Abstract
Background: Cognitive impairment associated with childhood malnutrition and stunting is generally considered irreversible.
Objective: The aim was to test a new nutritional supplement for the prevention and treatment of moderate-acute malnutrition (MAM) focused on enhancing cognitive performance.
Methods: An 11-wk, village-randomized, controlled pilot trial was conducted in 78 children aged 1–3 or 5–7 y living in villages in Guinea-Bissau. The supplement contained 291 kcal/d for young children and 350 kcal/d for older children and included 5 nutrients and 2 flavan-3-ol–rich ingredients not present in current food-based recommendations for MAM. Local bakers prepared the supplement from a combination of locally sourced items and an imported mix of ingredients, and it was administered by community health workers 5 d/wk. The primary outcome was executive function abilities at 11 wk. Secondary outcomes included additional cognitive measures and changes in z scores for weight (weight-for-age) and height (height-for-age) and hemoglobin concentrations at 11 wk. An index of cerebral blood flow (CBF) was also measured at 11 wk to explore the use of this measurement as a biological index of cognitive impairment.
Results: There were no significant differences in any outcome between groups at baseline. There was a beneficial effect of random assignment to the supplement group on working memory at 11 wk in children aged 1–3 y (P < 0.05). This difference contrasted with no effect in older children and was not associated with faster growth rate. In addition, CBF correlated with task-switching performance (P < 0.05).
Conclusions: These preliminary data suggest that cognitive impairment can be monitored with measurement of CBF. In addition, the findings provide preliminary data that suggest that it may be possible to improve poor cognitive performance in young children through changes in the nutritional formulation of supplementary foods used to prevent and treat MAM. Powered studies of the new supplement formulation are needed. This trial was registered at clinicaltrials.gov as NCT03017209.
Keywords: stunting, moderate-acute malnutrition (MAM), Guinea-Bissau, ready-to-use supplementary food, blended fortified foods, cognition, weight-for-age z score, height-for-age z score, cerebral blood flow, near-infrared spectroscopy
Introduction
Malnutrition and stunting occurring in early childhood are associated with a range of cognitive impairments, including deficits in executive functioning (e.g., attention span, working memory), which lead to poor school performance in later childhood (1–3). These functional problems can be traced to a range of structural and neurochemical deficiencies, including glial and dendritic development in areas of the brain that include the prefrontal cortex and hippocampal structures (4, 5). Moreover, interventions designed to prevent or treat malnutrition generally find little or no improvement in cognitive measures when administered solely as a nutrition supplement without other intervention components such as medical treatment or social enrichment (4, 6–9), which has led to the perception that the effects of childhood malnutrition on cognition are largely irreversible (10).
An alternative possible explanation for the long-term impairment in cognition associated with stunting and malnutrition is that recovery with food-based treatments is possible but that typical foods used for this purpose are not yet nutritionally optimal. Current recommendations for ready-to-use supplementary foods (RUSFs) and fortified blended foods (FBFs) do not include several nutrients that research in other populations has indicated are important for cognitive health. For example, choline is recognized as an essential nutrient required for synthesis of the neurotransmitter acetylcholine and maintenance of cell membranes (11), and low concentrations of dietary choline reduce brain volume in animal models, such as young pigs (12). In addition, stunting in early childhood is recognized to be associated with impaired cognition (13) and is also associated with low circulating choline (14). Nevertheless, this type of evidence does not meet high-quality standards, and choline is not currently included in WHO recommendations for the prevention and treatment of malnutrition. The trace elements chromium and molybdenum are also not included in current WHO requirements, and although there is little evidence for any effect on cognition in children, deficiency states in elderly and young adults have been suggested to be linked to impaired cognition (15–17). The omega-3 PUFAs EPA and DHA, and their relation to ω-6 FAs, are also not included in current recommendations for RUSFs and FBFs. However, ω-3 FAs are important components of neuronal membranes, and although their effects appear to be limited in healthy infants born at term (18), some studies in children and the elderly indicate that they may have broad cognitive benefits in nutritionally challenged populations, including in the promotion of neuronal growth and influencing signal processing and neural transmission (19–21).
There are also foods that contain classes of bioactive chemicals that are not currently categorized as essential nutrients but which cross the blood-brain barrier and have neuropsychological effects that may be beneficial for cognitive repair in the prevention and treatment of malnutrition (22–25). In particular, cocoa and green tea contain a class of polyphenols with an array of bioactions, including antioxidation, anti-inflammation, and glucoregulation, which have been documented to promote neurogenesis, reduce neuronal injury, and increase vasodilation and cerebral blood flow (CBF) in nonmalnourished individuals (26). To our knowledge, there have been no studies to date examining whether the consumption of cocoa or green tea could promote the recovery of cognitive damage resulting from childhood malnutrition, but the accumulating evidence from other populations justifies studies that explore their effectiveness. Cocoa and green tea also contain caffeine, which we hypothesized might have synergistic benefits for cognitive protection during treatment of malnutrition due to its recognized neurocognitive stimulatory effects (27).
We designed a nutritional formulation to improve cognitive function during nutrition interventions to prevent and treat stunting and moderate-acute malnutrition (MAM), on the basis of the considerations described above, and conducted a pilot study to obtain data for power calculations for a future powered trial on its impact on cognitive performance and growth. The work was conducted in village children in Guinea-Bissau, West Africa.
Methods
Study location and participants
Guinea-Bissau is a small, low-income country in West Africa with a population of 1.7 million. Families living in rural villages cultivate most of the food they eat, including rice (the staple food), millet, corn, sorghum, groundnuts, cassava, sweet potatoes, mangoes, and domestic animals. In addition, they catch wild foods, including fish and small mammals, and grow cashews to sell for additional rice and other popular foods, including sugar, oil, and bread.
The study was conducted in 2 Mandinka tribe villages in the Oio district of Guinea-Bissau, which is one of the poorest regions of the country with very high rates of stunting and MAM (28). The villages were a convenience sample that were comparable in terms of number of inhabitants, ethnicity, religion, and interest in study participation and were also sufficiently large to make study recruitment feasible. Inclusion criteria were as follows: parents were willing to have their child participate (no child refused) and the child was reported to be 2–3 y of age (20 children/village) or 6–7 y of age (20 children/village). MAM was not an inclusion criterion out of concern that the supplement could benefit children with nonobvious nutritional deficiencies (e.g., iron) and because in a previous study we found that enrolling only malnourished children resulted in nonadherence to an assessment-only control regimen (29). An additional inclusion criterion for the older age group was that the child was enrolled in first grade in the village elementary school. Exclusion criteria were as follows: if the child had ≥1 food allergy, if the child would not be in the village for the duration of the study, or if the child had severe acute malnutrition, as indicated by a midupper arm circumference (MUAC) in the red zone of a tape measure (in which case the parents were advised to take their child to the nearest tertiary clinic). Children were enrolled in the study on the basis of parental report of age. However, when parents presented participants' birth certificates at the end of the study, some children were found to be outside the original age range criteria. Children's ages were determined to be 1–3 and 5–7 y, and these data were retained in analyses with institutional review board (IRB) permission.
IRB permission to conduct the study and all of the measurements was provided by the National Committee of Ethics in Health, which is the relevant sub-body of the government of Guinea-Bissau, and informed consent was obtained in the local language. In addition, Tufts University provided permission for all measurements, except for near-infrared spectroscopy (NIRS); NIRS was approved as a substudy by the Massachusetts General Hospital IRB. A post hoc agreement was implemented to share de-identified data between the 2 US institutions. After an explanation of the study protocol, all mothers or legal guardians of children agreed to participate and provided their informed consent with a signature or thumbprint. The consent process took place in the presence of a member of the research team and a community health worker. Participating families received an allotment of rice as an expression of appreciation for their participation.
Study protocol
This 11-wk pilot study (registered at clinicaltrials.gov as NCT03017209) was a village-randomized controlled study comparing supplementation to an assessment-only control in 2 villages. Two age groups, 1–3 and 5–7 y, were studied (participant demographic information is reported in Table 1) and we hypothesized significant effects of the supplement on cognitive function in both age groups. The supplement was provided 5 d/wk for 11 wk to intervention children in the village community health center. The mothers or primary caretakers brought children to the community center and children were watched by the community health workers while they ate their food. If any supplement was not consumed, the consumed amount was recorded and the child was sent home with the remainder for use later in the day. Children who did not come to the community center were marked absent and received no supplement that day. The primary outcome was children's executive function abilities (working memory, reverse categorization) and secondary outcomes included growth, hemoglobin, and skin carotenoids. In addition, an index of CBF (CBFi) and an index of tissue absorption (μai) was measured at 12 wk by using an NIRS method called diffuse correlation spectroscopy (DCS) (30) to evaluate NIRS as a method potentially suitable for the assessment of cognitive function in large-population studies.
TABLE 1.
Summary statistics for anthropometric measures, skin carotenoids, grip strength, and hemoglobin at baseline and at 11 wk in children enrolled in the intervention and control sites1
| Intervention | Control | ||||||
|---|---|---|---|---|---|---|---|
| Baseline | 11 wk | Δ | Baseline | 11 wk | Δ | P | |
| Age 1–3 y | |||||||
| n | 20 | 18 | 18 | 18 | 17 | 17 | |
| Age, y | 2.4 ± 0.4 | 2.2 ± 0.0.4 | 0.1166 | ||||
| WAZ | −1.43 ± 0.94 | −1.30 ± 0.99 | 0.01 ± 0.35 | −1.20 ± 0.87 | −1.27 ± 0.86 | −0.07 ± 0.30 | 0.3965 |
| HAZ | −2.08 ± 1.37 | −2.03 ± 1.04 | 0.04 ± 0.70 | −1.80 ± 0.87 | −1.89 ± 0.81 | −0.07 ± 0.30 | 0.9746 |
| WHZ | −0.36 ± 0.81 | −1.30 ± 0.99 | −0.02 ± 0.61 | −0.29 ± 0.69 | −0.29 ± 0.77 | −0.03 ± 0.55 | 0.6155 |
| Arm circumference, cm | 14.93 ± 1.12 | 15.05 ± 1.11 | 0.13 ± 0.67 | 14.81 ± 1.14 | 15.09 ± 1.07 | 0.20 ± 0.48 | 0.7507 |
| Head circumference, cm | 47.04 ± 1.73 | 47.59 ± 1.53 | 0.35 ± 1.25 | 47.52 ± 1.94 | 47.46 ± 1.83 | −0.03 ± 0.79 | 0.6410 |
| Hemoglobin, g/dL | — | 12.29 ± 0.92 | — | — | 11.96 ± 0.72 | — | 0.3755 |
| Skin carotenoid content, Raman counts × 103 | 24.6 ± 7.2 (n = 15) | 30.7 ± 8.9 (n = 15) | 6.1 ± 8.4 (n = 15) | 34.8 ± 6.7 (n = 15) | 38.1 ± 10.6 (n = 14) | 3.4 ± 12.4 (n = 14) | 0.4697 |
| Age 5–7 y | |||||||
| n | 20 | 19 | 19 | 20 | 20 | 20 | |
| Age, y | 5.9 ± 0.3 | 6.1 ± 0.6 | 0.095 | ||||
| WAZ | −1.78 ± 0.92 | −1.77 ± 0.94 | 0.05 ± 0.24 | −1.28 ± 1.35 | −1.23 ± 1.05 | 0.05 ± 0.31 | 0.7255 |
| HAZ | −1.74 ± 0.99 | −1.72 ± 0.95 | 0.05 ± 0.13 | −1.13 ± 0.99 | −1.11 ± 1.31 | 0.02 ± 0.10 | 0.9708 |
| BMI z score | −0.96 ± 0.84 (n = 19) | −0.94 ± 0.80 | 0.02 ± 0.46 | −0.79 ± 0.76 | −0.74 ± 0.65 | 0.05 ± 0.48 | 0.3813 |
| Arm circumference, cm | 15.44 ± 1.30 | 15.30 ± 1.11 | −0.15 ± 0.57 | 15.78 ± 0.84 | 16.02 ± 0.92 | 0.24 ± 0.57 | 0.0280 |
| Head circumference, cm | 50.0 ± 1.38 (n = 17) | 49.58 ± 1.42 (n = 20) | −0.35 ± 0.74 (n = 17) | 50.13 ± 1.47 (n = 19) | 49.86 ± 1.56 (n = 19) | −0.20 ± 0.77 (n = 19) | 0.6410 |
| Hemoglobin, g/dL | — | 12.45 ± 0.87 | — | — | 12.49 ± 0.63 | — | 0.882 |
| Skin carotenoid content, Raman counts × 103 | 32.6 ± 12.1 | 35.8 ± 9.1 | 3.2 ± 10.2 | 37.4 ± 8.0 | 39.8 ± 8.4 | 2.4 ± 9.1 | 0.5227 |
Values are means ± SDs unless otherwise indicated. P values were adjusted for age in years and baseline values (change over time where available; otherwise, cross-sectional at 11 wk). HAZ, height-for-age z score; WAZ, weight-for-age z score; WHZ, weight-for-height z score.
Baseline assessments included demographic information, cognitive variables, anthropometric measurements (weight, height, head circumference, and MUAC), and skin carotenoids in all of the children. After baseline, villages were assigned a number and their treatment (intervention or control) was based on a randomization schema generated in SAS version 9.3 (SAS Institute). The villagers who were randomly assigned to the intervention group received the new supplement at the community health center 5 mornings/wk. Community health center workers distributed the supplement and tracked attendance and supplement consumption after receiving training by the research staff from the International Partnership for Human Development and the US team. When a child's caregiver was routinely someone other than the mother, that caregiver was allowed to bring the child for supplement distribution. Outcome assessments obtained at baseline were repeated at the end of the study period before the supplement distribution was completed. In addition, measurements of CBFi and tissue absorption with NIRS-DCS were only performed at the end of the study.
With regard to brain blood-flow assessments, we hypothesized that the measure of CBFi and µai by NIRS-DCS would provide an assessment of the level of neuronal maturation. Oxygen, delivered to the brain through the blood, is consumed by oxidative glucose metabolism to produce ATP, which provides energy to the neurons. Increases in neuronal activity cause increases in cerebral metabolism, which are associated with increases in blood volume and blood flow (so-called neurovascular coupling). The coupling between neuronal activity, cerebral oxygen metabolism, and CBF is present during functional activity, the resting state, and brain developmental maturation. In particular, the cerebral metabolic rate of oxygen (CMRO2) and the cerebral metabolic rate of glucose (CMRGlc) are known to increase with age in children and these increases match behavioral, neurophysiologic, and anatomic maturation that occur during development (31–33). Although these changes have been shown with positron emission tomography (PET), we recently replicated these findings in infants noninvasively with NIRS-DCS. For example, in premature infants we showed that CBF increases with postmenstrual age (34) and is higher in brain regions that are known to mature earlier (35). We also found that cerebral blood volume increases 3-fold during the first year of life across frontal, temporal, and parietal regions (36), which is consistent with increases in glucose metabolism observed with PET (37).
The supplementary food
The supplement formulation used in this study was designed as a substitute for typical RUSFs and FBFs, which are used in a variety of ways in low-income countries, including in supplementary feeding programs for mothers and children to prevent growth faltering, for community treatment of MAM, and in school feeding programs (38, 39). Due to practical considerations, the supplementary nutrients were delivered in a combination of a locally prepared biscuit and chewable micronutrient gummies (Children's MV-Alive; Nature's Way Products LLC; Vitamin Friend's Iron; Vitamin Friends LLC), but the future use of the formulation could include combinations of the ingredients and nutrients into a single mix or ready-to-use product. The general goals of the new supplement were as follows: 1) to increase anticipated nutrient intakes from home food to levels recommended by the WHO (18), 2) to increase nutrient intakes recommended by the Institute of Medicine for those nutrients that are not recommended by the WHO (see reference 19), and 3) to include cocoa and green tea as ingredients for their flavan-3-ol (and caffeine) contents. Specifically, the new nutrients included the following: choline provided at 50% adequacy due to the difficulty of incorporating the total recommended intake into a single food serving (11); fish oil containing the essential ω-3 PUFAs EPA and DHA (19); 2 additional micronutrients (molybdenum and chromium) that have been associated with reduced cognition in both elderly and younger adults (15–17) and are defined as essential nutrients in US national requirements for healthy children (40); high flavan-3-ol sources of cocoa and green tea (26); 50% greater amounts of vitamin K than WHO recommendations (41); and calcium amounts naturally existing in ingredients without additional fortification to facilitate iron absorption, taking into consideration both the negative effect of high amounts of calcium on nonheme iron absorption and the potential for added polyphenols to reduce absorption (42).
The biscuit was designed to provide 300 kcal/d for the older children and 250 kcal/d for the younger children and was prepared locally from a combination of the mix of new ingredients and the locally available ingredients peanuts, blended vegetable oil, honey, sugar, eggs, and dried powdered moringa (Moringa oleifera). The calculated macronutrient composition of the biscuit as formulated is given in Table 2. Village bakers were trained by the research staff to prepare the biscuits with the use of hygienic techniques, to keep records of production, and to deliver the baked goods to community health centers for distribution; quality control worked well except for occasional batches that were overcooked. Community health workers distributed the supplement in the morning, made records of attendance, observed consumption, and recorded amounts consumed. Both community health workers and bakers received a small stipend for their work. Oversight of quality control for supplement preparation and study recordkeeping was conducted in twice-weekly visits by a local study coordinator.
TABLE 2.
Daily macronutrient and essential FAs provided in the locally prepared biscuit supplement1
| A ge group | ||
|---|---|---|
| Nutrients | 1–3 y | 5–7 y |
| Energy, kcal | 291 | 350 |
| Protein, % of energy | 16.8 | 16.7 |
| Carbohydrate, % of energy | 29.4 | 28.6 |
| Fat, % of energy | 48.4 | 48.7 |
| EPA, mg | 309 | 309 |
| DHA, mg | 209 | 209 |
| Fiber, g | 2.0 | 2.3 |
The supplement was combined with a multivitamin gummy as described in Methods to meet the anticipated nutrient shortfalls in a typical home diet relative to WHO (18) and Institute of Medicine (19) nutrient targets, except for choline, which was provided at 50% adequacy, and calcium, which was not supplemented to promote absorption of other divalent cations such as iron.
Cognitive assessments
The cognitive assessments included 2 measures of executive functioning in early childhood: working memory and task-switching abilities. Specific tasks were chosen on the basis of their potential to be culturally adapted to the local villages, to be reliably conducted by the local research team, to require minimal instructions to the children, and to fit within a 15-min testing time frame (given the expected time needed for the other study measures and uncertainty of how long individual children in this specific setting would be interested in participating in the assessments).
The assessments were administered in a quiet room in the local village by a member of the local research team who had been trained by the senior researcher who designed the tests (PM) and followed a script designed by PM for all test implementation. Tasks were administered in the local language, either solely by the local research team or with an additional translator who repeated the local researchers' instructions and who was instructed not to interfere or provide additional task directions to the children. Children could participate alone or sit on the knee of their mother or a caregiver, as they preferred. Any other adults present were seated behind the child, were not told of the specific behaviors or measures of interest for each task, and were instructed to not help or provide answers to the child. If a child became fussy or upset, the individual task or entire assessment was stopped; caregivers were also instructed that they could stop the assessments at any time. All of the sessions were video-recorded for later blinded coding by coders who were unaware of participant randomization. Sessions were also reviewed by experienced test administrators for protocol adherence.
Working memory was assessed in both young and older children with the Spin the Pots task (43), and age modifications were guided by Hostinar et al. (44). Children were presented with an array of small opaque cups, each covered by a lid with a distinct color, pattern, or both, which were all placed on a circular base platform. At the start of the task, children were shown that the lids could be removed and that stickers could be hidden inside each cup. The test administrator hid stickers in a subset of the total number of cups (young children: 4 of 6 total cups; older children: 8 of 10 total cups) and then covered the entire array with an opaque cover. The test administrator then lifted the cover and allowed the child to search for a sticker. If the child found a sticker, the child could keep the sticker; if they did not find a sticker, the test administrator let the child see the bottom of the empty cup and told them that there was not a sticker there. The circular base was then covered and rotated 180°. The cover was then removed and the child was allowed to search again. This procedure continued until the child found all of the hidden stickers or until he or she reached a predetermined set of trials (young children: 12 trials; older children: 18 trials). Children's performance was assessed by the total number of stickers found as well as a categorical assessment of whether children found all of the hidden stickers.
Task-switching was assessed through a Reverse Categorization task modeled after Carlson et al. (45). The 1- to 3-y-old children were presented with a basket full of toy animals and told that the basket included both “mommy” animals and “baby” animals. They were then shown 2 boxes: 1 box had an image of an adult animal on the front of the box and the other box had an image of a baby animal on the front of the box. The test administrator directed the child's attention to the front of the boxes and labeled them the “mommy” and “baby” boxes. The test administrator then told the children that they were going to play a game in which they put “baby” animals in the “baby” box and “mommy” animals in the “mommy” box. The test administrator then demonstrated accurate sorting with 6 animals (3 mommies, 3 babies) during a pre-switch phase. The test administrator instructed the child, “This is a [baby/mommy] animal, so it goes in the [baby/mommy] box.” The child was then allowed to sort the next 6 animals (3 mommies, 3 babies). For each animal, the test administrator labeled the animal (“baby” or “mommy”) and asked the child where the animal should go. Children were praised for accurate sorting or corrected with a restatement of the rule for each of the 6 trials. Children were next told that they were now going to play a “silly game” in which they put all of the “mommy” animals in the “baby” box and all of the “baby” animals in the “mommy” box. During this post-switch phase, children were then allowed to sort 12 additional animals (6 mommies, 6 babies). The test administrator labeled the size and restated the rule for each animal; however, they did not correct inaccurate sorting. The procedure was similar for the 6-y-old children, with the following exceptions. During the pre-switch phase, the test administrator labeled the size of only the first 2 animals (e.g., mommy cow), subsequently only labeling the kind of animal (e.g., cow) for the remaining 4 animals. During the post-switch phase, the test administrator labeled only the kind, but not the size, of the animal. He also only reminded the children of the rule on the first and seventh trial. Similar to the 1- to 3-y-old children, 4- to 6-y-old children were corrected for errors during the pre-switch but not the post-switch phases. We coded the total number of accurate sorts during the post-study phase.
Two additional cognitive assessments were also conducted but are not reported or discussed here. An object-directed manual exploration task, which was conducted only in 2-y-old children, was designed to assess children's rate of habituation and novelty preferences and involved free play with a series of small animal toys. Review of the videotaped procedures showed that the test administrator had difficulty in adhering to the study protocol; therefore, the assessment was deemed ineligible for data analysis. A delay-of-gratification test, which was conducted only in the 6-y-old children, involved measuring how long children could wait to play with a novel set of toy cars and rollercoaster. Because almost none of the children were willing to wait any length of time, there was too little variability to assess differences between or changes within conditions. Both of these tasks occurred as the final cognitive assessments for the respective age groups, and thus could have no impact on the earlier cognitive assessments.
Anthropometric and biochemical assessments
Outcome assessments were performed by a group of trained medical or nursing staff from the Guinea-Bissau Ministry of Health who had no role in study design or supplement distribution. Nonfasting weight was measured at baseline and at 11 wk by using a digital calibrated scale that was accurate to ±0.1 kg (floor scale model 813; Seca). Height was assessed by using an upright stadiometer accurate to 0.1 cm (model 213; Seca). MUAC was taken at the midpoint between the acromion of the scapula and olecranon with a paper tape by using standardized methods (46).
Hemoglobin was measured in duplicate by pulse co-oximetry, a noninvasive technique validated for anemia screening (27) that uses a multiple-wavelength spectrophotometric sensor situated in a comfortable finger clip (Pronto-7; Masimo Corp.). Due to technical challenges in the field, the hemoglobin measurements could not be collected at baseline, so only 11-wk data are available.
Skin carotenoid content was measured in duplicate in the palm of each hand by resonance Raman spectroscopy (47, 48) to provide an index that could potentially serve as an independent marker of supplement adherence (because the supplement contained carotenoids). Measurements were made in the palm of the hand because the carotenoid concentration is high and differences in pigmentation among various skin types are minimal at this location, and the stratum corneum thickness of the palm (∼400 μm) is high compared with other skin sites. Measurements were made with a NuSkin scanner (Pharmanex Global Research), which uses a laser power of <10 mW and an exposure time of 30 s/measurement with an elliptical spot size of 2 × 3 mm.
CBF by NIRS
To assess cerebral hemodynamics, we used NIRS-DCS. DCS uses near-infrared light and, in addition to quantifying µai by measuring light attenuation at 785 nm, it also quantifies CBF (CBFi) by measuring the temporal fluctuations of the light speckle pattern generated by the dynamic scattering of RBCs (49, 50). Numerous studies in humans and in animals have shown that the CBFi agrees very well with CBF values measured with gold-standard methods (30, 51, 52). By simultaneously measuring both cerebral hemoglobin oxygenation (SO2) and CBFi in previous studies in infants we showed that CBFi is more tightly correlated with cerebral oxygen metabolism than SO2 (53). To measure both variables, a combination of 2 NIRS devices is needed. For this pilot study, we only had room for 1 device and chose the DCS, because CBF is a superior indicator of brain development (34, 35, 54) and brain health (55) compared with SO2.
For this study, we used a custom DCS device built at the Martinos Center at the Massachusetts General Hospital. The system includes 4 photon-counting avalanche photodiode detectors, 1 long coherence length laser at 785 nm, and a custom field-programmable gate array–based software correlator. Acquisition rate was set at 2 Hz per data point. Custom fiberoptics monitors were used to measure the children enrolled in the study. The light power at the probe was ∼20 mW and diffused over a 5-mm-diameter spot. This was well under American National Standards Institute limits for laser light exposure. Light was detected at 0.5-, 1.5-, 2-, and 2.5-cm distances of separation from the source. Because of the low light level detected at the largest distance, only 2 separations (1.5 and 2.0 cm) were used to calculate µai (see reference 56, Equation 2) and 1 separation (2 cm) was used to calculate CBFi (57). In particular, to obtain the blood flow index, we fit Equation 7 of Roche-Labarbe et al. (57) to the measured temporal autocorrelation functions at 2 cm. The short-separation (0.5 cm) DCS data were also analyzed and used to discriminate extracerebral blood flow (scalp BFi) from CBFi. A scattering coefficient of 5 cm−1 was assumed in all groups to calculate CBFi and μai (58). An average of 20 data points over the 10 s of acquisition was sufficient to eliminate noise from the intensity and autocorrelation curves. For these measurements, children were asked to sit still in a chair in front of a study staff member, in a room with low light, and to remain still for the whole examination period (1–2 min). Measurements consisted of positioning the optical monitor on the upper forehead of the participant and keeping it in place by hand for 20 s during data recording. Measurements were acquired in the left and right forehead corresponding to FP1 and FP2 in the electroencephalography 10–20 system, and if the child moved or the detected light signal was low measurements were repeated one more time. No pressure was applied to the monitor, which was sanitized between participants (59).
Data analyses
This was a pilot study designed to generate data for power calculations for a future powered trial, and the primary hypothesis was that random assignment to receive the new supplement would result in beneficial effects on cognition in both age groups. Participants with missing or implausible values were excluded from analyses. z Scores for weight-for-age, height-for-age, and weight-for-height were calculated with macros based on WHO child growth standards without any correction for height compared with length in children aged <2 y (60). All of the analyses were stratified by age group (young and older categories). Differences in baseline variables between the 2 supplement groups and control group were compared by using chi-square test for categorical variables; Student's t test for normally distributed, continuous variables; and Wilcoxon's Signed Rank test for non-normal continuous variables. Primary analyses were intention-to-treat based on the initial study group randomization. ANCOVA models adjusting for age and baseline values were used to compare change from baseline between the groups. Analyses were performed by using SAS version 9.3 (SAS Institute), and significance for all variables was set at a 2-sided P value of 0.05.
An independent coder, blinded to experimental condition, coded children's performance on the cognitive tests at the baseline and outcome testing points as well as any instances of experimental error or interference. A second coder, also blinded to experimental condition, coded one-third of the data set; interrater reliability was high (r > 0.9). Most of the participant data for baseline values in the 2-y-old children were deemed ineligible and are therefore not provided here. Some of these tests were excluded during coding because the test administrator could not speak the local language and the use of village interpreters resulted in some occasions in which the interpreter may have prompted the correct result (n = 10). In other cases, the 2-y-old children refused to participate or complete testing for the cognitive tasks (n = 27). In contrast, there were minimal data collection concerns with the 6-y-old children. We omitted 5 children from baseline analyses due to experimenter error and 1 child from baseline analyses due to potential translator interference. When tests were repeated at 11 wk, the test administrator was more experienced working with the 2-y-old children and in addition worked with a single trained interpreter. This allowed for data collection of acceptable quality for outcomes for most children. Therefore, in the analyses below, we report only comparisons between the intervention and control groups in the younger and older age groups at 11 wk of follow-up. An age-adjusted Poisson regression model was used to compare the number of stickers found between groups in separate models for younger and older children and for the number of correctly sorted animals in older children. An age-adjusted logic regression model was used to compare the proportion of children who found all the stickers.
Results
Baseline and change data for child anthropometric measures and skin carotenoid content and hemoglobin at 11 wk are shown in Table 1. None of the children dropped out of the study, but some did not come to the final outcome assessment and supplement attendance was 84% of possible days, with most children consuming most of the supplement under study observation. There were no significant differences between groups for any of the baseline variables, and both groups of children were undernourished as shown by negative mean z scores for all anthropometric variables. Mean values for z scores tended to be somewhat worse in the intervention group. Mean changes were not different in the intervention group compared with the control, except for a slightly (0.1 cm) greater arm circumference effect in the control subset of the older age group. It should be emphasized that the study was a pilot study and was underpowered to detect significant differences between groups.
Table 3 summarizes the cognitive data from both age groups. Results showed that, for the younger age group, random assignment to the supplemented group was associated with a large positive effect on children's working memory. In the Spin the Pots task, younger children in the intervention group found more stickers (mean: 3.44 compared with 2.88) and tended to be more likely to find all of the stickers than children in the control group (50% compared with 12.5%). The task-switching test is not reported for younger children due to lower numbers who completed the test. For the 6-y-old children, random assignment to the intervention group was not significantly associated with children's working memory or task-switching abilities; however, raw scores were high in both conditions for the Spin the Pots task, suggesting potential ceiling effects as discussed below. In the Reverse Categorization task, children performed equally well across conditions on the pre-switch trials (mean correct responses across 6 total pre-switch trials: intervention group = 5.05 trials, control group = 5.15 trials; P = NS), confirming that any differences that might have emerged on the post-switch trials as a result of the supplement would have reflected changes in task-switching abilities, rather than rule learning or general attention.
TABLE 3.
Summary statistics for the subset of children with cognitive test data at 11 wk1
| Intervention | Control | P | |
|---|---|---|---|
| Age 1–3 y | |||
| n | 16 | 16 | |
| Baseline age, y | 2.49 ± 0.40 | 2.23 ± 0.38 | |
| WAZ | −1.26 ± 1.03 | −1.25 ± 0.84 (n = 15) | 0.1526 |
| HAZ | −2.01 ± 1.11 | −1.85 ± 0.85 (n = 15) | 0.6707 |
| WHZ | −0.19 ± 0.92 | −0.31 ± 0.75 (n = 15) | 0.1272 |
| Cognition | |||
| Number of stickers found | 3.44 ± 0.63 | 2.88 ± 0.62 | 0.04352 |
| Found all stickers, % | 50.0 | 12.5 | 0.0763 |
| Age 5–7 y | |||
| n | 20 | 20 | |
| Baseline age, y | 5.87 ± 0.28 | 6.11 ± 0.55 | |
| WAZ | −1.78 ± 0.92 | −1.28 ± 0.99 | 0.7390 |
| HAZ | −1.74 ± 0.99 | −1.13 ± 1.35 | 0.4380 |
| BMI z score | −0.96 ± 0.84 (n = 19) | −0.79 ± 0.76 | 0.6027 |
| Cognition | |||
| Number of stickers found (maximum = 8) | 6.60 ± 0.99 | 7.05 ± 0.83 | 0.07552 |
| Found all stickers, % | 25.0 | 30 | 0.53843 |
| Correctly sorted animals (maximum = 12), % | 8.90 ± 2.99 | 9.40 ± 2.89 | 0.78092 |
Values are means ± SDs unless otherwise indicated. P values were derived by using ANCOVA, adjusted for age. HAZ, height-for-age z score; WAZ, weight-for-age z score; WHZ, weight-for-height z score.
An age-adjusted Poisson regression model was used.
An age-adjusted logistic regression model was used.
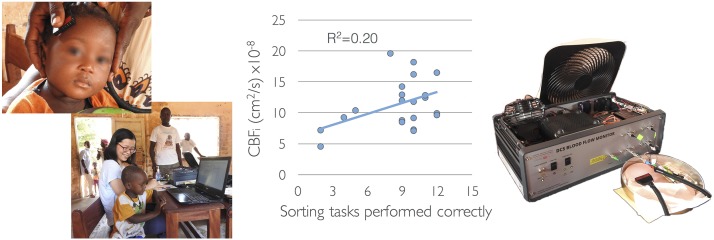
To test the utility of CBFi measurements in assessing cognitive abilities in children at risk of malnutrition, we performed data analysis combining the intervention and control children. Mean values for CBFi at 2 cm in the younger and older groups were 10.4 ± 0.5 and 11.0 ± 0.6 × 10−8 cm2/s, respectively. Scalp extracerebral blood flow index (BFi) was significantly lower than CBFi (1.6 ± 0.1 and 1.5 ± 0.1 × 10−8 cm2/s for young and older groups, respectively). Mean values for tissue absorbance (µai at 785 nm) in the younger and older groups were 0.18 ± 0.01 and 0.20 ± 0.01 cm−1, respectively. There was a significant correlation between the Reverse Categorization task and CBFi in the right frontal region in the older children (r = 0.44, P = 0.038). This correlation is shown in Figure 1 along with the depiction of village measurements. Some anthropometric measures correlated with CBFi in the younger and older groups. In particular, CBFi in the right frontal region of older children was negatively correlated with arterial oxygenation (r = −0.45; P = 0.034) and head circumference (r = −0.51; P = 0.016). In addition, CBFi in the younger children was negatively correlated with height-for-age z score (right CBFi: r = −0.43, P = 0.001; left CBFi: r = −0.49, P = 0.004). Tissue absorption did not correlate with any anthropometric measure.
FIGURE 1.
Relation between brain blood flow and number of sorting tasks performed correctly and illustration of measurements being conducted in a rural village in Guinea-Bissau. R2 represents the line of best fit. CBFi, cerebral blood flow index.
Discussion
This pilot randomized study tested a new nutritional formulation for use in undernourished and stunted children that, on theoretical grounds, could potentially contribute to structural and functional repair of brain structure and function. The results indicated improved executive function associated with short-term use of the solely food-based intervention, with significantly greater scores on a test of executive function found after just 11 wk of supplementation in the younger group of children compared with assessment-only controls. The results are based on an underpowered sample but justify further testing of the new formulation.
Like existing RUSFs and FBFs, the new formulation generally met WHO nutrient targets for renourishing children, including specified targets for protein, vitamins, and minerals. In addition, the new formulation contained 5 additional nutrients (DHA, EPA, choline, chromium, and molybdenum) that are not included in current WHO recommendations for malnutrition-related food products, and 2 ingredients (cocoa and green tea powder) that are high in the subclass of polyphenols that cross the blood-brain barrier and have potentially beneficial neurological effects in other population types such as elderly adults. Further, calcium amounts were reduced to facilitate iron absorption, given the known positive effect of iron on cognition and the widespread anemia in communities of the type studied. To our knowledge, none of the new nutrients, specific ingredients, and bioactive factors have previously been tested for use—either separately or in combination—in nutrient recommendations to prevent or treat malnutrition. They were included here on the basis of an evaluation of research studies mostly performed in nonmalnourished adults and children, which identified theoretical benefits including the promotion of neurogenesis and neuronal membrane growth and repair, enhanced neurotransmitter synthesis, reduced apoptosis, increased signal processing and transmission, decreased inflammation, and enhanced intestinal absorption and blood-brain transport of the neuroactive factors (11, 26, 61–64). Furthermore, the broad-reaching nature of these potential benefits suggested the potential for synergistic benefits.
The preliminary finding of an association of supplement consumption with improved working memory in younger children is noteworthy given evidence that links early working memory abilities to longer-term cognitive development and academic achievement both in healthy, typically developing children (65) as well as in children born prematurely for whom there is greater concern for cognitive impairments and delay (66). Although the apparently improved working memory was limited to the younger, 2-y-old children in the current study, the intervention lasted only 11 wk. In addition, the older children's high working memory abilities across both the intervention and control conditions (∼7 of 8 total stickers found across both conditions) suggest that this may represent ceiling performance under the current task parameters rather than a lack of effect at older ages, and increasing the working memory demands of the task (e.g., more hidden stickers, more empty cups, longer delays before searching) may yield a measure more sensitive to detect intervention-related change. Similarly, the fact that we failed to find an impact of the supplement on the other cognitive assessments may also be due to the parameters and sensitivity of the tasks or the relatively short duration of supplementation, rather than representative of the supplement's failure to affect these cognitive abilities. The need for cultural adaptation of the cognitive assessments in this pilot study, along with the lack of norms for this kind of population, may have resulted in tasks that lacked the sensitivity to detect significant changes between conditions. Given these limitations, future research would benefit from including additional time in their protocols before supplementation to pilot cognitive measures. However, the positive association of supplement consumption and improved working memory in younger children, and the positive relation of cognition with brain blood flow, warrant a more targeted investigation of working memory in a fully powered sample in both younger and older children.
Current neuroimaging tools to assess brain maturation are ethically unusable (PET), too expensive (MRI or fMRI), or require professionals or well-trained operators to perform functional studies that consist of presenting repeated stimuli to the children and measuring neurovascular evoked responses [electroencephalogram–event-related potential, functional NIRS (fNIRS)]. Several groups are currently testing the ability of fNIRS to assess cognitive function delays in malnourished children (67), although, to our knowledge, not yet in supplement interventions. These fNIRS studies rely on the evoked hemodynamic responses measured while children are performing cognitive tasks and require devices with multiple channels, placement of optical monitors on the head similar to electroencephalography caps, and collection of data for several minutes. Our approach makes it possible to quantify resting state cerebral perfusion noninvasively, in few seconds, with a portable device suitable for large studies. Thus, our approach can be done by nonexperts and fully scales to the large number of participants required for nutritional studies (e.g., in this study, we measured 78 children in 4 d). In addition, this pilot study showed the feasibility of measuring children in remote and low-resource area with the NIRS-DCS device powered with a portable generator and temperatures averaging 36–40°C. The test was well accepted by the children and their families and produced reasonable-quality data. The NIRS-DCS study was only conducted at the end of the nutritional intervention, which limited our ability to assess changes in hemodynamic variables as a consequence of the intervention, and future studies should perform measurements before and after supplementation.
With the use of NIRS-DCS methodology, the correlation between the right frontal CBF and the animal-sorting test in children aged 5–7 y showed that children who perform poorly on cognitive testing have lower than average CBFi. This is an important finding, which needs further validation in a larger population. The recovered variables (CBFi and µai) represent bulk hemodynamic values in a relative large volume (2 × 2 × 1.5 cm3) and, as in NIRS, scalp and skull indexes contribute to these values. As shown in this study, the large differences in blood flow indexes between the scalp and brain provide confidence that we can use the device in children ranging in age from 18 mo to 7 y without excessive extracerebral contamination. Nevertheless, the negative correlation between head circumference and CBFi in the older children suggests some scalp contamination. This problem can be addressed by using larger source-detector separations. In this study, we were limited by the relatively low power of the laser source. For future studies, we plan to increase the light power to 35 mW to increase the signal-to-noise ratio at larger separations. Another limitation of our study is that by using only DCS we could not estimate scattering coefficient but had to assume it to be constant. We are aware of the strong impact of the scattering coefficient in the quantification of CBF with DCS, and our calculated blood flow index reflects the product of blood flow and scattering. In future studies, we plan to use a combined frequency-domain NIRS and DCS system (68) to quantify the scattering rather than assuming it to be constant across children. The delivery of a noninvasive tool able to quantitatively assess brain function independently of neurodevelopmental tests (which are very challenging in field studies) would have a critical impact to directly assess the effect of a broad range of interventions to increase cognitive abilities and brain function in general.
If the cognitive function results found here are confirmed in a larger study, they would have major implications for the prevention and treatment of malnutrition, because cognitive impairment resulting from stunting and malnutrition is currently assumed to be mostly irreversible (1, 2) and nutrition-only trials have shown limited success [e.g., (9)]. It should be noted that the method of implementing the formulation described here, namely a food product prepared locally (from provided and local ingredients) and a vitamin supplement, is feasible for scaling. It would also be possible to incorporate all of the vitamins and minerals and unique nutrients into a single mix, which could be produced centrally and distributed, or to incorporate into ready-to-use foods by village bakers or other qualified individuals. Alternatively, the mix could be used to create shelf-stable food products, which could then be distributed to sites of consumption. All of these methods have the advantage that a portion of the ingredients are purchased locally, so they reduce importation relative to typical lipid-based pastes while also facilitating local businesses.
Concerning study weaknesses, the protocol was designed as a small pilot study to obtain data for power calculations and to examine the feasibility of preparing the supplement locally. Care was taken to use broadly comparable villages; however, as a 2-village cluster-randomized study, it could not distinguish a supplement effect from a village by supplement interaction. As such, the data should be considered preliminary and in need of verification in a powered trial. The lack of power is likely the reason for the finding of no significant effects of the supplement on growth and hemoglobin, which would need a much larger study of longer duration to yield detectable changes [e.g., (69)]. This pilot also used an assessment-only control that cannot distinguish between an effect of feeding on any specific influence of the supplement composition, whereas future studies should compare the new supplement with conventional products designed for the same purpose, namely supplemental nutrition for mothers and children for the prevention and treatment of stunting and MAM and for supplemental nutrition in school feeding programs. There were also translation issues for cognitive testing at baseline, which resulted in a loss of some data.
In summary, this study focused on developing methods for a future powered trial, because stunting and MAM remain widespread worldwide and are associated with lasting cognitive impairment. The data from the new approach to formulating supplementary foods suggested improved executive function in undernourished young children. The results are preliminary, but given their potential importance, they justify replication in a powered trial of longer duration.
Acknowledgments
We thank Madeleine Gamache for help in preparing the manuscript. The authors' responsibilities were as follows—WP and ABdS: led the study, which was conducted with local implementation by the International Partnership for Human Development; SBR: designed the supplement formulation with collaboration from ES and AK and drafted the manuscript for review by the other authors; PM: designed the cognitive testing methods and coded the videotaped sessions and blinded and interpreted those results; ES: designed and led the data safety and monitoring plan; MAF and K-CW: designed and built the DCS device and analyzed the DCS-NIRS data; MAF, P-YL, and KH: conducted the DCS-NIRS measurements and interpreted the DCS-NIRS results; OC: provided input on flavonoids and measured amounts in the supplement ingredients; EJJ: provided expertise on measuring skin carotenoids; ABdS: provided oversight of the field team and was responsible for obtaining local IRB permission and recruiting staff to conduct the study and villages to enroll in the study, assisted by RC, who also supervised supplement production and delivery; SBR: led development of a plan for staff training and quality control, which was implemented by ST, who also entered data for analyses, which were conducted by C Brown; C Balé and NS: provided comments and assisted in interpreting the results; and all authors: read and approved the final manuscript.
Abbreviations
- CBF
cerebral blood flow
- CBFi
cerebral blood flow index
- DCS
diffuse correlation spectroscopy
- FBF
fortified blended food
- fNIRS
functional near-infrared spectroscopy
- IRB
institutional review board
- MAM
moderate-acute malnutrition
- MUAC
midupper arm circumference
- NIRS
near-infrared spectroscopy
- PET
positron emission tomography
- RUSF
ready-to-use supplementary food
- SO2
cerebral hemoglobin oxygenation
- μai
tissue absorbance index
Footnotes
Supported by the Boston Foundation and a gift to Tufts University by Bill Schawbel. Masimo Corp. donated a Pronto-7 pulse co-oximeter for this study, Pharmanex Global Research provided a resonance Raman spectrometer, and Vitamin Friends LLC donated iron gummies.
Contributor Information
Susan B Roberts, Email: susan.roberts@tufts.edu.
Maria Angela Franceschini, Email: mari@nmr.mgh.harvard.edu.
Paul Muentener, Email: paul.muentener@tufts.edu.
References
- 1. World Bank Investing in early childhood development [Internet]. Washington (DC): The World Bank; 2006. [cited 2015 Dec 15]. Available from: econ.worldbank.org. [Google Scholar]
- 2. Kar BR, Rao SL, Chandramouli BA.. Cognitive development in children with chronic protein energy malnutrition. Behav Brain Funct 2008;4:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Crookston BT, Schott W, Cueto S, Dearden KA, Engle P, Georgiadis A, Lundeen EA, Penny ME, Stein AD, Behrman JR.. Postinfancy growth, schooling, and cognitive achievement: young lives. Am J Clin Nutr 2013;98:1555–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Waber DP, Eaglesfield D, Fitzmaurice GM, Bryce C, Harrison RH, Galler JR.. Cognitive impairment as a mediator in the developmental pathway from infant malnutrition to adolescent depressive symptoms in Barbadian youth. J Dev Behav Pediatr 2011;32:225–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Alamy M, Bengelloun WA.. Malnutrition and brain development: an analysis of the effects of inadequate diet during different stages of life in rat. Neurosci Biobehav Rev 2012;36:1463–80. [DOI] [PubMed] [Google Scholar]
- 6. Pollitt E, Saco-Pollitt C, Jahari A, Husaini MA, Huang J.. Effects of an energy and micronutrient supplement on mental development and behavior under natural conditions in undernourished children in Indonesia. Eur J Clin Nutr 2000;54(Suppl 2):S80–90. [DOI] [PubMed] [Google Scholar]
- 7. Grantham-McGregor SM, Powell CA, Walker SP, Himes JH.. Nutritional supplementation, psychosocial stimulation, and mental development of stunted children: the Jamaican study. Lancet 1991;338:1–5. [DOI] [PubMed] [Google Scholar]
- 8. Walker SP, Chang SM, Powell CA, Grantham-McGregor SM.. Effects of early childhood psychosocial stimulation and nutritional supplementation on cognition and education in growth-stunted Jamaican children: prospective cohort study. Lancet 2005;366:1804–7. [DOI] [PubMed] [Google Scholar]
- 9. Prado EL, Maleta K, Ashorn P, Ashorn U, Vosti SA, Sadalaki J, Dewey KG.. Effects of maternal and child lipid-based nutrient supplements on infant development: a randomized trial in Malawi. Am J Clin Nutr 2016;103:784–93. [DOI] [PubMed] [Google Scholar]
- 10. WHO The Healthy Growth Project. Geneva (Switzerland): WHO; 2016. [Google Scholar]
- 11. Zeisel SH, da Costa KA.. Choline: an essential nutrient for public health. Nutr Rev 2009;67):615–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mudd AT, Getty CM, Sutton BP, Dilger RN.. Perinatal choline deficiency delays brain development and alters metabolite concentrations in the young pig. Nutr Neurosci 2016;19:425–33. [DOI] [PubMed] [Google Scholar]
- 13. Walker SP, Wachs TD, Meeks Gardner J, Lozoff B, Wasserman GA, Pollitt E, Carter JA.. Child development: risk factors for adverse outcomes in developing countries. Lancet 2007;369:145–57. [DOI] [PubMed] [Google Scholar]
- 14. Semba RD, Zhang P, Gonzalez-Freire M, Moaddel R, Trehan I, Maleta KM, Ordiz MI, Ferrucci L, Manary MJ.. The association of serum choline with linear growth failure in young children from rural Malawi. Am J Clin Nutr 2016;104:191–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Daiello LA, Gongvatana A, Dunsiger S, Cohen RA, Ott BR; Alzheimer's Disease Neuroimaging Initiative . Association of fish oil supplement use with preservation of brain volume and cognitive function. Alzheimers Dement 2015;11:226–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sitaram N, Weingartner H, Gillin J.. Human serial learning: enhancement with arecholine and choline impairment with scopolamine. Science 1978;201:274–6. [DOI] [PubMed] [Google Scholar]
- 17. Brickman AM, Khan UA, Provenzano FA, Yeung L-K, Suzuki W, Schroeter H, Wall M, Sloan RP, Small SA.. Enhancing dentate gyrus function with dietary flavanols improves cognition in older adults. Nat Neurosci 2014;17:1798–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Simmer K, Patole SK, Rao SC.. Long-chain polyunsaturated fatty acid supplementation in infants born at term. Cochrane Database Syst Rev 2011;12:CD000376. [DOI] [PubMed] [Google Scholar]
- 19. Dyall SC.. Long-chain omega-3 fatty acids and the brain: a review of the independent and shared effects of EPA, DPA and DHA. Front Aging Neurosci 2015;7:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kuratko CN, Barrett EC, Nelson EB, Norman S.. The relationship of docosahexaenoic acid (DHA) with learning and behavior in healthy children: a review. Nutrients 2013;5:2777–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sheppard KW, Cheatham CL.. Executive functions and the omega-6-to-omega-3 fatty acid ratio: a cross-sectional study. Am J Clin Nutr 2017;105:32–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wightman EL, Haskell CF, Forster JS, Veasey RC, Kennedy DO.. Epigallocatechin gallate, cerebral blood flow parameters, cognitive performance and mood in healthy humans: a double-blind, placebo-controlled, crossover investigation. Hum Psychopharmacol 2012;27:177–86. [DOI] [PubMed] [Google Scholar]
- 23. Boespflug EL, Eliassen JC, Dudley JA, Shidler MD, Kalt W, Summer SS, Stein AL, Stover AN, Krikorian R.. Enhanced neural activation with blueberry supplementation in mild cognitive impairment. Nutr Neurosci 2017;21:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lamport DJ, Pal D, Moutsiana C, Field DT, Williams CM, Spencer JP, Butler LT.. The effect of flavanol-rich cocoa on cerebral perfusion in healthy older adults during conscious resting state: a placebo controlled, crossover, acute trial. Psychopharmacology (Berl) 2015;232:3227–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Krikorian R, Boespflug EL, Fleck DE, Stein AL, Wightman JD, Shidler MD, Sadat-Hossieny S.. Concord grape juice supplementation and neurocognitive function in human aging. J Agric Food Chem 2012;60:5736–42. [DOI] [PubMed] [Google Scholar]
- 26. Nehlig A... The neuroprotective effects of cocoa flavanol and its influence on cognitive performance. Br J Clin Pharmacol 2013;75:716–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hsu DP, French AJ, Madson SL, Palmer JM, Gidvani-Diaz V.. Evaluation of a noninvasive hemoglobin measurement device to screen for anemia in infancy. Matern Child Health J 2016;20:827–32. [DOI] [PubMed] [Google Scholar]
- 28. Batra P, Schlossman N, Balan I, Pruzensky W, Balan A, Brown C, Gamache MG, Schleicher MM, de Sa AB, Saltzman E, et al. A randomized controlled trial offering higher- compared with lower-dairy second meals daily in preschools in Guinea-Bissau demonstrates an attendance-dependent increase in weight gain for both meal types and an increase in mid-upper arm circumference for the higher-dairy meal. J Nutr 2016;146:124–32. [DOI] [PubMed] [Google Scholar]
- 29. Schlossman N, Brown C, Batra P, Braima de Sa A, Balan I, Balan A, Gamache MG, Wood L, Pruzensky W, Saltzman E, et al. A randomized controlled trial of two ready-to-use supplementary foods demonstrates benefit of the higher dairy supplement for reduced wasting in mothers, and differential impact in infants and children associated with maternal supplement response. Food Nutr Bull 2017;38:275–90. [DOI] [PubMed] [Google Scholar]
- 30. Buckley EM, Parthasarathy AB, Grant PE, Yodh AG, Franceschini MA.. Diffuse correlation spectroscopy for measurement of cerebral blood flow: future prospects. Neurophotonics 2014;1:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kennedy C, Sokoloff L.. An adaptation of the nitrous oxide method to the study of the cerebral circulation in children: normal values for cerebral blood flow and cerebral metabolic rate in childhood. J Clin Invest 1957;36:1130–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chugani HT, Phelps ME, Mazziotta JC.. Positron emission tomography study of human brain functional development. Ann Neurol 1987;22:487–97. [DOI] [PubMed] [Google Scholar]
- 33. Chugani HT.. A critical period of brain development: studies of cerebral glucose utilization with PET. Prev Med 1998;27:184–8. [DOI] [PubMed] [Google Scholar]
- 34. Roche-Labarbe N, Fenoglio A, Aggarwal A, Dehaes M, Carp SA, Franceschini MA, Grant PE.. Near-infrared spectroscopy assessment of cerebral oxygen metabolism in the developing premature brain. J Cereb Blood Flow Metab 2012;32:481–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lin PY, Roche-Labarbe N, Dehaes M, Fenoglio A, Grant PE, Franceschini MA.. Regional and hemispheric asymmetries of cerebral hemodynamic and oxygen metabolism in newborns. Cereb Cortex 2013;23:339–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Franceschini MA, Thaker S, Themelis G, Krishnamoorthy KK, Bortfeld H, Diamond SG, Boas DA, Arvin K, Grant PE.. Assessment of infant brain development with frequency-domain near-infrared spectroscopy. Pediatr Res 2007;61(5 Pt 1):546–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chugani HT, Phelps ME.. Maturational changes in cerebral function in infants determined by 18FDG positron emission tomography. Science 1986;231:840–3. [DOI] [PubMed] [Google Scholar]
- 38. McGovern-Dole The global effort to reduce child hunger and increase school attendance. Report to the United States Congress, fiscal years 2012#x20132014. USDA/Foreign Agricultural Service; 2016. [cited 2016 Aug 20] Available from: https://www.fas.usda.gov/sites/default/files/2016-07/8169534_mcgovern-dole_report_-_june_2016.pdf.
- 39. World Food Programme Specialized nutritious foods. Rome (Italy): World Food Programme; 2016.. [Google Scholar]
- 40. Institute of Medicine DRI: the essential guide to nutrient requirements. Washington (DC): Institute of Medicine; 2006. [Google Scholar]
- 41. Ferland G... Vitamin K and the nervous system: an overview of its actions. Adv Nutr 2012;3:204–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Abbaspour N, Hurrell R, Kelishadi R.. Review on iron and its importance for human health. J Res Med Sci 2014;19:164–74. [PMC free article] [PubMed] [Google Scholar]
- 43. Hughes C, Ensor R.. Executive function and theory of mind in 2 year olds: a family affair? Dev Neuropsychol 2005;28:645–68. [DOI] [PubMed] [Google Scholar]
- 44. Hostinar CE, Stellern SA, Schaefer C, Carlson SM, Gunnar MR.. Associations between early life adversity and executive function in children adopted internationally from orphanages. Proc Natl Acad Sci USA 2012;109(Suppl 2):17208–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Carlson SM, Mandell DJ, Williams L.. Executive function and theory of mind: stability and prediction from ages 2 to 3. Dev Psychol 2004;40:1105–22. [DOI] [PubMed] [Google Scholar]
- 46. Onyango AW, de Onis M.. WHO child growth standards: training course on child growth assessment. Geneva (Switzerland): WHO; 2008. [Google Scholar]
- 47. Hata TR, Scholz TA, Ermakov IV, McClane RW, Khachik F, Gellermann W, Pershing LK.. Non-invasive raman spectroscopic detection of carotenoids in human skin. J Invest Dermatol 2000;115:441–8. [DOI] [PubMed] [Google Scholar]
- 48. Ermakov IV, Ermakova MR, Gellermann W, Lademann J.. Noninvasive selective detection of lycopene and β-carotene in human skin using Raman spectroscopy. J Biomed Opt 2004;9:332–8. [DOI] [PubMed] [Google Scholar]
- 49. Boas DA, Yodh AG.. Spatially varying dynamical properties of turbid media probed with diffusing temporal light correlation. J Opt Soc Am A Opt Image Sci Vis 1997;14:192–215. [Google Scholar]
- 50. Cheung C, Culver JP, Takahashi K, Greenberg JH, Yodh AG.. In vivo cerebrovascular measurement combining diffuse near-infrared absorption and correlation spectroscopies. Phys Med Biol 2001;46:2053–65. [DOI] [PubMed] [Google Scholar]
- 51. Durduran T, Yodh AG.. Diffuse correlation spectroscopy for non-invasive, micro-vascular cerebral blood flow measurement. Neuroimage 2014;85(Pt 1):51–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Mesquita RC, Durduran T, Yu G, Buckley EM, Kim MN, Zhou C, Choe R, Sunar U, Yodh AG.. Direct measurement of tissue blood flow and metabolism with diffuse optics. Philos Trans A Math Phys Eng Sci 2011;369:4390–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Boas DA, Franceschini MA.. Haemoglobin oxygen saturation as a biomarker: the problem and a solution. Philos Trans A Math Phys Eng Sci 2011;369:4407–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Yoxall CW, Weindling AM.. Measurement of cerebral oxygen consumption in the human neonate using near infrared spectroscopy: cerebral oxygen consumption increases with advancing gestational age. Pediatr Res 1998;44:283–90. [DOI] [PubMed] [Google Scholar]
- 55. Dehaes M, Aggarwal A, Lin PY, Rosa Fortuno C, Fenoglio A, Roche-Labarbe N, Soul JS, Franceschini MA, Grant PE.. Cerebral oxygen metabolism in neonatal hypoxic ischemic encephalopathy during and after therapeutic hypothermia. J Cereb Blood Flow Metab 2014;34:87–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Matcher SJ, Kirkpatrick PJ, Nahid K, Cope M, Delpy DT.. Absolute quantification methods in tissue near-infrared spectroscopy. SPIE 1995;2389:486–95. [Google Scholar]
- 57. Roche-Labarbe N, Carp SA, Surova A, Patel M, Boas DA, Grant PE, Franceschini MA.. Noninvasive optical measures of CBV, StO(2), CBF index, and rCMRO(2) in human premature neonates' brains in the first six weeks of life. Hum Brain Mapp 2010;31:341–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Selb J, Boas DA, Chan ST, Evans KC, Buckley EM, Carp SA.. Sensitivity of near-infrared spectroscopy and diffuse correlation spectroscopy to brain hemodynamics: simulations and experimental findings during hypercapnia. Neurophotonics 2014;1:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Trans Cranial Technologies, Ltd 10/20 System positioning manual. Hong Kong; 2012. Available from: https://www.trans-cranial.com/local/manuals/10_20_pos_man_v1_0_pdf.pdf.
- 60. WHO WHO global database on child growth and malnutrition. Guinea-Bissau: WHO; 2014. [Google Scholar]
- 61. Calder PC.. Omega-3 fatty acids and inflammatory processes. Nutrients 2010;2:355–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Kuratko CN, Barrett EC, Nelson EB, Salem N Jr.. The relationship of docosahexaenoic acid (DHA) with learning and behavior in healthy children: a review. Nutrients 2013;5:2777–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Nehlig A, Daval JL, Debry G.. Caffeine and the central nervous system: mechanisms of action, biochemical, metabolic and psychostimulant effects. Brain Res Brain Res Rev 1992;17:139–70. [DOI] [PubMed] [Google Scholar]
- 64. Mueni E, Opiyo N, English M.. Caffeine for the management of apnea in preterm infants. Int Health 2009;1:190–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Alloway TP, Alloway RG.. Investigating the predictive roles of working memory and IQ in academic attainment. J Exp Child Psychol 2010;106:20–9. [DOI] [PubMed] [Google Scholar]
- 66. Mulder H, Pitchford NJ, Marlow N.. Processing speed and working memory underlie academic attainment in very preterm children. Arch Dis Child Fetal Neonatal Ed 2010;95:F267–72. [DOI] [PubMed] [Google Scholar]
- 67. Lloyd-Fox S, Papademetriou M, Darboe MK, Everdell NL, Wegmuller R, Prentice AM, Moore SE, Elwell CE.. Functional near infrared spectroscopy (fNIRS) to assess cognitive function in infants in rural Africa. Sci Rep 2014;4:4740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Carp SA, Farzam P, Redes N, Hueber DM, Franceschini MA.. Combined multi-distance frequency domain and diffuse correlation spectroscopy system with simultaneous data acquisition and real-time analysis. Biomed Opt Express 2017;8:3993–4006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Bhutta ZA, Ahmed T, Black RE, Cousens S, Dewey K, Giugliani E, Haider BA, Kirkwood B, Morris SS, Sachdev HP, et al. What works? Interventions for maternal and child undernutrition and survival. Lancet 2008;371:417–40. [DOI] [PubMed] [Google Scholar]