Abstract
Few studies have examined the induction of squamous metaplasia in human olfactory nasal tissue caused by tobacco use and the implications it may have for olfaction, particularly when there are pre-existing insults, such as chronic rhinosinusitis (CRS). Quantitative histopathological analyses were performed on Alcian blue and H&E stained sections of nasal biopsies taken from the upper aspect of the middle turbinate of CRS patients. CRS patients who were current smokers had a predominance of squamous metaplasia in the olfactory sensory epithelium, whereas CRS patients who were nonsmokers and were not exposed to secondhand cigarette smoke had a prevalence of goblet cell hyperplasia. In spite of this difference, the groups did not differ significantly in olfactory threshold sensitivity. The impact of primary cigarette smoke on olfaction and a possible role of squamous metaplasia in preserving olfactory neurogenesis are discussed.
Keywords: morphology, olfactory epithelium, tobacco smoke
It is well documented that remodeling of the upper and lower airways (i.e., trachea, bronchi, and lungs) into squamous metaplasia, with resulting pulmonary diseases, is closely associated with exposure to cigarette smoke (Coggins, 1998). In contrast, studies examining the impact of tobacco use on the nasal olfactory mucosa (OM) and olfaction are limited (Benninger, 1999). We examined nonsmokers and smokers who suffered from chronic rhinosinusitis (CRS), defined as exhibiting rhinosinusitis symptoms for >12 weeks (Benninger et al., 2003), to determine the impact of tobacco smoke on the OM and its potential effect on olfaction where there are co-existing inflammatory insults. We are unaware of any study suggesting that smoking leads to the development of CRS and studies examining the relationship between smoking and CRS have yielded variable results. A study of retrospective cases found that smoking predicted poorer post-operative recovery after endoscopic surgery for CRS (Briggs et al., 2004). However, no relationship was observed between smoking and predictive factors for CRS such as computed tomography (CT) evaluation and endoscopic examination (Smith et al., 2005).
Unlike respiratory tissues, the OM is composed of an apical layer of olfactory sustentacular cells and layers of olfactory sensory neurons (OSNs) that undergo continuous replacement via neurogenesis from a population of resident basal cells sitting above the basement membrane (Farbman, 1992). The axons of these sensory neurons project through the cribriform plate to the olfactory bulb, and relay action potentials triggered by receptor activation by odor stimuli, thereby performing the function of odor detection. Hence the OM allows us to examine the direct impact of tobacco use on the ability to smell, olfactory epithelial changes, olfactory neurogenesis, and morphological changes in the lamina propria, which is composed of olfactory nerve bundles, blood vessels, Bowman’s glands, mucus glands, and connective tissue. Preliminary findings from this study have been presented in abstract form (Yee et al., 2008b), and we have previously reported on the morphological patterns of the OE within this CRS population (Yee et al., 2008a). Here we present original data analyses focused on the impact of first-hand smoke exposure at the sensory, tissue and cellular levels..
All protocols described were approved by the Institutional Review Board of Thomas Jefferson University. Patients who met the criteria for CRS were recruited from the Otolaryngology-Head & Neck Surgery department at Thomas Jefferson University for a clinical study, and all subjects provided written informed consent prior to their inclusion in the study. Extensive medical history, subjective symptoms, psychological profile, quality of life and chemical exposure information was collected from each patient who also underwent nasal endoscopy and CT scan. For this study, we identified 15 patients who were ≤50 years old from the larger clinical study: seven female patients ranging in age from 25 – 39 yrs old (mean 30.14 ± 2.26) who were current smokers were compared with eight CRS patients (3M, 5F) ranging in age from 26 – 47 yrs old (mean 34.63 ± 2.71) who were nonsmokers and reported no exposure to secondhand cigarette smoke. Three non-smokers and three current smokers reported a history of asthma; however no CRS patients reported any history of chronic obstructive pulmonary disease, cystic fibrosis or emphysema nor had any sinus surgery. Patients diagnosed with nasal polyps were excluded from this study. There was no significant difference in age between nonsmokers and smokers (t-test, t = 1.25, p = 0.23). Phenylethyl alcohol (PEA) is commonly used as a specific olfactory stimulus that elicits little or no trigeminal response at any concentration (Wysocki et al., 2003). Olfactory thresholds for PEA were determined separately in each nostril. Procedural details are given in Pribitkin et al. (2003). A PEA dilution step ≥8 in our series (≤4.0 log vol/vol) was considered to be within normal olfactory threshold limits (Cowart et al., 1993).
A 1–2mm3 nasal biopsy from the upper aspect of the middle turbinate was obtained after local anesthetic from the nasal side with the poorest PEA score by EAP or DR in the physician’s examination room. The biopsy was immediately placed in 4% paraformaldehyde for 1–2 hr. These biopsies were a part of a larger clinical study and were processed for either frozen or paraffin sections to assay the optimal condition for various antibodies. Hence five nasal biopsies were processed for paraffin embedding and ten biopsies were processed for frozen sections. Paraffin sections were cut at 5µm thickness and frozen sections were cut at 10µm thickness through the entire biopsy, and both were mounted onto StarFrost adhesive slides (Mercedes Medical, Sarasota FL). Three paraffin sections or three frozen sections were selected equally spaced apart (i.e., 300 – 450µm depending on the number of slides generated) for each biopsy and were stained with Alcian blue (pH 3.0, Sigma, St. Louis, MO), hematoxylin (Fisher Scientific, Pittsburg PA) and eosin (Sigma). A standard immunohistochemical protocol was conducted with the olfactory marker protein (OMP) antibody, which specifically identifies mature OSNs (1:500 dilution, Waco, Richmond VA) (Keller and Margolis, 1975), and Alexa488 donkey anti-goat secondary (1:250 dilution, Molecular Probes, Eugene, OR).
Four distinctive olfactory mucosal (OM) morphologies were identified as characterized in a previous study (Yee et al., 2008a): normal pseudostratified epithelium, goblet cell hyperplasia, squamous metaplasia, and erosion. Brightfield images of the sections were digitally captured, the length (µm) of each OM morphology was quantitatively measured with ImagePro Plus (Media Cybernetics Inc., Silver Spring, MD) and the percent of total length calculated. While the tissue embedding method may alter overall dimensions of a biopsy, the relative measurements performed (i.e., percent of OM morphology) within each biopsy would be unlikely to be affected by tissue embedding method. Fluorescence images were captured with the Leica TCS SP2 Spectral Confocal Microscope (Leica Microsystems Inc., Mannheim, Germany) using UV and Ar lasers and appropriate excitation spectrums. Digital images were cropped, arranged and adjusted for contrast and sharpness using Photoshop CS (Adobe Systems, Inc., San Jose, California). Statistical analyses (i.e., Student t-tests and Pearson correlations) were run with Statistica (StatSoft, Inc., Tulsa, OK).
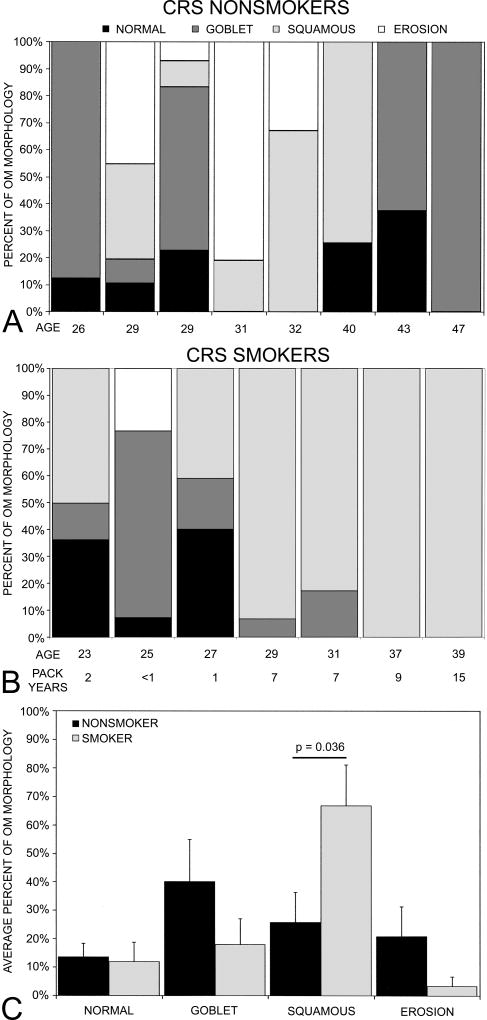
Squamous metaplasia caused distinctive morphological changes in the olfactory epithelium as previously described (Yee et al., 2008a): the absence of olfactory sustentacular cells; the presence of olfactory neurons in layers underneath squamous layers or just above the basal cell layer but without noticeable dendritic and axonal processes; and the hyperproliferation of basal cells. To further determine the impact of smoking on the OM, the percent distribution of each OM morphology was determined for individual biopsies. Figure 1 shows the percent distribution for individual CRS nonsmokers (Fig 1A) and CRS smokers (Fig 1B) in relation to age. The number of pack years is also shown for the smokers. The overall average percent distribution of different morphologies was then averaged for each group (Figure 1C). In CRS nonsmokers, goblet cell hyperplasia (40.0%) was the predominant OM morphology, followed by squamous metaplasia (25.70%), erosion (20.80%), and normal (13.52%). In CRS smokers, squamous metaplasia (66.74%) predominated and this pattern was significantly more prevalent in smokers than nonsmokers (t-test, t = 2.33, p<0.05). The distributions of goblet cell hyperplasia (18.00%, t = 1.29, p = 0.25), normal (11.92%, t = 0.19, p = 0.85), and erosion (3.33%, t = 1.48, p = 0.16) patterns were not significantly different between CRS smokers and nonsmokers. Consistent with the similar prevalence of normal OM, olfactory thresholds were similar between the two groups (smokers: mean PEA step = 9.2 ± 0.94 vs. nonsmokers mean PEA step = 8.38 ± 0.80; t-test, t = 0.67, p = 0.51). As a comparison, the average worst PEA step for seven healthy controls from the larger clinical study who were nonsmokers and of a similar age range to the CRS patients in the present study was 9.96 ± 0.85.
Figure 1.
The percent distribution of four different OM morphologies. The percent distribution of OM morphology (i.e., normal pseudostratified, goblet cell hyperplasia, squamous metaplasia and erosion) for A. individual CRS nonsmokers, B. individual CRS smokers in relation to age. The number of pack years is also included for CRS smokers.. C. The average percent distribution of the four OM morphologies for each group. Error bars = SEM.
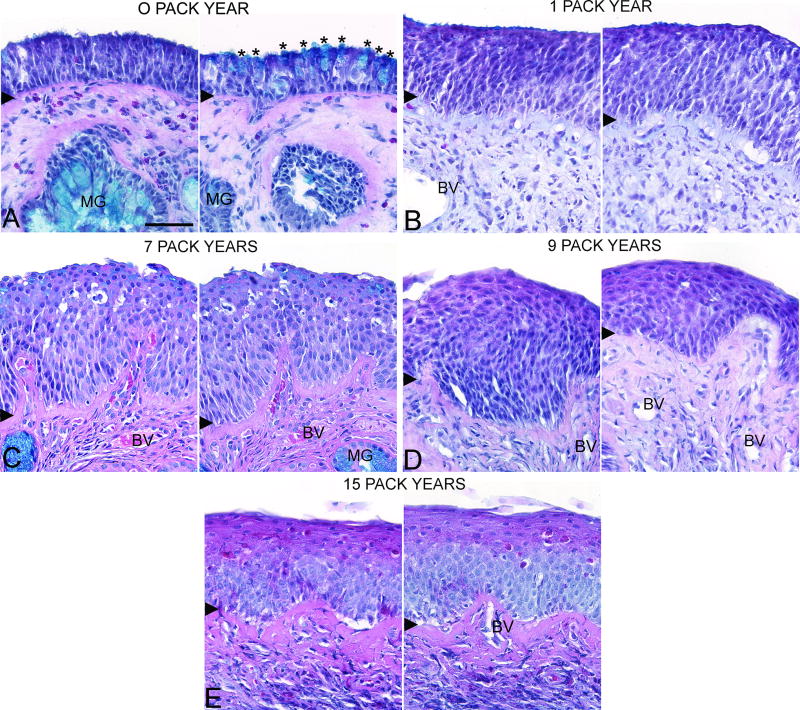
We also examined the relationship between OM morphology and pack years. The frequency of normal pseudostratified epithelium (r = −0.65, p = 0.11) and goblet cell hyperplasia tended to decrease with increasing pack years (r = −0.69, p = 0.09) although not significantly. The erosion pattern was only observed in the CRS smokers with <1 pack year exposure (i.e., those who had smoked no more that 4 cigarettes/day for less than a year) (r = −0.49, p = 0.27). On the other hand, increasing pack years significantly correlated with the increased frequency of squamous metaplasia (r = 0.86, p<0.01). Figure 2 shows the prevalence and degree of squamous metaplasia with increasing pack years as confirmed by histopathological examination of the tissue sections. We cannot be sure how the interaction between smoking and CRS influences the development or degree of squamous metaplasia.
Figure 2.
Olfactory mucosal histopathology of CRS smokers with different pack year histories. Sections were stained with Alcian blue, hematoxylin, and eosin and two olfactory mucosal regions from the same biopsy section were imaged with a 40× objective. A. <1 pack year with normal olfactory epithelium and goblet cell hyperplasia, B. 1 pack year, C. 7 pack years, D. 9 pack years, and E. 15 pack years. Asterisks = goblet cells, MG = mucus gland, BV = blood vessel, and arrowhead = basement membrane. Scale bar = 50µm.
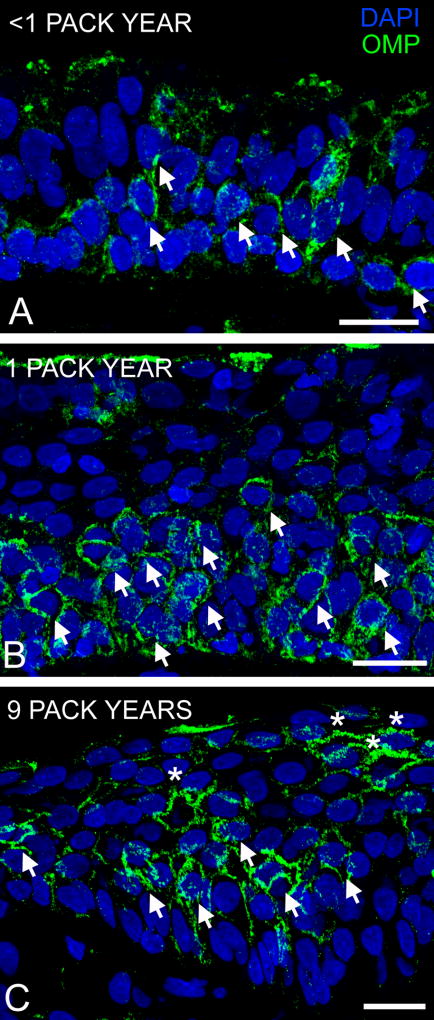
Olfactory neurogenesis was still active in these biopsies as demonstrated by the presence of OMP-immunoreactive OSNs (Fig 3). However the morphology of OSNs became abnormal with increasing squamous metaplasia and the inability to extend dendritic processes to the mucosal surface may lead to a high cell turnover rate similar to that observed after nasal occlusion in which sensory input is blocked (Farbman et al., 1988). No morphological assessment of the lamina propria was conducted in this study. There was no correlation between the PEA score and pack years (r = 0.03, p = 0.73), suggesting that the degree of squamous metaplasia is not directly reflective of olfactory abilities in CRS patients.
Figure 3.
Olfactory neurogenesis persisted in the squamous OM. Sections were labeled with antibody to olfactory marker protein (OMP) and imaged with a 40× objective. A. <1 pack year: the presence of OMP-immunoreactive (OMP-ir, green) olfactory sensory neurons (OSNs, arrows) in the olfactory epithelium, B. 1 pack year: layers of OMP-ir OSNs are present, and C. 9 pack years: the presence of abnormally-shaped OMP-ir OSNs (asterisk) near the apical squamous layer. DAPI = blue. Scale bar = 20µm.
Results from our study together with published data reveal similarities between nasal and bronchial biopsies of current smokers in that smoking induced squamous metaplasia in both epithelial tissues. A correlation between the frequency of squamous metaplasia and tobacco use was also shown in human bronchial biopsies (Peters et al., 1993). Squamous metaplasia may be a protective mechanism of epithelial tissue after chemical insult and injury. In a recent study of naphthalene-induced bronchial tissue injury in mice, the subsequent squamous metaplasia remodeling was shown to act as a protective barrier, allowing neighboring epithelial cells to infiltrate the injured area, differentiate and repair the ciliated respiratory epithelium (Park et al., 2006). Hence, squamous metaplasia in the OM may provide a similar cellular mechanism, preserving continuous neurogenesis as observed by the presence of mature olfactory sensory neurons underneath the squamous layers. Although translocation of epithelial cells has not been reported in OM, we found evidence of a shift towards goblet cell hyperplasia in the nasal biopsies of CRS patients who had quit smoking for more than 10 years (data not shown) that was similar to the percent of goblet cell hyperplasia observed in CRS nonsmokers. This suggests a plausible process for the preservation of olfactory function in the face of CRS and repair of the OM after cessation of smoking.
The effects of squamous metaplasia may not be limited to the epithelium as demonstrated by the interaction between the airway epithelial and mesenchyme of COPD patients (Araya et al., 2007). Squamous metaplastic epithelium secreted IL-1β that activated airway fibroblasts, which in turn increased TGF-β production, suppressed epithelial proliferation, initiated self-amplification of TGF-β, and increased thickening of airway walls. The expression of TGF-β1 has been found in nasal tissue of CRS patients and was related to fibrosis although the histopathology of the epithelium was not mentioned (Watelet et al., 2004). We did not conduct morphological assessments (e.g., degree of fibrosis) in the lamina propria for this study.
The lack of a difference in olfactory sensitivity between CRS smokers and nonsmokers may seem paradoxical; however, we do not know the extent of squamous metaplasia throughout the entire nasal cavity of current smokers. Olfactory epithelium is found on the upper aspect of the middle turbinate, where our biopsies were taken, on the superior turbinate, and in the olfactory cleft. Based on patients’ olfactory performance, there are evidently sufficient regions of olfactory epithelium containing functional olfactory sensory neurons, perhaps even in OM regions of the middle turbinate with milder forms of squamous metaplasia, to sustain olfactory sensitivity. It is unknown if squamous metaplasia as induced by tobacco smoke would also occur in these levels of the nasal cavity. In summary, squamous metaplasia can be induced by chemical insult in olfactory epithelial tissue and may assist in preserving olfactory neurogenesis and cellular repair and recovery.
Acknowledgments
We are grateful to our CRS patients who volunteered for this study. This study was supported by the National Institute on Deafness and Other Communication Disorders DC006760 (G.K. Beauchamp), National Institute of Mental Health MH-080193 (CGH) and NSF DBI-0216310 (NER).
References
- Araya J, Cambier S, Markovics JA, Wolters P, Jablons D, Hill A, Finkbeiner W, Jones K, Broaddus VC, Sheppard D, Barzcak A, Xiao Y, Erle DJ, Nishimura SL. Squamous metaplasia amplifies pathologic epithelial-mesenchymal interactions in COPD patients. J Clinical Invest. 2007;117:3551–3562. doi: 10.1172/JCI32526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benninger MS. The impact of cigarette smoking and environmental tobacco smoke on nasal and sinus disease: a review of the literature. Am J Rhinol. 1999;13:435–38. doi: 10.2500/105065899781329683. [DOI] [PubMed] [Google Scholar]
- Benninger MS, Ferguson BJ, Hadley JA, Hamilos DL, Jacobs M, Kennedy DW, Lanza DC, Marple BF, Osguthorpe JD, Stankiewicz JA, Anon J, Denney J, Emanuel I, Levin H. Adult chronic rhinosinusitis: definitions, diagnosis, epidemiology, and pathophysiology. Otolaryngol–Head Neck Surg. 2003;129:S1–S32. doi: 10.1016/s0194-5998(03)01397-4. [DOI] [PubMed] [Google Scholar]
- Briggs RD, Wright ST, Cordes S, Calhoun KH. Smoking in chronic rhinosinusitis: a predictor of poor long-term outcome after endoscopic sinus surgery. Laryngoscope. 2004;114:126–128. doi: 10.1097/00005537-200401000-00022. [DOI] [PubMed] [Google Scholar]
- Coggins CR. A review of chronic inhalation studies with mainstream cigarette smoke in rats and mice. Toxicol Pathol. 1998;28:307–14. doi: 10.1177/019262339802600301. [DOI] [PubMed] [Google Scholar]
- Cowart BJ, Flynn-Rodden K, McGeady SJ, Lowry LD. Hyposmia in allergic rhinitis. J Allergy Clin Immunol. 1993;91:747–51. doi: 10.1016/0091-6749(93)90194-k. [DOI] [PubMed] [Google Scholar]
- Farbman AI. Cell Biology of Olfaction. Cambridge University Press; Cambridge, England: 1992. [Google Scholar]
- Farbman AI, Brunjes PC, Rentfro L, Michas J, Ritz S. The effect of unilateral naris occlusion on cell dynamics in the developing rat olfactory epithelium. J Neurosci. 1988;9:3290–3295. doi: 10.1523/JNEUROSCI.08-09-03290.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller A, Margolis FL. Immunological studies of the rat olfactory marker protein. J Neurochem. 1975;24:1101–1106. doi: 10.1111/j.1471-4159.1975.tb03883.x. [DOI] [PubMed] [Google Scholar]
- Lee JJ, Liu D, Lee JS, Curie JM, Khuri FR, Ibarguen H, Morice RC, Walsh G, Ro JY, Broxson A, Hong WK, Hittelman WN. Long-term input of smoking on lung epithelial proliferation in current and past smokers. J NCI. 2001;93:1081–88. doi: 10.1093/jnci/93.14.1081. [DOI] [PubMed] [Google Scholar]
- Park K-S, Wells JM, Zorn AM, Wert SE, Laubach VE, Fernandez LG, Whitsett JA. Transdifferentiation of ciliated cells during repair of the respiratory epithelium. Am J Respir Cell Mol Biol. 2005;34:151–57. doi: 10.1165/rcmb.2005-0332OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters EJ, Morice R, Benner SE, Lippman S, Lukeman J, Lee JS, Ro JY, Hong WK. Squamous metaplasia of the bronchial mucosa and its relationshiop to smoking. Chest. 1993;103l:1429–32. doi: 10.1378/chest.103.5.1429. [DOI] [PubMed] [Google Scholar]
- Pribitkin EA, Rosenthal MD, Cowart BJ. Prevalence and causes of severe taste loss in a chemosensory clinic population. Ann Otol Rhinol Laryngol. 2003;112:971–78. doi: 10.1177/000348940311201110. [DOI] [PubMed] [Google Scholar]
- Smith TL, Mendolia-Loffredo S, Loehrl TA, Sparapani R, Laud PW, Nattinger AB. Predictive factors and outcomes in endoscopic sinus surgery for chronic rhinosinusitis. Laryngoscope. 2005;115:2199–2205. doi: 10.1097/01.mlg.0000182825.82910.80. [DOI] [PubMed] [Google Scholar]
- Watelet JB, Claeys C, Perez-Novo C, Gevaert P, van Cauwenberge P, Bachert C. Transforming growth factor beta 2 in nasal remodeling: differences between chronic rhinosinusitis and nasal polyposis. Am J Rhinol. 2004;18:267–272. [PubMed] [Google Scholar]
- Wysocki CJ, Cowart BJ, Radil T. Nasal trigeminal chemosensitivity across the adult life span. Percept Psychophys. 2003;65:115–122. doi: 10.3758/bf03194788. [DOI] [PubMed] [Google Scholar]
- Yee KK, Pribitkin EA, Cowart BJ, Rosen D, Feng P, Rawson NE. Analysis of the olfactory mucosa in chronic rhinosinusitis. Ann NY Acad Sci (accepted) 2008a doi: 10.1111/j.1749-6632.2009.04364.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee KK, Pribitkin EA, Cowart BJ, Vainius AA, Klock CT, Rawson NE. Impact of cigarette smoke on olfactory damage in patients with chronic rhinosinusitis. Abstract XV International Symposium on Olfaction and Taste. 2008b [Google Scholar]