Abstract
Background
Individuals on renal replacement therapy (RRT) have increased fracture risk, but risk in less advanced chronic kidney disease (CKD) is unclear.
Objective
To investigate CKD associations with hip fracture incidence and mortality.
Design
Record linkage cohort study Grampian Laboratory Outcomes Mortality and Morbidity Study II.
Setting
Single health region in Scotland.
Participants
All individuals (≥15 years) with sustained CKD stages 3–5 and those on RRT, and a 20% random sample of those with normal renal function, in the resident population in 2003.
Outcome measures
Outcomes were (1) incident hip fracture measured with (A) admissions or (B) deaths, with at least 5.5 years follow-up and (2) post-hip fracture mortality. Unadjusted and adjusted, incident rate ratios (IRRs) and mortality rate ratios were calculated using Poisson regression.
Results
Of 39 630 individuals identified in 2003 (41% males, mean age 63.3 years), 19 537 had CKD stages 3–5, 345 were on RRT and 19 748 had normal estimated glomerular filtration rate (eGFR). Hip fracture incidence, measured by admissions, was increased in CKD stages 3–5 (compared with normal eGFR), both overall (adjusted IRR 1.49 (95% CI 1.24 to 1.79)) and for individual CKD stages 3a, 3b and 4. Hip fracture incidence, measured using deaths, was increased in those with CKD stages 3b and 4. Post-hip fracture mortality was only increased in CKD stage 4. There was only a small number of individuals and events for CKD stage 5, resulting in insufficient statistical power.
Conclusion
Hip fracture incidence was higher in CKD stages 3–5 compared with normal eGFR. Post-hip fracture mortality was only increased in CKD stage 4. Reducing hip fracture incidence in CKD through regular fall and fracture risk review should reduce overall deaths after hip fracture in the population.
Keywords: chronic kidney disease, cohort, death, hip fracture, incidence, mortality
Strengths and limitations of this study.
A large, population-based cohort using valid methods for assessing comorbidity and identifying individuals with chronic kidney disease (CKD).
Reporting hip fracture incidence using both admission and death records, allowing direct comparison with studies that have used either of these methods and highlighting the importance of methods of measurement.
Using at least two serum creatinine measurements to identify CKD, thus reducing the risk of identifying episodes of acute kidney injury as CKD.
Use of hospital episode data to ascertain hip fracture, acknowledging that a small number of hip fractures may not lead to hospital admission.
We only considered hip fractures acknowledging that CKD may be associated with other fracture sites; however, hip fractures would have more complete admission data.
Introduction
Hip fractures are a major public health issue recognised in national clinical guidelines, and more common in women and the elderly.1 2 In the UK, there is an estimated 70 000–90 000 hip fractures annually.1 Hip fracture is associated with high mortality; up to one-third of people die within a year.3 The associated morbidity and economic cost is large, with the annual cost of hip fractures estimated at ~£2 billion for the UK.1
Chronic kidney disease (CKD) is also a major and growing healthcare challenge, with an estimated global prevalence of 11%–13%, again higher in the elderly and women.4 People with CKD are at increased risk of morbidity, mortality and progressive kidney function decline, leading to renal replacement therapy (RRT).5–7 A well-recognised complication of RRT is renal bone disease. The association between RRT and risk of hip fracture is well established, with a high incidence of hip fractures.8 Mortality at 1-year post-hip fracture is 55%–64% for dialysis patients,8 9 a 2.7-fold increase compared with the non-fractured dialysis population.8 9
Although changes in bone metabolism are seen in patients with CKD long before the initiation of RRT,10 the evidence of hip fracture risk in CKD is uncertain, particularly at less advanced stages. Some studies report on renal function measured after hip fracture has occurred,11 12 others restrict to those at high risk of fracture.13 Recent studies reporting hip fracture incidence in those with prior documented CKD have been conflicting, some showing an association14–16 and others not.17 In addition, an increased risk of hip fracture-related deaths has been reported only among those with more advanced CKD.18 Mortality post-hip fracture is high regardless of renal function,3 although CKD has been linked to higher post-hip fracture mortality in hospital19 and at 1 year20 compared with those with normal renal function. Overall, however, many previous studies reporting the effect of CKD on the incidence of hip fracture or mortality post-hip fracture are limited by: older populations15 16 18 20; CKD based on only one measurement of renal function15 17 18 20 or reliance on administrative coding for identification of CKD.16 19
With an increasing and ageing population, both the prevalence of CKD and hip fracture incidence will rise, together with associated health and social care costs. As a result of uncertainty about the association between CKD and hip fracture, current guidelines on fracture risk management may be poorly targeted. Therefore, the study aim was to clarify the association of CKD with hip fracture incidence and mortality.
Methods
Population
The Grampian Laboratory Outcomes Mortality and Morbidity Study II (GLOMMS-II) cohort consists of residents (≥15 years) in a single health region in the Northeast of Scotland (population ~0.5 million) in 2003. All with at least one abnormal measure of kidney function (estimated glomerular filtration rate (eGFR) <60 mL/min/1.73 m2); a 20% random sample with normal kidney function; a 20% random sample with no measurement of kidney function and all on maintenance RRT (dialysis and transplants) were included, as described previously.21–23 A single biochemistry service processes all blood samples (inpatient, outpatient and community) for the region. For this study, individuals with sustained CKD stages 3–5 (not dialysis or transplant), on maintenance RRT (dialysis and transplants), and those with normal kidney function are reported only.
The study protocol was reviewed by Information Services Division Privacy Advisory Committee, NHS Grampian Caldicott Guardian, North of Scotland Research Ethics Service and University of Aberdeen College Ethics Review Board (CERB). The study was carried out in accordance with the principles of the Declaration of Helsinki.
Exposure
Renal status was assessed at entry to study. CKD was defined and staged according to Kidney Disease: Improving Global Outcomes (KDIGO).24 25 The index value and date were defined as the first abnormal kidney function measure, (eGFR <60 mL/min/1.73 m2), calculated from a creatinine measured between 1 January and 31 December 2003 (see footnote in table 1).26 To fulfil the criteria of CKD, the next eGFR at least 3 months after index (or if there were no samples after 3 months postindex, the eGFR prior to 3 months before the index) also had to be <60 mL/min/1.73 m2. In those with normal kidney function throughout 2003 (eGFR ≥60 mL/min/1.73 m2), the last value and date were taken as the index.
Table 1.
Characteristics of individuals with and without a hip fracture admission
| Characteristic | Hip fracture, n (%) | No hip fracture, n (%) |
| Total no of patients: 39 630 | 1084 (2.7) | 38 546 (97.3) |
| Renal status* | ||
| RRT | 8 (0.7) | 337 (0.9) |
| CKD stages 3–5 | 915 (84.4) | 18 622 (48.3) |
| CKD stage 5 | 2 (0.2) | 180 (0.5) |
| CKD stage 4 | 77 (7.1) | 1321 (3.4) |
| CKD stage 3b | 320 (29.5) | 4941 (12.8) |
| CKD stage 3a | 516 (47.6) | 12 180 (31.6) |
| Normal eGFR | 161 (14.9) | 19 587 (50.8) |
| Sex | ||
| Males | 231 (21.3) | 16 137 (41.9) |
| Females | 853 (78.7) | 22 409 (58.1) |
| Age, mean (95% CI), years | 80.3 (79.7 to 80.8) |
62.8 (62.6 to 63.0) |
| 15–44 | 10 (0.9) | 7278 (18.9) |
| 45–54 | 13 (1.2) | 4533 (11.8) |
| 55–64 | 42 (3.9) | 6203 (16.1) |
| 65–74 | 172 (15.9) | 8805 (22.8) |
| 75–84 | 516 (47.6) | 8583 (22.3) |
| ≥85 | 331 (30.5) | 3144 (8.2) |
| Creatinine, median (IQR), µmol/L | 99.5 (85.5–121.1) |
88.7 (73.6–109.2) |
| eGFR (MDRD), mean (95% CI), mL/min/1.73 m2† | 50.6 (49.5 to 51.7) |
66.5 (66.3 to 66.8) |
| Proteinuria‡ | ||
| Normal | 79 (7.3) | 2888 (7.5) |
| Microalbuminuric | 47 (4.3) | 957 (2.5) |
| Macroalbuminuric | 13 (1.2) | 683 (1.8) |
| Not measured | 945 (87.2) | 34 018 (88.3) |
| ACR, median (IQR), mg/mmol | 1.0 (0.9–5.0) | 1.0 (0.9–3.0) |
| PCR, median (IQR), mg/mmol | 21.5 (10.0–49.0) |
25.0 (10.0–79.0) |
| Charlson Comorbidity Index score | ||
| 0 | 699 (64.5) | 29 547 (76.7) |
| 1–2 | 297 (27.4) | 7156 (18.6) |
| 3–4 | 69 (6.4) | 1301 (3.4) |
| ≥5 | 19 (1.8) | 542 (1.4) |
| Urban rural classification§ | ||
| Large urban | 448 (41.3) | 13 512 (35.1) |
| Other urban | 155 (14.3) | 5536 (14.4) |
| Accessible small towns | 99 (9.1) | 3096 (8.0) |
| Remote small towns | 112 (10.3) | 3146 (8.2) |
| Accessible rural | 159 (14.7) | 6851 (17.8) |
| Remote rural | 106 (9.8) | 3664 (9.5) |
| SIMD (quintile)§ | ||
| 1 (most deprived) | 87 (8.0) | 2414 (6.3) |
| 2 | 182 (16.8) | 5794 (15.0) |
| 3 | 286 (26.4) | 8994 (23.3) |
| 4 | 260 (24.0) | 9377 (24.3) |
| 5 (least deprived) | 264 (24.4) | 9226 (23.9) |
*Renal status based on KDIGO CKD stages: overall CKD stages 3–5, index eGFR <60 mL/min/1.73 m2; individual stages 3a, 3b, 4 and 5 CKD, index eGFR 59–45, 44–30, 29–15 and <15 mL/min/1.73 m2. CKD stage 5 does not include dialysis or transplants.
†eGFR was calculated using the four-variable isotope dilution mass spectrometry aligned MDRD formula as is used clinically in the region.
‡Proteinuria status defined as: not measured: no test prior to index date; normoalbuminuric: measured, not microalbuminuric /macroalbuminuric; microalbuminuric: ≥2.5 mg/mmol ACR for men, ≥3.5 mg/mmol ACR for women or PCR ≥15 for both; macroalbuminuric: ≥30 mg/mmol ACR for both men and women or ≥50 mg/mmol PCR.
§SIMD quintile and urban rural classification were unknown for 2746 individuals because of missing postal codes. Where relevant, missing data were included in the analysis as a separate category.
ACR, albumin to creatinine ratio; CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; MDRD, Modification of Diet in Renal Disease; RRT, renal replacement therapy (dialysis and transplants); SIMD, Scottish Index of Multiple Deprivation; KDIGO, Kidney Disease: Improving Global Outcomes.
Covariates
Other covariates included age, sex, proteinuria, comorbidity, rurality and deprivation. Proteinuria was assessed using the last urinary albumin to creatinine or protein to creatinine ratio measured prior to index, and categorised as not measured, normoalbuminuric, microalbuminuric or macroalbuminuric (see footnote in table 1). Comorbidities were identified from hospital episode records for the 5 years prior to 2003 (International Classification of Diseases, 10th Revision (ICD-10) coding). A modified Charlson Comorbidity Index score was calculated27 28 (see footnote in online supplementary table 1). Postal code-based rurality29 and deprivation30 categories were also obtained (see table 1 and footnote in online supplementary table 1).
bmjopen-2017-020312supp001.pdf (174.9KB, pdf)
Follow-up
From index date in 2003, follow-up was available to 30 June 2009, giving an observation period of at least 5.5 years.
Outcomes
The primary outcome was hip fracture incidence, measured using (1) hip fracture admission (from hospital admissions data, where hip fracture was recorded as the main or additional diagnosis (ICD-10 code S72)) and (2) hip fracture-related death (from national death records, where hip fracture was recorded as a main or other cause of death). The secondary outcome was post-hip fracture mortality in individuals who had a hip fracture admission, both (1) all-cause mortality (ACM) and (2) specifically hip fracture-related mortality (HFM) where hip fracture was recorded as main or other cause of death.
Exclusions
Those who had a hip fracture or died on index date, and non-residents of Grampian were excluded.
Data linkage
The Community Health Index number, a unique patient identifier used throughout the Scottish healthcare system, was used to link GLOMMS-II with hospital episode and death registry data using deterministic matching. The deidentified dataset was hosted by Grampian Data Safe Haven, allowing secure controlled access for researchers while ensuring data security.31
Statistical analyses
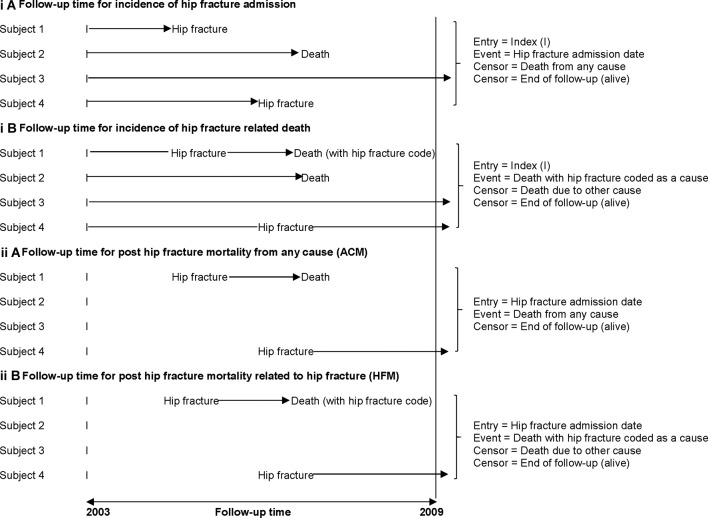
Hip fracture incidence rates were calculated using both (1) hip fracture admissions and (2) hip fracture deaths, as ‘markers’ for hip fracture occurrence. Follow-up time for hip fracture admissions (figure 1i(A)) was calculated from index date (study entry) to the date of the first hospital admission for hip fracture, censoring at death or end of follow-up. Follow-up time for hip fracture deaths (figure 1i(B)) was calculated from index date to date of hip fracture-related death, censoring at end of follow-up or date of death from other causes. Unadjusted and adjusted incident rate ratios (IRRs) for the effect of CKD on hip fracture incidence were calculated using Poisson regression with an offset of person-years of exposure. Models for hip fracture admission incidence were developed using forward selection of variables, both using variables with significant effect on univariate analysis and specifically for available risk factors for fractures (see footnote in table 2) identified from national guidelines.32 Variables were included if they improved model performance (likelihood ratio test). The final model for hip fracture death incidence was adjusted for age and sex only.
Figure 1.
Entry to the study, follow-up time and censoring for (i) hip fracture incidence and (ii) post-hip fracture mortality. The index date was defined as the first abnormal eGFR (<60 mL/min/1.73 m2) from a creatinine measured between 1 January and 31 December 2003. Where all eGFR values in 2003 were normal, the last value and date were taken as the index. Primary outcome, hip fracture incidence measured using (A) hip fracture admission (from hospital admissions data, where hip fracture was recorded as the main or additional diagnosis (ICD-10 code S72)) and (B) hip fracture-related death (from national death records, where hip fracture was recorded as a main or other cause of death). Secondary outcome, post-hip fracture mortality in individuals who had a hip fracture admission, both (A) all-cause mortality (ACM) and (B) specifically hip fracture-related mortality (HFM) where hip fracture was recorded as main or other cause of death. eGFR, estimated glomerular filtration rate; ICD-10, International Classification of Diseases, 10th Revision.
Table 2.
Incidence of hip fracture in CKD, measured with (a) admissions and (b) deaths
| Renal status | Total | Events n | PY follow to up (1000) | Rate per 1000 PY (95% CI) |
Unadjusted IRR (95% CI) |
Age/sex adjusted IRR (95% CI) |
Adjusted IRR (95% CI) final model* |
SIGN final model† |
| (a) Association of CKD and incidence of hip fracture admission | ||||||||
| CKD stages 3–5‡ | 19 537 | 915 | 91.4 | 10.0 (9.4 to 10.7) | 6.90 (5.83 to 8.15) | 1.58 (1.32 to 1.90) | 1.49 (1.24 to 1.79) | 1.53 (1.27 to 1.84) |
| CKD stage 5‡ | 182 | 2 | 0.6 | 3.3 (0.8 to 13.2) | 2.27 (0.56 to 9.16) | 0.78 (0.19 to 3.15) | 0.69 (0.17 to 2.78) | |
| CKD stage 4 | 1398 | 77 | 4.8 | 15.9 (12.7 to 19.9) | 10.96 (8.36 to 14.39) | 1.98 (1.49 to 2.63) | 1.74 (1.30 to 2.33) | |
| CKD stage 3b | 5261 | 320 | 22.6 | 14.1 (12.7 to 15.8) | 9.74 (8.06 to 11.77) | 1.83 (1.49 to 2.24) | 1.70 (1.38 to 2.09) | |
| CKD stage 3a | 12 696 | 516 | 63.3 | 8.2 (7.5 to 8.9) | 5.61 (4.70 to 6.70) | 1.47 (1.21 to 1.77) | 1.40 (1.16 to 1.70) | |
| Normal eGFR | 19 748 | 161 | 110.9 | 1.5 (1.2 to 1.7) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) |
| (b) Association of CKD and incidence of hip fracture-related death | ||||||||
| CKD stages 3–5‡ | 19 537 | 198 | 92.8 | 2.1 (1.9 to 2.5) | 7.91 (5.39 to 11.61) | 1.27 (0.85 to 1.88) | ||
| CKD stage 5‡ | 182 | 0 | 0.6 | – | – | – | ||
| CKD stage 4 | 1398 | 26 | 4.9 | 5.3 (3.6 to 7.8) | 19.59 (11.59 to 33.13) | 2.28 (1.32 to 3.92) | ||
| CKD stage 3b | 5261 | 78 | 23.1 | 3.4 (2.7 to 4.2) | 12.53 (8.22 to 19.08) | 1.57 (1.01 to 2.44) | ||
| CKD stage 3a | 12 696 | 94 | 64.1 | 1.5 (1.2 to 1.8) | 5.43 (3.60 to 8.19) | 1.03 (0.68 to 1.58) | ||
| Normal eGFR | 19 748 | 30 | 111.2 | 0.3 (0.2 to 0.4) | 1.00 (Reference) | 1.00 (Reference) | ||
*Final model adjusted for age, sex, Charlson Comorbidity Index score, deprivation, proteinuria and rurality (all variables remained in model).
†SIGN model developed using age, sex, diabetes, connective tissue disease, chronic pulmonary disease, dementia, chronic liver disease, non-haematological malignancy, haematological malignancy, metastatic solid tumour, peptic ulcer disease, HIV/AIDS, obesity (ICD-10 code E66) and smoking status (ICD-10 code Z72.0, F17.2, Z71.6). We did not have available: drug therapy, certain other coexisting diseases, low BMD (bone mineral density), alcohol use, previous fracture, parental history of osteoporosis and early menopause. The SIGN final (best) model was adjusted for age, sex, diabetes, chronic pulmonary disease, dementia and chronic liver disease.
‡CKD stage 5 does not include dialysis or transplants.
CKD, chronic kidney disease; ICD-10, 10th revision of International Classification of Diseases; IRR, incident rate ratio; PY, patient-years; SIGN, Scottish Intercollegiate Guidelines Network.
Post-hip fracture mortality follow-up time was calculated from the date of a hip fracture-related hospital admission to date of death, censoring at end of follow-up for ACM (figure 1ii(A)), and for HFM, date of death from other causes also (figure 1ii(B)). Mortality rates were calculated. Unadjusted and adjusted mortality rate ratios (MRRs) were calculated using Poisson regression. The final adjusted model for each outcome (ACM and HFM) was developed as above. Assumptions were checked including proportionality over time. As there was a low number of individuals and events in the RRT group, this group was reported in the descriptive statistics only, but not included in the hip fracture or mortality post-hip fracture risk analyses. Where measures of effect were calculated, 95% CIs were reported. Analyses were performed using StataV.13.0.
Results
Baseline characteristics
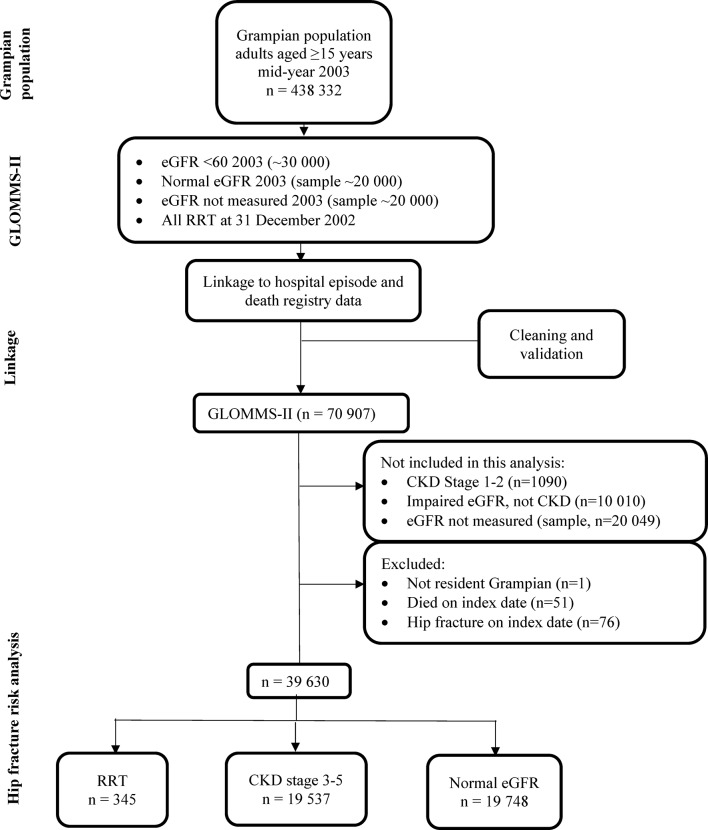
The flow diagram for generating GLOMMS-II and identifying individuals for this study is shown in figure 2. There were 19 537 individuals with CKD stages 3–5, 345 on RRT and 19 748 with normal eGFR (20% random sample). Those with CKD were older (mean age 74.8 (95% CI 74.6 to 74.9) years), and more likely to be female (64.9%) than those with normal eGFR (52.1 (95% CI 51.8 to 52.3) years, 52.8%) or on RRT (54.4 (95% CI 52.7 to 56.2) years, 44.9%) respectively. Individuals with CKD had more comorbidity than those with normal eGFR (33.3% vs 13.8% with Charlson Comorbidity Index score ≥1) (online supplementary table 1).
Figure 2.
Flow diagram of study population from GLOMMS-II. CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; GLOMMS-II, Grampian Laboratory Outcomes Morbidity and Mortality Study II; RRT, renal replacement therapy (dialysis and transplants).
Hip fracture incidence (primary outcome)
Hip fracture incidence was assessed using both (1) hip fracture admissions and (2) hip fracture-related deaths as measures of occurrence.
There were 915 hip fracture admissions for individuals with CKD stages 3–5, 8 among those on RRT and 161 for those with normal eGFR. Compared with those with no hip fracture admission, individuals with a hip fracture were more likely to be female, older, have more comorbidity and have CKD stages 3–5 (table 1). As there were few fracture admissions among individuals on RRT, these individuals’ results are not reported further.
CKD stages 3–5 overall was associated with an increased incidence of hip fracture admission compared with those with normal eGFR, rates of 10.0 (95% CI 9.4 to 10.7) and 1.5 (95% CI 1.2 to 1.7) per 1000 patient-years (PY), respectively. The unadjusted hip fracture admission IRR for CKD stages 3–5 overall compared with normal eGFR was 6.90 (95% CI 5.83 to 8.15) (table 2a). This was increased in both men and women, unadjusted IRR 5.28 (95% CI 3.89 to 7.16) and 6.85 (95% CI 5.59 to 8.38), respectively (online supplementary table 2). The increased risk was present for every age group, but more marked in younger age groups, the unadjusted IRR (CKD stages 3–5 vs normal eGFR) was 7.63 (95% CI 1.54 to 37.82) and 1.48 (95% CI 1.14 to 1.91) for those aged 15–44 and 75–84 years, respectively (online supplementary table 2). After adjusting for age, sex, comorbidity, deprivation, proteinuria and rurality (final model), hip fracture risk remained increased for CKD stages 3–5 overall (IRR 1.49 (95% CI 1.24 to 1.79)) and for CKD stages 3a, 3b and 4 individually. There were, however, only small numbers of individuals and events for CKD stage 5. The model adjusting for available fracture risk factors identified from national guidelines32 showed similar results (table 2a).
Hip fracture incidence was also assessed with hip fracture-related deaths as a measure of occurrence. Few individuals died with a hip fracture-related death; 1.0% of those with CKD stages 3–5 and 0.2% of those with normal eGFR. This equates to 2.1 (1.9–2.5) versus 0.3 (0.2–0.4) per 1000 PY respectively; an eightfold difference. After adjusting for age and sex (table 2b), the overall IRR for CKD stages 3–5 effect was 1.27 (0.85–1.88). However, individual CKD stages 3b and 4 were still associated with hip fracture incidence when measured with death. Fifteen individuals had no hip fracture admission recorded, but had a hip fracture-related death.
Post-hip fracture mortality (secondary outcome)
Among the 915 individuals with CKD stages 3–5 and 161 with normal eGFR who had a hip fracture admission, there were subsequently 666 and 83 all-cause; and 183 and 26 hip fracture-related deaths, respectively. Four patients died on hip fracture admission date and therefore excluded from further analysis. Compared with those alive at the end of follow-up, individuals who died were older (mean age 82.6 (82.1–83.2)) vs 74.7 (73.6–75.8) years), had more comorbidity (Charlson Comorbidity Index score ≥1, 42.0% vs 21.4%) and more had CKD stages 3–5 (89.0% vs 75.9%).
Post-hip fracture ACM rates for those who had a hip fracture admission were 488.0 (452.4–526.6) and 308.9 (249.1–383.0) per 1000 PY for those with CKD stages 3–5 and normal eGFR, respectively (table 3a). CKD stages 3–5 overall were initially associated with ACM. However, after adjusting for age, sex, comorbidity, deprivation, proteinuria and rurality (final model), only the post-hip fracture ACM MRR for CKD stage 4 was increased (2.04 (1.44–2.89)).
Table 3.
Post-hip fracture admission mortality in CKD, both (a) all-cause mortality and (b) hip fracture-related mortality
| Renal status | Total with hip fracture | Events, n | PY follow-up (1000) | Rate per 1000 PY (95% CI) |
Unadjusted MRR (95% CI) |
Adjusted* MRR (95% CI) final model |
| (a) All-cause mortality post-hip fracture | ||||||
| CKD stages 3–5† | 915 | 666 | 1.4 | 488.0 (452.4 to 526.6) | 1.58 (1.26 to 1.99) | 1.09 (0.85 to 1.39) |
| CKD stage 5b† | 2 | 2 | 0.0 | 764.1 (191.1 to 3055.3) | 2.47 (0.61 to 10.06) | 1.04 (0.24 to 4.51) |
| CKD stage 4 | 77 | 71 | 0.1 | 885.3 (701.6 to 1117.1) | 2.87 (2.09 to 3.93) | 2.04 (1.44 to 2.89) |
| CKD stage 3b | 320 | 240 | 0.4 | 542.2 (477.7 to 615.3) | 1.76 (1.37 to 2.25) | 1.11 (0.85 to 1.45) |
| CKD stage 3a | 516 | 353 | 0.8 | 420.7 (379.0 to 466.9) | 1.36 (1.07 to 1.73) | 1.02 (0.80 to 1.32) |
| Normal eGFR | 161 | 83 | 0.3 | 308.9 (249.1 to 383.0) | 1.00 (Reference) | 1.00 (Reference) |
| (b) Hip fracture mortality post-hip fracture | ||||||
| CKD stages 3–5† | 915 | 183 | 1.4 | 134.1 (116.0 to 155.0) | 1.39 (0.92 to 2.09) | 0.79(0.51 to 1.22) |
| CKD stage 5† | 2 | 0 | 0.0 | – | – | – |
| CKD stage 4 | 77 | 24 | 0.1 | 299.3 (200.6 to 446.5) | 3.09 (1.78 to 5.39) | 1.88 (1.02 to 3.47) |
| CKD stage 3b | 320 | 72 | 0.4 | 162.7 (129.1 to 204.9) | 1.68 (1.07 to 2.63) | 0.85 (0.53 to 1.37) |
| CKD stage 3a | 516 | 87 | 0.8 | 103.7 (84.0 to 127.9) | 1.07 (0.69 to 1.66) | 0.71 (0.45 to 1.12) |
| Normal eGFR | 161 | 26 | 0.3 | 96.8 (65.9 to 142.1) | 1.00 (Reference) | 1.00 (Reference) |
The number of hip fracture deaths are not the same in tables 3b and 2b because:
1. Four individuals with CKD stages 3–5 died on date of hip fracture admission and are not included in the number of deaths in table 3.
2. A further 15 individuals had no record of a hip fracture admission but had a hip fracture-related death, and are therefore not included in the population for post-hip fracture mortality. Of these, 11 had CKD stages 3–5, with an average age of 87. Some died within a few days of index date. They had multiple and varied other causes of death, hip fracture was not the first or second cause for any. Four had normal eGFR, with an average age of 86. All died within 3 days of index date, and did not have hip fracture recorded as first, second or third cause of death.
*Adjusted for age, sex, Charlson Comorbidity Index score, deprivation, proteinuria and rurality (all variables remained in model).
†CKD stage 5 does not include dialysis or transplants.
CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; MRR, mortality rate ratio; PY, patient-years.
Post-hip fracture HFM rates for individuals who had a hip fracture admission were 134.1 (95% CI 116.0 to 155.0) and 96.8 (95% CI 65.9 to 142.1) per 1000 PY for those with CKD stages 3–5 overall and normal eGFR, respectively (table 3b). The unadjusted HFM MRR for CKD stages 3–5 overall was 1.39 (95% CI 0.92 to 2.09). After adjusting for age, sex, comorbidity, deprivation, proteinuria and rurality (final model), CKD stages 3–5 overall remained not associated with post-hip fracture HFM (MRR 0.79 (95% CI 0.51 to 1.22)), however, CKD stage 4 remained associated (adjusted MRR 1.88 (95% CI 1.02 to 3.47)).
Discussion
The incidence of hip fracture measured by admissions was increased in those with CKD stages 3–5 overall, and for individual CKD stages 3a, 3b and 4 compared with those with normal eGFR, particularly in younger age groups. The incidence of hip fracture measured by deaths was also increased in those with CKD stages 3b and 4. Post-hip fracture mortality, however, whether ACM or HFM, was not increased in CKD stages 3–5 overall, although it was for CKD stage 4. There were, however, only small numbers of individuals and events for CKD stage 5.
The mechanisms underlying the association between CKD and fractures are likely to be at the metabolic level, due to abnormalities in the parathyroid–calcium– phosphate axis as a result of reduced kidney function.33 In addition, CKD may be a marker for frailty, leading to falls and an increased risk of fracture.
In keeping with some14 16 but not all17 reports, we have shown that hip fracture incidence is increased in individuals with CKD. We showed this effect is best demonstrated when hip fracture incidence is measured with admissions but also present when measured with deaths. We demonstrated that this increased risk was present across all ages; few previous studies included all age ranges16 34 and many only included the elderly.15 35–37 We have shown that despite both CKD and hip fractures being more common in women, this effect of CKD on hip fracture incidence was also raised in men, something that has rarely been demonstrated.34 We demonstrated that worse CKD stage was associated with increased fracture admission risk (even after accounting for confounders), as reported by Naylor et al.14 However, we found the effect attenuated and not associated in CKD stage 5 due to less statistical power since, unlike Naylor et al 14 we excluded RRT patients from stage 5 and reported RRT as a separate group. Our finding that hip fracture incidence when measured with deaths was only increased among those with more advanced CKD (stages 3b and 4, but not 3a), supports a previous study in patients ≥75 years.18
We found that post-hip fracture mortality, among those who had a hip fracture admission, was little affected by CKD stages 3–5 overall, except stage 4. Studies in general38 and RRT populations39 have reported elevated subsequent mortality in individuals who suffer a hip fracture compared with their age-matched non-fractured peers. However, to our knowledge, few studies have investigated the effect of CKD on mortality post-hip fracture.19 20 40–42 Three reported increased mortality risk with worse renal function, either eGFR20 or creatinine,41 42 and one found no mortality risk with worse creatinine after adjusting for confounders.40 All of these studies, however, only included older patients and were based on single admission creatinine or eGFR, thus, deranged creatinine could be due to acute kidney injury (AKI). A further study relied on hospital admission coding for hip fractures with surgical repair, and a concurrent code for CKD or otherwise,19 thus limiting CKD ascertainment. Our finding of an effect with stage 4 CKD is consistent with the literature generally—that mortality risk increases with renal function decline. However, in our study the lack of effect with stage 5 is likely due to less statistical power.
This study had several strengths. To our knowledge, this is the first study to report hip fracture incidence using both admission and death records, allowing direct comparison with studies that have used either of these methods. The lack of a consistent effect of CKD on fracture incidence when measured by admission versus death demonstrates the importance of methods of measurement in assessing such an outcome. Fractures are more likely to be recorded during an admission than at death, since for admission, a fracture is likely to be a major event, whereas for a death, many other factors may have contributed, and time between fracture and death recording may be lengthy. In our cohort, 1076 individuals had a hip fracture recorded from an admission compared with only 228 having a hip fracture recorded at death. GLOMMS-II, a large, population-based cohort including adults ≥15 years, uses valid methods for assessing comorbidity,43 identifying individuals with CKD,22 and has data linkage to long-term outcome data. Importantly, all biochemistry is provided by a single biochemistry service, whether inpatient, outpatient or community. This minimises loss of baseline and follow-up data, avoids selection biases in recruitment, and enables assessment of the relationship between CKD and hip fracture over the full range of adult ages and CKD stages 3–5 groups. The use of at least two serum creatinine measurements to identify CKD (as per KDIGO),24 25 is a great strength compared with the majority of previous studies, which used only one measurement, therefore risking identifying episodes of AKI as CKD15; AKI itself being a risk factor for mortality and often complicates hospital admissions.23 Access to biochemistry results allowed us to demonstrate the effect of CKD more completely than studies where CKD diagnosis is based on healthcare coding alone.16 19
However, our study had limitations. We assessed hip fracture from hospital episode data, and while most patients with a hip fracture are admitted to hospital, we recognise that a very small number may not be admitted. However, we postulate this would be low numbers (we noted only 15 individuals who died with hip fracture as cause of death without a prior hip fracture admission). We only considered hip fractures, acknowledging that CKD may be associated with other fracture sites, but assuming that hip fractures would have more complete admission data, and have been used as a tracer condition for fragility fractures at other sites.44 The GLOMMS-II cohort inception was 2003, with follow-up to 2009. However, national data suggest that age-standardised and sex-standardised rates of hip fracture admissions have been stable from 2003 for over a decade.45 In addition, although new treatments have become available since 2003, treatment of hyperparathyroidism in patients with less advanced CKD, who make up the majority of this cohort, has changed little. We did not take into account the competing risk of death, which has been reported to result in an overestimation of the excess risk of hip fracture,16 however, we have demonstrated the high fracture burden seen. Therefore, hip fracture avoidance is important and specifically measures to address modifiable risk factors in this population. We were unable to adjust for some factors that have been associated with fracture risk, including previous fracture and low bone mineral density (see footnote under table 2), however, whatever an individual’s risk factors are, we have demonstrated the high risk for the population as a whole. Although we did not include individuals with CKD stage 1 or 2, 94% of those with normal eGFR did not have proteinuria measured. Therefore, we cannot rule out the possibility that some individuals in the normal eGFR comparison group could be CKD stage 1 or 2. Finally, in this analysis, renal status was assessed at baseline, thus, progression of CKD has not been taken into account. We would therefore recommend that the relationship between CKD progression and risk of fracture be investigated in future research.
The findings of this study are directly applicable to Scottish healthcare, and likely applicable across the UK and many worldwide settings. Vitamin D exposure may be lower at extreme latitudes, and may contribute to fracture rate. However, in our study, the exposed (CKD) and unexposed (normal eGFR) groups were from the same population, and Vitamin D exposure would be equivalent, therefore, the relative risk is likely to be relevant internationally. The results summarise the workload faced by health services as a result of CKD-related bone disease. Our finding that individuals with CKD, particularly more advanced, are at higher risk of hip fracture should encourage better assessment and management of risk factors for fracture and falls, including in younger patients with CKD. Risk factors for fracture include modifiable risk factors (alcohol use, smoking, body mass, bone mineral density and medication) and non-modifiable risk factors (age, gender, previous fracture and early menopause). Frailty has been associated with falls46 and fractures46 47 in both the general and dialysis populations; and falls are common in those on dialysis.48 Falls risk assessment should therefore be integral to fracture risk assessment,32 and perhaps CKD reviews should therefore include consideration of interventions that might improve impaired balance and fracture risks. A reduction in hip fracture incidence would potentially reduce the number of fracture-related deaths overall.
Conclusion
By using different measures of hip fracture incidence and mortality, we have demonstrated why other studies have shown mixed associations between CKD and hip fracture. Hip fracture incidence was higher in individuals with CKD compared with those with normal eGFR particularly where measured with admissions. However, following a hip fracture, CKD did not increase post-hip fracture mortality except in those with CKD stage 4. Nonetheless, a reduction in hip fracture incidence in those with CKD would reduce the number of deaths after hip fracture in the population.
Supplementary Material
Acknowledgments
We thank Information Services Division Scotland, Scottish Renal Registry and NHS Grampian who provided data. We also thank the Grampian Data Safe Haven, who hosted the data and provided data management support and the linkage service, and also the staff of NHS Grampian Renal Unit. We also acknowledge the support from The Farr Institute of Health Informatics Research, Scotland. The Farr Institute is supported by a 10-funder consortium: Arthritis Research UK, the British Heart Foundation, Cancer Research UK, the Economic and Social Research Council, the Engineering and Physical Sciences Research Council, the Medical Research Council, the National Institute of Health Research, the National Institute for Social Care and Health Research (Welsh Assembly Government), the Chief Scientist Office (Scottish Government Health Directorates), the Wellcome Trust (MRC Grant nos: Scotland MR/K007017/1).
Footnotes
Contributors: Study design: AM, CB, NF and LR. Data analysis: LR, AM and HN. Interpretation of data: LR, CB, NF, SG, RH, GP and AM. Drafting manuscript: LR, AM. All authors revised manuscript content and approved the final version of the manuscript and take responsibility for the integrity of the data analysis.
Funding: This work was supported by NHS Grampian Endowment (grant no: 14/30). A Chief Scientist Office for Scotland grant (grant no: CZH/4/656) funded the set-up of the cohort.
Competing interests: LR was supported by NHS Grampian Endowment (grant no: 14/30).
Patient consent: Not required.
Ethics approval: University of Aberdeen College Ethics Review Board.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: Deidentified data used for this study are held by Grampian Data Safe Haven. These data are available provided the necessary permissions have been obtained. Further information is available at http://www.abdn.ac.uk/iahs/facilities/grampian-data-safe-haven.php and requests for data may be made to Professor Corri Black on behalf of Grampian Data Safe Haven, corri.black@abdn.ac.uk.
References
- 1. National Institute for Health and Care Excellence. The management of hip fracture in adults. London: National Clinical Guideline Centre, 2011. [Google Scholar]
- 2. Scottish Intercollegiate Guidelines Network (SIGN). Management of hip fracture in older people. A national clinical guideline. Edinburgh: Scottish Intercollegiate Guidelines Network, 2009. [Google Scholar]
- 3. Royal College of Physicians. National Hip Fracture Database (NHFD) annual report 2016. London: Royal College of Physicians, 2016. [Google Scholar]
- 4. Hill NR, Fatoba ST, Oke JL, et al. Global prevalence of chronic kidney disease - a systematic review and meta-analysis. PLoS One 2016;11:e0158765 10.1371/journal.pone.0158765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Matsushita K, van der Velde M, Astor BC, et al. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet 2010;375:2073–81. 10.1016/S0140-6736(10)60674-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Marks A, Fluck N, Prescott GJ, et al. Definitions of progression in chronic kidney disease–predictors and relationship to renal replacement therapy in a population cohort with a 6 year follow-up. Nephrol Dial Transplant 2014;29:333 10.1093/ndt/gft393 [DOI] [PubMed] [Google Scholar]
- 7. McCullough PA, Li S, Jurkovitz CT, et al. Chronic kidney disease, prevalence of premature cardiovascular disease, and relationship to short-term mortality. Am Heart J 2008;156:277–83. 10.1016/j.ahj.2008.02.024 [DOI] [PubMed] [Google Scholar]
- 8. Coco M, Rush H. Increased incidence of hip fractures in dialysis patients with low serum parathyroid hormone. Am J Kidney Dis 2000;36:1115–21. 10.1053/ajkd.2000.19812 [DOI] [PubMed] [Google Scholar]
- 9. Mittalhenkle A, Gillen DL, Stehman-Breen CO. Increased risk of mortality associated with hip fracture in the dialysis population. Am J Kidney Dis 2004;44:672–9. [PubMed] [Google Scholar]
- 10. Steddon S, Sharples E. Renal association clinical practice guideline in mineral and bone disorders in CKD. Nephron Clin Pract 2011;118(Suppl 1):c145–52. 10.1159/000328066 [DOI] [PubMed] [Google Scholar]
- 11. Kinsella S, Chavrimootoo S, Molloy MG, et al. Moderate chronic kidney disease in women is associated with fracture occurrence independently of osteoporosis. Nephron Clin Pract 2010;116:c256–62. 10.1159/000317207 [DOI] [PubMed] [Google Scholar]
- 12. Nickolas TL, McMahon DJ, Shane E. Relationship between moderate to severe kidney disease and hip fracture in the United States. J Am Soc Nephrol 2006;17:3223–32. 10.1681/ASN.2005111194 [DOI] [PubMed] [Google Scholar]
- 13. Dukas L, Schacht E, Stähelin HB. In elderly men and women treated for osteoporosis a low creatinine clearance of <65 ml/min is a risk factor for falls and fractures. Osteoporos Int 2005;16:1683–90. 10.1007/s00198-005-1903-7 [DOI] [PubMed] [Google Scholar]
- 14. Naylor KL, McArthur E, Leslie WD, et al. The three-year incidence of fracture in chronic kidney disease. Kidney Int 2014;86:810–8. 10.1038/ki.2013.547 [DOI] [PubMed] [Google Scholar]
- 15. Daya N, Voskertchian A, Schneider ALC, et al. Kidney function and fracture risk: the Atherosclerosis Risk in Communities (ARIC) study. Am J Kidney Dis 2016;67:218–26. 10.1053/j.ajkd.2015.06.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pérez-Sáez MJ, Prieto-Alhambra D, Barrios C, et al. Increased hip fracture and mortality in chronic kidney disease individuals: the importance of competing risks. Bone 2015;73:154–9. 10.1016/j.bone.2014.12.020 [DOI] [PubMed] [Google Scholar]
- 17. Elliott MJ, James MT, Quinn RR, et al. Estimated GFR and fracture risk: a population-based study. Clin J Am Soc Nephrol 2013;8:1367–76. 10.2215/CJN.09130912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nitsch D, Mylne A, Roderick PJ, et al. Chronic kidney disease and hip fracture-related mortality in older people in the UK. Nephrol Dial Transplant 2009;24:1539–44. 10.1093/ndt/gfn678 [DOI] [PubMed] [Google Scholar]
- 19. Kim SM, Long J, Montez-Rath M, et al. Hip fracture in patients with non-dialysis-requiring chronic kidney disease. J Bone Miner Res 2016;31:1803–9. 10.1002/jbmr.2862 [DOI] [PubMed] [Google Scholar]
- 20. Khan SK, Rushton SP, Courtney M, et al. Elderly men with renal dysfunction are most at risk for poor outcome after neck of femur fractures. Age Ageing 2013;42:76–81. 10.1093/ageing/afs152 [DOI] [PubMed] [Google Scholar]
- 21. Marks A, Fluck N, Prescott GJ, et al. Looking to the future: predicting renal replacement outcomes in a large community cohort with chronic kidney disease. Nephrol Dial Transplant 2015;30:1507–17. 10.1093/ndt/gfv089 [DOI] [PubMed] [Google Scholar]
- 22. Robertson LM, Denadai L, Black C, et al. Is routine hospital episode data sufficient for identifying individuals with chronic kidney disease? A comparison study with laboratory data. Health Informatics J 2016;22:383–96. 10.1177/1460458214562286 [DOI] [PubMed] [Google Scholar]
- 23. Sawhney S, Marks A, Fluck N, et al. Intermediate and long-term outcomes of survivors of acute kidney injury episodes: a large population-based cohort study. Am J Kidney Dis 2017;69:18–28. 10.1053/j.ajkd.2016.05.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl 2013;3:1–150. [Google Scholar]
- 25. National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis 2002;39:s1. [PubMed] [Google Scholar]
- 26. Levey AS, Coresh J, Greene T, et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med 2006;145:247–54. [DOI] [PubMed] [Google Scholar]
- 27. Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373–83. [DOI] [PubMed] [Google Scholar]
- 28. Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care 2005;43:1130–9. [DOI] [PubMed] [Google Scholar]
- 29. The Scottish Government. Urban rural classification. http://www.gov.scot/Topics/Statistics/About/Methodology/UrbanRuralClassification (accessed Aug 2017).
- 30. The Scottish Government. Scottish index of multiple deprivation. http://www.scotland.gov.uk/Topics/Statistics/SIMD (accessed Aug 2017).
- 31. University of Aberdeen. Grampian data safe haven. 2017. http://www.abdn.ac.uk/iahs/facilities/grampian-data-safe-haven.php.
- 32. Scottish Intercollegiate Guidelines Network (SIGN). Management of osteoporosis and the prevention of fragility fractures. Edinburgh: Scottish Intercollegiate Guidelines Network, 2015. [Google Scholar]
- 33. Pimentel A, Ureña-Torres P, Zillikens MC, et al. Fractures in patients with CKD-diagnosis, treatment, and prevention: a review by members of the European Calcified Tissue Society and the European Renal Association of Nephrology Dialysis and Transplantation. Kidney Int 2017;92:1343–55. 10.1016/j.kint.2017.07.021 [DOI] [PubMed] [Google Scholar]
- 34. Dooley AC, Weiss NS, Kestenbaum B. Increased risk of hip fracture among men with CKD. Am J Kidney Dis 2008;51:38–44. 10.1053/j.ajkd.2007.08.019 [DOI] [PubMed] [Google Scholar]
- 35. Ensrud KE, Parimi N, Fink HA, et al. Estimated GFR and risk of hip fracture in older men: comparison of associations using cystatin C and creatinine. Am J Kidney Dis 2014;63:31–9. 10.1053/j.ajkd.2013.05.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ensrud KE, Parimi N, Cauley JA, et al. Cystatin C and risk of hip fractures in older women. J Bone Miner Res 2013;28:1275–82. 10.1002/jbmr.1858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Fried LF, Biggs ML, Shlipak MG, et al. Association of kidney function with incident hip fracture in older adults. J Am Soc Nephrol 2007;18:282–6. 10.1681/ASN.2006050546 [DOI] [PubMed] [Google Scholar]
- 38. Abrahamsen B, van Staa T, Ariely R, et al. Excess mortality following hip fracture: a systematic epidemiological review. Osteoporos Int 2009;20:1633–50. 10.1007/s00198-009-0920-3 [DOI] [PubMed] [Google Scholar]
- 39. Tentori F, McCullough K, Kilpatrick RD, et al. High rates of death and hospitalization follow bone fracture among hemodialysis patients. Kidney Int 2014;85:166–73. 10.1038/ki.2013.279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Björkelund KB, Hommel A, Thorngren KG, et al. Factors at admission associated with 4 months outcome in elderly patients with hip fracture. Aana J 2009;77:49–58. [PubMed] [Google Scholar]
- 41. Mosfeldt M, Pedersen OB, Riis T, et al. Value of routine blood tests for prediction of mortality risk in hip fracture patients. Acta Orthop 2012;83:31–5. 10.3109/17453674.2011.652883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lewis JR, Hassan SK, Wenn RT, et al. Mortality and serum urea and electrolytes on admission for hip fracture patients. Injury 2006;37:698–704. 10.1016/j.injury.2006.04.121 [DOI] [PubMed] [Google Scholar]
- 43. Soo M, Robertson LM, Ali T, et al. Approaches to ascertaining comorbidity information: validation of routine hospital episode data with clinician-based case note review. BMC Res Notes 2014;7:253 10.1186/1756-0500-7-253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kassim Javaid M, Chana J, Cooper C. Hip fracture as the tracer condition. Best Pract Res Clin Rheumatol 2013;27:711–5. 10.1016/j.berh.2014.03.003 [DOI] [PubMed] [Google Scholar]
- 45. Smith P, Ariti C, Bardsley M. Focus on hip fracture. Trends in emergency admissions for fractured neck of femur, 2001 to 2011. London: The Health Foundation and Nuffield Trust, 2013. [Google Scholar]
- 46. McAdams-DeMarco MA, Suresh S, Law A, et al. Frailty and falls among adult patients undergoing chronic hemodialysis: a prospective cohort study. BMC Nephrol 2013;14:224–369. 10.1186/1471-2369-14-224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Delgado C, Shieh S, Grimes B, et al. Association of self-reported frailty with falls and fractures among patients new to dialysis. Am J Nephrol 2015;42:134–40. 10.1159/000439000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. López-Soto PJ, De Giorgi A, Senno E, et al. Renal disease and accidental falls: a review of published evidence. BMC Nephrol 2015;16:176–015. 10.1186/s12882-015-0173-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2017-020312supp001.pdf (174.9KB, pdf)