artery smooth muscle cells (HCASMCs) were cultured in EBM-2 supplemented with an EGM-2 bullet kit, EBM-2 supplemented with an EGM-2MV bullet kit, and SmGM-2 supplemented with a SmGM-2 bullet kit (Lonza, Basel, Switzerland), respectively. Human umbilical vein smooth muscle cells (HUVSMCs) and bovine aortic endothelial cells (BAECs) were obtained from Cell Applications, Inc. (San Diego, CA, USA) and cultured in growth medium according to the manufacturer’s instruction. All cells were cultured at 37°C and 5% CO2. For a vascular cell co-culture model, ECs were co-cultured with vascular SMCs on a Transwell(R) (24 mm) (Corning Inc., NY, USA) to allow a formation of MEJs, as previously described.11,24 SMCs were first seeded on the lower part of the Transwell insert and cultured for 24 hours. In the same Transwell insert, ECs were cultured on the upper part of the insert and grown for an additional 24 hours. Following this initial period of growth, KLF2 or KLF4 expression was manipulated by adenoviral infection.
Statistics
All data are presented as mean ± standard error of the mean (SEM). Statistical analyses were done using the two-tailed Student’s t-test and one-way analysis of variance (ANOVA) with Bonferroni’s post hoc test to analyze the difference between two groups and among the groups, respectively. A p-value of 0.05 or less was considered significant.
Results
KLF2 and KLF4 induce HBA expression in the endothelium
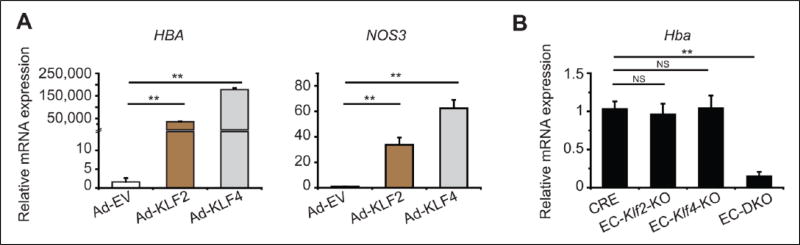
Given the importance of endothelial KLF2/KLF4 in maintaining endothelial homeostasis, endothelial identity, and vascular integrity, we first investigated whether KLF2/KLF4 mediated endothelial HBA expression. Adenoviral overexpression of KLF2 or KLF4 strongly induced HBA mRNA in HCAECs (Figure 1A). Increases in KLF2 and KLF4 mRNA expression were also confirmed in HCAECs (Figure S1, A and B). As a positive control,18 mRNA expression of NOS3 was examined and found to be significantly enhanced (Figure 1A). Further, the effect of KLF2 or KLF4 deficiency on Hba mRNA expression was examined in ECs isolated from adult mice with tamoxifen-inducible endothelial Klf2 (EC-Klf2-KO), Klf4 (EC-Klf4-KO), or both Klf2 and Klf4 (EC-DKO) knockout. While Hba levels were not significantly affected by the loss of either KLF2 or KLF4, loss of both factors resulted in a marked decrease of Hba expression levels compared to CRE control mice (Figure 1B).
Figure 1.
Hemoglobin alpha mRNA expression in the EC by KLF2/KLF4. (A) Overexpression of either KLF2 or KLF4 induced an expression of hemoglobin subunit alpha (HBA) and endothelial nitric oxide synthase (NOS3) mRNA in human coronary artery ECs. Representative data of three independent experiments are shown. (B) Cardiac microvascular ECs were isolated from EC-specific deletion of Klf2 (EC-Klf2-KO), Klf4 (EC-Klf4-KO), both Klf2 and Klf4 (EC-DKO), and CRE control (n=8–9 per genotype; each n was pooled from two mice). EC-DKO showed attenuated mRNA expression levels of Hba. Data are presented as mean ± standard error of the mean (SEM) values. **p < 0.01.) Ad-EV, control (empty) adenovirus; Ad-KLF2, adenovirus carrying Kruppel-like factor 2; Ad-KLF4, adenovirus carrying Kruppel-like factor 4; CRE, Cdh5(PAC)-CreERT2; EC, endothelial cell; NS, not significant.
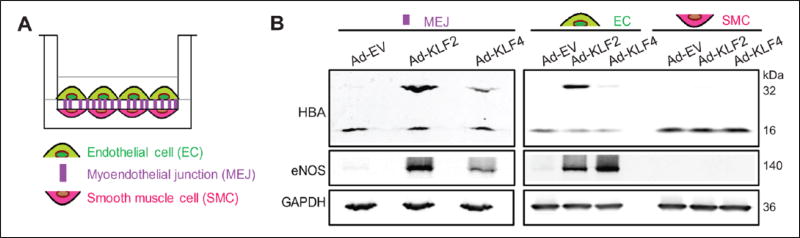
Next, we confirmed the induction of HBA as well as eNOS proteins by KLF2 or KLF4 in ECs which were either cultured alone (Figure S2, A and B) or co-cultured with vascular SMCs (Figure 2, A and B; Figure S3, A–C). For the latter study, HCAECs were co-cultured with HCASMCs on a Transwell insert to allow a formation of the MEJ.11 These cell types were chosen as they have previously been shown to allow for optimal MEJ formation. Adenovirus carrying KLF2, KLF4 or empty vector control was then introduced into the ECs on the top of the Transwell insert. EC, MEJ, and SMC proteins were collected according to a previously reported method.11,24 A protein at the molecular weight of approximately 32 kDa was recognized by anti-hemoglobin alpha antibody in the EC and MEJ that were infected with adenovirus carrying KLF2 or KLF4, but not empty vector (Figure 2, A and B; Figure S3, A–C). The size of this protein band is close to that of dimeric hemoglobin and mass spectrometry analyses confirmed the presence of HBA (Table S3). In addition, expression of hemoglobin beta protein was not detectable in the EC and MEJ (data not shown), suggesting a specificity of endothelial hemoglobin alpha expression by KLF2/KLF4. Collectively, these observations support a role for KLF2 and KLF4 to promote HBA expression in the EC and the MEJ.
Figure 2.
Hemoglobin alpha protein expression in the endothelial cell by KLF2/KLF4. (A) Schematic picture showing a Transwell(R) co-culture of HCAECs/HCASMCs. (B) Representative western blot analysis obtained from HCAECs/HCASMCs co-culture where the endothelial cells were overexpressed with either KLF2 or KLF4 (n=3–4 independent experiments). Ad-EV, control (empty) adenovirus; Ad-KLF2, adenovirus carrying Kruppel-like factor 2; Ad-KLF4, adenovirus carrying Kruppel-like factor 4; HBA, hemoglobin subunit alpha; eNOS, endothelial nitric oxide synthase; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; HCAECs, human coronary artery endothelial cells; HCASMCs, human coronary artery smooth muscle cells.
The transactivation of the human hemoglobin alpha gene promoter in ECs by KLF2/KLF4
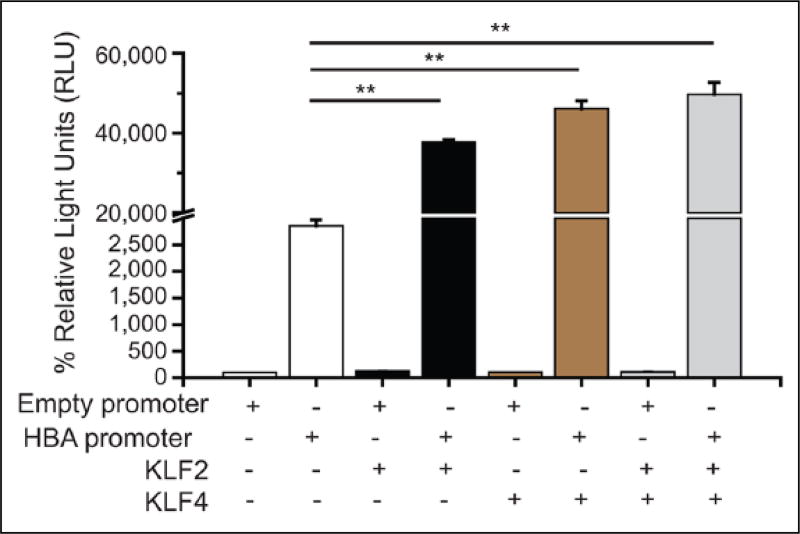
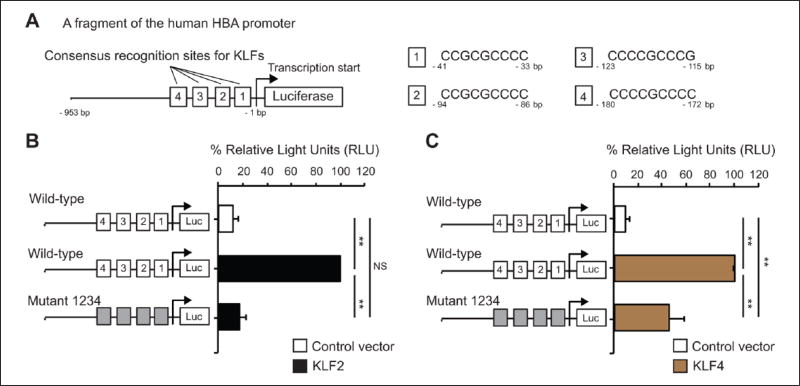
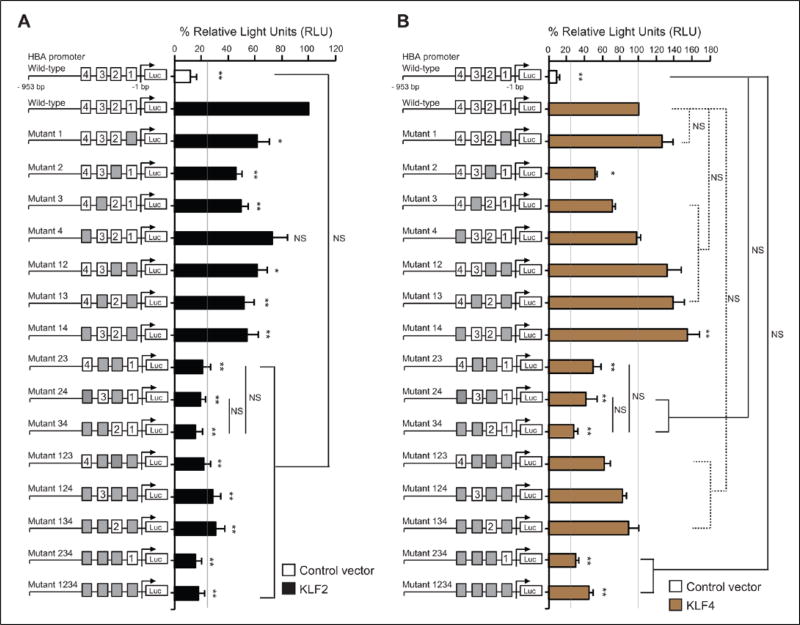
To determine if KLF2/KLF4 could directly regulate the HBA promoter, we performed a luciferase reporter assay in BAECs, which have high transfection efficiency when compared to human ECs. As shown in Figure 3, co-transfection of either KLF2 or KLF4 or both KLFs significantly increased the HBA promoter activity. Previous reports identified KLFs as DNA-binding transcription factors that bind to GC-, GT-, and CACCC-box motifs in gene promoters and other regulatory elements in order to mediate gene expression.17,20–23 We therefore aimed to pinpoint the KLF recognition sites (GC-, GT-, and CACCC) of the HBA promoter that are critical for KLF2/KLF4 binding to augment the transactivation of the promoter in the endothelium. We first focused on four previously reported4,5 KLF-binding sites of the HBA promoter (Figure 4A, site 1, −33 to −41 bp; site 2, −86 to −94 bp; site 3: −115 to −123 bp; site 4, −172 to −180 bp), which were mutated to prevent KLF-binding. In our study, the luciferase reporter was driven by a 953 bp fragment of the HBA promoter plasmid. We found that the promoter induction by KLF2 (Figure 4B) or KLF4 (Figure 4C) was significantly diminished with mutation of all four KLF-binding sites (Mutant 1234).
Figure 3.
KLF2/KLF4 mediate transactivation of the HBA promoter. Luciferase reporter assay in bovine aortic endothelial cells (n=3–4 independent experiments). The HBA promoter activity was significantly enhanced in the cells when the HBA promoter was co-transfected with KLF2 and/or KLF4. Data are presented as mean ± standard error of the mean (SEM) values. **p < 0.01. KLF2, Kruppel-like factor 2 plasmid; KLF4, Kruppel-like factor 4 plasmid; HBA, hemoglobin subunit alpha.
Figure 4.
KLF2/KLF4 fail to mediate transactivation of the mutant HBA promoter. (A) Schematic picture of the luciferase reporter driven by a fragment (953 bp, counting from the transcription start site) of the human HBA promoter. Four positions of consensus recognition sites for KLFs are indicated by white rectangles. (B and C) The mutant HBA promoter lacking four KLF-binding sites was co-expressed with either KLF2 (B) or KLF4 (C) (n=3–4 independent experiments). Gray boxes denote mutant KLF-binding sites on the HBA promoter. Data are presented as mean ± SEM values. **p < 0.01. KLF2, Kruppel-like factor 2 plasmid; KLF4, Kruppel-like factor 4 plasmid; HBA, hemoglobin subunit alpha; Luc, luciferase reporter; NS, not significant.
Next, to pinpoint the site(s) that is critical for KLF-mediated transactivation, we generated single and multiple mutations within the four KLF-binding sites on the HBA promoter. In the presence of KLF2 (Figure 5A) or KLF4 (Figure 5B), the HBA promoter mutants lacking at least two of the three sites (sites 2, 3, and 4) had the lowest luciferase activity when compared to other mutants, suggesting that three (sites 2, 3 and 4) out of the four KLF binding sites on the HBA promoter are critical for KLF2 and KLF4 to transactivate the expression of endothelial HBA. Interestingly, co-expression of KLF4 with the luciferase reporter driven by the HBA promoter mutant 14 led to a significant increase in the luciferase signals when compared to co-expression of KLF4 with the reporter driven by the wild-type HBA promoter (Figure 5B). These observations suggest that binding sites 1 and 4 together are not required for KLF4 – but suppress the ability of KLF4 – to activate the endothelial HBA expression. Altogether, KLF2/KLF4 promotes HBA expressions in the endothelium by transactivating the HBA promoter.
Figure 5.
Critical KLF-binding sites on the HBA promoter. Luciferase activity of wild-type or mutant HBA promoter constructs co-expressed with either KLF2 (A) or KLF4 (B) were observed in bovine aortic endothelial cells (n=3–4 independent experiments). Gray boxes represent mutant KLF-binding sites on the HBA promoter. Data are presented as mean ± standard error of the mean (SEM) values. *p < 0.05 and **p < 0.01 when compared with a co-expression of wild-type HBA promoter with KLF2 or KLF4. KLF2, Kruppel-like factor 2 plasmid; KLF4, Kruppel-like factor 4 plasmid; HBA, hemoglobin subunit alpha; Luc, luciferase reporter; NS, not significant.
Direct binding of KLF4 to the endogenous hemoglobin alpha promoter in the EC
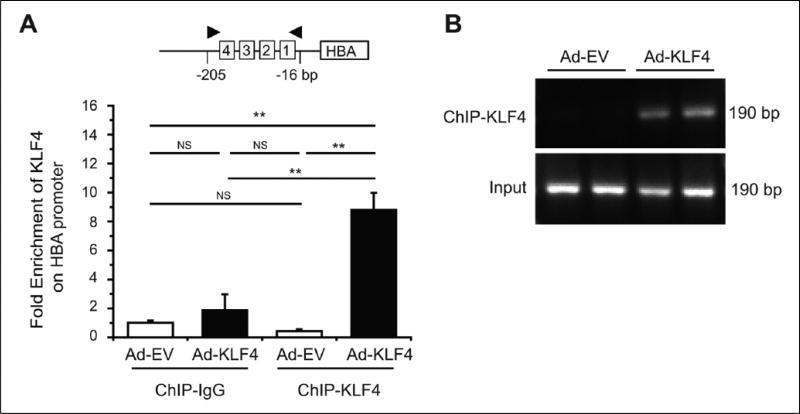
Next, we assessed whether KLF4 directly bound to the hemoglobin alpha promoter in the endothelium by chromatin immunoprecipitation (ChIP). We focused on KLF4 in ChIP studies because of the availability of a ChIP-validated antibody. We found a significant enrichment of KLF4 (Figure 6A) on the promoter region of HBA in HUVECs overexpressing KLF4. No enrichment was observed with control IgG. The specificity and efficiency of quantitative PCR primers were confirmed by agarose gel electrophoresis showing single amplicon of 190 bp (Figure 6B). These results indicate that KLF4 directly binds to the HBA promoter in the EC.
Figure 6.
Direct binding of KLF4 on the HBA promoter in the endothelial cell. (A) Enrichment of KLF4 on the HBA promoter was observed by chromatin immunoprecipitation (ChIP) coupled with quantitative PCR (qPCR) in human umbilical vein endothelial cells that were infected with KLF4 adenovirus for 48 hours (n=3 independent experiments). (B) Representative image of agarose gel electrophoresis showed the efficiency and specificity of the qPCR HBA primer pair which produced single amplicon of 190 bp (n=3 independent experiments). Data are presented as mean ± SEM values. **p < 0.01. Ad-EV, control (empty) adenovirus; Ad-KLF4, adenovirus carrying Kruppel-like factor 4; HBA, hemoglobin subunit alpha; NS, not significant.
Discussion
The fact that hemoglobins can exist outside of red blood cells has been appreciated for many years, with recent studies suggesting that their functions extend beyond acting as oxygen carriers to include regulating NO scavenging and blood pressure. An important gap in our understanding centers on how the expression of hemoglobin is controlled in non-erythroid cell types, including endothelial cells. Here we show that KLF2 and/or KLF4 regulate endothelial HBA expression by binding directly to and transactivating the HBA promoter.
Transcriptional regulation of HBA in ECs by KLFs is most likely similar to mechanisms of regulation in erythroid cells. KLFs have been shown to be responsible for hemoglobin expression in erythroid and non-erythroid cells. In erythroid cells, KLF4 is shown to recognize a CACCC motif in the promoter region of HBA and gamma-globin (HBG) genes.4,6 Specifically, Marini et al. reported four KLF-binding sites on the HBA promoter that are important for KLF4-mediated hemoglobin alpha expression in erythroid cells.4 A previous study using microarray analysis in ECs showed that HBA is in the top five genes regulated by KLF2.25 Our study shows for the first time that both KLF2 and KLF4 transactivate HBA expression in the EC, and that three of the four KLF-binding sites (sites 2, 3, and 4) are critical for KLF2/KLF4-mediated HBA expression. Besides KLF2 and KLF4, KLF3 has also been shown to promote the adult alpha-globin genes in erythroid cells; however, it has been shown to suppress HBA in non-erythroid cells.5 KLF1 and KLF2 promote beta-globin gene expression in embryos.26 This raises the possibility that a member of the KLF family activates expression of hemoglobin in other non-erythroid cells in addition to ECs.
The free alpha hemoglobin molecule is unstable and precipitates.27 In erythroid cells, alpha and beta hemoglobin subunits are held together by non-covalent bonds.28 In our study, we observed a protein of 32 kDa in the EC and the MEJ, which represents dimeric HBA protein. A dimerization of HBA observed in our study may be caused by aggregation of an excess amount of free hemoglobin proteins in the cells. It will be interesting to further explore how dimeric hemoglobin is assembled and the functional importance of hemoglobin dimer. Furthermore, we detected a monomeric HBA form in ECs, MEJs, and SMCs, which is similar to observations by Straub et al.11 However, significant changes in expression levels of dimeric, but not monomeric, HBA were observed. We speculate that, when HBA protein levels reach a high enough concentration in ECs, it may spontaneously form dimers. An additional explanation is that KLFs induce proteins that confer a post-translational modification to facilitate dimerization that is resistant to denaturation. This also explains why the monomeric HBA bands appeared similar between control (empty vector) and KLF-infected samples, as the extra amount of HBA has formed dimers.
Conclusion
Collectively, our work provides novel information regarding the transcriptional regulation of endothelial expression of hemoglobin with implications for vascular function and homeostasis. KLF2 and KLF4 promote eNOS expression, which subsequently leads to increased NO production and vasodilation.16 Here we show that KLF2 and KFL4 induce endothelial HBA expression. Previous reports demonstrated that endothelial HBA can capture NO and thereby prevent diffusion of NO from ECs to SMCs, promoting vasoconstriction.13 Thus, by virtue of their ability to increase eNOS and HBA, KLFs may form an autoregulatory loop to control vasoreactivity and key physiological functions such as blood pressure and tissue perfusion. The results of this study provide a better understanding of transcriptional regulation of endothelial HBA, a fine-tuner of NO diffusion to SMCs that contributes to maintenance of vascular homeostasis. Hence, our findings have the potential to provide the foundation for developing novel therapies aimed at ameliorating cardiovascular diseases.
Supplementary Material
Acknowledgments
We thank Dr Alfred Hausladen and Dr Douglas Hess for providing technical advice and troubleshooting.
Funding
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: this work was supported by NIH Grants R01HL110630-01, R01HL112486, R01HL086548, R01HL119195 (to MKJ), R00HL-11290402, R01HL-133864 and R01HL-128304 (to ACS) and by AHA grants 12SDG12050558 (to YL), 12SDG12070077 (to XL) and 16GRNT27250146 (to ACS).
Footnotes
Declaration of conflicting interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
The supplementary material is available at http://vmj.sagepub.com/supplemental
References
- 1.Saha D, Patgaonkar M, Shroff A, et al. Hemoglobin expression in nonerythroid cells: Novel or ubiquitous? Int J Inflam. 2014;2014:803237. doi: 10.1155/2014/803237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schechter AN. Hemoglobin research and the origins of molecular medicine. Blood. 2008;112:3927–3938. doi: 10.1182/blood-2008-04-078188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li B, Ding L, Li W, et al. Characterization of the transcriptome profiles related to globin gene switching during in vitro erythroid maturation. BMC Genomics. 2012;13:153. doi: 10.1186/1471-2164-13-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marini MG, Porcu L, Asunis I, et al. Regulation of the human HBA genes by KLF4 in erythroid cell lines. Br J Haematol. 2010;149:748–758. doi: 10.1111/j.1365-2141.2010.08130.x. [DOI] [PubMed] [Google Scholar]
- 5.Funnell AP, Vernimmen D, Lim WF, et al. Differential regulation of the alpha-globin locus by Kruppel-like Factor 3 in erythroid and non-erythroid cells. BMC Mol Biol. 2014;15:8. doi: 10.1186/1471-2199-15-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kalra IS, Alam MM, Choudhary PK, et al. Kruppel-like Factor 4 activates HBG gene expression in primary erythroid cells. Br J Haematol. 2011;154:248–259. doi: 10.1111/j.1365-2141.2011.08710.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aird WC. Phenotypic heterogeneity of the endothelium: I. Structure, function, and mechanisms. Circ Res. 2007;100:158–173. doi: 10.1161/01.RES.0000255691.76142.4a. [DOI] [PubMed] [Google Scholar]
- 8.Feletou M, Vanhoutte PM. Endothelial dysfunction: A multifaceted disorder (The Wiggers Award Lecture) Am J Physiol Heart Circ Physiol. 2006;291:H985–H1002. doi: 10.1152/ajpheart.00292.2006. [DOI] [PubMed] [Google Scholar]
- 9.Aird WC. Endothelium as an organ system. Crit Care Med. 2004;32:S271–S279. doi: 10.1097/01.ccm.0000129669.21649.40. [DOI] [PubMed] [Google Scholar]
- 10.Lim SS, Vos T, Flaxman AD, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2224–2260. doi: 10.1016/S0140-6736(12)61766-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Straub AC, Lohman AW, Billaud M, et al. Endothelial cell expression of haemoglobin alpha regulates nitric oxide signalling. Nature. 2012;491:473–477. doi: 10.1038/nature11626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rahaman MM, Reinders FG, Koes D, et al. Structure guided chemical modifications of propylthiouracil reveal novel small molecule inhibitors of cytochrome b5 reductase 3 that increase nitric oxide bioavailability. J Biol Chem. 2015;290:16,861–16,872. doi: 10.1074/jbc.M114.629964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Straub AC, Butcher JT, Billaud M, et al. Hemoglobin alpha/eNOS coupling at myoendothelial junctions is required for nitric oxide scavenging during vasoconstriction. Arterioscler Thromb Vasc Biol. 2014;34:2594–2600. doi: 10.1161/ATVBAHA.114.303974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Félétou M. The Endothelium: Part 1: Multiple Functions of the Endothelial Cells—Focus on Endothelium-Derived Vasoactive Mediators. San Rafael, CA: Morgan & Claypool Life Sciences; 2011. [PubMed] [Google Scholar]
- 15.Straub AC, Zeigler AC, Isakson BE. The myoendothelial junction: Connections that deliver the message. Physiology (Bethesda) 2014;29:242–249. doi: 10.1152/physiol.00042.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Atkins GB, Jain MK. Role of Kruppel-like transcription factors in endothelial biology. Circ Res. 2007;100:1686–1695. doi: 10.1161/01.RES.0000267856.00713.0a. [DOI] [PubMed] [Google Scholar]
- 17.Jain MK, Sangwung P, Hamik A. Regulation of an inflammatory disease: Kruppel-like factors and atherosclerosis. Arterioscler Thromb Vasc Biol. 2014;34:499–508. doi: 10.1161/ATVBAHA.113.301925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sangwung P, Zhou G, Nayak L, et al. KLF2 and KLF4 control endothelial identity and vascular integrity. JCI Insight. 2017;2:e91700. doi: 10.1172/jci.insight.91700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McConnell BB, Yang VW. Mammalian Kruppel-like factors in health and diseases. Physiol Rev. 2010;90:1337–1381. doi: 10.1152/physrev.00058.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.SenBanerjee S, Lin Z, Atkins GB, et al. KLF2 Is a novel transcriptional regulator of endothelial proinflammatory activation. J Exp Med. 2004;199:1305–1315. doi: 10.1084/jem.20031132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin Z, Kumar A, SenBanerjee S, et al. Kruppel-like factor 2 (KLF2) regulates endothelial thrombotic function. Circ Res. 2005;96:e48–e57. doi: 10.1161/01.RES.0000159707.05637.a1. [DOI] [PubMed] [Google Scholar]
- 22.Hamik A, Lin Z, Kumar A, et al. Kruppel-like factor 4 regulates endothelial inflammation. J Biol Chem. 2007;282:13,769–13,779. doi: 10.1074/jbc.M700078200. [DOI] [PubMed] [Google Scholar]
- 23.Zhou G, Hamik A, Nayak L, et al. Endothelial Kruppel-like factor 4 protects against atherothrombosis in mice. J Clin Invest. 2012;122:4727–4731. doi: 10.1172/JCI66056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heberlein KR, Straub AC, Best AK, et al. Plasminogen activator inhibitor-1 regulates myoendothelial junction formation. Circ Res. 2010;106:1092–1102. doi: 10.1161/CIRCRESAHA.109.215723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Parmar KM, Larman HB, Dai G, et al. Integration of flow-dependent endothelial phenotypes by Kruppel-like factor 2. J Clin Invest. 2006;116:49–58. doi: 10.1172/JCI24787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alhashem YN, Vinjamur DS, Basu M, et al. Transcription factors KLF1 and KLF2 positively regulate embryonic and fetal beta-globin genes through direct promoter binding. J Biol Chem. 2011;286:24,819–24,827. doi: 10.1074/jbc.M111.247536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abdullah UYH, Al-Attraqchi AGF, Ibrahim HM, et al. The free alpha-hemoglobin: A promising biomarker for β-thalassemia. J Mol Biomark Diagn. 2014;5:197. [Google Scholar]
- 28.Bunn HF. Subunit assembly of hemoglobin: An important determinant of hematologic phenotype. Blood. 1987;69:1–6. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.