Summary
Background
Rilotumumab is a fully human monoclonal antibody that selectively targets the ligand of the MET receptor, hepatocyte growth factor (HGF). We aimed to assess the efficacy, safety, and pharmacokinetics of rilotumumab combined with epirubicin, cisplatin, and capecitabine, and to assess potential biomarkers, in patients with advanced MET-positive gastric or gastro-oesophageal junction adenocarcinoma.
Methods
This multicentre, randomised, double-blind, placebo-controlled, phase 3 study was done at 152 centres in 27 countries. We recruited adults (aged ≥18 years) with unresectable locally advanced or metastatic gastric or gastro-oesophageal junction adenocarcinoma, an Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1, MET-positive tumours (≥25% of tumour cells with membrane staining of ≥1+ staining intensity), and evaluable disease, who had not received previous systemic therapy. Eligible patients were randomly assigned (1:1) via a computerised voice response system to receive rilotumumab 15 mg/kg intravenously or placebo in combination with open-label chemotherapy (epirubicin 50 mg/m2 intravenously; cisplatin 60 mg/m2 intravenously; capecitabine 625 mg/m2 orally twice daily) in 21-day cycles for up to ten cycles. After completion of chemotherapy, patients continued to receive rilotumumab or placebo monotherapy until disease progression, intolerability, withdrawal of consent, or study termination. Randomisation was stratified by disease extent and ECOG performance status. Both patients and physicians were masked to study treatment assignment. The primary endpoint was overall survival, analysed by intention to treat. We report the final analysis. This study is registered with ClinicalTrials.gov, number NCT01697072.
Findings
Between Nov 7, 2012, and Nov 21, 2014, 609 patients were randomly assigned to rilotumumab plus epirubicin, cisplatin, and capecitabine (rilotumumab group; n=304) or placebo plus epirubicin, cisplatin, and capecitabine (placebo group; n=305). Study treatment was stopped early after an independent data monitoring committee found a higher number of deaths in the rilotumumab group than in the placebo group; all patients in the rilotumumab group subsequently discontinued all study treatment. Median follow-up was 7·7 months (IQR 3·6–12·0) for patients in the rilotumumab group and 9·4 months (5·3–13·1) for patients in the placebo group. Median overall survival was 8·8 months (95% CI 7·7–10·2) in the rilotumumab group compared with 10·7 months (9·6–12·4) in the placebo group (stratified hazard ratio 1·34, 95% CI 1·10–1·63; p=0·003). The most common grade 3 or worse adverse events in the rilotumumab and placebo groups were neutropenia (86 [29%] of 298 patients vs 97 [32%] of 299 patients), anaemia (37 [12%] vs 43 [14%]), and fatigue (30 [10%] vs 35 [12%]). The frequency of serious adverse events was similar in the rilotumumab and placebo groups (142 [48%] vs 149 [50%]). More deaths due to adverse events occurred in the rilotumumab group than the placebo group (42 [14%] vs 31 [10%]). In the rilotumumab group, 33 (11%) of 298 patients had fatal adverse events due to disease progression, and nine (3%) had fatal events not due to disease progression. In the placebo group, 23 (8%) of 299 patients had fatal adverse events due to disease progression, and eight (3%) had fatal events not due to disease progression.
Interpretation
Ligand-blocking inhibition of the MET pathway with rilotumumab is not effective in improving clinical outcomes in patients with MET-positive gastric or gastro-oesophageal adenocarcinoma.
Funding
Amgen.
Introduction
Together, gastric and oesophagogastric cancers are the second leading cause of cancer death worldwide.1 Platinum-based and fluoropyrimidine-based chemotherapy regimens are the standard of care for advanced disease; however, no one regimen is preferred. In the REAL-2 study, the combination regimen of epirubicin, cisplatin, and capecitabine was as effective as other chemotherapy regimens for the treatment of advanced oesophagogastric adenocarcinoma.2
The hepatocyte growth factor (HGF) and its receptor MET are important for tumour cell proliferation, migration, and survival in patients with oesophagogastric adenocarcinoma.3,4 In these patients, 24–74% of cases show MET expression, depending on cohort selection, age of tissue section, antibody (monoclonal vs polyclonal), staining procedure, and, notably, interpretation and scoring of the immunohistochemistry analysis;5–9 however, these materials and procedures are not standardised. MET gene amplification with consequent protein overexpression is far less frequent than MET overexpression, and, despite being reported in up to 23% of cases,10 depending on the definition of amplification, most studies show an approximate 5% incidence of MET amplification in patients with newly diagnosed metastatic oesophagogastric adenocarcinoma.6,7,9–11 MET or HGF overexpression correlates with tumour invasion, metastasis, disease stage, and shorter survival; therefore, agents targeting HGF and MET are considered good therapeutic candidates.6,12,13
Rilotumumab (previously AMG 102) is a fully human, monoclonal antibody that selectively targets HGF.14 Rilotumumab functions by blocking downstream cell proliferation, migration, and survival pathways, inhibiting HGF-dependent tumour growth in vivo.15,16 In a phase 2 study of rilotumumab versus placebo in combination with epirubicin, cisplatin, and capecitabine in the first-line treatment of 121 patients with gastric or gastro-oesophageal cancer, longer progression-free survival was observed in the rilotumumab group independent of MET status.17 Both progression-free survival (hazard ratio [HR] 0·46, 95% CI 0·25–0·85, p=0·013) and overall survival (HR 0·46, 0·24–0·87; p=0·016) were longer with rilotumumab in a preplanned subgroup analysis of patients with MET-positive tumours (64% of samples evaluable), defined as having at least 25% of tumour cells with membrane staining at an intensity of 1+ or greater.17 No pharmacodynamic interactions with chemotherapy were reported in earlier studies.18
We report the final analysis results of RILOMET-1, a phase 3 study that assessed rilotumumab versus placebo in combination with epirubicin, cisplatin, and capecitabine as first-line therapy for patients with advanced MET-positive gastric or gastro-oesophageal junction adenocarcinoma.
Methods
Study design and participants
RILOMET-1 was a multicentre, randomised, double-blind, placebo-controlled, phase 3 study done at 152 centres in 27 countries (appendix p 7). Patients were eligible if they were aged 18 years or older, had pathologically confirmed, unresectable, locally advanced or metastatic gastric or gastro-oesophageal junction adenocarcinoma, an Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1, MET-positive tumours as determined by immunohistochemistry (defined as ≥25% of tumour cells with membrane staining of ≥1+ intensity), and evaluable disease per Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1. Patients were excluded if they had HER2-positive tumours, or if they had received previous systemic therapy for advanced disease. Patients who had completed neoadjuvant or adjuvant chemotherapy within the previous 6 months, who had squamous cell histology, or who had a left ventricle ejection fraction of less than 50% were also excluded. See appendix (pp 2–5) for full list of inclusion and exclusion criteria. On the basis of the median survival time of 8·9 months for patients in the placebo plus epirubicin, cisplatin, and capecitabine group in the phase 2 trial,17 patients in this trial had a life expectancy of approximately 9 months. The study protocol was approved by independent ethics committees or institutional review boards at participating institutions; all patients gave written informed consent.
Randomisation and masking
Patients were randomly assigned (1:1) to receive rilotumumab plus epirubicin, cisplatin, and capecitabine or placebo plus epirubicin, cisplatin, and capecitabine. The randomisation list was generated by fixed stratified permuted block randomisation, with a block size of four, and prepared by an individual who was independent of the study team. Treatment allocation was assigned via a computerised interactive voice response system. Randomisation was stratified according to disease extent (locally advanced vs metastatic disease) and ECOG performance status (0 vs 1). The patients were enrolled by the study investigators. Both patients and physicians were masked to treatment assignment. Rilotumumab was provided as a sterile, colourless to slightly yellow solution in 10 mL vials. Placebo was provided as a sterile protein-free solution similar in appearance and vial size to rilotumumab and stored under the same conditions as rilotumumab. Administration of epirubicin, cisplatin, and capecitabine was planned to be open label.
Procedures
On day 1 of each 21-day cycle, patients received rilotumumab (15 mg/kg intravenously) or placebo, epirubicin (50 mg/m2 intravenously), and cisplatin (60 mg/m2 intravenously). Patients self-administered capecitabine (625 mg/m2 orally twice daily) throughout the cycle. Chemotherapy was given for a maximum of ten cycles. After completion of chemotherapy cycles, patients continued on rilotumumab or placebo monotherapy (one dose every 21 days) until disease progression, intolerability, withdrawal of consent, or study termination. If chemotherapy administration was delayed because of toxicity, administration of rilotumumab or placebo was also delayed. If rilotumumab or placebo was delayed, chemotherapy was administered as scheduled. If chemotherapy, rilotumumab, or placebo was discontinued but the patient did not meet withdrawal criteria for toxicity (ie, grade ≥3 neutropenia, cardiac failure, bilirubin >1·5 × the upper limit of normal [ULN], aspartate aminotransferase or alanine aminotransferase ≥2 × ULN, creatinine clearance <30 mL/min, or neurotoxicity), continuation of other treatment was permitted. Patients who missed more than two consecutive scheduled administrations of rilotumumab or placebo were required to have their cases reviewed by the medical monitor before resuming administration. Grade 4 infusion reaction, any grade arterial thrombosis, symptomatic grade 4 venous thrombosis, or vascular ischaemic adverse event resulted in the discontinuation of the investigational product. Patient crossover between groups was not allowed.
A 25% dose reduction of epirubicin was allowed in patients with grade 3 neutropenia associated with infection or fever, and a 50% dose reduction was allowed in patients with grade 4 neutropenia associated with infection or fever. A 50% dose reduction of cisplatin was allowed in patients with grade 2 neuropathy or ototoxicity; cisplatin was to be discontinued in patients with grade 3 or worse neuropathy or ototoxicity. Epirubicin was discontinued if a patient had symptoms of cardiac failure and had a left ventricular ejection fraction of less than 50%. If total bilirubin increased to more than 1·5 times the ULN or if aspartate aminotransferase or alanine aminotransferase increased to more than 2·0 times the ULN, epirubicin was discontinued until concentrations were less than or equal to 1·5 times the ULN. If total bilirubin increased to more than 1·5 times the ULN, capecitabine was omitted until concentrations were less than or equal to 1·5 times the ULN. All components of the epirubicin, cisplatin, and capecitabine chemotherapy backbone were discontinued in patients with creatinine clearance of less than 30 mL/min. Patients had the right to withdraw from the study at any time and for any reason without prejudice to their future medical care by the physician or at the institution; reasons for removal of patients from the study could include decision by the sponsor, withdrawal of consent, death, or loss to follow-up.
Haematology, blood chemistry, and creatinine clearance were assessed at screening, in every cycle, and at the safety follow-up visit. Urinalysis with microscopy was assessed at screening, in cycle 1, every three cycles thereafter, and at the safety follow-up visit. Anti-rilotumumab antibodies were assessed for binding by a bridging immunoassay and samples positive for binding were assessed for neutralising activity by a cell-based assay in cycles 1, 3, and 7, every eight cycles after the completion of chemotherapy, and at the safety follow-up visit.
Blood samples for preplanned HGF biomarker analyses were collected before rilotumumab or placebo administration during cycles 1, 3, and 5, and then 30 days after the final dose. Adverse events were recorded and classified according to the Common Terminology Criteria for Adverse Events, version 3.0. Adverse events were reviewed at every clinic visit. Investigators were responsible for ensuring that all adverse events observed by the investigator or reported by the patient that occurred on or after the first treatment with protocol-specified therapy until 30 (+3) days after the last administration of protocol-specified therapy were reported. Adverse events were reported continuously using electronic case report forms. Serious adverse events were reported to the study sponsor within 24 h of the investigator becoming aware of the event. Fatal adverse events were not assessed for whether they were related to treatment, but rather whether they were or were not due to disease progression.
Radiological imaging (CT or MRI) of all sites of known disease for tumour response assessment was done at baseline, week 13 (± 1), and every 12 (± 1) weeks thereafter per RECIST version 1.1; investigators could perform earlier scans if clinically indicated.
Rilotumumab pharmacokinetic blood samples were collected from all patients. For patients participating in non-intensive pharmacokinetic assessments, blood samples were collected pre-dose and after infusion on day 1 of cycles 1, 3, 5, and 7. For patients participating in intensive pharmacokinetic assessments, blood samples were collected pre-dose, 5 min before the end of infusion, and 2, 24, 168, and 336 h after the start of infusion cycle 1; pre-dose on day 1 of cycle 2; pre-dose and after infusion on day 1 of cycles 3, 5, and 7; and 30 days after the final dose. We assessed maximum observed drug concentration during a dosing interval (Cmax) and minimum observed drug concentration during a dosing interval (Cmin).
Tumour MET-positivity by immunohistochemistry (ie, ≥25% of tumour cells with membrane staining of ≥1+ staining intensity) was an inclusion criterion for the study. MET immunohistochemistry analyses were prespecified and performed centrally using formalin-fixed paraffin-embedded tumour blocks or tumour tissue sections that were provided by the investigators at screening. MET protein expression was determined using the automated, investigational-use only Dako MET immunohistochemistry assay and the MET4 antibody (Dako North America, Carpinteria, CA, USA), the same assay and antibody used in the phase 2 study.17 Percentage positive (ie, extensity) was defined as the sum of the percentage of tumour cells that were positive for MET expression intensities, defined as 1+ (weak membrane staining), 2+ (moderate intensity membrane staining), and 3+ (strong intensity membrane staining). H (histology) score19,20 was defined as the sum of the percentage of cell staining at each intensity level greater than 0 multiplied by the intensity level—ie, (1 × [% of cells stained at intensity 1]) + (2 × [% of cells stained at intensity 2]) + (3 × [% of cells stained at intensity 3]).
As prespecified in the protocol, MET gene amplification was assessed in exploratory analyses by fluorescence in-situ hybridisation (FISH) using the research-use only MET/CEN-7 IQFISH Probe Mix (Dako North America) in combination with the K5799 Histology FISH Accessory Kit (Dako North America). MET gene amplification was defined as a MET:CEN7 ratio of 2·0 or greater. Other dichotomisations were tested (average MET copies of ≥5 vs average MET copies <5 and percentage of tumour nuclei with ≥15 MET copies in ≥10% of counted nuclei vs percentage of nuclei with ≥15 MET copies in <10% of counted nuclei).
Outcomes
The primary endpoint was overall survival. Secondary endpoints were progression-free survival, overall survival at 12 months, time to progression, objective response, duration of response, time to response, disease control, safety, and pharmacokinetics. Exploratory endpoints included the assessment of potential biomarkers and their association with clinical outcome.
The primary endpoint of overall survival was defined as the time from randomisation to death. Patients who had not died by analysis cutoff or who were lost to follow-up were censored at their last contact date. Progression-free survival was defined as the time from randomisation to the earlier of disease progression (per RECIST version 1.1) or death. Overall survival at 12 months was defined as the Kaplan-Meier estimate of the proportion of patients alive at 12 months. Objective response was defined as the proportion of patients with a complete response or partial response per RECIST version 1.1. Disease control was defined as the proportion of patients achieving complete response, partial response, or stable disease per RECIST version 1.1. The stable disease classification required patients to have had a response of stable disease at least 11 weeks after the first dose of rilotumumab or placebo.
Statistical analysis
The hypothesis tested in this study was that rilotumumab in combination with epirubicin, cisplatin, and capecitabine would significantly improve overall survival compared with placebo in combination with epirubicin, cisplatin, and capecitabine in the first-line treatment of patients with MET-positive advanced gastric or gastro-oesophageal junction adenocarcinoma. A previous analysis of a phase 1b/2 study17 found the HR comparing overall survival between the rilotumumab and placebo groups was 0·29 in patients with high MET expression; however, because the sample size was small for use in designing a phase 3 study, bootstrap sampling of the data was used to compensate. Examination of the distribution after completion of bootstrapping showed that the median HR was 0·27 and the 95th percentile was 0·69. Use of the 95th percentile as the hypothesised HR in the RILOMET-1 study design was determined to be a justifiably robust approach to mitigate the risks associated with using the phase 2 result. The study was planned to achieve at least 90% power for rilotumumab versus placebo at an overall one-sided 0·025 significance level when the overall survival HR was 0·69; enrolment of 450 patients was estimated to yield approximately 319 overall survival events. The protocol was amended on Feb 10, 2014, and the power calculations in the statistical analysis plan were subsequently revised after the steering committee agreed to increase the number of patients enrolled to 600 to allow testing of a higher MET cutoff (≥50% of tumour cells with membrane staining of 1+ or greater) if statistical analyses did not find a significant difference in overall survival based on the 25% MET cutoff.
Preplanned, unblinded safety reviews by the independent data monitoring committee were scheduled after 40 and 120 randomly assigned patients had completed one cycle of protocol-specified therapy. Safety reviews were subsequently done every 6 months thereafter.
Analyses of overall survival and progression-free survival included all randomly assigned patients; patients were analysed according to randomly allocated treatment (intention to treat). Analyses of objective response and disease control included all patients with at least one unidimensionally measurable lesion at baseline per RECIST version 1.1. Safety analyses included all patients who received at least one dose of rilotumumab or placebo; patients were analysed according to treatment received. The non-intensive pharmacokinetics analysis included all randomly assigned patients who received at least one dose of rilotumumab or epirubicin, cisplatin, and capecitabine, and had at least one pharmacokinetic sample collected; the intensive pharmacokinetics analysis was done in patients at selected sites. The antirilotumumab antibody analysis was done in all patients who had evaluable antibody data. Biomarker analyses were done in all patients who received at least one dose of rilotumumab or placebo and who had evaluable biomarker data.
For time-to-event endpoints, Kaplan-Meier estimates and 95% CIs were calculated. The primary comparisons of overall survival and progression-free survival were done with log-rank tests stratified by the randomisation factors (disease extent and ECOG performance status). HRs for overall survival and progression-free survival were estimated with Cox proportional hazards models stratified by the randomisation factors. A Kolmogorovtype supremum test was used to test the proportional hazards assumption for randomised treatment for the comparison of rilotumumab plus epirubicin, cisplatin, and capecitabine versus placebo plus epirubicin, cisplatin, and capecitabine based on an analysis of cumulative martingale-based residuals overall and within the randomisation strata.21 Odds ratios for objective response and disease control were calculated with the Cochran-Mantel-Haenszel test stratified by the randomisation factors.
In prespecified analyses, overall survival and progression-free survival were analysed according to predefined subgroups based on baseline characteristics and MET percentage expression extensity tertiles (ie, the percentage of tumour cell staining at any intensity level >1+). Overall survival and progression-free survival were modelled using a Cox proportional hazards model stratified by randomisation factors. For each treatment group, MET expression as a continuous variable was included in the models as an independent variable to assess the prognostic value of MET expression as a continuous variable. The interaction of MET with treatment was analysed to assess whether MET was predictive of treatment effect. For categorical variables such as MET cutoffs, MET was defined as high or low according to the cutoff. For each treatment group, the high or low categorical variable was included as an independent variable in a Cox model stratified by the randomisation stratification factors to assess prognostic value. For each level of the categorical variable, treatment was included as a categorical variable in a Cox model stratified by the randomisation stratification factors to assess treatment effect in each level of the categorical MET value. The interaction between the categorical value and treatment was then assessed to see whether the categorical value was predictive of treatment effect. H score was similarly assessed as a continuous variable and the maximum staining intensity and staining intensity above or below normal were similarly assessed as categorical.
Analyses were done with SAS version 9.3. This study is registered with ClinicalTrials.gov, number NCT01697072.
Role of the funding source
The funder provided study drugs and collaborated with investigators on the study design, data collection, data analysis, data interpretation, writing of the report, and the decision to submit the paper for publication. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
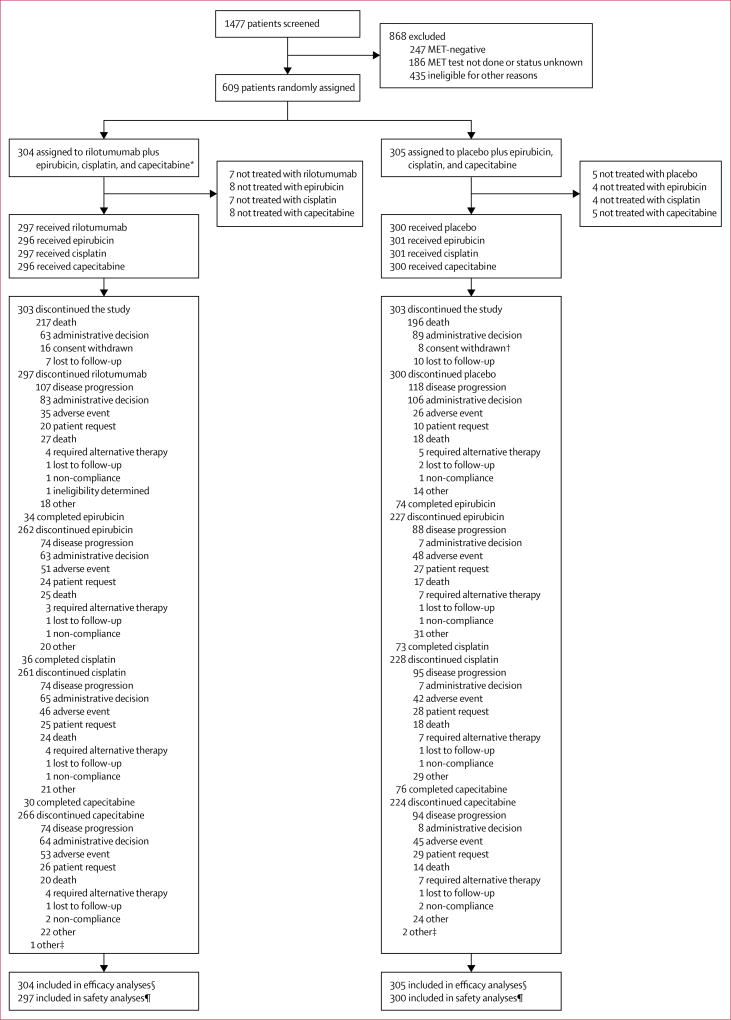
Between study initiation (Nov 7, 2012) and termination (Nov 21, 2014), 1477 patients were screened; 1291 completed successful biomarker testing, of whom 1043 (81%) were deemed to be MET positive. 868 screened patients were excluded because they were MET negative, MET testing was not done or the MET status was unknown, or because they were ineligible for other prespecified reasons (appendix p 6). 608 (47%) of the 1291 patients completed the required biomarker testing successfully and met the other enrolment criteria and were enrolled, and one patient who was MET negative was enrolled in error; these 609 patients were randomly assigned to receive rilotumumab plus epirubicin, cisplatin, and capecitabine (rilotumumab group; n=304) or placebo plus epirubicin, cisplatin, and capecitabine (placebo group; n=305; figure 1). Median follow-up was 7·7 months (IQR 3·6–12·0) for patients in the rilotumumab group and 9·4 months (5·3–13·1) for patients in the placebo group. Demographics and baseline characteristics were generally balanced across treatment groups (table 1). Notably, although not specifically controlled for by stratification, the distribution of WHO histology classification was balanced between the treatment groups (table 1). The number of randomly assigned patients by country and treatment is shown in the appendix (p 7).
Figure 1. Trial profile.
*One patient in the rilotumumab group was MET negative and enrolled in error. †One patient withdrew consent and then died (after which their death was reported and included in the survival analyses); this patient is listed under consent withdrawn. ‡End of study visit data not available for three patients. §Efficacy analyses included all randomly assigned patients. ¶Safety analyses included all randomly assigned patients who received at least one dose of rilotumumab or placebo; one patient randomly assigned to the placebo group received one dose of rilotumumab in one cycle in error, so was analysed as part of the rilotumumab group for the safety analyses.
Table 1.
Demographics and baseline characteristics
| Rilotumumab plus epirubicin, cisplatin, and capecitabine (n=304) |
Placebo plus epirubicin, cisplatin, and capecitabine (n=305) |
|
|---|---|---|
| Age (years) | 61 (28–84) | 59 (26–81) |
| >65 years | 111 (37%) | 94 (31%) |
|
| ||
| Sex | ||
| Male | 205 (67%) | 220 (72%) |
| Female | 99 (33%) | 85 (28%) |
|
| ||
| Ethnicity | ||
| Hispanic or Latino | 23 (8%) | 24 (8%) |
| Other | 281 (92%) | 281 (92%) |
|
| ||
| Race | ||
| White | 290 (95%) | 288 (94%) |
| Asian | 4 (1%) | 2 (<1%) |
| Black or African American | 3 (1%) | 5 (2%) |
| Native Hawaiian or other Pacific Islander | 1 (<1%) | 0 |
| Other | 6 (2%) | 10 (3%) |
|
| ||
| Region | ||
| Western Europe, South Africa, Australia | 121 (40%) | 129 (42%) |
| Eastern Europe (including Turkey) | 141 (46%) | 130 (43%) |
| North America | 26 (9%) | 27 (9%) |
| South America | 16 (5%) | 19 (6%) |
|
| ||
| ECOG performance score | ||
| 0 | 117 (39%) | 115 (38%) |
| 1 | 187 (62%) | 189 (62%) |
| 2 | 0 | 1 (<1%)* |
|
| ||
| Primary tumour location | ||
| Distal oesophageal | 24 (8%) | 39 (13%) |
| Gastro-oesophageal junction | 53 (17%) | 71 (23%) |
| Gastric | 227 (75%) | 195 (64%) |
|
| ||
| Histological type (WHO classification) | ||
| Tubular adenocarcinoma | 22 (7%) | 22 (7%) |
| Mucinous adenocarcinoma | 8 (3%) | 11 (4%) |
| Papillary adenocarcinoma | 2 (<1%) | 1 (<1%) |
| Signet-ring carcinoma | 36 (12%) | 36 (12%) |
| Adenocarcinoma | 236 (78%) | 235 (77%) |
|
| ||
| Metastatic disease | 284 (93%) | 283 (93%) |
|
| ||
| Liver metastases | 118 (39%) | 136 (45%) |
|
| ||
| Disease stage at initial diagnosis | ||
| I | 11 (4%) | 3 (1%) |
| II | 18 (6%) | 17 (6%) |
| III | 42 (14%) | 42 (14%) |
| IV | 230 (76%) | 240 (79%) |
| Missing | 3 (1%) | 3 (1%) |
|
| ||
| Previous surgery† | 48 (16%) | 48 (16%) |
|
| ||
| Previous chemotherapy‡ | 24 (8%) | 31 (10%) |
|
| ||
| Previous radiotherapy | 15 (5%) | 27 (9%) |
|
| ||
| H score§ | 96 (56) | 93 (56) |
Data are median (range), n (%), or mean (SD). ECOG=Eastern Cooperative Oncology Group.
This patient was enrolled in error.
Surgery included previous gastrectomy or oesophagectomy.
Chemotherapy included previous adjuvant or neoadjuvant chemotherapy.
Histology score (calculated as 1×[% cells 1+] + 2×[% cells 2+]+ 3×[% cells 3+]). The biomarker analyses only included patients who had received at least one dose of study treatment and had evaluable biomarker data (rilotumumab, n=298; placebo, n=299; overall, n=597).
Study treatment was stopped early after an independent data monitoring committee found a higher number of deaths in the rilotumumab group compared with the placebo group during a planned safety review (94 deaths in the rilotumumab group vs 75 deaths in the placebo group; data cutoff Sept 22, 2014) and it was determined that protocol-defined futility criteria were likely to have been met.22 As a result, patients in the rilotumumab group discontinued all study treatment, whereas those in the placebo group were allowed to continue on chemotherapy alone. All patients were invited to stay on-study to complete safety follow-up visits 30 days after stopping treatment as well as to complete long-term survival follow-up visits, until the study closed on Aug 18, 2015. The database was locked on Sept 11, 2015.
The median number of infusions per patient was higher in the placebo group than in the rilotumumab group (appendix p 8). The median number of cycles of rilotumumab or placebo were 4·0 (IQR 2·0–7·0) and 6·0 (3·0–8·0), respectively, in the combined treatment period (ie, treatment with rilotumumab or placebo in combination with chemotherapy) and 4·0 (IQR 2·0–6·0) and 2·5 (1·0–6·0), respectively, in the monotherapy period (ie, treatment with rilotumumab or placebo after completion of chemotherapy). The overall median relative dose intensity of rilotumumab (defined as the ratio of the actual cumulative dose of rilotumumab to the protocol-specified cumulative dose over the specified period) over the full study was 0·98 (range 0·1–1·1). An attempt to collect survival status was made within 3 months of study close for each patient still on study at that time. More patients were classified as having discontinued treatment because of disease progression (107 [35%] in the rilotumumab group vs 118 [39%] in the placebo group) than because of adverse events (35 [12%] vs 26 [9%]).
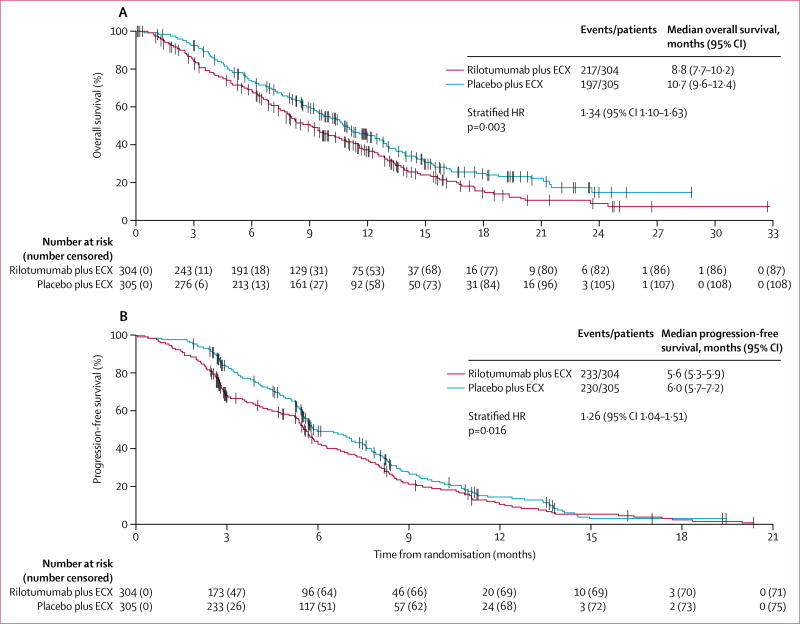
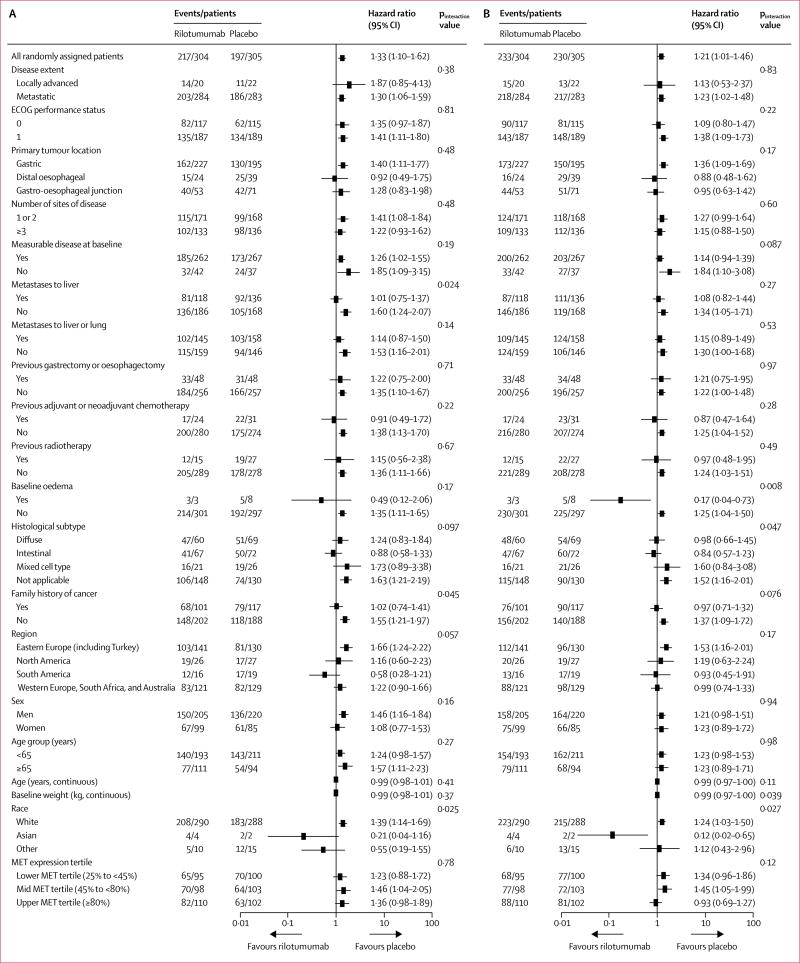
At the time of the final analysis, 217 (71%) of 304 patients in the rilotumumab group and 197 (65%) of 305 patients in the placebo group had died, and 233 (77%) patients in the rilotumumab group and 230 (75%) patients in the placebo group had a progression-free survival event of disease progression or death. Median overall survival and progression-free survival were shorter in the rilotumumab group than in the placebo group (overall survival: 8·8 months [95% CI 7·7–10·2] vs 10·7 months [9·6–12·4], HR 1·34 [95% CI 1·10–1·63]; progression-free survival: 5·6 months [5·3–5·9] vs 6·0 months [5·7–7·2], HR 1·26 [1·04–1·51]; figure 2). The stratified Kaplan-Meier estimate of overall survival at 12 months was 36·0% (95% CI 30·3–41·7) in the rilotumumab group and 45·1% (39·2–50·8) in the placebo group (p=0·032). In overall survival and progression-free survival subgroups defined by baseline characteristics, no subgroups were found to benefit from the addition of rilotumumab to chemotherapy (figure 3).
Figure 2. Overall survival (A) and progression-free survival (B).
ECX=epirubicin, cisplatin, and capecitabine. HR=hazard ratio.
Figure 3. Overall survival (A) and progression-free survival (B) subgroup analysis.
All hazard ratios given in this figure are unstratified. ECOG=Eastern Cooperative Oncology Group.
Disease progression was reported in 116 (38%) of 304 patients in the rilotumumab group and 141 (46%) of 305 patients in the placebo group. Median time from randomisation to disease progression was 6·05 months (95% CI 5·68–7·95) in the rilotumumab group and 7·06 months (5·88–7·85) in the placebo group (stratified HR 1·24, 95% CI 0·96–1·59; p=0·097).
In patients with measurable disease at baseline, the proportion of patients who achieved an objective response was lower in the rilotumumab group than in the placebo group (table 2). Median time from randomisation to first complete response or partial response was very similar in the two groups of the study: 2·79 months (IQR 2·69–2·99) in the rilotumumab group and 2·76 months (2·63–2·96) in the placebo group.
Table 2.
Overall response
| Rilotumumab plus epirubicin, cisplatin, and capecitabine (n=262) |
Placebo plus epirubicin, cisplatin, and capecitabine n=267) |
|
|---|---|---|
| Best overall response* | ||
| Complete response | 3 (1%) | 8 (3%) |
| Partial response | 75 (29%) | 111 (42%) |
| Stable disease† | 62 (24%) | 70 (26%) |
| Progressive disease | 42 (16%) | 37 (14%) |
| Non-complete response or non-progressive disease‡ | 1 (<1%) | 0 |
| Not evaluable | 8 (3%) | 8 (3%) |
| Missing§ | 71 (27%) | 33 (12%) |
| Objective response (95% CI)¶ | 29·8% (24·3–35·7) | 44·6% (38·5–50·8) |
| Stratified odds ratio (95% CI) | 0·53 (0·37–0·76) | ·· |
| p value | 0·0005 | ·· |
| Disease control (95% CI)‖ | 53·4% (47·2–59·6) | 70·8% (64·9–76·2) |
| Stratified odds ratio (95% CI) | 0·47 (0·33–0·68) | ·· |
| p value | <0·0001 | ·· |
Data are n (%) unless otherwise stated. Response analysis set includes all patients with at least one unidimensionally measurable lesion at baseline per Response Evaluation Criteria in Solid Tumors version 1.1 (RECIST 1.1).
Defined as best observed disease response per RECIST 1.1, excluding tumour assessments after initiation of new anticancer therapy, surgical resection, or an assessment of disease progression.
Classification required patients to have stable disease at least 11 weeks after the first dose of protocol-specified therapy.
Non-complete response or non-progressive disease was defined as measurable disease without target lesions at baseline and no new lesions developed during therapy.
The first scan after baseline was scheduled to occur at week 12±7 days. Of the 104 patients with missing response results, 58 ended the study before the first scan was expected (death, n=43; consent withdrawal, n=12; other, n=3). An additional seven patients (rilotumumab, n=4; placebo, n=3) ended the study during the scan window but before the scan occurred (death, n=5 [rilotumumab, n=3; placebo, n=2]; lost to follow-up, n=1 [rilotumumab]; consent withdrawal, n=1 [placebo]), and 39 patients remained on study but missed the scan window (rilotumumab, n=24; placebo, n=15).
Objective response defined as complete response plus partial response.
Disease control defined as complete response plus partial response plus stable disease.
Disease progression or death following objective response was reported in 54 (69%) of 78 patients in the rilotumumab group and 82 (69%) of 119 patients in the placebo group; 24 (31%) patients in the rilotumumab group and 37 (31%) in the placebo group were censored. The median time from the date of first assessment of objective response to disease progression or death was 5·65 months (IQR 3·22–8·41) in the rilotumumab group and 5·29 months (2·79–8·34) in the placebo group (stratified HR 0·95, 95% CI 0·65–1·35.
Treatment-emergent adverse events occurring in 10% or more patients are shown in table 3 (see appendix pp 9–19 for grade 1–2 treatment-emergent adverse events occurring in ≥10% of patients, and all grade 3 or worse treatment-emergent adverse events). Treatment-emergent adverse events occurring with the greatest difference between groups are shown in the appendix (p 20). The most common grade 3–5 adverse events in the rilotumumab and placebo groups were neutropenia (86 [29%] of 298 patients vs 97 [32%] of 299 patients), anaemia (37 [12%]) vs 43 [14%]), and fatigue (30 [10%] vs 35 [12%]). The frequency of serious adverse events was similar in the rilotumumab and placebo groups (table 3) The most common serious adverse events in the rilotumumab and placebo groups were anaemia (seven [2%] of 298 patients vs 21 [7%] of 299 patients), vomiting (14 [5%] vs 12 [4%]), and febrile neutropenia (12 [4%] vs 11 [4%]). Adverse events resulting in withdrawal of study drug in the safety analysis set occurred in 41 (14%) of 298 patients in the rilotumumab group and 30 (10%) of 299 patients in the placebo group; these data differ from the proportions of patients who were classified as having discontinued treatment due to adverse events, because of discrepancies in data collection and classification. The most common adverse events resulting in withdrawal of study drug in the rilotumumab and placebo groups were pulmonary embolism (four [1%] of 298 patients vs three [1%] of 299 patients), fatigue (none vs four [1%]), and asthenia (two [1%] vs one [<1%]). Adverse events resulting in a dose change in study drug in the safety analysis set occurred in three (1%) patients in the rilotumumab group and in no patients in the placebo group. 73 patients died due to adverse events (42 [14%] of 298 patients in the rilotumumab group vs 31 [10%] of 299 patients in the placebo group), most related to disease progression (table 3).
Table 3.
Summary of treatment-emergent adverse events occurring in 10% or more patients in the safety analysis set
| Rilotumumab plus epirubicin, cisplatin, and capecitabine (n=298) |
Placebo plus epirubicin, cisplatin, and capecitabine (n=299) |
|
|---|---|---|
| Treatment-emergent adverse events (all grades) | 283 (95%) | 288 (96%) |
| Serious adverse events | 142 (48%) | 149 (50%) |
| Fatal adverse events | 42 (14%) | 31 (10%) |
| Due to disease progression | 33 (11%) | 23 (8%) |
| Not due to disease progression | 9 (3%) | 8 (3%) |
| Treatment-emergent adverse events occurring in ≥10% of patients | ||
| Nausea | 128 (43%) | 153 (51%) |
| Neutropenia | 111 (37%) | 126 (42%) |
| Anaemia | 97 (33%) | 125 (42%) |
| Fatigue | 103 (35%) | 100 (33%) |
| Vomiting | 100 (34%) | 98 (33%) |
| Alopecia | 74 (25%) | 94 (31%) |
| Decreased appetite | 73 (24%) | 74 (25%) |
| Diarrhoea | 61 (20%) | 81 (27%) |
| Palmar-plantar erythrodysaesthesia syndrome | 59 (20%) | 81 (27%) |
| Constipation | 67 (22%) | 62 (21%) |
| Peripheral oedema | 88 (30%) | 39 (13%) |
| Asthenia | 57 (19%) | 53 (18%) |
| Abdominal pain | 49 (16%) | 54 (18%) |
| Hypomagnesaemia | 46 (15%) | 36 (12%) |
| Weight decreased | 23 (8%) | 49 (16%) |
| Hypokalaemia | 32 (11%) | 33 (11%) |
| Dizziness | 32 (11%) | 27 (9%) |
| Peripheral neuropathy | 24 (8%) | 33 (11%) |
| Leucopenia | 22 (7%) | 31 (10%) |
| Hypoalbuminaemia | 34 (11%) | 10 (3%) |
Treatment-emergent adverse event defined as any adverse event that occurred or worsened after the first dose of protocol-required therapy and before 30 days after the last dose of protocol-required therapy. Safety analysis set includes all patients who received at least one dose of rilotumumab or placebo; patients were analysed according to treatment received.
In patients with evaluable serum samples (rilotumumab group, n=282; placebo group, n=290), four patients (two in each group) surprisingly tested positive for anti-rilotumumab-binding antibodies at baseline; however, no additional anti-rilotumumab-binding antibodies were detected after treatment. Additionally, no neutralising anti-rilotumumab antibodies were detected in any patients.
316 (52%) of 609 patients were included in the pharmacokinetics analyses (rilotumumab group, n=278; placebo group, n=38). The intensive pharmacokinetic analysis set included 91 patients (rilotumumab group, n=55; placebo group, n=36). After a single intravenous infusion of 15 mg/kg rilotumumab in combination with epirubicin, cisplatin, and capecitabine, the mean Cmax for rilotumumab was 227 µg/mL (SD 59·1) and the mean area under the curve from time zero to time of last quantifiable concentration (AUClast) was 54 200 h × µg/mL (SD 12 200). After multiple infusions of rilotumumab in combination with epirubicin, cisplatin, and capecitabine, the mean end-of-infusion concentrations of rilotumumab were 205 (SD 63), 308 (114), 320 (97), and 344 (104) µg/mL in cycles 1, 3, 5, and 7, respectively; and the mean trough concentrations (pre-dose) of rilotumumab were 90 (SD 37), 115 (56), and 132 (50) µg/mL in cycles 3, 5 and 7, respectively. Rilotumumab exposure seemed to near steady state after cycle 3.
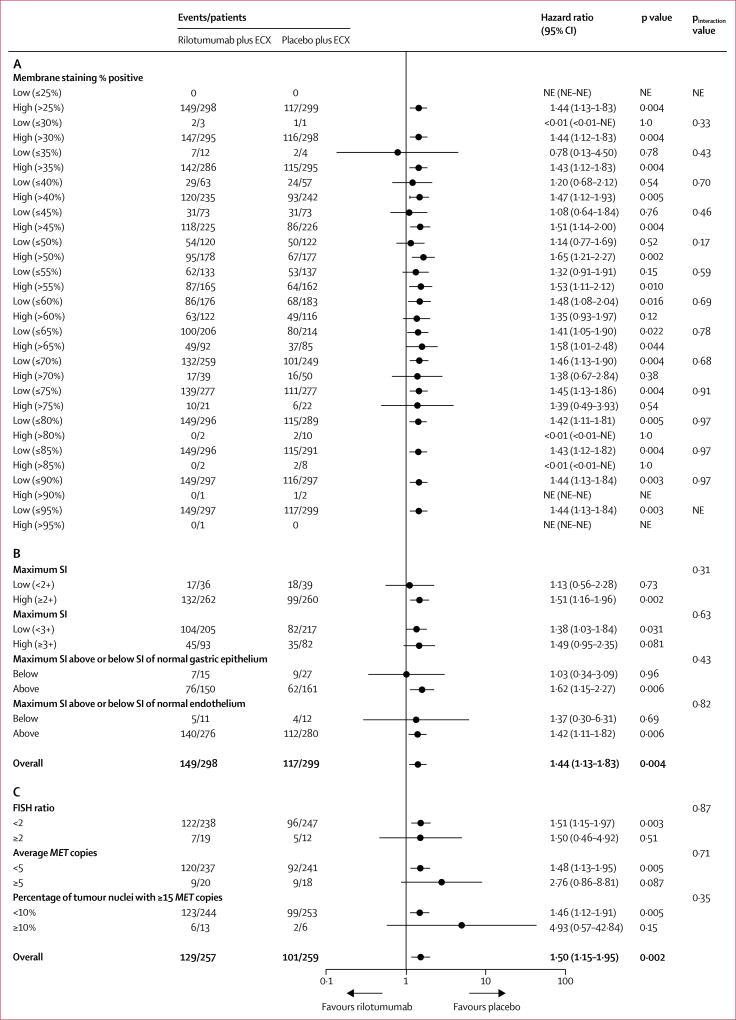
597 patients were included in the MET protein expression analysis (rilotumumab group, n=298; placebo group, n=299; primary analysis data cutoff; appendix p 21); one patient in the rilotumumab group included in this analysis was MET negative (enrolled in error). Mean tumour cell percentage membrane staining (ie, extensity; 63% [SD 24·5] in the rilotumumab group vs 61% [24·9] in the placebo group) and mean H score (96·0 [SD 56·2] vs 92·7 [55·8]) were balanced between groups. MET expression extensity did not correlate with prognosis (appendix p 24). After adjusting for MET expression extensity as a continuous variable, the HR for treatment effect on overall survival was 1·44 (95% CI 1·13–1·83) and the HR for progression-free survival was 1·25 (1·01–1·55), which are similar to the stratified overall survival and progression-free survival primary analyses. No significant interactions between MET expression extensity and treatment on overall survival (figure 4) or progression-free survival (appendix p 25) were noted. When the population was split by maximum staining intensity score (≥2 + vs <2 and ≥3+ vs <3 +), no score identified a significant interaction with treatment on overall survival (figure 4) or progression-free survival (appendix p 25). H score analysed as a continuous variable was not found to be prognostic in either treatment group, or predictive of treatment effect, for either overall survival (both groups: H score HR 1·00, 95% CI 1·00–1·00; pinteraction=0·88) or progression-free survival (both groups: H score HR 1·00, 1·00–1·00; pinteraction=0·93). When the population was split by maximum staining intensity score above or below the staining intensity of normal gastric epithelium and normal endothelium, neither dichotomisation identified a significant interaction with treatment on overall survival (figure 4) or progression-free survival (appendix p 25).
Figure 4. Treatment effect on overall survival by biomarker subgroup.
Forest plot assessing treatment effect on overall survival by (A) MET expression level measured as extent of tumour cell membrane staining (%) at staining intensity of 1+ or greater, (B) MET expression level measured as maximum staining intensity score, and (C) MET gene amplification level. ECX=epirubicin, cisplatin, and capecitabine. NE=not estimable. SI=staining intensity.
516 patients were included in the MET gene amplification analysis (rilotumumab group, n=257; placebo group, n=259). Few patients harboured MET amplifications (19 [7%] patients in the rilotumumab group vs 12 [5%] patients in the placebo group). Mean average MET copy number (4·0 [SD 5·3] in the rilotumumab group vs 3·3 [2·7] in the placebo group) and mean MET/CEN7 ratio (1·6 [SD 2·4] vs 1·2 [0·9]) were generally balanced between groups. No significant interactions between MET amplification and treatment on overall survival and progression-free survival were noted (figure 4; appendix p 25). Significant treatment effects on overall survival and progression-free survival favouring placebo were noted in the MET unamplified group (figure 4; appendix p 25). Additionally, there was no significant prognostic effect of MET amplification on overall survival (HR 1·01 [95% CI 0·93–1·10] in the rilotumumab group vs 1·04 [0·86–1·26] in the placebo group) or progression-free survival (HR 1·01 [0·94–1·09] vs 0·97 [0·77–1·22]) in either treatment group, although numbers were small for this analysis.
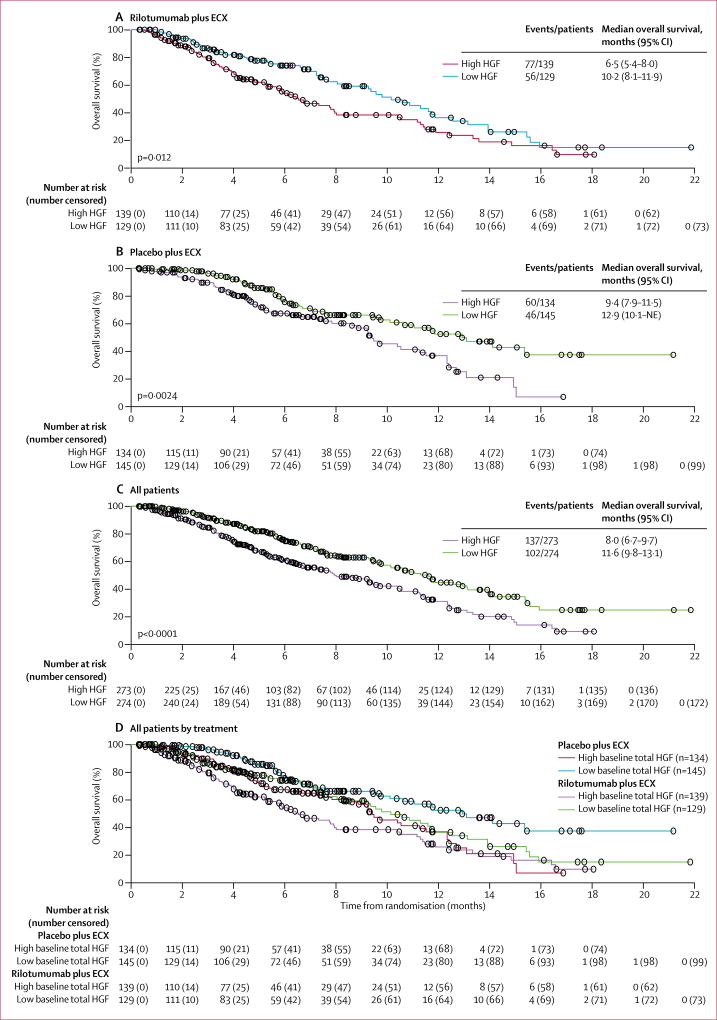
547 patients were included in the HGF analysis (rilotumumab group, n=268; placebo group, n=279). High baseline serum HGF levels (ie, >median) were associated with worse overall survival than were low baseline serum HGF levels (ie, ≤median) in each treatment group when analysed separately and in all patients when analysed together (figure 5). Similarly, high baseline serum HGF levels were associated with worse progression-free survival than were low baseline serum HGF levels in all patients (p=0·0006) and in each treatment group (rilotumumab, p=0·0605; placebo, p=0·0012; appendix p 26). However, no evidence of an interaction of baseline HGF level on treatment effect was observed (overall survival, pinteraction=0·54; progression-free survival, pinteraction=0·38).
Figure 5. Overall survival by treatment group and for entire patient population by baseline HGF level.
Log-rank p values are shown. High HGF was defined as an HGF concentration of greater than the median value; low HGF was defined as an HGF concentration of less than or equal to the median value. ECX=epirubicin, cisplatin, and capecitabine. HGF=hepatocyte growth factor. NE=not estimable.
Discussion
The addition of rilotumumab to epirubicin, cisplatin, and capecitabine was of no benefit to patients with MET-positive gastro-oesophageal cancer regarding overall survival or progression-free survival, with no patient subgroups (as defined by demographics or biomarkers) appearing to benefit. Median overall survival was shorter in the rilotumumab group than the placebo group (8·8 months vs 10·7 months; HR 1·34; 95% CI 1·10–1·63). Study treatment was stopped early after an independent data monitoring committee found a higher number of deaths in the rilotumumab group than in the placebo group; patients in the rilotumumab group subsequently discontinued all study treatment, including epirubicin, cisplatin, and capecitabine, to avoid the possibility of negative interaction between the rilotumumab present after the last treatment and epirubicin, cisplatin, and capecitabine.
By contrast with the MET-positive subset of the phase 2 study in which patients who received rilotumumab (n=41) had a median overall survival of 10·6 months compared with 5·7 months for patients who received placebo (n=17),17 rilotumumab was strikingly ineffective and shortened overall survival compared with that for placebo in this larger phase 3 study. Although discordance from phase 2 to phase 3 studies is unfortunately a frequent occurrence,23 and not entirely surprising in this case in view of the small number of patients in the phase 2 MET-positive subset analysis, several conceivable explanations and considerations are noteworthy.
First, there was no evidence that treatment with rilotumumab resulted in reduced chemotherapy drug concentrations, and no antagonistic pharmacokinetic effect was observed with the combination of rilotumumab with standard epirubicin, cisplatin, and capecitabine chemotherapy in either the phase 2 or phase 3 studies (data not shown). Second, there was a modest difference in the number of patients screened who were considered MET positive between the phase 2 and 3 studies (64% vs 81%, respectively),17 for unknown reasons. Tumour biopsy frequency versus surgical resection frequency, as well as the biopsy frequency of primary tumour sites versus metastatic sites, were similar between the two studies, as were all other clinicopathological characteristics assessed (data not shown). Therefore, potential intrapatient spatial molecular heterogeneity (ie, molecular differences between cells in one part of a tumour and cells in another part) to explain this finding is unlikely. Prescreening with available commercial MET immunohistochemistry assays is not likely to have led to higher patient MET-positivity than in the phase 2 study and, subsequently, selection bias. Overall, no substantial imbalances were seen in the proportion of patients with higher MET-staining intensity and extensity cutoffs between the populations and treatment groups of the phase 2 and phase 3 studies, suggesting that the two trial populations were largely similar (appendix p 22).17
Third, although no new safety signals for rilotumumab were reported in this trial, more patients discontinued rilotumumab and all three cytotoxic therapies because of adverse events in the rilotumumab group than in the placebo group. It is also possible that the observed, yet expected, anti-MET class-effect toxicities, namely oedema and hypoalbuminaemia,24 mirrored clinical progression that consequently led to an increased rate of premature discontinuation of standard active epirubicin, cisplatin, and capecitabine therapy. This suggestion is somewhat supported by the lower median number of cycles of each cytotoxic chemotherapy administered and the decreased frequency of chemotherapy-related toxicity in the rilotumumab group compared with the placebo group. Alternatively, a general antagonistic effect on the efficacy of cytotoxic chemotherapy by rilotumumab leading to earlier progression and death, potentially by exacerbating signalling feedback loops, might also account for the worse results, only appreciated in this larger study. It is possible that cross-talk between MET and other signalling pathways influenced the expression of other receptor proteins, which might have contributed to this observation. Indeed, several other studies of molecules targeting the MET pathway via ligand blocking antibodies have reported negative results;25,26 the lack of a positive effect of targeting the MET pathway has been particularly noted in patients who are negative for MET expression in biomarker-stratified trials.17,27 These findings suggest that biomarker assays and biomarker-positive definitions are essential in selecting optimal patients for anti-MET therapy in any future studies.
Furthermore, it is possible that the MET immunohistochemistry assay inadequately selected the optimal patient population for treatment, with 81% of patients screened considered positive for MET expression. Clearly, the optimal MET biomarker assay, scoring, and positivity criteria for ligand-blocking inhibitors, if any, remain undefined, as exemplified by two large studies (RILOMET-1 and METGastric28) in this disease using two different antibodies and divergent scoring systems. Unfortunately, neither study successfully enriched for a population that benefited from MET ligand-blocking antibodies.
The phase 3 METGastric study, which assessed onartuzumab, stipulated a higher MET-expression cutoff for inclusion than did our study (ie, immunohistochemistry ≥1+ intensity at ≥50% extensity) and included not only membranous staining but also cytoplasmic staining.28 Moreover, in a preplanned co-primary endpoint analysis in patients with a higher expression intensity (≥2+ intensity at ≥50% extensity), the HR for overall survival was 0·64 in favour of onartuzumab (p=0·062).26,28 Unfortunately, the METGastric study closed early because a parallel phase 2 trial failed to identify an obvious predictive cutoff.26 Furthermore, the proportion of patients with higher expression intensity (≥2+ intensity at ≥50% extensity) was 38% (214 of 562 patients) in the METGastric study, compared with only 21% (128 of 609 patients) in our study. Whether these more stringent criteria or other novel biomarker assays or criteria, such as mass spectrometry,29 coupled with an adequately powered analysis, would result in improved outcomes in a more select group thus remains unknown. Nevertheless, on the basis of the results from the available phase 3 studies, ligand-blocking antibodies are clearly not effective in gastro-oesophageal cancers with predominantly low levels of MET expression.
Finally, the mechanism of MET pathway activation as well as the mechanism of therapeutic inhibition must be considered as possible biological explanations for the negative results seen in this study. The MET axis is genomically activated most frequently by MET gene amplification, accounting for about 5% of gastro-oesophageal adenocarcinoma.9 This driver event is a consequence of MET overexpression several times higher than mere overexpression in the absence of MET amplification and results in constitutive autodimerisation, activation, and oncogene addiction of the MET receptor, irrespective of ligand presence.9 Moreover, MET amplification is reported to be a biomarker of poor prognosis and is the biomarker with the most evidence for predictive benefit from anti-MET therapy via receptor-specific inhibitors, including tyrosine kinase inhibitors and bivalent antibodies that cause receptor internalisation and degradation.29 By contrast, a ligand-blocking approach in this setting would be predicted to be ineffective,7,8,29 and this was seen in our study in the rilotumumab group. In our study, MET amplification by FISH using various parameters was balanced between treatment groups as expected; few patients harboured MET amplification (7% in the rilotumumab group vs 5% in the placebo group), and no significant interactions between MET amplification and overall and progression-free survival were seen in either treatment group. Additionally, the alternative and more ubiquitous activation of the MET axis via HGF-induced receptor activation in the absence of MET amplification, although appealing from a drug development perspective and more suited for ligand-blocking therapeutics, was not supported by our findings nor those of the METGastric study. This lack of efficacy is most likely to be a result of the absence of oncogene addiction, by contrast with the MET amplification situation, in which multiple oncogenic pathways are upregulated in the cell, and isolated inhibition of MET is generally insufficient to inhibit tumour growth and is easily circumvented.30 Notably, high HGF expression in this study was associated with worse prognosis irrespective of treatment group. Unfortunately, rilotumumab treatment did not seem to reverse the negative prognostic effect of high HGF compared with placebo even in patients with high HGF expression. The combination of HGF neutralising agents with other therapies that target other key pathways might lead to improved results;31,32 however, this approach requires further investigation.
Several previous studies that assessed MET expression using various antibodies and scoring cutoffs have suggested a negative prognostic effect for MET expression;5,6,8 however, in our study, higher MET expression extensity did not correlate with poorer prognosis. In the phase 2 trial of rilotumumab plus epirubicin, cisplatin, and capecitabine,17 patients in the MET-positive placebo group (≥25% membrane staining at any intensity, n=17) had shorter median overall survival than patients in the MET-negative placebo group (<25% membrane staining at any intensity, n=11). This comparison could not be assessed in the present phase 3 study because MET-negative patients were excluded from enrolment. Again, no prognostic effect was seen at various, albeit arbitrary, expression extensity cutoff points from 25% to 95% with the MET-positive patients enrolled in this study (about 80% of patients screened). The assays that are used to define a MET-positive population probably need to evolve if further testing of agents that target the MET pathway are going to be pursued. In an assessment of the prognostic value of MET expression in a large independent cohort of patients with gastro-oesophageal adenocarcinoma using MET immunohistochemistry, FISH, and selected reaction monitoring mass spectrometry (SRM), each of these three assays demonstrated a negative prognostic effect on overall survival when positive by each method’s criteria.29 However, after adjusting for known prognostic variables (eg, age, stage, and tumour grade), only positivity by FISH (about 5% of cases) and SRM (about 13% of cases) retained significance of an independent negative prognostic effect, whereas positivity by MET immunohistochemistry (about 40% of cases in that study) did not.29
Overall, RILOMET-1 was a negative study. Future studies of MET inhibitors should anticipate class-effect toxicities and incorporate strategies to differentiate these from clinical disease progression. Additional insights into the role of the MET pathway in gastric cancer, a careful assessment of the outcomes in this and other studies, and a greater understanding of predictive biomarkers would be required before embarking on any further development of MET pathway inhibitors in gastric cancer.
Research in context.
Evidence before this study
Platinum-based and fluoropyrimidine-based chemotherapy regimens are the standard treatment options for patients with advanced gastric cancer; however, early preclinical studies indicated that rilotumumab might be beneficial for these patients. We searched PubMed for clinical studies in gastric cancer that assessed rilotumumab treatment in patients with advanced disease. Specific search terms were “rilotumumab”, “AMG102”, “AMG 102”, and “gastric”. We included all English language articles published before Feb 23, 2017.
A phase 2 study assessed rilotumumab plus chemotherapy versus placebo plus chemotherapy in the first-line setting in patients with advanced gastric or oesophagogastric junction adenocarcinoma. Rilotumumab plus chemotherapy met its primary endpoint (progression-free survival) in the intention-to-treat analysis set and in the predefined subset of patients with MET overexpression, supporting initiation of a confirmatory phase 3 study in patients with MET-positive gastric or gastro-oesophageal junction cancer.
Added value of this study
RILOMET-1 is, to our knowledge, the first phase 3 study to assess rilotumumab in combination with epirubicin, cisplatin, and capecitabine chemotherapy as first-line treatment in patients with MET-positive gastric or gastro-oesophageal junction cancer. By contrast with the results of the phase 2 study, in this phase 3 study, the addition of rilotumumab versus placebo to first-line chemotherapy did not increase overall survival in patients with advanced gastro-oesophageal cancer with predominantly low levels of MET expression. Furthermore, no benefit was seen in the small subset of patients with tumours with MET amplification, or in any other preplanned biomarker or clinicopathological subgroups.
Implications of all the available evidence
Our results suggest that ligand-blocking inhibition of the MET pathway in MET-expressing tumours with rilotumumab is not effective in improving clinical outcomes in patients with gastro-oesophageal cancer. It is unlikely that there is a role for this agent in the treatment of gastric cancer given the current limited understanding of the contribution of the MET pathway to tumour development.
Acknowledgments
We acknowledge Meghan Johnson and James Balwit (Complete Healthcare Communications, a CHC Group company, West Chester, PA, USA), whose work was funded by Amgen, and Micah Robinson (Amgen, Thousand Oaks, CA, USA) for assistance in writing this manuscript. DVTC is funded by the National Institutes of Health, USA (NIH K23 CA178203-01A1). DC is funded by the National Institute for Health Biomedical Research Centre based at the Royal Marsden Hospital and Institute of Cancer Research, London, UK.
DVTC reports grants and personal fees from Amgen and Genentech. NCT reports personal fees from Amgen and Roche. S-EA-B reports grants and personal fees from Roche, Celgene, and Lilly; grants from Vifor Pharma, Medac, Hospira, and Novartis; and personal fees from Merck, Bristol-Myers Squibb, Nordic Pharma, and Servier. MT reports serving on an advisory board and receiving honoraria from Lilly, Celgene, Bristol-Myers Squibb, and Merck. CT, AA, and RS report employment by and stock ownership in Amgen. YZ reports previous employment by and stock ownership in Amgen. TH and RT report previous employment by and previous stock ownership in Amgen. DC reports grants from Amgen, AstraZeneca, Bayer, Celgene, Merrimack, MedImmune, Merck Serono, and Sanofi.
Footnotes
See Online/Comment http://dx.doi.org/10.1016/S1470-2045(17)30714-3
See Online for appendix
Contributors
DVTC, NCT, ID, AMM, S-EA-B, DHI, and DC contributed to study conception and design, patient data collection or acquisition, and data analysis and interpretation. ST, EG, BK, IB, MAT, AAU, MT, and FDV contributed to patient data collection or acquisition. CT, RT, AA, TH, and RS contributed to data analysis and interpretation. YZ contributed to study conception and design, and data analysis and interpretation. All authors contributed to the writing and editing of the manuscript and approved the manuscript for submission. DC, the corresponding author, had full access to all the data in the study and had the final responsibility for the decision to submit for publication.
Declaration of interests
All other authors declare no competing interests.
References
- 1.WHO International Agency for Research on Cancer. [accessed June 5, 2017];Cancer fact sheets. http://gco.iarc.fr/today/fact-sheets-cancers?cancer=29&type=0&sex=0.
- 2.Cunningham D, Starling N, Rao S, et al. Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med. 2008;358:36–46. doi: 10.1056/NEJMoa073149. [DOI] [PubMed] [Google Scholar]
- 3.Trusolino L, Bertotti A, Comoglio PM. MET signalling: principles and functions in development, organ regeneration and cancer. Nat Rev Mol Cell Biol. 2010;11:834–48. doi: 10.1038/nrm3012. [DOI] [PubMed] [Google Scholar]
- 4.Kawakami H, Okamoto I. MET-targeted therapy for gastric cancer: the importance of a biomarker-based strategy. Gastric Cancer. 2016;19:687–95. doi: 10.1007/s10120-015-0585-x. [DOI] [PubMed] [Google Scholar]
- 5.Drebber U, Baldus SE, Nolden B, et al. The overexpression of c-met as a prognostic indicator for gastric carcinoma compared to p53 and p21 nuclear accumulation. Oncol Rep. 2008;19:1477–83. [PubMed] [Google Scholar]
- 6.Nakajima M, Sawada H, Yamada Y, et al. The prognostic significance of amplification and overexpression of c-met and c-erb B-2 in human gastric carcinomas. Cancer. 1999;85:1894–902. doi: 10.1002/(sici)1097-0142(19990501)85:9<1894::aid-cncr3>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 7.Lennerz JK, Kwak EL, Ackerman A, et al. MET amplification identifies a small and aggressive subgroup of esophagogastric adenocarcinoma with evidence of responsiveness to crizotinib. J Clin Oncol. 2011;29:4803–10. doi: 10.1200/JCO.2011.35.4928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Catenacci DV, Cervantes G, Yala S, et al. RON (MST1R) is a novel prognostic marker and therapeutic target for gastroesophageal adenocarcinoma. Cancer Biol Ther. 2011;12:9–46. doi: 10.4161/cbt.12.1.15747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Catenacci DV, Liao WL, Thyparambil S, et al. Absolute quantitation of Met using mass spectrometry for clinical application: assay precision, stability, and correlation with MET gene amplification in FFPE tumor tissue. PLoS One. 2014;9:e100586. doi: 10.1371/journal.pone.0100586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Taniguchi T, Kitamura M, Arai K, et al. Increase in the circulating level of hepatocyte growth factor in gastric cancer patients. Br J Cancer. 1997;75:673–77. doi: 10.1038/bjc.1997.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jardim DL, Tang C, Gagliato Dde M, et al. Analysis of 1,115 patients tested for MET amplification and therapy response in the MD Anderson Phase I Clinic. Clin Cancer Res. 2014;20:6336–45. doi: 10.1158/1078-0432.CCR-14-1293. [DOI] [PubMed] [Google Scholar]
- 12.Taniguchi K, Yonemura Y, Nojima N, et al. The relation between the growth patterns of gastric carcinoma and the expression of hepatocyte growth factor receptor (c-met), autocrine motility factor receptor, and urokinase-type plasminogen activator receptor. Cancer. 1998;82:2112–22. [PubMed] [Google Scholar]
- 13.Peters S, Adjei AA. MET: a promising anticancer therapeutic target. Nat Rev Clin Oncol. 2012;9:314–26. doi: 10.1038/nrclinonc.2012.71. [DOI] [PubMed] [Google Scholar]
- 14.Burgess T, Coxon A, Meyer S, et al. Fully human monoclonal antibodies to hepatocyte growth factor with therapeutic potential against hepatocyte growth factor/c-Met-dependent human tumors. Cancer Res. 2006;66:1721–29. doi: 10.1158/0008-5472.CAN-05-3329. [DOI] [PubMed] [Google Scholar]
- 15.Jun HT, Sun J, Rex K, et al. AMG 102, a fully human anti-hepatocyte growth factor/scatter factor neutralizing antibody, enhances the efficacy of temozolomide or docetaxel in U-87 MG cells and xenografts. Clin Cancer Res. 2007;13:6735–42. doi: 10.1158/1078-0432.CCR-06-2969. [DOI] [PubMed] [Google Scholar]
- 16.Gao CF, Xie Q, Zhang YW, et al. Therapeutic potential of hepatocyte growth factor/scatter factor neutralizing antibodies: inhibition of tumor growth in both autocrine and paracrine hepatocyte growth factor/scatter factor:c-Met-driven models of leiomyosarcoma. Mol Cancer Ther. 2009;8:2803–10. doi: 10.1158/1535-7163.MCT-09-0125. [DOI] [PubMed] [Google Scholar]
- 17.Iveson T, Donehower RC, Davidenko I, et al. Rilotumumab in combination with epirubicin, cisplatin, and capecitabine as first-line treatment for gastric or oesophagogastric junction adenocarcinoma: an open-label, dose de-escalation phase 1b study and a double-blind, randomised phase 2 study. Lancet Oncol. 2014;15:1007–18. doi: 10.1016/S1470-2045(14)70023-3. [DOI] [PubMed] [Google Scholar]
- 18.Gordon MS, Sweeney CJ, Mendelson DS, et al. Safety, pharmacokinetics, and pharmacodynamics of AMG 102, a fully human hepatocyte growth factor-neutralizing monoclonal antibody, in a first-in-human study of patients with advanced solid tumors. Clin Cancer Res. 2010;16:699–710. doi: 10.1158/1078-0432.CCR-09-1365. [DOI] [PubMed] [Google Scholar]
- 19.Detre S, Saclani Jotti G, Dowsett M. A “quickscore” method for immunohistochemical semiquantitation: validation for oestrogen receptor in breast carcinomas. J Clin Pathol. 1995;48:876–78. doi: 10.1136/jcp.48.9.876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Greil R, Fasching B, Weger A, Loidl P. Investigation of nuclear c-MYC oncoprotein expression in human hematopoiesis: suitability of a rapid and reliable semiquantitative evaluation system. Lab Invest. 1992;66:251–60. [PubMed] [Google Scholar]
- 21.Lin DY, Wei LJ, Ying Z. Checking the Cox model with cumulative sums of martingale-based residuals. Biometrika. 1993;80:557–72. [Google Scholar]
- 22.PR Newswire. Amgen announces termination of all Amgen-sponsored clinical studies of rilotumumab in advanced gastric cancer. [accessed June 5 2017];2014 Nov 24; http://www.prnewswire.com/news-releases/amgen-announces-termination-of-all-amgen-sponsored-clinical-studies-of-rilotumumab-in-advanced-gastric-cancer-300000103.html.
- 23.Ratain MJ. Phase II oncology trials: let’s be positive. Clin Cancer Res. 2005;11:5661–62. doi: 10.1158/1078-0432.CCR-05-1046. [DOI] [PubMed] [Google Scholar]
- 24.Morley R, Cardenas A, Hawkins P, et al. Safety of onartuzumab in patients with solid tumors: experience to date from the onartuzumab clinical trial program. PLoS One. 2015;10:e0139679. doi: 10.1371/journal.pone.0139679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Spigel DR, Edelman MJ, O’Byrne K, et al. Results from the phase III randomized trial of onartuzumab plus erlotinib versus erlotinib in previously treated stage IIIB or IV non-small-cell lung cancer: METLung. J Clin Oncol. 2017;35:412–20. doi: 10.1200/JCO.2016.69.2160. [DOI] [PubMed] [Google Scholar]
- 26.Shah MA, Cho JY, Tan IB, et al. A randomized phase II study of FOLFOX with or without the MET inhibitor onartuzumab in advanced adenocarcinoma of the stomach and gastroesophageal junction. Oncologist. 2016;21:1085–90. doi: 10.1634/theoncologist.2016-0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Spigel DR, Ervin TJ, Ramlau RA, et al. Randomized phase II trial of onartuzumab in combination with erlotinib in patients with advanced non-small-cell lung cancer. J Clin Oncol. 2013;31:4105–14. doi: 10.1200/JCO.2012.47.4189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shah MA, Bang YJ, Lordick F, et al. Effect of fluorouracil, leucovorin, and oxaliplatin with or without onartuzumab in HER2-negative, MET-positive gastroesophageal adenocarcinoma: the METGastric randomized clinical trial. JAMA Oncol. 2017;3:620–27. doi: 10.1001/jamaoncol.2016.5580. Proc Am Soc Clin Oncol 2015; 33 (suppl): abstract 4012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Catenacci DVT, Ang A, Liao WL, et al. MET tyrosine kinase receptor expression and amplification as prognostic biomarkers of survival in gastroesophageal adenocarcinoma. Cancer. 2017;123:1061–70. doi: 10.1002/cncr.30437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Corso S, Ghiso E, Cepero V, et al. Activation of HER family members in gastric carcinoma cells mediates resistance to MET inhibition. Mol Cancer. 2010;9:121. doi: 10.1186/1476-4598-9-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang L, Fu L. Mechanisms of resistance to EGFR tyrosine kinase inhibitors. Acta Pharm Sin B. 2015;5:390–401. doi: 10.1016/j.apsb.2015.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Madoz-Gurpide J, Zazo S, Chamizo C, et al. Activation of MET pathway predicts poor outcome to cetuximab in patients with recurrent or metastatic head and neck cancer. J Transl Med. 2015;13:282. doi: 10.1186/s12967-015-0633-7. [DOI] [PMC free article] [PubMed] [Google Scholar]