Commentary
Seizure Detection by Critical Care Providers Using Amplitude-Integrated Electroencephalography and Color Density Spectral Array in Pediatric Cardiac Arrest Patients.
Du Pont-Thibodeau G, Sanchez SM, Jawad AF, Nadkarni VM, Berg RA, Abend NS, Topjian AA. 2017;18:363–369.
OBJECTIVES: Determine the accuracy and confidence of critical care medicine providers to identify seizures using amplitude-integrated electroencephalography versus amplitude-integrated electroencephalography combined with color density spectral array electroencephalography (aEEG + CDSA). DESIGN: Tutorial and questionnaire. SETTING: PICU. SUBJECTS: Pediatric critical care providers (attendings, fellows, and nurses). INTERVENTIONS: A standardized powerpoint tutorial on amplitude-integrated electroencephalography and color density spectral array followed by classification of 100 amplitude-integrated electroencephalography images and 100 amplitude-integrated electroencephalography combined with color density spectral array as displaying seizures or not displaying seizures. MEASUREMENTS AND MAIN RESULTS: Electroencephalography tracings were obtained from children monitored with continuous electroencephalography after cardiac arrest. The gold standard for seizure identification was continuous electroencephalography interpretation by a pediatric electroencephalographer. The same electroencephalography tracings were used to generate images containing only amplitude-integrated electroencephalography or aEEG + CDSA. Twenty-three critical care medicine providers underwent a 30-minute tutorial on amplitude-integrated electroencephalography and color density spectral array interpretation. They were then asked to determine if there were seizures on 100 amplitude-integrated electroencephalography images and 100 aEEG + CDSA. Amplitude-integrated electroencephalography seizure detection sensitivity was 77% (95% CI, 73%–80%), specificity of 65% (95% CI, 62%–67%), negative predictive value of 88% (95% CI, 86%–90%), and positive predictive value of 46% (95% CI, 43%–49%). For aEEG + CDSA, sensitivity was 77% (95% CI, 74%–81%), specificity of 68% (95% CI, 66%–71%), negative predictive value of 89% (95% CI, 87%–90%), and positive predictive value of 49% (95% CI, 46%–52%). Sensitivity for status epilepticus detection was 77% (95% CI, 71%–82%) with amplitude-integrated electroencephalography and 75% (95% CI, 69%–81%) with aEEG + CDSA. The addition of color density spectral array to amplitude-integrated electroencephalography did not improve seizure detection. However, 87% of critical care medicine providers qualitatively felt that combining both modalities increased their ability to detect seizures. CONCLUSIONS: Amplitude-integrated electroencephalography and aEEG + CDSA offer reasonable sensitivity and negative predictive value for seizure detection by critical care medicine providers. aEEG + CDSA did not improve seizure detection over amplitude-integrated electroencephalography alone although critical care medicine providers felt more confident using both tools combined. Amplitude-integrated electroencephalography and color density spectral array require further evaluation as a tool for screening for seizures and should only be used in conjunction with professional continuous electroencephalography review.
Since many epilepsy centers do not provide continuous monitoring of ongoing video-EEG studies, automated seizure detection algorithms are important for prompt notification of care providers in inpatient settings. There has been increasing research to improve sensitivity and specificity of such algorithms for other purposes as well, including responsive neurostimulation devices and automatic parsing of electrocorticography (1). Automated detection research started in the 1970s but continued to be scattered and scanty until this last decade, when it surged (Figure). Despite numerous attempts—including ones that utilize time–frequency decompositions, amplitudes, synchronization likelihood (2), and signal complexity such as Gabor atom density (3), among others—the gold standard continues to be direct interpretation of the full-montage EEG by an expert electroencephalographer.
FIGURE.
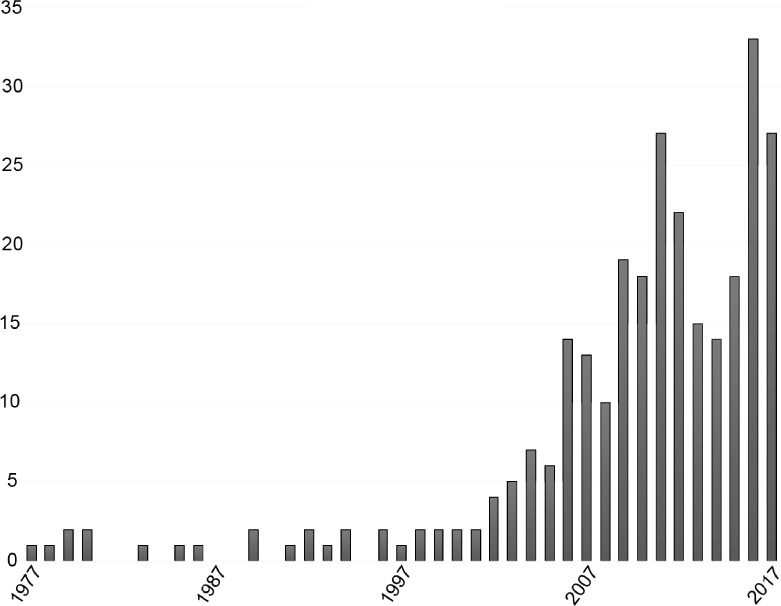
Numbers of publications per year over the past 4 decades according to a PubMed search using the terms “EEG and Epilepsy and automated detection.”
An important focus of automated seizure detection is in the ICU setting where expert interpretation of the EEG is often not continuously available. Up to 92% of seizures in the ICU are clinically silent (4), and seizure occurrence is a determinant of prognosis. The success of seizure treatment is also dependent on seizure duration, with longer seizures becoming increasingly more refractory to pharmacotherapy. Thus, early detection and treatment can improve the outcome. In modern ICUs, however, while vital functions are monitored continuously, brain functions are not. The routine “neurochecks” are only intermittent and are not sensitive for detecting subclinical seizures. In up to 82% of monitored neurological patients, continuous EEG will have an impact on medical decision making (5), but only four of every five centers in the United States have in-house continuous EEG monitoring available, and according to Du Pont-Thibodeau et al., more than one-third of Canadian centers do not have access to continuous EEG remotely.
A well-investigated method of seizure detection is amplitude-integrated EEG (aEEG), which plots the amplitude of the EEG as the ordinate versus compressed time as the abscissa. Another method is color density spectral array (CDSA), which uses the Fourier transformation, a time–frequency decomposition method, to present the EEG power (defined as the square of the EEG amplitude divided by the frequency) as a color spectrum with the ordinate corresponding to the EEG frequency and the abscissa to time. In both tools, the x-axis, which denotes the time, displays hours of compressed EEG in a single image. Du Pont-Thibodeau et al. used a standardized PowerPoint tutorial to educate health-care workers about aEEG and CDSA as methods of seizure detection in postanoxic pediatric patients. The participants were five nurses, 12 fellows, and six attending physicians, most of whom had not received any training in EEG. The tutorial included basics of continuous EEG as well as aEEG and CDSA interpretation with examples of seizure detection and movement artifacts. The authors defined “seizures” as events lasting at least 10 seconds, or shorter if in association with a clinical change with evolution patterns in morphology, voltage, and frequency. “Status epilepticus” was defined as a single event of at least 30 minutes or recurrent seizures of 30 minutes or longer adding up to a total of 30 minutes or longer within a 1-hour period.
After the tutorial, the participants received a questionnaire that required the classification of aEEG and aEEG + CDSA as having seizures or no seizures. The authors presented 100 images of aEEG, each followed by aEEG combined with the matching CDSA image. Afterwards, the participants were asked about their level of confidence of interpreting aEEG and aEEG + CDSA images and whether both tools increased their ability to detect seizures compared with aEEG alone.
The authors found no difference in the accuracy of seizure detection among the nurses, fellows, and attending physicians by calculating the kappa statistic. The sensitivity of seizure detection using aEEG was 77% and specificity was 65%. This was comparable to the accuracy of seizure detection using aEEG + CDSA, which had a sensitivity of 77% and a specificity of 68%. For aEEG, the false-positive rate was 35% and false-negative rate was 23%. For aEEG combined with CDSA, the false-positive rate was 32% and false-negative rate was 23%. Additionally, the sensitivity of detecting status epilepticus was 77% using aEEG alone, compared with 75% for aEEG + CDSA. Of note, 87% of the participants felt that combining both modalities improved their ability to detect seizures.
This study is important because many seizures in an ICU setting occur without clear clinical manifestations, and they influence the prognosis significantly. It has been suggested that reviewing continuous EEG data at least twice a day can minimize the chances of missing seizures (6). For nonexpert readers, compressing EEG data into relatively easily interpretable plots is superior to viewing the raw EEG. Indeed, a prospective cohort study that used a PowerPoint lecture about recognition of epileptiform discharges among residents, fellows, nurses, and EEG technicians concluded that identification of epileptiform discharges by these bedside caregivers continued to be startlingly low (7). The overall mean correct response rate in that study only increased to 67% for the post-test from a rate of 61% for the pretest. However, a more recent study that used 5–6 hours of training of nonexpert physicians and ICU nurses in interpreting simultaneously displayed aEEG and density spectral arrays achieved a better outcome (8). In that study, the sensitivity of seizure detection was 88 to 99 percent with a specificity of 89 to 95 percent, interrater agreement was high, and no difference in performance was noted between physicians and nurses.
It is interesting that, despite the reported false-positive and false-negative rates in the study by Du Pont-Thibodeau et al., training the participants, most of whom had no prior EEG education, was fairly brief. Perhaps with more training—for example, via online modules followed by brief quizzes and certification—the results could improve markedly. The ultimate practical goal is not merely to educate bedside caregivers in EEG interpretation but to improve patient outcomes. In that sense, since suspected seizures identified by these care providers should prompt reversion to the gold standard (i.e., assessment of the raw EEG by an expert reader), any effort to increase the rate of detection compared to the status quo is meritorious.
Supplementary Material
References
- 1. Baldassano SN, Brinkmann BH, Ung H, Blevins T, Conrad EC, Leyde K, Cook MJ, Khambhati AN, Wagenaar JB, Worrell GA, Litt B.. Crowd-sourcing seizure detection: Algorithm development and validation on human implanted device recordings. 2017; 140: 1680– 1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ponten SC, Ronner HE, Strijers RLM, Visser MC, Peerdeman SM, Vandertop WP, Beishuizen A, Girbes AR, Stam CJ.. Feasibility of online seizure detection with continuous EEG monitoring in the intensive care unit. 2010; 19: 580– 586. [DOI] [PubMed] [Google Scholar]
- 3. Jouny CC, Bergey GK.. Characterization of early partial seizure onset: Frequency, complexity and entropy. 2012; 123: 658– 669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Claassen J, Mayer SA, Kowalski RG, Emerson RG, Hirsch LJ.. Detection of electrographic seizures with continuous EEG monitoring in critically ill patients. 2004; 62: 1743– 1748. [DOI] [PubMed] [Google Scholar]
- 5. Jordan KG. Continuous EEG and evoked potential monitoring in the neuroscience intensive care unit. 1993; 10: 445– 475. [DOI] [PubMed] [Google Scholar]
- 6. Wittman JJ, Hirsch LJ.. Continuous electroencephalogram monitoring in the critically ill. 2005; 2: 330– 341. [DOI] [PubMed] [Google Scholar]
- 7. Leira EC, Bertrand ME, Hogan RE, Cruz-Flores S, Wyrwich KW, Albaker OJ, Holzemer EM.. Continuous or emergent EEG: Can bedside caregivers recognize epileptiform discharges? 2004; 30: 207– 212. [DOI] [PubMed] [Google Scholar]
- 8. Dericioglu N, Yetim E, Bas DF, Bilgen N, Caglar G, Arsava EM, Topcuoglu MA.. Non-expert use of quantitative EEG displays for seizure identification in the adult neuro-intensive care unit. 2015; 109: 48– 56. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


