Abstract
Sucrose:sucrose 1-fructosyl transferase (1-SST) is the key enzyme initiating fructan synthesis in Asteraceae. Using reverse transcriptase-PCR, we isolated the cDNA for 1-SST from Taraxacum officinale. The cDNA-derived amino acid sequence showed very high homology to other Asteracean 1-SSTs (Cichorium intybus 86%, Cynara scolymus 82%, Helianthus tuberosus 80%), but homology to 1-SST from Allium cepa (46%) and Aspergillus foetidus (18%) was much lower. Fructan concentrations, 1-SST activities, 1-SST protein, and mRNA concentrations were compared in different organs during vegetative and generative development of T. officinale plants. Expression of 1-SST was abundant in young roots but very low in leaves. 1-SST was also expressed at the flowering stages in roots, stalks, and receptacles. A good correlation was found between northern and western blots showing transcriptional regulation of 1-SST. At the pre-flowering stage, 1-SST mRNA concentrations and 1-SST activities were higher in the root phloem than in the xylem, resulting in the higher fructan concentrations in the phloem. Fructan localization studies indicated that fructan is preferentially stored in phloem parenchyma cells in the vicinity of the secondary sieve tube elements. However, inulin-like crystals occasionally appeared in xylem vessels.
Although a majority of flowering plant species store starch or Suc, approximately 15% (Hendry, 1993) use fructan as their main reserve carbohydrate. Fructans are Fru-based oligo- or polysaccharides. The precise type present and the DP are species and even tissue specific. Inulin-type fructans consist of linear β (2→1)-linked fructofuranosyl units and occur in dicotyledonous species such as Cichorium intybus, Helianthus tuberosus, and Taraxacum officinale. Monocotyledonous species often contain levans consisting of linear β-(2→6)-linked fructofuranosyl units or more complex branched graminans (Shiomi, 1989; Livingston et al., 1993; Vijn et al., 1997). Essentially, all of these fructans represent extensions of Suc by Suc-derived fructosyl units (Wiemken et al., 1995). Aside from a function as a long- or short-term reserve carbohydrate, other, perhaps more specific, roles in plants remain elusive; however, data suggest a correlation with drought and/or frost tolerance (Hendry, 1993; Pilon-Smits et al., 1995; Livingston and Henson, 1998).
In inulin-producing plants, fructan synthesis involves two distinct enzymes: Suc:Suc 1-fructosyl transferase (1-SST) and fructan:fructan 1-fructosyl transferase (1-FFT) (Edelman and Jefford, 1968; Koops and Jonker, 1996; Lüscher et al., 1996; Van den Ende and Van Laere, 1996a). In a first step, the key enzyme 1-SST (EC 2.4.1.99; G-F + G-F → G-F-F + G) produces the trisaccharide 1-kestose and Glc. In a second step, 1-FFT (EC 2.4.1.100; G-Fn + G-Fm ⇔ G-F[n − 1] + G-F[m + 1]) with n > 1, m ≥ 1) elongates the Fru chain by catalyzing the transfer of a Fru residue from one fructan molecule to another.
Several cDNAs encoding plant fructan-synthesizing enzymes have recently been cloned: 1-SST and 1-FFT from C. intybus (De Halleux and Van Cutsem, 1997; J.P. Goblet, L. Canon, and P.J. Van Cutsem, unpublished data), Cynara scolymus (Hellwege et al., 1997, 1998), H. tuberosus (van der Meer et al., 1998), 1-SST, fructan:fructan 6-fructosyl transferase (6G-FFT) from Allium cepa (Vijn et al., 1997, 1998), and Suc:fructan 6-fructosyl transferase (6-SFT) from Hordeum vulgare (Sprenger et al., 1995). However, none of these reports addressed the level of regulation by comparing mRNA concentrations, enzyme concentrations, and enzyme activities.
Based on vacuole isolation from protoplasts, fructans are believed to be localized in the vacuole (Wagner et al., 1983; Wiemken et al., 1986; Darwen and John, 1989). However, the exclusive vacuolar localization of fructan metabolism has recently been questioned, and both the presence of fructan and β-fructosidase activity have been reported in apoplastic fluid (Livingston and Henson, 1998). Moreover, in the leaves of the monocot Agave deserti, it has been shown that low-DP fructan is synthesized in phloem parenchyma cells and subsequently loaded, probably in a symplastic way, into the phloem. Surprisingly, aside from the presence of low DP fructan, very high activities of fructan exohydrolases were also found in the phloem sap (Wang and Nobel, 1998). The localization of fructan in sink tissues is not straightforward. In roots of Gomphrena marcocephala and a number of Asteracean representatives of the Brazilian cerrado, fructan can be detected in xylem parenchyma cells and even inside xylem vessels (Ernst, 1991; Vieira and Figueiredo-Ribeiro, 1993), suggesting a possible translocation of fructan via the xylem at specific developmental stages (e.g. early-spring regrowth).
This paper describes a multidisciplinary approach to investigating the tissue-specific and developmental regulation of 1-SST, the key enzyme initiating fructan biosynthesis in Asteraceae, and the histological distribution of fructan in sink tissue. We chose the Asteracean species T. officinale as a model plant because of the more convenient size of its mature roots and its less complex anatomical structure compared with C. intybus. Moreover, we could take advantage of the fact that root phloem and xylem can easily be separated in T. officinale.
RESULTS
Cloning and Sequencing of T. officinale 1-SST cDNA
As schematically presented in Figure 1, a partial T. officinale 1-SST cDNA was obtained by RT-PCR with SSTa (derived from C. intybus 1-SST) and CTERM (conserved in all vacuolar fructosyl transferases and invertases; Vijn et al., 1997). After sequencing this partial 1-SST cDNA, we constructed P1 and P2, two specific primers. These primers were combined with MASSTT (5′ end SST-specific primer; see also Fig. 2) and oligo dT to obtain the sequence of the whole cDNA.
Figure 1.
Scheme of the 1-SST cDNA from T. officinale. The cDNA contains a single open reading frame of 1,896 bp. The first part of this open reading frame is a putative leader sequence (black) of 267 bp. A 3′ 176-bp untranslated part is also present (line). Primers used during RT-PCR and PCR are indicated with arrows. The 1-SST probe was prepared using the primers MASSTT and P3. For details, see “Materials and Methods.”
Figure 2.
Multiple alignment of 1-SSTs from Asteraceae. Putative glycosylation sites are underlined. 1-SST cDNAs have MASSTT (bold underlined) as their 5′ part. Based on the N-terminal sequence of the mature C. intybus 1-SST protein (Van den Ende et al., 1996b), the leader sequence of C. intybus 1-SST is indicated (italic). Consensus line: asterisks (*) indicate identical residues; colons (:) indicate conserved subsitutions; and periods (.) indicate semi-conserved substitutions.
T. officinale 1-SST cDNA contains a single open reading frame of 632 codons and a 176-bp untranslated 3′ region (Fig. 1). Comparison of the cDNA-derived amino acid sequence with the N-terminal sequence of the mature C. intybus 1-SST enzyme (Van den Ende et al., 1996b) suggests that the primary translation product in T. officinale has an 89-amino acid signal peptide that is post-translationally removed (Figs. 1 and 2). Furthermore, the 632 amino acids constituting the mature protein contain six potential N-glycosylation sites (N-X-S/T: see Fig. 2). If the primary translation product is processed as in C. intybus, the estimated molecular mass of the mature T. officinale 1-SST protein is 61.5 kD, which is nearly identical to C. intybus (61.4 kD). The higher Mr of 68,800 found by matrix-assisted laser-desorption ionization time of flight mass spectroscopy of the C. intybus enzyme (Van den Ende et al., 1996b) was probably due to extensive glycosylation. The calculated pI of T. officinale deduced protein (4.9) was very close to the experimental value found for C. intybus 1-SST (Van den Ende et al., 1996b).
At the amino acid level, the sequence shows very high homology to 1-SSTs from other Asteraceae (86% to C. intybus 1-SST, 82% to Cynara scolymus 1-SST, and 80% to Helianthus tuberosus 1-SST). Homology to 1-FFTs from Asteraceae (53%–56%) was higher than homology to 1-SSTs from Allium cepa (46%) and Aspergillus foetidus (18%). Homology to 6-SFT from Hordeum vulgare and 6G-FFT from A. cepa were 42% and 44%, respectively. General homology to plant vacuolar invertases from dicotyledonous species was between 46% and 53%. Homologies were calculated using the Clustal W program.
Fructan and Fructan Enzymes during Development
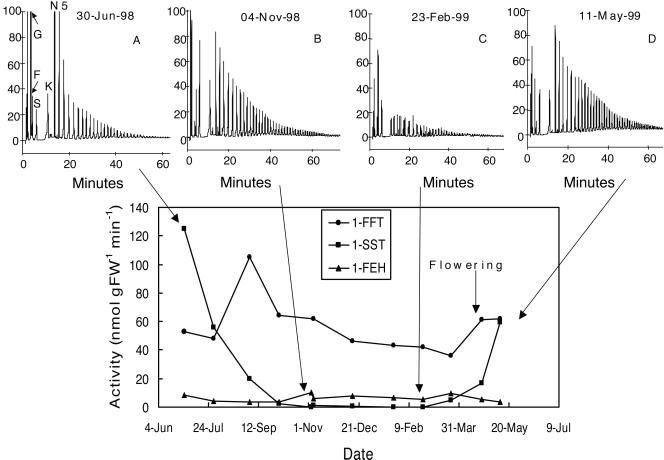
The activity of 1-SST was very high in young T. officinale roots (June 1998), but decreased steadily throughout the growing season to become very low from October 1998 up to March 1999 (Fig. 3). Activities of 1-SST in the roots increased again in April 1999 (flowering stage) and further on during the post-flowering stage (May 1999). The activity of 1-FFT was rather constant throughout the same period. The activity of 1-FEH was low and roughly constant throughout the growing season.
Figure 3.
Changes of fructan-metabolizing enzymatic activities in roots of T. officinale throughout the 1st year of growth, overwintering, and the 2nd year flowering. Fructan patterns are presented at four different dates. G, Glc; F, Fru; S, Suc; K, 1-kestose; N, 1,1-nystose; 5, DP5 fructan.
The evolution of carbohydrates throughout the growing season is illustrated by some typical chromatography patterns. In young roots with high 1-SST activity, the Suc concentration (substrate for 1-SST) was low and the Glc concentration (1-SST product) was high (Fig. 3A). Low-DP fructan were dominant. Between June and November (Fig. 3B), higher DP fructan and Suc increased, and Glc decreased. Between November and February (Fig. 3C), the Fru concentration greatly increased, the fructan concentration decreased, and an alternative series (Fn) of fructan became apparent. During the following spring (Fig. 3D), fructan synthesis resumed. Overall, there was a good correlation between 1-SST activity and Glc concentration (data not shown).
Carbohydrates, 1-SST, and Invertase Activities in Parts of a Flowering T. officinale
Since 1-SST was the only enzyme strongly fluctuating during the growing season (Fig. 3), we focused on 1-SST activity in different parts of a flowering T. officinale plant. Acid invertase activity was also assayed for its putative role in young, strongly growing tissues (Sturm and Chrispeels, 1990) and because it competes with 1-SST for Suc as substrate. The fructan concentration, fructan DP, and 1-SST activity were higher in root phloem compared with root xylem (Table I). The fructan DP, fructan concentration, and 1-SST activity were moderate in the receptacle and stalk. In the leaf, 1-SST activity was low, being higher in the veins than in the leaf parenchyma; the Glc concentration was also much higher in the veins. In the receptacle, and especially in the stalk, extremely high acid invertase activities were detected (Table I), coinciding with high Glc and Fru concentrations. Only in the root tissue did the 1-SST activity exceed the invertase activity. Except for the stalk, with its high acid invertase activity, higher 1-SST activities in general coincide with higher fructan concentrations and higher maximal fructan DP (Table I).
Table I.
Concentrations of Glc, Fru, Suc, and different classes of fructans (μmol g fresh wt−1) and activities of 1-SST and acid invertase (units g fresh wt−1 min−1) in different tissues of a flowering T. officinale plant
| Xy | Ph | Lp | Lv | St | Re | |
|---|---|---|---|---|---|---|
| Glc | 5.9 | 7.2 | 4.7 | 75 | 108 | 34 |
| Fru | 8.7 | 4.9 | 5.6 | 10 | 70 | 56 |
| Suc | 11 | 8.2 | 8.4 | 9.4 | 1.8 | 21 |
| DP3-20 | 70 | 86 | 3.5 | 7.6 | 3.1 | 31 |
| DP21-50 | 22 | 48 | 0.1 | 0.2 | 0 | 1.4 |
| DP >50 | 1.1 | 3.6 | 0 | 0 | 0 | 0 |
| Total fructan | 93.1 | 137.6 | 3.6 | 7.8 | 3.1 | 32.4 |
| Maximal DP | 70 | 83 | 25 | 25 | 18 | 42 |
| 1-SST | 54 | 87 | 1 | 7 | 11 | 23 |
| Invertase | 20 | 17 | 8.3 | 68 | 820 | 262 |
Xy, Secondary root xylem; Ph, secondary root phloem; Lp, leaf intervenal parts; Lv, leaf veins; St, stalk; Re, receptacle.
1-SST mRNA and 1-SST Protein Concentration (Northern and Western Analysis)
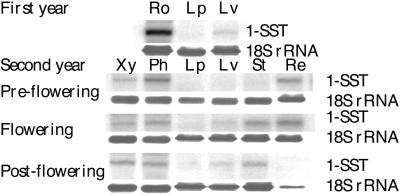
1-SST was abundantly expressed in young roots during the first year of growth (Fig. 4). 1-SST was also expressed, although less abundantly, in roots of flowering plants (second year of growth), especially in the phloem. Some 1-SST mRNA could be detected in the leaf veins but none in the leaf parenchyma. In the inflorescence stalk and leaf veins, 1-SST mRNA was only detected in the flowering and post-flowering stages. Expression was also clear in the young and fully grown receptacles, but fell to zero in the post-flowering stage.
Figure 4.
Northern blot containing total RNA from several tissues of T. officinale during different developmental stages: Ro, total root tissue; Lp, leaf intervenal parts; Lv, leave veins; Xy, secondary root xylem; Ph, secondary root phloem; St, stalk; Re, receptacle. Partial T. officinale 1-SST cDNA or 18-S C. intybus was used as a probe.
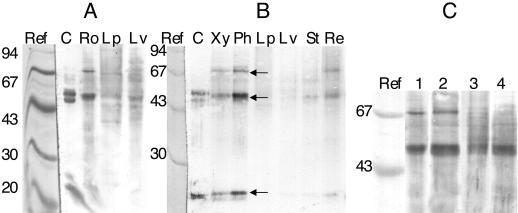
We used a cross-reacting C. intybus 1-SST polyclonal antibody to quantify the amount of 1-SST protein in crude extracts of T. officinale (Fig. 5). Bands of 70, 48, and 22 kD (arrows in Fig. 5B) appeared in roots of young (Fig. 5A) and flowering (Fig. 5B) T. officinale plants, especially in the phloem of the latter (Fig. 5B). In leaves, 1-SST protein was clearly present in vascular tissue, but the concentration was very low in leaf parenchyma (Fig. 5A). 1-SST was also clearly present in the receptacle and stalk. Figure 5C shows a detail of a western blot on which root phloem crude extracts from different developmental stages were compared. A 70-kD band was clearly present in young or pre-flowering roots (lanes 1 and 2), but this band was absent in the flowering and post-flowering stages (lanes 3 and 4), while the 48-kD band remained prominent throughout.
Figure 5.
Western blot developed with C. intybus 1-SST polyclonal antibody. A, Young (1st year) T. officinale plant. B, Flowering T. officinale plant. Arrows show the 70-, 48-, and 22-kD bands. C, Root secondary phloem of young (1), pre-flowering (2), flowering (3), and senescing (4) stages. Ref, Mr markers stained with Coomassie; C, concanavalin A fraction containing C. intybus 1-SST; Ro, total root tissue; Lp, leaf intervenal parts; Lv, leave veins; Xy, secondary root xylem; Ph, secondary root phloem; St, stalk; Re, receptacle.
Fructan Localization in T. officinale Roots
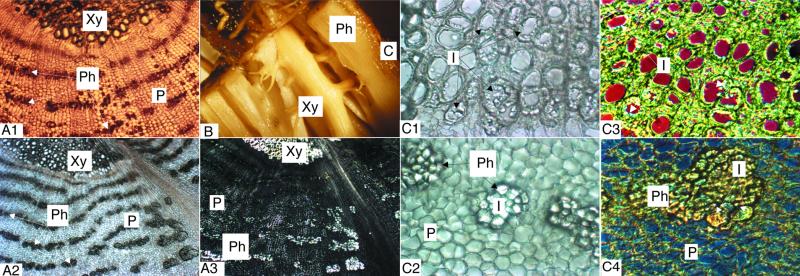
During the analyses above we had already taken advantage of the fact that root phloem and xylem of T. officinale roots can be easily separated (Fig. 6B). Figure 6A1 shows a FAA-fixed cryosection illustrating the general organization of xylem and phloem tissues in the root. Sections of roots fixed in 11.73 m ethanol show precipitation of inulin crystals (Fig. 6, A2 and A3), mainly over the secondary root phloem parenchyma (Fig. 6, C2 and C4). However, inulin crystals also lit up within lignified and suberized metaxylem vessels (Fig. 6, C1 and C3).
Figure 6.
Photographs showing fructan localization in T. officinale roots. A, Transverse cryosections of T. officinale root vascular tissue (magnification, ×22). Light microscopic view of an FAA- (A1) or 11.73 m ethanol fixed section (A2) and a section as in A2 but photographed under polarized light (A3). B, Anatomy of a fresh T. officinale root. Xylem and phloem can be easily separated from each other. C, Localization of inulin in xylem (C1 and C3) and phloem (C2 and C4) from the vascular tissue of a T. officinale root. Transverse cryosections (magnification, ×55). All tissues were fixed in 11.73 m ethanol for 2 d. Tissue slices 1 and 2 were photographed under a light microscope. Inulin crystals light up in the lignified metaxylem vessels (C1) and in clusters close to and surrounding the phloem tissue (C2). Figures C3 and C4 were obtained using a differential interference contrast microscope. Again, inulin crystals occupy the edge of the xylem vessels (C3) or appear as clusters surrounding the phloem (C4). This technique shows the crystals as striped structures marked by a color depending on the wavelength used. For all photographs: C, cortex; I, inulin; P, phloemparenchyma; Ph, phloem; Xy, xylem. All pictures were taken from a flowering T. officinale root.
DISCUSSION
We used a PCR-based method to isolate full-length T. officinale 1-SST cDNA (Fig. 1) by taking advantage of the conserved sequence MASSTT at the extreme 5′ edge of the cDNA. T. officinale 1-SST cDNA appeared very homologous to other Asteracean 1-SST cDNAs, except for the more variable leader sequence (Fig. 2) included in the 1-SST probe, making it highly specific.
By comparing carbohydrates and enzyme activities throughout the growing season (Fig. 3), it was evident that 1-SST determines fructan synthesis in T. officinale roots. As in C. intybus (Van den Ende and Van Laere, 1996b), 1-SST activity gradually decreased between June and October, while 1-FFT remained more or less constant. For unknown reasons and in contrast to C. intybus (Van den Ende et al., 1996a; Van den Ende and Van Laere, 1996b), the fructan breakdown observed in autumn was not correlated with increased 1-FEH activity in the extracts.
In spring, simultaneously with the pre-flowering and flowering stages (April), 1-SST activities started to increase again and fructan synthesis resumed in the roots (Fig. 3). Apparently, energy for the flowering process is supplied by leaf photosynthate and not by the breakdown of fructan in the root, and both root and inflorescence can be considered as competitive sinks.
The physiological role of fructan in the roots would then perhaps be limited to a rapid resumption of growth in early spring, allowing more successful competition for space with neighboring species. Another possibility is that fructans form a safeguard against grazing, as suggested by De Roover et al. (1999).
The rather extensive depolymerization of fructans in early autumn, when energy demands for growth become marginal, is rather puzzling. Perhaps the Hex, Suc, and low-DP fructan in overwintering roots have a function as cryo-protectors. The possible role of fructans in freezing tolerance has recently been re-emphasized in winter oat by Livingston and Henson (1998). These authors reported the presence of fructans as well as invertase and FEH activity in the apoplastic fluid. They suggested that low-DP carbohydrates could reach extremely high concentrations in small layers of liquid water in the apoplast, thereby preventing further ice adhesions and possibly providing protection to cell walls and membranes.
Similar to our results, data from Vieira and Figueiredo-Ribeiro (1993) and Ernst (1991) support the presence of fructan in xylem vessels (Fig. 6), although tyloses cannot be excluded in this case. In contrast to starch, fructan (especially lower DP fructan) is highly soluble in water. Loading of apoplastic fructan oligomers into the xylem in early spring would be a logical and fast energy supply for the regrowth of new leaves, perhaps providing a selective advantage over starch-storing species.
The transportability of oligofructans in phloem of Agave deserti has already been demonstrated (Wang and Nobel, 1998). Fructans would fit nicely into the polymer-trapping model for symplastic loading of photosynthates (Turgeon, 1991). In this context it is worth mentioning that the concentration of fructan and the activity of 1-SST are much higher in leaf veins than in mesophyll, although fructan concentrations remain much lower than in roots (Table I). Fructan synthesis in phloem parenchyma cells might be the driving force maintaining a steep Suc gradient facilitating Suc transport to the vascular tissues.
In T. officinale roots, fructans preferentially accumulate near the sites of phloem unloading (Fig. 6, A2, C2, and C4). More and larger fructans, higher 1-SST activity, 1-SST mRNA, and 1-SST protein concentrations are found in the root phloem compared with the root xylem (Table I; Figs. 4 and 5B).
All of these observations support the determining role of 1-SST in sink strength, as convincingly demonstrated by Améziane et al. (1995) and Druart (1999). Moreover, when roots are abruptly forced from a sink to a source organ, 1-SST activity strongly decreases and 1-FEH activity strongly increases (De Roover et al., 1999; Van den Ende et al., 1999). Exogenously supplied Suc induces 1-SST in detached C. intybus leaves and intact C. intybus rootlets (Vijn et al., 1997; W. Van den Ende*, A. Michiels, D. Van Wonterghem, R. Vergauwen, and A. Van Laere, unpublished results). A putative role of 1-SST as a sink strength determinant is in agreement with the observation that 1-SST is expressed in the receptacle at the pre-flowering and flowering stages. After flowering, 1-SST mRNA disappears in the receptacle simultaneously with the onset of senescing processes, causing a decrease in the rRNA concentration (Fig. 4).
A close correlation between northern (Fig. 4) and western blots (Fig. 5, A and B) shows that the 1-SST gene is mainly regulated at the level of transcription. However, it cannot be ruled out completely that there are additional post-transcriptional regulatory processes. It is known that C. intybus 1-SST, 1-FFT (Van den Ende et al., 1996b, 1996c), and other fructosyl transferases (Sprenger et al., 1995; Vijn et al., 1997, 1998) and some plant invertases (Unger et al., 1994) are heterodimers originating from the cleavage of a single polypeptide. It was demonstrated that the subunits are not produced during the purification process (Arai et al., 1991; Van den Ende et al., 1996c), but rather that both monomeric and heterodimeric forms can be present in vivo. T. officinale 1-SST clearly is a heterodimer (48- and 22-kD bands, Fig. 5). The monomeric form is present in very young and pre-flowering stages but completely disappears throughout the flowering and post-flowering stages (Fig. 5C). Similar results were obtained for a mung bean and carrot invertase: full-length protein predominated in very young seedlings, whereas fragments were more abundant in later developmental stages (Arai et al., 1991; Unger et al., 1994). Apparently, the ratio of the monomeric to the heterodimeric form is developmentally regulated, but the physiological significance of this remains elusive.
In conclusion, we isolated T. officinale 1-SST cDNA and used it as a probe for northern analysis to reveal differential expression on a tissue-specific and developmental basis. Comparison of these results with western analysis and activity measurements strongly suggested that the gene is mainly regulated at the transcriptional level. Fructan localization studies mainly showed a link with phloem vascular tissue. All of our results are consistent with a putative function for 1-SST as a sink strength determinant.
MATERIALS AND METHODS
Plant Material
In the spring of 1997, we harvested seeds from a single wild-type Taraxacum officinale plant that grew highly isolated (small border between a field and a wood) from other T. officinale plants, which strongly favored self-reproduction and genetically homogenous offspring. Seeds from this single plant were sown on a local field in early April, 1998. At several developmental stages (June 1998–May 1999), 10 plants were uprooted, leaves were cut off, and the roots were washed and cut into small pieces. During the youngest stages, not enough root material was present to perform all of the analyses on individual roots. Therefore, tissues from 10 plants were combined in one extract throughout the season. For some data, we studied the variations in enzyme activities and carbohydrate concentrations between 10 individual roots. In all cases the coefficient of variation was well below 15%.
We carefully isolated the stalk, receptacle, intervenal leaf parenchyma, leaf vascular tissue, root secondary phloem, and root secondary xylem from flowering plants (April, 1999). Samples for carbohydrates and RNA were frozen in liquid nitrogen and stored at −80°C prior to analysis. Enzymatic activity measurements were performed on fresh material.
The same experiments were also performed with a commercial T. officinale (cv Pissenlit amélioré) and very similar results were obtained.
Extraction, Carbohydrate Analysis, and Activity Measurements
Carbohydrates were extracted and analyzed by anion exchange chromatography-pulsed-amperometric detection as described by Van den Ende et al. (1998). Activities of 1-SST, invertase, 1-FFT, and fructan 1-exohydrolase (1-FEH) were determined by incubation with an appropriate substrate and analysis of the products by anion exchange chromatography-pulsed-amperometric detection as described previously (Van den Ende et al., 1998; De Roover et al., 1999). Activities are expressed in nanomoles per gram fresh weight per minute.
Cloning of T. officinale 1-SST
Based on the N-terminal amino acid sequence of the purified Cichorium intybus 1-SST protein (Van den Ende et al., 1996b) and the cDNA from industrial C. intybus 1-SST (De Halleux and Van Cutsem, 1997), we constructed a sense primer SSTa (5′-AATGCTGATGTTGAGTGGCAACG-3′). A conserved C-terminal amino acid sequence FNNATG (Vijn et al., 1997; van der Meer et al., 1998) was used to construct a degenerate antisense primer CTERM (5′-CCNGTNGCRTTGTTRAA-3′). Using these two primers, we performed one-step reverse transcriptase (RT)-PCR (Access System, Promega, Madison, WI) on total RNA (RNeasy Plant Mini Kit, Qiagen USA, Valencia, CA) from young T. officinale roots containing high 1-SST activity. The RT step was performed at 46°C. Subsequently, PCR was performed under the following conditions: initial denaturation 94°C, 3 min; followed by 30 cycles of 94°C, 1 min; 46°C, 1 min; and 72°C, 2 min. Final extension was at 72°C, 7 min. The obtained 1,500-bp PCR fragment was ligated in the TOPO-XL PCR vector and transformed to competent E. coli cells according to the manufacturer's instructions (TOPO-XL PCR cloning kit, Invitrogen, Carlsbad, CA). Single bacterial colonies were selected, cultured on liquid medium, and plasmid was extracted using Wizard Plus SV Minipreps (Promega). A number of clones were sequenced on an automatic DNA-sequencing apparatus and a dye-terminator cycle-sequencing kit (ABI-PRISM, Eurogentec, Seraing, Belgium).
From this sequence a sense (P1, 5′-ATGGGGACTGGATAATGATCATGG-3′) and antisense version (P2, 5′-CCATGATCATTATCCAGTCCCCAT-3′) of a specific internal primer were constructed. Subsequently, the 5′ end of the cDNA was amplified during RT-PCR using a degenerate sense primer derived from MASSTT, the conserved N-terminal amino acid sequence of 1-SST propeptides, and P2 as antisense primer. The 3′ end of the cDNA was amplified by using P1 and oligo dT primer. For both reactions, the RT step was performed at 48°C and PCR was as above except that annealing was at 57°C. Finally, both the 5′ and 3′ cDNA parts were subcloned and several clones were sequenced on both strands as described above. The sequence was deposited in the EMBL sequence library (accession no. AJ250634).
Preparation of T. officinale 1-SST and 18S rRNA Probes
A clone containing the 5′ part of 1-SST cDNA was used as a template to amplify a 450-bp PCR fragment with MASSTT as the sense primer and a specific antisense primer, P3 (5′-TAGATGGAACCAATTGATCA-3′). PCR conditions were as described above for the whole 5′ part of the cDNA. The PCR product was further purified on a PCR-Wizard column (Promega), and then 50 ng was labeled by a random-primed method using the DNA-labeling T7 QuickPrime Kit (Pharmacia Biotech, Piscataway, NJ) and 50 μCi [α-32P]dCTP as described in Feinberg and Vogelstein (1984).
Based on conserved sequences in 18S rRNAs, two specific rRNA primers were constructed (5′-AGACTGTGAAACTGCGAATGG-3′ and 5′-TTGTCACTACCTCCCCGTGT-3′). We used these primers in RT-PCR on C. intybus total RNA to amplify a 400-bp fragment, which was subcloned and sequenced as described above. Subsequently, the plasmid construct was used as a template to produce a PCR fragment (identical primers) that was labeled as described above.
RNA Analysis (Northern Blotting)
RNA from 1 g of frozen roots and leaves was extracted using an RNA extraction kit (TRI REGEANT, MRC Inc., Cincinatti). Total RNA (10 μg) was denatured in 12.55 m formamide, 2.2 m formaldehyde, and 20 mm 3-(N-morpholino)-propanesulfonic acid (MOPS) buffer, pH 7.0 (also contianing 5 mm Na-acetate and 0.1 mm EDTA) at 65°C for 5 min and fractionated on a 1.2% (w/v) agarose gel containing 2.2 m formaldehyde in MOPS buffer. Subsequent blotting and hybridization were as described previously (De Roover et al., 2000). To ensure that equal amounts of RNA were loaded in each well, the membrane was also hybridized with the radiolabeled C. intybus 18S rRNA probe.
Western Blotting
Frozen plant material was ground in a mortar with ice-cold acetate extraction buffer (Van den Ende et al., 1998). The 5-min 10,000g supernatant was collected and used for western blotting and for the determination of protein content according to the method of Bradford (1976) using bovine serum albumin as a standard. Preparation of polyclonal antibodies, electrophoresis, membrane transfer, and staining were as described by Van den Ende et al. (1996b).
Microscopy
For fixation and sectioning, T. officinale roots were fixed in 11.73 m ethanol or FAA containing 11.73 m ethanol, 0.87 m acetic acid, and 0.67 m formaldehyde for at least 48 h at room temperature. The fixed material was frozen on the metal cutting table of a cryotome (Reichert, Vienna, Austria). Cryosections (20–50 μm) were mounted on microscope slides with Haupts adhesive (Van Cottem and Fryns-Claessens, 1972), and viewed in a light microscope with polarization filter (Leitz, Midland, Ontario, Canada) and differential interference contrast optics (Reichert).
ACKNOWLEDGMENT
We thank E. Nackaerts for his technical assistance.
Footnotes
This work was supported by FSR Flanders (grant no. G.0328.98).
LITERATURE CITED
- Améziane R, Limami MA, Noctor G, Morot-Gaudry JF. Effect of nitrate concentration during growth on carbon partitioning and sink strength in chicory. J Exp Bot. 1995;46:1423–1428. [Google Scholar]
- Arai M, Mori H, Imaseki H. Roles of sucrose-metabolizing enzymes in growth of seedlings: purification of acid invertase from growing hypocotyls of mung bean seedlings. Plant Cell Physiol. 1991;32:1291–1298. [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Darwen CWE, John P. Localisation of the enzymes of fructan metabolism in vacuoles isolated by a mechanical method from tubers of Jerusalem artichoke (Helianthus tuberosus[L.]) Plant Physiol. 1989;89:658–663. doi: 10.1104/pp.89.2.658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Halleux S, Van Cutsem P. Cloning and sequencing of the 1-SST cDNA from chicory root (accession no. U81520) (PGR 97-036) Plant Physiol. 1997;113:1003. [Google Scholar]
- De Roover J, Van den Branden K, Van Laere A, Van den Ende W (2000) Drought induces fructan synthesis and 1-SST (sucrose:sucrose 1-fructosyl transferase) in roots and leaves of chicory seedlings (Cichorium intybus L.). Planta (in press) [DOI] [PubMed]
- De Roover J, Van Laere A, Van den Ende W. Effect of defoliation on fructan pattern and fructan metabolizing enzymes in young chicory plants (Cichorium intybusL.) Physiol Plant. 1999;106:158–163. [Google Scholar]
- Druart N. La mise en place de la tubérisation chez la chicorée (Cichorium intybus L.): evolution des metabolisms azoté et carboné. PhD thesis. Lille, France: Université de Sciences et Technologies de Lille; 1999. [Google Scholar]
- Edelman J, Jefford TG. The mechanism of fructosan metabolism in higher plants as exemplified in Helianthus tuberosus. New Phytol. 1968;67:517–531. [Google Scholar]
- Ernst M. Histochemische Untersuchungen auf Inulin, Stärke and Kallose bei Helianthus tuberosusL. (Topinambur) Angew Botanik. 1991;65:319–330. [Google Scholar]
- Feinberg AP, Vogelstein B. Addendum: a technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1984;137:266. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- Hellwege EM, Gritscher D, Willmitzer L, Heyer AG. Transgenic potato tubers accumulate high levels of 1-kestose and nystose: functional identification of a sucrose:sucrose 1-fructosyltransferase of artichoke (Cynara scolymus) blossom discs. Plant J. 1997;12:1057–1065. doi: 10.1046/j.1365-313x.1997.12051057.x. [DOI] [PubMed] [Google Scholar]
- Hellwege EM, Raap M, Gritscher D, Willmitzer L, Heyer AG. Differences in chain length distribution of inulin from Cynara scolymus and Helianthus tuberosusare reflected in a transient plant expression system using the respective 1-FFT cDNAs. FEBS Lett. 1998;427:25–28. doi: 10.1016/s0014-5793(98)00386-x. [DOI] [PubMed] [Google Scholar]
- Hendry G. Evolutionary origins and natural functions of fructans: a climatological biogeographic and mechanistic appraisal. New Phytol. 1993;123:3–14. [Google Scholar]
- Koops AJ, Jonker HH. Purification and characterisation of the enzymes of fructan biosynthesis in tubers of Helianthus tuberosusColombia: II. Purification of sucrose:sucrose 1-fructosyltransferase and reconstitution of fructan synthesis in vitro with purified SST and FFT. Plant Physiol. 1996;110:1167–1175. doi: 10.1104/pp.110.4.1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingston DP, Chatterton NJ, Harrison PA. Structure and quantity of fructan oligomers in oat (Avenaspp.) New Phytol. 1993;123:725–734. [Google Scholar]
- Livingston DP, Henson CA. Apoplastic sugars fructans fructan exohydrolase and invertase in winter oat: responses to second-phase cold hardening. Plant Physiol. 1998;116:403–408. [Google Scholar]
- Lüscher M, Erdin C, Sprenger N, Hochstrasser U, Boller T, Wiemken A. Inulin synthesis by a combination of purified fructosyltransferases from tubers of Helianthus tuberosus. FEBS Lett. 1996;385:39–42. doi: 10.1016/0014-5793(96)00343-2. [DOI] [PubMed] [Google Scholar]
- Pilon-Smits EAH, Ebskamp MJM, Jeuken MJW, Weisbeek PJ, Smeekens SCM. Improved performance of transgenic fructan-accumulating tobacco under drought stress. Plant Physiol. 1995;107:125–130. doi: 10.1104/pp.107.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiomi N. Properties of fructosyltransferases involved in the synthesis of fructan in Liliaceous plants. J Plant Physiol. 1989;134:151–155. [Google Scholar]
- Sprenger N, Bortlik K, Brandt A, Boller T, Wiemken A. Purification, cloning, and functional expression of sucrose:fructan 6-fructosyltransferase, a key enzyme of fructan synthesis in barley. Proc Natl Acad Sci USA. 1995;92:11652–11656. doi: 10.1073/pnas.92.25.11652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturm A, Chrispeels M. cDNA cloning of carrot extracellular β-fructosidase and its expression in response to wounding and infection. Plant Cell. 1990;2:1107–1119. doi: 10.1105/tpc.2.11.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turgeon R. Symplastic phloem loading and the sink-source transition in leaves: a model. In: Bonnemain JL, Delrot S, Lucas WJ, Dainty J, editors. Recent Advances in Phloem Transport and Assimilate Compartmentation. Nantes, France: Quest Editions; 1991. pp. 18–22. [Google Scholar]
- Unger C, Hardegger M, Lienhard S, Sturm A. CDNA cloning of carrot (Daucus carota) soluble acid β-fructofuranosidases and comparison with the cell wall isoenzyme. Plant Physiol. 1994;104:1351–1357. doi: 10.1104/pp.104.4.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Cottem W, Fryns-Claessens E. Plantenanatomie in Practijk. J. Lier, Belgium: Van In; 1972. [Google Scholar]
- Van den Ende W, De Roover J, Van Laere A. Effect of nitrogen concentration on fructan and fructan metabolizing enzymes in young chicory plants (Cichorium intybus) Physiol Plant. 1999;105:2–8. [Google Scholar]
- Van den Ende W, Mintiens A, Speleers H, Onuoha A, Van Laere A. The metabolism of fructans in roots of Cichorium intybusL. during growth storage and forcing. New Phytol. 1996a;132:555–563. doi: 10.1111/j.1469-8137.1996.tb01874.x. [DOI] [PubMed] [Google Scholar]
- Van den Ende W, Van Hoenacker G, Moors S, Van Laere A. Effect of osmolytes on the fructan pattern in feeder roots produced during forcing of chicory (Cichorium intybusL.) J Plant Physiol. 1998;153:290–298. [Google Scholar]
- Van den Ende W, Van Laere A. De-novo synthesis of fructans from sucrose in vitro by a combination of two purified enzymes (sucrose:sucrose fructosyl transferase and fructan:fructan fructosyl transferase) from chicory roots (Cichorium intybusL.) Planta. 1996a;200:335–342. [Google Scholar]
- Van den Ende W, Van Laere A. Fructan synthesizing and degrading activities in chicory roots (Cichorium intybusL.) during growth, storage and forcing. J Plant Physiol. 1996b;149:43–50. [Google Scholar]
- Van den Ende W, Van Wonterghem D, Verhaert P, Dewil E, De Loof A, Van Laere A. Purification and characterization of 1-SST, the key enzyme initiating fructan biosynthesis in young chicory roots (Cichorium intybusL.) Physiol Plant. 1996b;98:455–466. [Google Scholar]
- Van den Ende W, Van Wonterghem D, Verhaert P, Dewil E, Van Laere A. Purification and characterization of fructan:fructan fructosyl transferase from chicory roots (Cichorium intybusL.) Planta. 1996c;199:493–502. [Google Scholar]
- van der Meer I, Koops AJ, Hakkert JC, van Tunen AJ. Cloning of the fructan biosynthesis pathway of Jerusalem artichoke. Plant J. 1998;15:489–500. doi: 10.1046/j.1365-313x.1998.00230.x. [DOI] [PubMed] [Google Scholar]
- Vieira CCJ, Figueiredo-Ribeiro RCL. Fructose-containing carbohydrates in the tuberous roots of Gomphrena marcocephalaSt.-Hil. (Amaranthaceae) at different phonological phases. Plant Cell Environ. 1993;16:919–928. [Google Scholar]
- Vijn I, van Dijken A, Lüscher M, Bos A, Smeets E, Weisbeek P, Wiemken A, Smeekens S. Cloning of sucrose:sucrose 1-fructosyltransferase from onion and synthesis of structurally defined molecules from sucrose. Plant Physiol. 1998;117:1507–1513. doi: 10.1104/pp.117.4.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijn I, van Dijken A, Sprenger N, van Dun K, Weisbeek P, Wiemken A, Smeekens S. Fructan of the inulin neoseries is synthesized in transgenic chicory plants (Cichorium intybus L.) harbouring onion (Allium cepaL.) fructan:fructan 6G fructosyltransferase. Plant J. 1997;11:387–398. doi: 10.1046/j.1365-313x.1997.11030387.x. [DOI] [PubMed] [Google Scholar]
- Wagner W, Keller F, Wiemken A. Fructan metabolism in cereals: induction in leaves and compartmentation in protoplasts and vacuoles. Z Pflanzenphysiol. 1983;112:359–372. [Google Scholar]
- Wang N, Nobel PS. Phloem transport of fructans in the Crassulacean acid metabolism species Agave deserti. Plant Physiol. 1998;116:709–712. doi: 10.1104/pp.116.2.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiemken A, Frehner M, Keller F, Wagner W. Fructan metabolism, enzymology and compartmentation. Curr Top Plant Biochem Physiol. 1986;5:17–37. [Google Scholar]
- Wiemken A, Sprenger N, Boller T. Fructans: an extension of sucrose by sucrose. In: Pontis HG, Salerno GL, Echeverria EJ, editors. Current Topics in Plant Physiology. Vol. 14. Rockville, MD: American Society of Plant Physiologists; 1995. pp. 179–189. [Google Scholar]