Abstract
Decades of research, including the 1996 Nobel Prize in Medicine, confirm the evolutionary and immunological importance of CD8 T lymphocytes (TCD8+) that target peptides bound by the highly variable major histocompatibility complex class I (MHC-I) proteins. However, their perceived importance has varied dramatically over the past decade. Regardless, there remains myriad reasons to consider the diversity of MHC-I alleles and the TCD8+ that target them as enormously important in infectious disease research. Thus, understanding these molecules in the best animal models of human disease could be a necessity for optimizing the translational potential of these models. Knowledge of macaque MHC has substantially improved their utility for modeling HIV and could aid in modeling other viruses as well, both in the context of distribution of alleles across treatment groups in vaccine trials and in deciphering mechanisms of immune control of pathogens for which specific MHC alleles demonstrate differential impacts on disease.
Keywords: Immune system, MHC, Virus, Macaque
Macaques, due primarily to their relative evolutionary closeness to humans, provide an ideal animal model for a number of infectious diseases. Analysis of the fully sequenced rhesus macaque genome suggests that humans and macaques diverged approximately 25 million years ago (Rhesus Macaque Genome et al., 2007). On average, the nucleotide sequence of the macaque genome is between 90 and 95% homologous to that of the human genome. The macaque immune system is likewise similar to that of humans and, with notable exceptions, responds similarly to a number of infectious agents. It stands to reason, then, that the underlying genetic architecture of the human and macaque immune systems must be largely overlapping. One of the most important genome loci involved in immunity to pathogens is the major histocompatibility complex (MHC). This region encodes the classical MHC-I and MHC-II genes as well as non-classical MHC genes such as MHC-E, which has received much recent attention (Davis et al., 2016; Hansen et al., 2016; Pietra et al., 2010; van Meijgaarden et al., 2015) and others. It also contains genes that encode proteins involved in a number of other immunological processes, such as the Transporter associated with Antigen Processing (TAP) and Tumor Necrosis Factor alpha (TNF-α) (Horton et al., 2004; Shiina et al., 2009).
Each haplotype on the human MHC-I locus contains a single A, B and C allele. In contrast, the MHC-I locus in macaques is astoundingly complex, with each individual haplotype expressing multiple A alleles and potentially more than 10 B alleles (Otting et al., 2005). However, the majority of these alleles are expressed at very low levels if at all, and the major alleles, those expressed at the highest level, are the ones most involved in immune responses to pathogens (Budde et al., 2011). Thus, in terms of functionality, the MHC-I region in macaques parallels that of humans more than it appears upon sequencing. Figure 1 depicts the MHC-I region from the human and macaque genomes.
Figure 1.
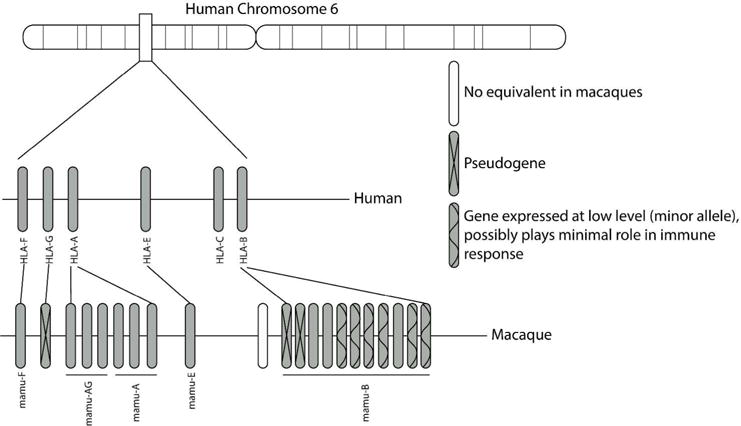
The organization of the MHC-I region in the human and macaque genome. Each human chromosome expresses a single A, B, C, E and F allele. Macaques express equivalent E and F alleles but the A and B loci have expanded resulting in expression of multiple alleles from each locus. However, many of these alleles are expressed at very low levels and are termed minor alleles. These minor alleles appear to play a minimal role in the antiviral immune response.
MHC-I molecules serve to present endogenously generated peptides to circulating CD8+ T lymphocytes (TCD8+). These peptides can be derived from self-proteins, including those expressed in an altered state during tumorigenesis, or from intracellular pathogens such as viruses. The MHC-I gene family is among the most polymorphic in the human genome. Given the predominant function of these molecules in peptide presentation, it’s not surprising that the sequence differences that define different alleles are most commonly located in the peptide binding region of the proteins, suggesting natural selection to maximize the ability to bind a diverse array of peptides from equally diverse pathogens (Hughes and Nei, 1988).
Interestingly, a number of particular human MHC-I alleles are statistically correlated with enhanced capacity to control chronic viral infections, including HIV (Goulder and Watkins, 2008) and Hepatitis C Virus (HCV) (Neumann-Haefelin et al., 2010), although HCV shows stronger associations with MHC-II alleles (Scotto et al., 2003), and surprisingly with acute hantavirus infections (Torresilla et al., 2013). Likewise, particular MHC-I alleles in macaques infected with simian immunodeficiency virus (SIV) show strong correlations with significantly reduced viral load (Budde et al., 2012; Loffredo et al., 2007; O’Connor et al., 2010; Yant et al., 2006).
Understanding MHC associated control of viral replication provides a framework for rational design of vaccines that induce TCD8+ cells. The ultimate goal of vaccines in general is to prevent infection at the port of entry, suggesting that neutralizing antibodies should be the most critical immunological component of a vaccine. However, one of the most effective vaccines ever created, the Yellow Fever vaccine 17D, induces a broad immunological response that includes antibodies and TCD8+ cells (Muyanja et al., 2014; Querec et al., 2009; Wieten et al., 2016), all of which likely contribute to viral prevention and clearance. In addition, a number of vaccines could be enhanced by induction of effective TCD8+ cells, which can target all viral proteins, rather than just the envelope glycoprotein, the predominant target of neutralizing antibodies. In the case of HIV, the Envelope protein is highly variable, rendering the induction of broadly neutralizing antibodies, those that neutralize isolates of the virus that might be encountered in the real world, incredibly difficult. Thus, a vaccine that can target the conserved regions of other viral proteins would be of enormous benefit. Similarly, there is considerable interest in creation of vaccines that can target phylogenetically divergent but related viruses. Many related virus harbor substantial conservation in their capsid and polymerase proteins but lack such sequence conservation in their surface glycoproteins. Hence, a broadly effective vaccine in such a context must almost certainly induce TCD8+ cells.
Here, I argue for an integrative approach to understanding the mechanisms of immunological control of viral infections based on similarities between macaque and human genetic associations with such control. The most striking association between an MHC-I allele and control of HIV-1 replication is that of HLA-B27 (Goulder and Watkins, 2008). HLA-B27 presents HIV-1 derived peptides that conform to a very specific sequence motif. Specifically, position 2 of the peptide is nearly always an arginine (R) and position 9 (or the C terminal residue) is most often a leucine (L). Interestingly, position 1 is very frequently an R or a K, similar acidic residues, which forms a di-acidic motif that impacts peptide stability (Herberts et al., 2006). HLA-B27 is also associated with control of HCV (Neumann-Haefelin et al., 2010) and Puumala hantavirus (Mustonen et al., 1998), suggesting a generalized and highly effective mechanism of control of these very divergent viruses. Of note, the MHC-I allele Mamu-B*08, expressed by a subset of rhesus macaques of Indian origin, is associated with very strong control of SIV replication (Loffredo et al., 2007; Mudd et al., 2012a; Mudd et al., 2012b). Mamu-B*08 and HLA-B27 have nearly identical peptide binding preferences (Loffredo et al., 2009). Together, these data suggest that TCD8+ responses that target virus-derived peptides bound by these MHC-I molecules are uniquely potent at restricting viral replication. Intriguingly, primary isolates of hantaviruses induce strikingly similar patterns of disease in macaques that they do in humans, suggesting an ideal animal model for identifying correlates of TCD8+ mediated control of viral replication across multiple viruses and multiple species (human and macaque). One could envision a study wherein macaques that express or do not express Mamu-B*08 are infected with these hantaviruses and their capacity to control the virus compared. Immunological parameters of the antiviral TCD8+ could be compared in a systems approach, including transcriptomics and cytokine production upon antigen encounter. These data could be compared between macaques infected with SIV, Puumala virus and humans with HIV-1 and Puumala virus. Such a dataset could suggest immunological mechanisms of control.
TCD8+ cells are clearly important in control of viral infections but they are often overlooked in the context of vaccines. I would argue that we simply have yet to identify the most critical aspects of an effective TCD8+ response. At the heart of such an examination is an in-depth understanding of the MHC-I allelic repertoire expressed by both humans and the animal models used to model particular infectious diseases and the peptides they produce.
Acknowledgments
This work is dedicated to the memory of Dr. Austin Hughes, who’s work in the evolution of MHC inspired the author to pursue a career studying how MHC diversity impacts immune responses.
BIBLIOGRAPHY
- Budde ML, Greene JM, Chin EN, Ericsen AJ, Scarlotta M, Cain BT, Pham NH, Becker EA, Harris M, Weinfurter JT, O’Connor SL, Piatak M, Jr, Lifson JD, Gostick E, Price DA, Friedrich TC, O’Connor DH. Specific CD8+ T cell responses correlate with control of simian immunodeficiency virus replication in Mauritian cynomolgus macaques. J Virol. 2012;86:7596–7604. doi: 10.1128/JVI.00716-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budde ML, Lhost JJ, Burwitz BJ, Becker EA, Burns CM, O’Connor SL, Karl JA, Wiseman RW, Bimber BN, Zhang GL, Hildebrand W, Brusic V, O’Connor DH. Transcriptionally abundant major histocompatibility complex class I alleles are fundamental to nonhuman primate simian immunodeficiency virus-specific CD8+ T cell responses. J Virol. 2011;85:3250–3261. doi: 10.1128/JVI.02355-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis ZB, Cogswell A, Scott H, Mertsching A, Boucau J, Wambua D, Le Gall S, Planelles V, Campbell KS, Barker E. A Conserved HIV-1-Derived Peptide Presented by HLA-E Renders Infected T-cells Highly Susceptible to Attack by NKG2A/CD94-Bearing Natural Killer Cells. PLoS pathogens. 2016;12:e1005421. doi: 10.1371/journal.ppat.1005421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goulder PJ, Watkins DI. Impact of MHC class I diversity on immune control of immunodeficiency virus replication. Nat Rev Immunol. 2008;8:619–630. doi: 10.1038/nri2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen SG, Wu HL, Burwitz BJ, Hughes CM, Hammond KB, Ventura AB, Reed JS, Gilbride RM, Ainslie E, Morrow DW, Ford JC, Selseth AN, Pathak R, Malouli D, Legasse AW, Axthelm MK, Nelson JA, Gillespie GM, Walters LC, Brackenridge S, Sharpe HR, Lopez CA, Fruh K, Korber BT, McMichael AJ, Gnanakaran S, Sacha JB, Picker LJ. Broadly targeted CD8(+) T cell responses restricted by major histocompatibility complex E. Science. 2016;351:714–720. doi: 10.1126/science.aac9475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herberts CA, Neijssen JJ, de Haan J, Janssen L, Drijfhout JW, Reits EA, Neefjes JJ. Cutting edge: HLA-B27 acquires many N-terminal dibasic peptides: coupling cytosolic peptide stability to antigen presentation. J Immunol. 2006;176:2697–2701. doi: 10.4049/jimmunol.176.5.2697. [DOI] [PubMed] [Google Scholar]
- Horton R, Wilming L, Rand V, Lovering RC, Bruford EA, Khodiyar VK, Lush MJ, Povey S, Talbot CC, Jr, Wright MW, Wain HM, Trowsdale J, Ziegler A, Beck S. Gene map of the extended human MHC. Nat Rev Genet. 2004;5:889–899. doi: 10.1038/nrg1489. [DOI] [PubMed] [Google Scholar]
- Hughes A, Nei M. Pattern of nucleotide substitution at major histocompatibility complex class I loci reveals overdominant selection. Nature. 1988;335:167–170. doi: 10.1038/335167a0. [DOI] [PubMed] [Google Scholar]
- Loffredo J, Maxwell J, Qi Y, Glidden C, Borchardt G, Soma T, Bean A, Beal D, Wilson N, Rehrauer W, Lifson J, Carrington M, Watkins D. Mamu-B*08-positive macaques control simian immunodeficiency virus replication. J Virol. 2007;81:8827–8832. doi: 10.1128/JVI.00895-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loffredo J, Sidney J, Bean A, Beal D, Bardet W, Wahl A, Hawkins O, Piaskowski S, Wilson N, Hildebrand W, Watkins D, Sette A. Two MHC class I molecules associated with elite control of immunodeficiency virus replication, Mamu-B*08 and HLA-B*2705, bind peptides with sequence similarity. J Immunol. 2009;182:7763–7775. doi: 10.4049/jimmunol.0900111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mudd PA, Ericsen AJ, Burwitz BJ, Wilson NA, O’Connor DH, Hughes AL, Watkins DI. Escape from CD8(+) T cell responses in Mamu-B*00801(+) macaques differentiates progressors from elite controllers. J Immunol. 2012a;188:3364–3370. doi: 10.4049/jimmunol.1102470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mudd PA, Martins MA, Ericsen AJ, Tully DC, Power KA, Bean AT, Piaskowski SM, Duan L, Seese A, Gladden AD, Weisgrau KL, Furlott JR, Kim YI, Veloso de Santana MG, Rakasz E, Capuano S, 3rd, Wilson NA, Bonaldo MC, Galler R, Allison DB, Piatak M, Jr, Haase AT, Lifson JD, Allen TM, Watkins DI. Vaccine-induced CD8+ T cells control AIDS virus replication. Nature. 2012b;491:129–133. doi: 10.1038/nature11443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustonen J, Partanen J, Kanerva M, Pietila K, Vapalahti O, Pasternack A, Vaheri A. Association of HLA B27 with benign clinical course of nephropathia epidemica caused by Puumala hantavirus. Scand J Immunol. 1998;47:277–279. doi: 10.1046/j.1365-3083.1998.00302.x. [DOI] [PubMed] [Google Scholar]
- Muyanja E, Ssemaganda A, Ngauv P, Cubas R, Perrin H, Srinivasan D, Canderan G, Lawson B, Kopycinski J, Graham AS, Rowe DK, Smith MJ, Isern S, Michael S, Silvestri G, Vanderford TH, Castro E, Pantaleo G, Singer J, Gillmour J, Kiwanuka N, Nanvubya A, Schmidt C, Birungi J, Cox J, Haddad EK, Kaleebu P, Fast P, Sekaly RP, Trautmann L. Immune activation alters cellular and humoral responses to yellow fever 17D vaccine. J Clin Invest. 2014;124:3147–3158. doi: 10.1172/JCI75429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann-Haefelin C, Timm J, Schmidt J, Kersting N, Fitzmaurice K, Oniangue-Ndza C, Kemper MN, Humphreys I, McKiernan S, Kelleher D, Lohmann V, Bowness P, Huzly D, Rosen HR, Kim AY, Lauer GM, Allen TM, Barnes E, Roggendorf M, Blum HE, Thimme R. Protective effect of human leukocyte antigen B27 in hepatitis C virus infection requires the presence of a genotype-specific immunodominant CD8+ T-cell epitope. Hepatology. 2010;51:54–62. doi: 10.1002/hep.23275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor SL, Lhost JJ, Becker EA, Detmer AM, Johnson RC, Macnair CE, Wiseman RW, Karl JA, Greene JM, Burwitz BJ, Bimber BN, Lank SM, Tuscher JJ, Mee ET, Rose NJ, Desrosiers RC, Hughes AL, Friedrich TC, Carrington M, O’Connor DH. MHC heterozygote advantage in simian immunodeficiency virus-infected Mauritian cynomolgus macaques. Science translational medicine. 2010;2:22ra18. doi: 10.1126/scitranslmed.3000524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otting N, Heijmans C, Noort R, de Groot N, Doxiadis G, van Rood J, Watkins D, Bontrop R. Unparalleled complexity of the MHC class I region in rhesus macaques. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:1626–1631. doi: 10.1073/pnas.0409084102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietra G, Romagnani C, Manzini C, Moretta L, Mingari MC. The emerging role of HLA-E-restricted CD8+ T lymphocytes in the adaptive immune response to pathogens and tumors. J Biomed Biotechnol. 2010;2010:907092. doi: 10.1155/2010/907092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Querec T, Akondy R, Lee E, Cao W, Nakaya H, Teuwen D, Pirani A, Gernert K, Deng J, Marzolf B, Kennedy K, Wu H, Bennouna S, Oluoch H, Miller J, Vencio R, Mulligan M, Aderem A, Ahmed R, Pulendran B. Systems biology approach predicts immunogenicity of the yellow fever vaccine in humans. Nat Immunol. 2009;10:116–125. doi: 10.1038/ni.1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhesus Macaque Genome S, Analysis C, Gibbs RA, Rogers J, Katze MG, Bumgarner R, Weinstock GM, Mardis ER, Remington KA, Strausberg RL, Venter JC, Wilson RK, Batzer MA, Bustamante CD, Eichler EE, Hahn MW, Hardison RC, Makova KD, Miller W, Milosavljevic A, Palermo RE, Siepel A, Sikela JM, Attaway T, Bell S, Bernard KE, Buhay CJ, Chandrabose MN, Dao M, Davis C, Delehaunty KD, Ding Y, Dinh HH, Dugan-Rocha S, Fulton LA, Gabisi RA, Garner TT, Godfrey J, Hawes AC, Hernandez J, Hines S, Holder M, Hume J, Jhangiani SN, Joshi V, Khan ZM, Kirkness EF, Cree A, Fowler RG, Lee S, Lewis LR, Li Z, Liu YS, Moore SM, Muzny D, Nazareth LV, Ngo DN, Okwuonu GO, Pai G, Parker D, Paul HA, Pfannkoch C, Pohl CS, Rogers YH, Ruiz SJ, Sabo A, Santibanez J, Schneider BW, Smith SM, Sodergren E, Svatek AF, Utterback TR, Vattathil S, Warren W, White CS, Chinwalla AT, Feng Y, Halpern AL, Hillier LW, Huang X, Minx P, Nelson JO, Pepin KH, Qin X, Sutton GG, Venter E, Walenz BP, Wallis JW, Worley KC, Yang SP, Jones SM, Marra MA, Rocchi M, Schein JE, Baertsch R, Clarke L, Csuros M, Glasscock J, Harris RA, Havlak P, Jackson AR, Jiang H, Liu Y, Messina DN, Shen Y, Song HX, Wylie T, Zhang L, Birney E, Han K, Konkel MK, Lee J, Smit AF, Ullmer B, Wang H, Xing J, Burhans R, Cheng Z, Karro JE, Ma J, Raney B, She X, Cox MJ, Demuth JP, Dumas LJ, Han SG, Hopkins J, Karimpour-Fard A, Kim YH, Pollack JR, Vinar T, Addo-Quaye C, Degenhardt J, Denby A, Hubisz MJ, Indap A, Kosiol C, Lahn BT, Lawson HA, Marklein A, Nielsen R, Vallender EJ, Clark AG, Ferguson B, Hernandez RD, Hirani K, Kehrer-Sawatzki H, Kolb J, Patil S, Pu LL, Ren Y, Smith DG, Wheeler DA, Schenck I, Ball EV, Chen R, Cooper DN, Giardine B, Hsu F, Kent WJ, Lesk A, Nelson DL, O’Brien W E, Prufer K, Stenson PD, Wallace JC, Ke H, Liu XM, Wang P, Xiang AP, Yang F, Barber GP, Haussler D, Karolchik D, Kern AD, Kuhn RM, Smith KE, Zwieg AS. Evolutionary and biomedical insights from the rhesus macaque genome. Science. 2007;316:222–234. doi: 10.1126/science.1139247. [DOI] [PubMed] [Google Scholar]
- Scotto G, Fazio V, D’Alessandro G, Monno L, Saracino A, Palumbo E, Angarano G. Association between HLA class II antigens and hepatitis C virus infection. J Biol Regul Homeost Agents. 2003;17:316–321. [PubMed] [Google Scholar]
- Shiina T, Hosomichi K, Inoko H, Kulski JK. The HLA genomic loci map: expression, interaction, diversity and disease. J Hum Genet. 2009;54:15–39. doi: 10.1038/jhg.2008.5. [DOI] [PubMed] [Google Scholar]
- Torresilla C, Larocque E, Landry S, Halin M, Coulombe Y, Masson J, Mesnard J, Barbeau B. Detection of the HIV-1 minus strand-encoded Antisense Protein and its association with autophagy. J Virol. 2013 doi: 10.1128/JVI.00225-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Meijgaarden KE, Haks MC, Caccamo N, Dieli F, Ottenhoff TH, Joosten SA. Human CD8+ T-cells recognizing peptides from Mycobacterium tuberculosis (Mtb) presented by HLA-E have an unorthodox Th2-like, multifunctional, Mtb inhibitory phenotype and represent a novel human T-cell subset. PLoS pathogens. 2015;11:e1004671. doi: 10.1371/journal.ppat.1004671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieten RW, Jonker EF, van Leeuwen EM, Remmerswaal EB, Ten Berge IJ, de Visser AW, van Genderen PJ, Goorhuis A, Visser LG, Grobusch MP, de Bree GJ. A Single 17D Yellow Fever Vaccination Provides Lifelong Immunity; Characterization of Yellow-Fever-Specific Neutralizing Antibody and T-Cell Responses after Vaccination. PloS one. 2016;11:e0149871. doi: 10.1371/journal.pone.0149871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yant L, Friedrich T, Johnson R, May G, Maness N, Enz A, Lifson J, O’Connor D, Carrington M, Watkins D. The high-frequency major histocompatibility complex class I allele Mamu-B*17 is associated with control of simian immunodeficiency virus SIVmac239 replication. J Virol. 2006;80:5074–5077. doi: 10.1128/JVI.80.10.5074-5077.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]


