Abstract
Although ethylene regulates a wide range of defense-related genes, its role in plant defense varies greatly among different plant-microbe interactions. We compared ethylene's role in plant response to virulent and avirulent strains of Xanthomonas campestris pv. vesicatoria in tomato (Lycopersicon esculentum Mill.). The ethylene-insensitive Never ripe (Nr) mutant displays increased tolerance to the virulent strain, while maintaining resistance to the avirulent strain. Expression of the ethylene receptor genes NR and LeETR4 was induced by infection with both virulent and avirulent strains; however, the induction of LeETR4 expression by the avirulent strain was blocked in the Nr mutant. To determine whether ethylene receptor levels affect symptom development, transgenic plants overexpressing a wild-type NR cDNA were infected with virulent X. campestris pv. vesicatoria. Like the Nr mutant, the NR overexpressors displayed greatly reduced necrosis in response to this pathogen. NR overexpression also reduced ethylene sensitivity in seedlings and mature plants, indicating that, like LeETR4, this receptor is a negative regulator of ethylene response. Therefore, pathogen-induced increases in ethylene receptors may limit the spread of necrosis by reducing ethylene sensitivity.
Plant response to pathogen infection can determine both the extent of pathogen growth and the amount of damage caused by it. During a compatible interaction, a virulent pathogen spreads from the point of entry and causes cell damage far beyond the site of infection. During an incompatible interaction, cell death is limited to the site of infection and colonization of the plant by the avirulent pathogen is greatly reduced. An incompatible interaction often results in a hypersensitive response in which damage is limited to the rapid death of a small number of cells (Goodman and Novacky, 1994).
Several differences between compatible and incompatible interactions may explain how the plant limits both pathogen growth and cell death. One of the first differences is the greater increase in reactive oxygen intermediates observed during an incompatible interaction (Keppler et al., 1989; Orlandi et al., 1992). This oxidative burst may kill the pathogen directly (Keppler et al., 1989; Wu et al., 1995) or limit its spread by killing infected plant cells (Greenberg et al., 1994) and inducing cross-linkage of cell wall proteins (Bradley et al., 1992; Brisson et al., 1994). During an incompatible interaction, cell walls are also strengthened through increased deposition of hydroxy-Pro-rich glycoproteins (Showalter et al., 1985), callose (Parker et al., 1993), and lignin (Moerschbacher et al., 1990). Infection with an avirulent pathogen often causes a stronger and more rapid increase in pathogenesis-related (PR) proteins (Linthorst, 1991), which may enhance resistance to fungi (Broglie et al., 1991; Zhu et al., 1994). Increased synthesis of other antimicrobial compounds such as phytoalexins (Hain et al., 1993), thionins (Epple et al., 1995), and defensins (Penninckx et al., 1996) observed during incompatible interactions may also limit pathogen growth. Many of these resistance responses are also a component of compatible interactions, but occur much later in the progression of the disease (Staskawicz et al., 1995). Therefore, whether infection results in a compatible or an incompatible interaction may be determined more by the speed of the response than by qualitative differences between these interactions.
Synthesis of the plant hormones salicylic acid (SA), ethylene, and jasmonic acid increases greatly during many incompatible interactions (Malamy et al., 1990; Boller, 1991; Penninckx et al., 1996). These hormones regulate a wide range of defense-related genes, making them likely candidates as signals that coordinate plant response to pathogens (Penninckx et al., 1998; Thomma et al., 1998). SA in particular is essential in mounting the resistance response in many plant-pathogen interactions (Delaney et al., 1994). Ethylene regulates several genes involved in defense responses, including those encoding PR proteins such as chitinases, β-1,3-glucanases, and PR1 (Deikman, 1997), phytoalexin synthesis enzymes (Ecker and Davis, 1987), defensin (Penninckx et al., 1996), and hydroxy-Pro-rich glycoproteins (Toppan et al., 1982). Since ethylene induces the expression of many defense-related genes, increasing ethylene synthesis during infection would be one way of initiating a defense response. Increased ethylene synthesis in infected tissue has been reported for a wide range of pathogens (Boller, 1991). For some, such as tobacco mosaic virus in tobacco (Nicotiana tabacum) and Uromyces phaseoli in bean (Phaseolus vulgaris), the increase in ethylene levels during an incompatible interaction is greater and more rapid than during a compatible interaction (Montalbini and Elstner, 1977; De Laat and van Loon, 1983). This increase in ethylene synthesis may be one way that the plant activates a more rapid defense response after infection with an avirulent pathogen.
Ethylene responses can also be regulated by changes in ethylene perception. Several genes encoding ethylene receptors have been isolated from Arabidopsis (Chang et al., 1993; Hua et al., 1995, 1998) and tomato (Lycopersicon esculentum Mill.) (Wilkinson et al., 1995; Lashbrook et al., 1998; Tieman and Klee, 1999). In Arabidopsis, loss-of-function mutations in four of these receptor genes, ETR1, ETR2, EIN4, and ERS2, have been identified. Plants containing all four of these mutations showed strong constitutive ethylene responses, demonstrating that these receptors are negative regulators of the ethylene response (Hua and Meyerowitz, 1998). In tomato, five different members of an ethylene receptor gene family, LeETR1, LeETR2, NR, LeETR4, and LeETR5, have been isolated. In transgenic tomato plants, reduced expression of one of these receptor genes, LeETR4, also resulted in constitutive ethylene responses such as leaf epinasty and flower senescence, indicating that a reduction in receptor level causes an increase in ethylene sensitivity (Tieman et al., 2000). Although the effect of increasing ethylene receptor levels has not been reported previously, the evidence cited above suggests that an increase in receptors would reduce sensitivity. Therefore, plants may be capable of reducing sensitivity of specific tissues through the induction of receptor gene expression. To determine whether greater ethylene receptor gene expression does in fact reduce ethylene sensitivity, we analyzed transgenic tomato plants overexpressing the wild-type NR gene.
In contrast to the loss-of-function mutants, Arabidopsis plants containing dominant mutations in the ethylene receptor genes are insensitive to ethylene. For one of these mutants, etr1-1, this insensitivity is due to the inability of the mutant ETR1 protein to bind ethylene (Schaller and Bleecker, 1995). In wild-type plants, binding of ethylene by the receptor is thought to inactivate its function as a negative regulator, allowing the ethylene response to occur. Since the mutant receptors are unable to bind ethylene, they cannot be inactivated and remain constitutive suppressors of the ethylene response (Hua and Meyerowitz, 1998). The tomato NR gene is homologous to ETR1 and other Arabidopsis ethylene receptor genes and, like ETR1, the wild-type NR protein is able to bind ethylene (G.E. Schaller, F. Rodriguez, and A.B. Bleecker, personal communication). The Never ripe (Nr) mutant displays ethylene insensitivity in several developmental processes, including hypocotyl elongation, flower senescence, and fruit ripening (Lanahan et al., 1994).
Analysis of ethylene-insensitive plants in several different species has demonstrated a role for ethylene in both compatible and incompatible interactions, yet the effect of ethylene insensitivity on pathogenesis varies greatly among pathogens. In tomato, the ethylene-insensitive Nr mutant showed increased tolerance to virulent strains of Fusarium oxysporum, Pseudomonas syringae pv. tomato, and Xanthomonas campestris pv. vesicatoria (Lund et al., 1998). In Arabidopsis, the ethylene-insensitive ein2 mutant displayed increased tolerance to virulent strains of the bacterial pathogens P. syringae pv. tomato and pv. maculicola as well as X. campestris pv. campestris (Bent et al., 1992). However, ethylene-insensitive transgenic tobacco (Nicotiana tabacum) plants expressing a mutant form of the Arabidopsis ETR1 ethylene receptor gene were more susceptible to the soil-borne fungal pathogen Pythium sylvaticum (Knoester et al., 1998). Soybean (Glycine max) mutants with reduced ethylene sensitivity displayed less severe symptoms in response to virulent P. syringae pv. glycinea and Phytopthora sojae, but more severe symptoms to Septoria glycines and Rhizoctonia solani (Hoffman et al., 1999).
The role of ethylene in incompatible interactions also appears to vary from one pathogen to another. Ethylene-insensitive Arabidopsis mutants maintained their resistance to P. syringae pv. tomato (Bent et al., 1992), Peronospora parasitica, and Alternaria brassicicola (Thomma et al., 1999). Likewise, ethylene-insensitive transgenic tobacco plants were resistant to an incompatible strain of tobacco mosaic virus (Knoester et al., 1998), and soybean mutants with reduced ethylene sensitivity maintained resistance to avirulent P. syringae pv. glycinea (Hoffman et al., 1999). However, resistance to the avirulent fungal pathogen P. sojae was compromised in these soybean mutants (Hoffman et al., 1999), and an ethylene-insensitive Arabidopsis mutant was more susceptible to a normally avirulent strain of the fungus Botrytis cinerea (Thomma et al., 1999). Therefore, ethylene is involved in the resistance response for some plant-pathogen interactions.
We compared the role of ethylene in compatible and incompatible interactions with X. campestris pv. vesicatoria, the causal agent of bacterial spot in tomato and pepper (Capsicum annuum). Wild-type and ethylene-insensitive Nr mutant plants were infected with virulent (Xv 93-1) and avirulent (Xv 87-7) strains of this pathogen. Transgenic tomato plants overexpressing the wild-type NR protein were also infected to determine the effect of increased NR expression on disease development.
RESULTS
Response to X. campestris pv. vesicatoria Infection in Wild-Type and Nr Mutant Plants
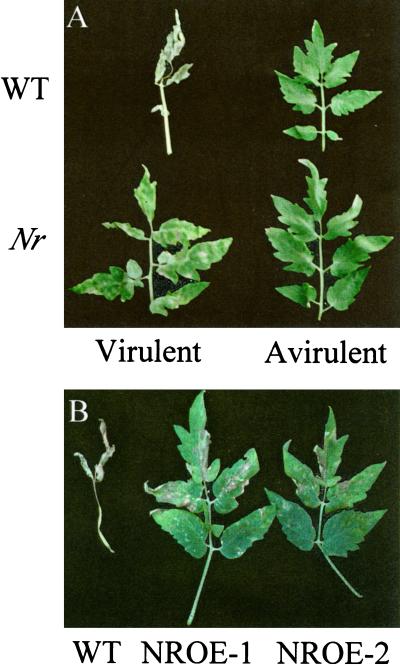
Both wild-type and Nr plants infected with the compatible strain of X. campestris pv. vesicatoria first developed water-soaked lesions on leaves 5 to 6 d after inoculation (DAI). Chlorosis began in the wild-type plants 10 to 12 DAI. In the wild-type plants, this area of chlorosis enlarged and was followed by complete necrosis of entire leaflets 13 to 14 DAI. As reported previously (Lund et al., 1998), in the Nr mutant chlorosis and the spread of necrosis were greatly reduced (Fig. 1A). Wild-type and Nr plants inoculated with the incompatible X. campestris pv. vesicatoria strain Xv 87-7 developed small, light-brown lesions on the abaxial surface of the leaf 3 to 4 DAI. These lesions darkened and increased in quantity up to 5 DAI, but did not increase in size or quantity after this time. There was no further symptom development except for small areas of necrosis that developed along the margins of a few leaflets (Fig. 1A).
Figure 1.
Disease severity in tomato leaves 14 DAI with X. campestris pv. vesicatoria. A, Four-week-old wild-type (WT) and ethylene-insensitive Nr mutant plants were inoculated with virulent and avirulent strains of the pathogen. B, Four-week-old wild-type and NROE-1 and NROE-2 were infected with a virulent strain of the pathogen.
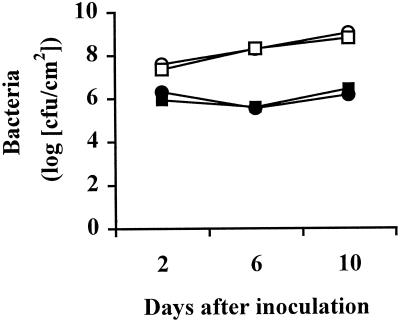
Levels of virulent X. campestris pv. vesicatoria increased approximately 50-fold in both wild-type and Nr plants in the period from 2 to 10 DAI, and there was no difference in bacterial populations between the wild-type and the mutant (Fig. 2). Populations of the avirulent strain Xv 87-7 were approximately 100-fold lower than those of the virulent strain in both the wild-type and the mutant during this same time period, indicating that there was no change in resistance of the Nr plants to this strain.
Figure 2.
Growth of X. campestris pv. vesicatoria in leaves of wild-type and Nr tomato plants. Four-week-old plants were inoculated with virulent and avirulent strains of the pathogen. se bars are smaller than the symbols. ○, Wild-type virulent; ●, wild-type avirulent; □, Nr virulent; ▪, Nr avirulent.
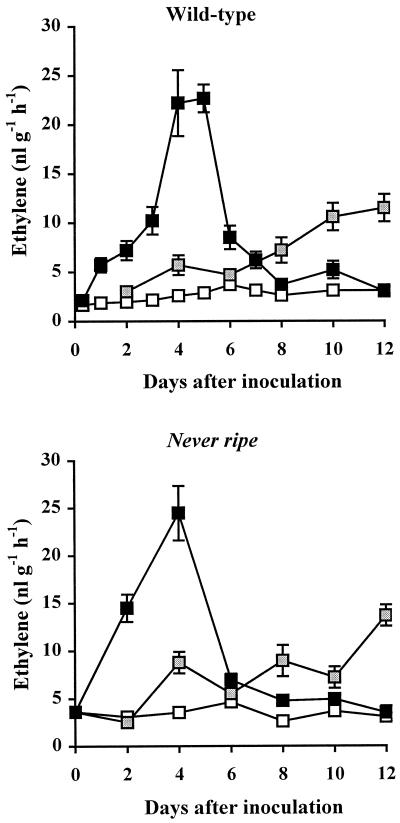
An earlier and greater increase in ethylene levels occurred in plants infected with an avirulent strain of X. campestris pv. vesicatoria compared with plants infected with a virulent strain. Ethylene began to increase 8 to 24 h after inoculation with the avirulent strain and peaked 4 to 5 DAI, with ethylene levels eight times higher than in mock-inoculated plants (Fig. 3). In plants infected with the virulent strain, ethylene levels did not begin to increase until 4 DAI, and did not increase more than 4-fold by 12 DAI. Similar levels of ethylene were observed in infected wild-type and Nr mutant plants (Fig. 3).
Figure 3.
Ethylene synthesis in leaves of wild-type and Nr mutant plants inoculated with X. campestris pv. vesicatoria. Four-week-old plants were inoculated with virulent and avirulent strains of the pathogen. □, Control; ░⃞, virulent; ▪, avirulent.
PR Gene and Ethylene Biosynthesis Gene Expression
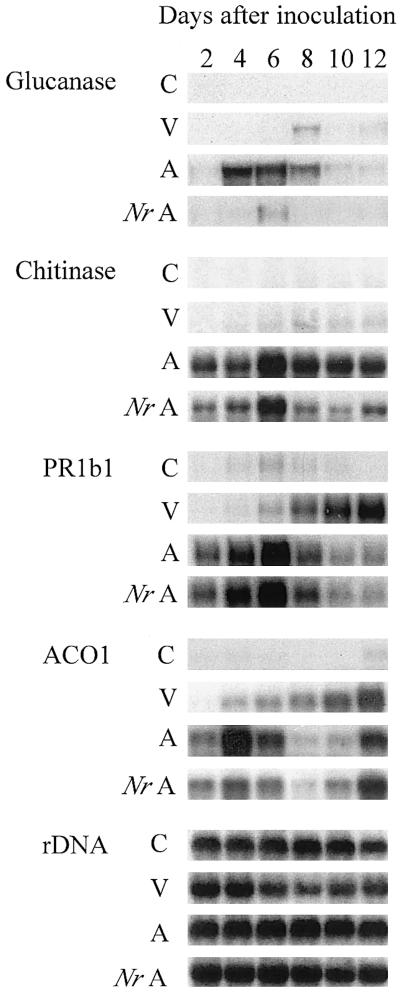
The expression of PR genes and an ethylene biosynthetic gene was measured after inoculation with virulent and avirulent strains of X. campestris pv. vesicatoria. RNA levels of three basic, intracellular PR genes, PR1b1, chitinase, and β-1,3-glucanase, began to increase 1 to 2 DAI (data for 1 DAI not shown) after inoculation with the avirulent strain (Fig. 4). In contrast, PR1b1 gene expression did not increase until 8 DAI in response to the virulent strain, and there was little or no induction of chitinase and β-1,3-glucanase. The expression pattern of a wound-inducible 1-aminocyclopropane-1-carboxylic acid (ACC) oxidase gene (ACO1) was similar to that of PR1b1 except that there was an increase in expression 12 DAI in response to the avirulent strain. Induction of β-1,3-glucanase by the avirulent strain was almost completely inhibited in the Nr mutant, and induction of chitinase and ACO1 gene expression by the avirulent strain was reduced in the Nr mutant compared with wild type. PR1b1 mRNA levels were the same in wild-type and mutant plants (Fig. 4).
Figure 4.
Pathogenesis-related and ethylene biosynthesis gene expression in leaves of wild-type and Nr mutant tomato plants inoculated with X. campestris pv. vesicatoria. Four-week-old plants were inoculated with virulent and avirulent strains of the pathogen. RNA levels were determined by RNA gel-blot analysis. Plants are wild type unless otherwise indicated. C, Control; V, virulent; A, avirulent.
LeETR Gene Expression
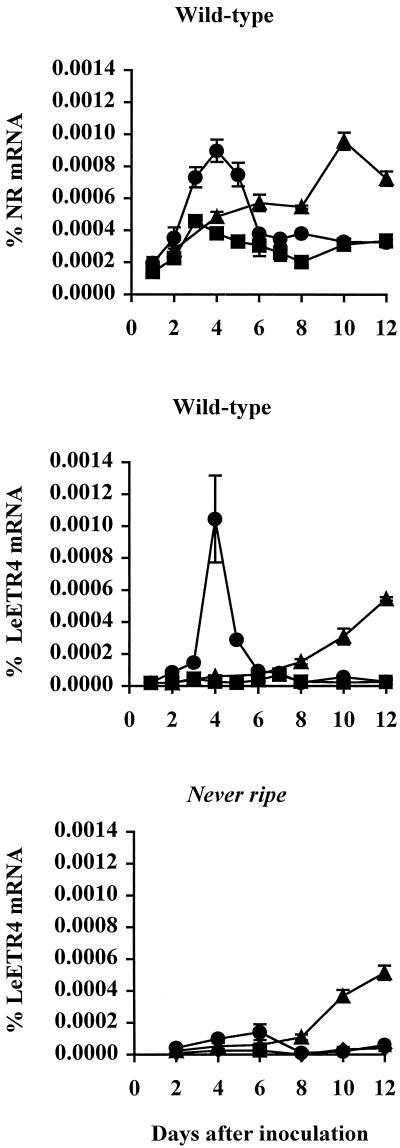
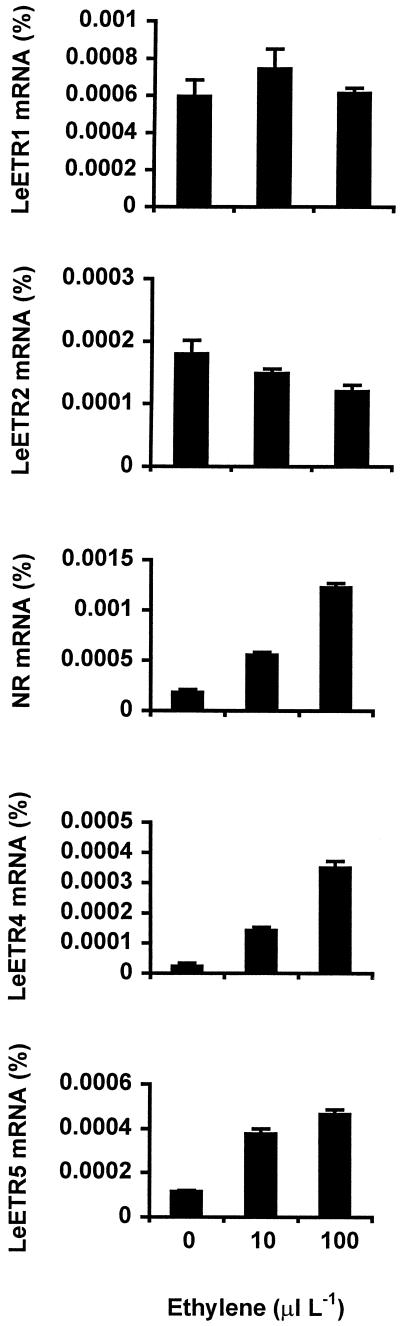
Expression of five members of the tomato ethylene receptor gene family, LeETR1, LeETR2, NR, LeETR4, and LeETR5, was measured in leaves of plants infected with virulent and avirulent strains of X. campestris pv. vesicatoria. NR and LeETR4 mRNA levels began to increase 2 DAI in response to the avirulent strain and peaked 4 DAI, when there was a 3-fold increase in NR mRNA and an approximately 30-fold increase in LeETR4 (Fig. 5). A similar level of induction of NR and LeETR4 expression was observed in response to the virulent strain, but did not begin until 8 DAI. The induction of LeETR4 expression by the avirulent strain was greatly reduced in the Nr mutant, but there was no difference in induction by the virulent strain (Fig. 5). NR gene expression was similar in Nr mutant and wild-type plants (data not shown). There were no significant changes in mRNA levels of LeETR1, LeETR2, and LeETR5 during disease progression (data not shown). Expression of NR, LeETR4, and LeETR5 in leaves was induced by exogenous ethylene, with LeETR4 and NR showing the greatest induction (Fig. 6). Exogenous ethylene did not affect LeETR1 and LeETR2 mRNA levels.
Figure 5.
Expression of the tomato ethylene receptor genes LeETR4 and NR in leaves following inoculation with X. campestris pv. vesicatoria. Four-week-old plants were inoculated with virulent and avirulent strains of the pathogen. Percent mRNA was quantified by RNase protection assays as described in “Materials and Methods.” ▪, Control; ▴, virulent; ●, avirulent.
Figure 6.
Ethylene receptor gene expression in tomato leaves in response to exogenous ethylene. Four-week-old wild-type (cv Pearson) plants were treated with ethylene for 1 h. Percent mRNA of ethylene receptor genes was quantified by RNase protection assays as described in “Materials and Methods.”
Ethylene Sensitivity of NR-Overexpressing Lines
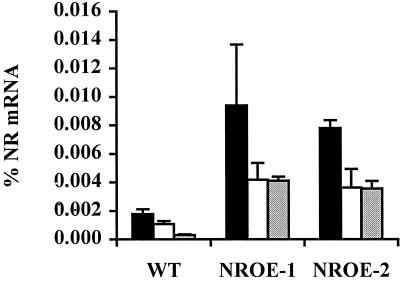
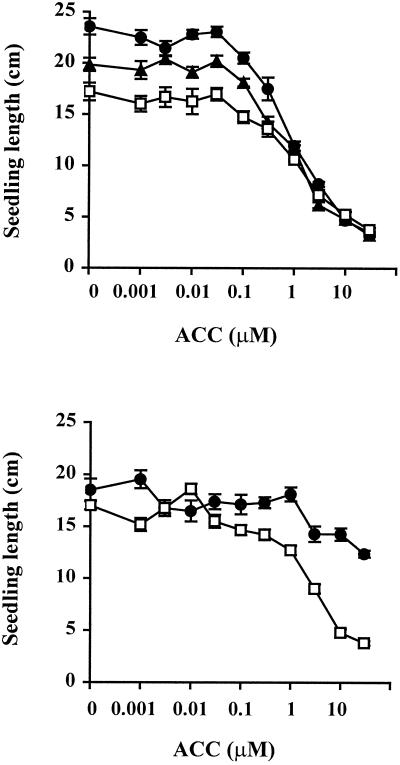
Transgenic tomato plants overexpressing a cDNA of the wild-type NR gene were analyzed to determine the effect of NR expression on ethylene sensitivity. NR mRNA levels were 4- to 10-fold higher than the wild type in seedlings, stems, and leaves of two independent transgenic lines overexpressing the tomato ethylene receptor gene NR (NROE-1 and NROE-2) (Fig. 7). However, NR expression was lower in fruit of the transgenic lines, apparently due to co-suppression of the native gene (data not shown). To determine the effect of increased NR levels on ethylene sensitivity, seedlings were grown in the dark on medium containing varying amounts of the ethylene precursor ACC. ACC is converted to ethylene by the plant and reduces hypocotyl and root elongation in wild-type tomato seedlings (Lanahan et al., 1994). Etiolated seedlings of both NR-overexpressing lines were taller and had longer roots than the wild type, even in the absence of exogenous ethylene (Fig. 8).
Figure 7.
Expression of the ethylene receptor gene NR in stems (black bars), etiolated seedlings (white bars), and leaves (gray bars) of wild-type (WT) and NROE-1 and NROE-2 tomato plants. Percent mRNA was quantified by RNase protection assays as described in “Materials and Methods.”
Figure 8.
Triple response assay of NROE-1 and NROE-2, Nr mutant, and wild-type tomato seedlings. Seedling length is the sum of hypocotyl and root length. Seedlings were grown in the dark for 2 weeks on 1% (w/v) agar containing varying concentrations of ACC. Seedlings in the top panel are cv Floradade (●, NROE-1; ▴, NROE-2; □, wild type); seedlings in the bottom panel are cv Pearson (●, Nr; □, wild type).
Growing seedlings in the presence of the ethylene action inhibitor 1-methylcyclopropene (Sisler and Serek, 1997) reduced this difference in hypocotyl and root length (data not shown), indicating that it was dependent on ethylene sensitivity. At levels of ACC below 0.1 μm, ACC treatment did not reduce seedling length, and NR-overexpressing lines remained longer than the wild type. Concentrations of ACC above 0.1 μm reduced seedling elongation in all three lines, and at 1 μm there was no difference in seedling length between the transgenic lines and the wild type (Fig. 8). Nr mutant seedlings were longer than wild type, even at ACC concentrations above 10 μm, indicating that they were less sensitive to ethylene than the NR-overexpressing lines (Fig. 8).
Like hypocotyl elongation, stem elongation is regulated by endogenous ethylene and can be inhibited by exogenous ethylene (Abeles et al., 1992). Ethylene insensitivity results in increased stem elongation, as illustrated by the greater internode length and plant height of the Nr mutant (Table I). To further analyze the ethylene sensitivity of the NR overexpressors, stem elongation was measured in 9-week-old greenhouse-grown plants. At this age the plants had approximately 12 internodes and had begun to flower. Like the Nr mutant, the NR-overexpressing lines are taller and have longer internodes than wild-type plants, indicating that they have reduced sensitivity to ethylene (Table I).
Table I.
Stem elongation in 9-week-old tomato plants
| Plant | Internode No. | Average Internode Length | Plant Height |
|---|---|---|---|
| cm | |||
| WT (cv Floradade) | 12.6 ± 0.4 | 5.7 ± 0.3 | 84 ± 2 |
| NROE-1 | 12.4 ± 0.2 | 6.8 ± 0.4** | 94 ± 3** |
| NROE-2 | 12.7 ± 0.4 | 6.9 ± 0.4* | 99 ± 4** |
| WT (cv Pearson) | 20.4 ± 0.2 | 8.5 ± 0.4 | 167 ± 4 |
| Nr | 18.0 ± 0.3** | 11.0 ± 0.4** | 176 ± 3* |
NROE-1, NROE-2, Independent transgenic lines overexpressing the ethylene receptor gene NR; WT, wild type. Transgenic lines are cv Floradade, Nr mutant is cv Pearson. *, Significantly different from wild type (P ≤ 0.05); **, significantly different from wild type (P ≤ 0.01).
Pathogen Response of NR-Overexpressing Lines
Two independent NR-overexpressing lines were infected with virulent and avirulent strains of X. campestris pv. vesicatoria. In general, symptom development in the NR overexpressors was similar to that in the Nr mutant. There was no visible difference in symptoms between wild-type and NR-overexpressing lines in response to infection with the avirulent strain (data not shown). However, NR-overexpressing plants infected with the virulent strain displayed greatly reduced necrosis 14 DAI relative to wild type (Fig. 1B). As with the Nr mutant, reduced necrosis in the NR overexpressors was a result of tolerance (rather than resistance) to the pathogen, since there was no difference in bacterial growth between wild-type and transgenic plants (data not shown).
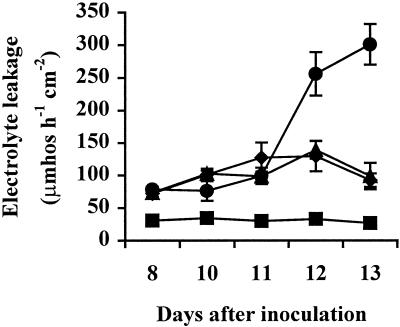
Electrolyte leakage from leaf tissue of inoculated plants was also assayed to quantify the extent of disease damage. At 12 and 13 DAI, electrolyte leakage was significantly higher in wild-type plants, and this increased membrane permeability was accompanied by chlorosis and the spread of necrosis (Fig. 9). The NR overexpressors showed only limited chlorosis at this stage. By 14 DAI, the wild-type leaves were completely necrotic, while the NR overexpressors showed only small areas of necrosis. By 16 DAI, leaves of the NR-overexpressing lines contained large areas of necrosis as well, illustrating that overexpression of NR delayed necrosis but did not completely prevent it. Therefore, tolerance to X. campestris pv. vesicatoria was not as strong in the NR overexpressors as it was in the Nr mutant, which showed little necrosis even 16 DAI (data not shown). There were no significant differences in PR gene expression between wild-type and NR-overexpressing lines (data not shown).
Figure 9.
Electrolyte leakage from tomato leaves infected with a virulent strain of X. campestris pv. vesicatoria. Plants were 4 weeks old at the time of inoculation. ▪, Wild-type control; ●, wild-type virulent; ▴, NROE-1 virulent; ♦, NROE-2 virulent.
DISCUSSION
In tomato, symptom development in response to avirulent X. campestris pv. vesicatoria strain Xv 87-7 is a hypersensitive response involving the formation of small, distinct lesions that do not spread. Since response to this pathogen has not been characterized at the molecular level, we measured PR gene expression in plants infected with virulent and avirulent strains. Expression of PR1b1, β-1,3-glucanase, and chitinase increased more quickly during the incompatible interaction than during the compatible interaction, indicating a faster response to the avirulent strain (Fig. 4). A faster induction of PR genes also occurs during a hypersensitive response to other tomato pathogens such as Cladosporium fulvum (van Kan et al., 1992) and Pseudomonas syringae (Jia and Martin, 1999), as well as during several other incompatible plant-pathogen interactions (Linthorst, 1991). Therefore, response to X. campestris pv. vesicatoria strain Xv 87-7 at the molecular level appears typical of hypersensitive responses to other pathogens. Since all L. esculentum genotypes tested are resistant to this strain, it could serve as a useful tool for studying the hypersensitive response in the wide range of transgenic and mutant tomato lines that lack resistance genes to other pathogens.
Increases in ethylene synthesis (Fig. 3) and ACO1 expression (Fig. 4) also indicated a more rapid response to the avirulent strain than to the virulent strain. Earlier increases in ethylene synthesis have been observed for several other incompatible interactions (Montalbini and Elstner, 1977; De Laat and van Loon, 1983; Boller, 1991), suggesting that ethylene may be one of the signals that initiates the faster defense response. However, no increase in ethylene synthesis occurred during the first 8 h after inoculation with the avirulent strain; therefore, ethylene does not appear to play a role in the earliest resistance responses to X. campestris pv. vesicatoria. Furthermore, the peak in ethylene synthesis occurred relatively late in the disease progression, at the time of lesion formation and spread, suggesting that ethylene could be involved in regulating the spread of cell death during infection. Since ACO1 expression was reduced in the Nr mutant but ethylene levels were not, other enzymes appear to be involved in regulating ethylene synthesis in response to this pathogen.
Chitinase and β-1,3-glucanase expression was reduced in the ethylene-insensitive Nr mutant (Fig. 4) and was correlated with endogenous ethylene levels during infection (Fig. 3 and Fig. 4), indicating that the induction of these genes is ethylene regulated. However, there were no differences in disease symptoms (Fig. 1) or bacterial populations (Fig. 2) between the wild-type and mutant plants infected with the avirulent strain. Therefore, these enzymes do not appear to be critical for resistance to this pathogen. A similar result was observed in the ethylene-insensitive ein2 mutant of Arabidopsis in response to the fungal pathogen Alternaria brassicicola. Pathogen-induced expression of three PR genes was eliminated or greatly reduced in the mutant plants, but resistance to this pathogen was maintained (Thomma et al., 1999). Ethylene-insensitive Arabidopsis (Bent et al., 1992; Thomma et al., 1999), tobacco (Knoester et al., 1998), and soybean (Hoffman et al., 1999) have all shown normal resistance to a range of different avirulent pathogens, while ethylene insensitivity increased susceptibility to B. cinerea in Arabidopsis (Thomma et al., 1999) and to P. sojae in soybean (Hoffman et al., 1999). Therefore, while ethylene does regulate specific components of the defense response, the importance of those components in plant resistance varies greatly from one pathogen to another.
Expression of two tomato ethylene receptor genes, NR and LeETR4, was induced during infection, suggesting that these genes may play a role in pathogen response (Fig. 5). Induction of LeETR4 gene expression by the avirulent pathogen was reduced in the Nr mutant (Fig. 5), indicating that this induction during the incompatible interaction is ethylene regulated. LeETR4 expression was induced by exogenous ethylene and was closely correlated with endogenous ethylene synthesis during infection (Figs. 3 and 6), further indicating that LeETR4 mRNA levels are regulated by ethylene. It is interesting that LeETR4 mRNA levels were not reduced in Nr mutant plants infected with virulent X. campestris pv. vesicatoria, demonstrating that additional signals control LeETR4 expression during the compatible interaction. A similar result was observed in tomato in response to P. syringae pv. tomato, in which the ethylene action inhibitor norbornadiene blocked induction of glucanase and osmotin expression during an incompatible interaction, but had no effect on expression during the compatible interaction (Thara et al., 1999).
NR gene expression was also induced by exogenous ethylene and was correlated with endogenous ethylene during infection (Figs. 3 and 6). However, induction of NR expression by X. campestris pv. vesicatoria was similar in mutant and wild-type plants, indicating that this induction is not ethylene dependent. Although the level of ethylene induction was similar for LeETR4 and NR, the induction of LeETR4 during infection was much greater. Furthermore, LeETR5 mRNA levels also increased in response to exogenous ethylene, but not during infection. These data indicate that the ethylene inducibility of these genes is only one component of pathogen induction.
The effect of pathogen infection on LeETR protein levels has not been determined. The abundance of NR protein is correlated with transcript levels in both wild-type and NR antisense plants (D.M. Tieman, unpublished data); antibodies to the other LeETR proteins are not yet available. Although increased expression of LeETR4 and NR mRNA during infection indicates that protein levels may also increase, it is not yet known what role post-transcriptional regulation plays in determining ethylene receptor abundance.
In Arabidopsis, loss-of-function mutations in four ethylene receptor genes greatly increased sensitivity to ethylene, identifying these genes as negative regulators of the ethylene response (Hua and Meyerowitz, 1998). Similarly, decreased LeETR4 expression in antisense tomato lines caused constitutive ethylene responses such as leaf epinasty and accelerated flower senescence (Tieman et al., 2000). Therefore, a reduction in ethylene receptor levels increases sensitivity to ethylene. According to this model, an increase in receptor levels would be expected to decrease sensitivity. In fact, NR-overexpressing lines are less sensitive to ethylene, as indicated by increased stem elongation in mature plants (Table I) and increased hypocotyl elongation in etiolated seedlings (Fig. 8).
Based on seedling response to ACC, the NR-overexpressing lines are not as ethylene insensitive as the Nr mutant. It has been suggested that the mutant receptors cannot be inactivated due to their inability to bind ethylene (Schaller and Bleecker, 1995). This model would explain why the NR-overexpressing seedlings show no difference in length at higher ACC concentrations, while the Nr mutant is longer than wild type even at the highest concentrations tested (Fig. 8). At high ethylene levels, the additional wild-type NR protein in the overexpressors would be inactivated, while the mutant protein would continue to suppress the ethylene response. The reduced ethylene sensitivity of the NR overexpressors also indicates that NR, like LeETR4, is a negative regulator of ethylene response. Therefore, the induction of LeETR4 and NR gene expression observed in response to X. campestris pv. vesicatoria infection would decrease the ethylene sensitivity of infected tissue.
Given the function of LeETR4 and NR as negative regulators of ethylene response, it is intriguing that these genes are ethylene inducible. Treatment with 10 ppm ethylene for 1 h induced expression of both genes approximately 10-fold (Fig. 6), indicating that one of the plant's responses to increased ethylene levels is a fairly rapid reduction in ethylene sensitivity. Ethylene induction of these genes may serve to regulate the magnitude and duration of ethylene responses. Regulation of ethylene action at the level of both synthesis and perception would allow for an initial response to increased ethylene levels to be quickly dampened by greater LeETR expression. As receptor levels increase, more ethylene would be required to inactivate these suppressors and continue the response.
A strong induction in NR expression also occurs during tomato fruit ripening and is highly correlated with a large increase in ethylene synthesis (Lashbrook et al., 1998). In tissues with autocatalytic ethylene synthesis, such as ripening fruit, an additional level of regulation may be necessary to control the ethylene response. Similar dampening mechanisms exist for other hormones such as auxin, in which increases in endogenous indole-3-acetic acid levels are accompanied by conjugation to inactive forms (Cohen and Bandurski, 1982), and SA, in which pathogen-induced increases are accompanied by conjugation to SA glucosides (Malamy et al., 1992). These mechanisms provide a means of inducing a rapid but brief hormone response, allowing a large initial increase in hormone synthesis while preventing a prolonged activation of these responses.
Like the Nr mutant (Fig. 1A), transgenic plants overexpressing wild-type NR displayed tolerance to virulent X. campestris pv. vesicatoria, as evidenced by reduced necrosis (Fig. 1B) and greater membrane integrity (Fig. 9) in infected NR-overexpressing lines. Therefore, an increase in the expression of the wild-type NR gene is sufficient to confer this tolerance, indicating that the plant may be able to control its response to pathogens through the regulation of this and other ethylene receptor genes. Specifically, an increase in LeETR expression may help to limit the spread of necrosis in response to infection, as it did in the NR overexpressing lines. Induction of the LeETR genes during an incompatible interaction may play a similar role, limiting cell death to the site of infection by decreasing the ethylene sensitivity of the surrounding tissue. Although the induction of LeETR4 expression was blocked in the Nr mutant and the spread of cell death was still limited, this tissue is already insensitive to ethylene and would not require the induction of LeETR4 expression to limit necrosis. Analysis of disease progression in LeETR4 and NR antisense lines will be necessary to determine whether blocking the induction of these genes alters the extent of cell death.
MATERIALS AND METHODS
Plant Material
The homozygous Nr tomato (Lycopersicon esculentum Mill.) mutant and wild-type cv Pearson lines are isogenic (Rick and Butler, 1956). The NR-overexpressing transgenic lines were produced through Agrobacterium tumefaciens-mediated transformation of cv Floradade (McCormick et al., 1986). A NR cDNA under transcriptional control of the figwort mosaic virus promoter (Richins et al., 1987) was inserted into the tomato genome, along with a glyphosate-resistance gene as a selectable marker. Insertion of the transgene was confirmed by PCR amplification of the glyphosate resistance gene. Primary transformants were self-pollinated to produce lines that were homozygous for the transgene.
Inoculations and Disease Development
Four-week-old tomato plants were inoculated with Xanthomonas campestris pv. vesicatoria strains Xv 93-1 (virulent) and Xv 87-7 (avirulent). X. campestris pv. vesicatoria strain Xv 87-7 is avirulent on all L. esculentum genotypes tested, but virulent on pepper cv Early Calwonder (Canteros, et al., 1991). Xv 87-7 contains the avirulence gene avrBs3-2; conjugation of a virulent strain (Xv 75-3) with this gene converts the strain to avirulent in tomato (Bonas et al., 1993). Inoculations were performed by dipping plants for 15 s into an inoculum containing 1 × 108 colony forming units (cfu)/mL and 0.025% (v/v) Silwet 77 (Lehle Seeds, Round Rock, TX) in sterile tap water. Control plants were dipped in sterile tap water containing 0.025% (v/v) Silwet 77. Plants were grown under standard greenhouse conditions. Electrolyte leakage and bacterial growth were measured as described previously (Lund et al., 1998). All experiments were repeated at least twice.
Ethylene samples were collected by placing single leaflets from the third or fourth leaf from the base of the plant into 5-mL containers and incubating at room temperature for 1 h. Ethylene concentration from a 1-mL sample was determined by gas chromatograph (model 5890, Hewlett-Packard, Palo Alto, CA).
RNA Isolation and Quantification
RNA was isolated from leaflets of the third and fourth leaf from the base of the plant; these were the two youngest fully expanded leaves at the time of inoculation. For the NR-overexpressing lines, RNA was also isolated from 2-week-old etiolated seedlings grown on 1% (w/v) agar and the 10th internode (from the base of the plant) of 9-week-old greenhouse-grown plants. RNA was extracted in SDS-phenol and purified by LiCl precipitation. Northern-blot analysis was performed as described using 10 μg of total RNA (Kneissl and Deikman, 1996). All DNA probes were labeled with 32P by random primer labeling, as described by Sambrook et al. (1989). The template for PR1b1 was a 348-bp PCR fragment (Lund et al., 1998). A 655-bp PCR fragment from a basic intracellular β-1,3-glucanase (GenBank accession no. M80608; van Kan et al., 1992) was amplified using the forward primer 5′-TCTTGCCCCATTTCAACTTC and the reverse primer 3′-GTCCCAAACTCTTTCAGACACC. The template for the basic intracellular chitinase (GenBank accession no. Z15140; Danhash et al., 1993) was isolated from a Lambda Zap II cDNA library prepared from phosphate-stressed tomato roots. The template for ACO1 (ethylene biosynthesis gene ACC Oxidase 1) was a full-length cDNA (GenBank accession no. X04792; Holdsworth et al., 1987). Blots were probed with labeled 18S rDNA from Zamia floridana to ensure equal levels of total RNA. RNase protection assays were performed with 20 μg of total RNA using gene-specific probes as described previously (Lashbrook et al., 1998; Tieman and Klee, 1999).
Ethylene and ACC Treatment
Ethylene treatments were conducted by sealing 4-week-old wild-type tomato plants (cv Pearson) in glass containers and adding ethylene to 10 or 100 μL L−1. Control plants were sealed in glass containers containing potassium permanganate, an ethylene absorbant. Triple response assays were performed by germinating surface-sterilized seed on 1% (w/v) agar containing varying concentrations of ACC. Seedlings were grown in the dark for 2 weeks at room temperature.
ACKNOWLEDGMENTS
We wish to thank Dr. Jack Wilkinson for the NR overexpressor constructs, Jeanne Layton at Monsanto for producing the NR-overexpressing transgenic plants, and Dr. Mark Taylor for care and maintenance of these plants. We also thank Gerry Minsavage for maintenance of bacterial strains and assistance in growing plants for inoculation. Finally, we thank Kerrie Powell for cloning of the chitinase cDNA.
Footnotes
This work was supported in part by the National Science Foundation (grant no. IBN–9728133 to H.J.K.) and the U.S. Department of Agriculture (grant no. 95–37304–2326 to H.J.K.). This is Florida Agricultural Experiment Station journal series no. R–07335.
LITERATURE CITED
- Abeles FB, Morgan PW, Saltveit ME. Ethylene in Plant Biology. Ed 2. San Diego: Academic Press; 1992. pp. 264–296. [Google Scholar]
- Bent AF, Innes RW, Ecker JR, Staskawicz BJ. Disease development in ethylene-insensitive Arabidopsis thaliana infected with virulent and avirulent Pseudomonas and Xanthomonaspathogens. Mol Plant-Microbe Interact. 1992;5:372–378. doi: 10.1094/mpmi-5-372. [DOI] [PubMed] [Google Scholar]
- Boller T. Ethylene in pathogenesis and disease resistance. In: Matoo AK, Suttle JC, editors. The Plant Hormone Ethylene. Boca Raton, FL: CRC Press; 1991. pp. 293–314. [Google Scholar]
- Bonas U, Conrads-Strauch J, Balbo I. Resistance in tomato to Xanthomonas campestris pv vesicatoria is determined by alleles of the pepper-specific avirulence gene avrBs3. Mol Gen Genet. 1993;238:261–269. doi: 10.1007/BF00279555. [DOI] [PubMed] [Google Scholar]
- Bradley DJ, Kjellbom P, Lamb CJ. Elicitor- and wound-induced oxidative cross-linking of a proline-rich plant cell wall protein: a novel, rapid defense response. Cell. 1992;70:21–30. doi: 10.1016/0092-8674(92)90530-p. [DOI] [PubMed] [Google Scholar]
- Brisson LF, Tenhaken R, Lamb CJ. Function of oxidative cross-linking of cell wall structural proteins in plant disease resistance. Plant Cell. 1994;6:1703–1712. doi: 10.1105/tpc.6.12.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broglie K, Chet I, Jolliday M, Cressman R, Biddle P, Knowlton S, Mauvais CJ, Broglie R. Transgenic plants with enhanced resistance to the fungal pathogen Rhizoctonia solani. Science. 1991;254:1194–1197. doi: 10.1126/science.254.5035.1194. [DOI] [PubMed] [Google Scholar]
- Canteros B, Minsavage G, Bonas U, Pring D, Stall R. A gene from Xanthomonas campestris pv. vesicatoria that determines avirulence in tomato is related to avrBs3. Mol Plant-Microbe Interact. 1991;4:628–632. doi: 10.1094/mpmi-4-628. [DOI] [PubMed] [Google Scholar]
- Chang C, Kwok SF, Bleecker AB, Meyerowitz EM. Arabidopsis ethylene response gene ETR1-similarity of product to two-component regulators. Science. 1993;262:539–544. doi: 10.1126/science.8211181. [DOI] [PubMed] [Google Scholar]
- Cohen JD, Bandurski RS. Chemistry and physiology of the bound auxins. Annu Rev Plant Physiol. 1982;33:403–430. [Google Scholar]
- Danhash N, Wagemakers CAM, van Kan JAL, de Wit PJGM. Molecular characterization of four chitinase cDNAs obtained from Cladosporium-infected tomato. Plant Mol Biol. 1993;22:1017–1029. doi: 10.1007/BF00028974. [DOI] [PubMed] [Google Scholar]
- Deikman J. Molecular mechanisms of ethylene regulation of gene transcription. Physiol Plant. 1997;100:561–566. [Google Scholar]
- De Laat AMM, van Loon LC. The relationship between stimulated ethylene production and symptom expression in virus-infected tobacco leaves. Physiol Plant Pathol. 1983;22:261–273. [Google Scholar]
- Delaney TP, Uknes S, Vernooij B, Friedrich L, Weymann K, Negrotto D, Gaffney T, Gut-Rella M, Kessmann H, Ward E, Ryals J. A central role of salicylic acid in plant disease resistance. Science. 1994;266:1247–1250. doi: 10.1126/science.266.5188.1247. [DOI] [PubMed] [Google Scholar]
- Ecker JR, Davis RW. Plant defense genes are regulated by ethylene. Proc Natl Acad Sci USA. 1987;84:5202–5206. doi: 10.1073/pnas.84.15.5202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epple P, Apel K, Bohlmann H. An Arabidopsis thalianathionin gene is inducible via a signal transduction pathway different from that for pathogenesis-related proteins. Plant Physiol. 1995;109:813–820. doi: 10.1104/pp.109.3.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman RN, Novacky AJ. The Hypersensitive Reaction in Plants to Pathogens: A Resistance Response. APS Press, St. Paul. 1994. pp. 117–119. [Google Scholar]
- Greenberg JT, Guo A, Klessig DF, Ausubel FM. Programmed cell death in plants: a pathogen-triggered response activated coordinately with multiple defense functions. Cell. 1994;77:551–563. doi: 10.1016/0092-8674(94)90217-8. [DOI] [PubMed] [Google Scholar]
- Hain R, Reif H, Krause E, Langebartels R, Kindl H, Vonram B, Wiese W, Schmelzer E, Schreier PH, Stocker RH, Stenzel K. Disease resistance results from foreign phytoalexin expression in a novel plant. Nature. 1993;361:153–156. doi: 10.1038/361153a0. [DOI] [PubMed] [Google Scholar]
- Hoffman T, Schmidt JS, Zheng X, Bent AF. Isolation of ethylene-insensitive soybean mutants that are altered in pathogen susceptibility and gene-for-gene disease resistance. Plant Physiol. 1999;119:935–949. doi: 10.1104/pp.119.3.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holdsworth MJ, Bird CR, Ray J, Schuch W, Grierson D. Structure and expression of an ethylene-related mRNA from tomato. Nucleic Acids Res. 1987;15:731–739. doi: 10.1093/nar/15.2.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua J, Chang C, Sun Q, Meyerowitz EM. Ethylene insensitivity conferred by Arabidopsis ERSgene. Science. 1995;269:1712–1714. doi: 10.1126/science.7569898. [DOI] [PubMed] [Google Scholar]
- Hua J, Meyerowitz EM. Ethylene responses are negatively regulated by a receptor gene family in Arabidopsis thaliana. Cell. 1998;94:261–271. doi: 10.1016/s0092-8674(00)81425-7. [DOI] [PubMed] [Google Scholar]
- Hua J, Sakai H, Nourizadeh S, Chen QG, Bleecker AB, Ecker JR, Meyerowitz EM. EIN4 and ERS2 are members of the putative ethylene receptor gene family in Arabidopsis. Plant Cell. 1998;10:1321–1332. doi: 10.1105/tpc.10.8.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia YL, Martin GB. Rapid transcript accumulation of pathogenesis-related genes during an incompatible interaction in bacterial speck disease-resistant tomato plants. Plant Mol Biol. 1999;40:455–465. doi: 10.1023/a:1006213324555. [DOI] [PubMed] [Google Scholar]
- Keppler LD, Baker CJ, Atkinson MM. Activated oxygen production during a bacteria induced hypersensitive reaction in tobacco suspension cells. Phytopathology. 1989;79:974–978. [Google Scholar]
- Kneissl ML, Deikman J. The tomato E8gene influences ethylene biosynthesis in fruit but not in flowers. Plant Physiol. 1996;112:537–547. doi: 10.1104/pp.112.2.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoester M, van Loon LC, van den Heuvel J, Hennig J, Bol JF, Linthorst HJM. Ethylene-insensitive tobacco lacks non-host resistance against soil-borne fungi. Proc Natl Acad Sci USA. 1998;95:1933–1937. doi: 10.1073/pnas.95.4.1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanahan MB, Yen H-C, Giovannoni JJ, Klee HJ. The Never ripemutation blocks ethylene perception in tomato. Plant Cell. 1994;6:521–530. doi: 10.1105/tpc.6.4.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lashbrook CC, Tieman DM, Klee HJ. Differential regulation of the tomato ETR gene family throughout plant development. Plant J. 1998;15:243–252. doi: 10.1046/j.1365-313x.1998.00202.x. [DOI] [PubMed] [Google Scholar]
- Linthorst HJM. Pathogenesis-related proteins in plants. Crit Rev Plant Sci. 1991;10:123–150. [Google Scholar]
- Lund ST, Stall RE, Klee HJ. Ethylene regulates the susceptible response to pathogen infection in tomato. Plant Cell. 1998;10:371–382. doi: 10.1105/tpc.10.3.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malamy J, Carr JP, Klessig DF, Raskin I. Salicylic acid: a likely endogenous signal in the resistance response of tobacco to viral infection. Science. 1990;250:1002–1004. doi: 10.1126/science.250.4983.1002. [DOI] [PubMed] [Google Scholar]
- Malamy J, Hennig J, Klessig DF. Temperature-dependent induction of salicylic acid and its conjugates during the resistance response to tobacco mosaic virus infection. Plant Cell. 1992;4:359–366. doi: 10.1105/tpc.4.3.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick S, Neidermeyer J, Fry J, Barnason A, Horsch R, Fraley R. Leaf disc transformation of cultivated tomato (L. esculentum) using Agrobacterium tumefaciens. Plant Cell Rep. 1986;5:81–84. doi: 10.1007/BF00269239. [DOI] [PubMed] [Google Scholar]
- Moerschbacher BM, Noll U, Gorrichon L, Reisener HJ. Specific inhibition of lignification breaks hypersensitive resistance of wheat to stem rust. Plant Physiol. 1990;93:465–470. doi: 10.1104/pp.93.2.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montalbini P, Elstner EF. Ethylene evolution by rust-infected, detached bean (Phaseolus vulgaris L.) leaves susceptible and hypersensitive to Uromyces phaseoli(Pers.) Wint. Planta. 1977;135:301–306. doi: 10.1007/BF00384904. [DOI] [PubMed] [Google Scholar]
- Orlandi EW, Hutcheson SW, Baker CJ. Early physiological responses associate with race-specific recognition in soybean leaf tissue and cell suspensions treated with Pseudomonas syringae pv. glycinea. Physiol Mol Plant Pathol. 1992;40:173–180. [Google Scholar]
- Parker JE, Szabo V, Staskawicz B, Lister C, Dean C, Daniels MJ, Jones J. Phenotypic characterizationand molecular mapping of the Arabidopsis thaliana locus RPP5, determining disease resistance to Peronospora parasitica. Plant J. 1993;4:821–831. [Google Scholar]
- Penninckx IAMA, Eggermont K, Terras FRG, Thomma BPHJ, De Samlanx GW, Buchala A, Metraux J-P, Manners JM, Broekaert WF. Pathogen-induced systemic activation of a plant defensin gene in Arabidopsisfollows a salicylic acid-independent pathway. Plant Cell. 1996;8:2309–2323. doi: 10.1105/tpc.8.12.2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penninckx IAMA, Thomma BPHJ, Buchala A, Metraux J-P, Broekaert WF. Concomitant activation of jasmonate and ethylene response pathways is required for induction of a plant defensin gene in Arabidopsis. Plant Cell. 1998;10:2103–2113. doi: 10.1105/tpc.10.12.2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richins RD, Scholthof HB, Shepard RJ. Sequence of the figwort mosaic virus DNA (caulimovirus group) Nucleic Acid Res. 1987;15:8451–8466. doi: 10.1093/nar/15.20.8451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rick C, Butler L. Phytogenetics of the tomato. Adv Gen. 1956;8:267–382. [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: A Laboratory Manual. Ed 2. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- Schaller GE, Bleecker AB. Ethylene binding sites generated in yeast expressing the Arabidopsis ETR1gene. Science. 1995;270:1809–1811. doi: 10.1126/science.270.5243.1809. [DOI] [PubMed] [Google Scholar]
- Showalter AM, Bell JN, Cramer CL, Bailey JA, Varner JE, Lamb CJ. Accumulation of hydroxyproline-rich glycoprotein mRNAs in response to fungal elicitor and infection. Proc Natl Acad Sci USA. 1985;82:6551–6555. doi: 10.1073/pnas.82.19.6551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sisler EC, Serek M. Inhibitors of ethylene responses in plants at the receptor level: recent developments. Physiol Plant. 1997;100:577–582. [Google Scholar]
- Staskawicz BJ, Ausubel FM, Baker BJ, Ellis JG, Jones JDG. Molecular genetics of plant disease resistance. Science. 1995;268:661–667. doi: 10.1126/science.7732374. [DOI] [PubMed] [Google Scholar]
- Thara VK, Tang X, Gu YQ, Martin GB, Zhou JM. Pseudomonas syringae pv tomato induces the expression of tomato EREBP-like genes Pti4 and Pti5independent of ethylene, salicylate and jasmonate. Plant J. 1999;20:475–483. doi: 10.1046/j.1365-313x.1999.00619.x. [DOI] [PubMed] [Google Scholar]
- Thomma BPHJ, Eggermont K, Penninckx IAMA, Mauch-Mani B, Vogelsang R, Cammue BPA, Broekaert WF. Separate jasmonate-dependent and salicylate-dependent defense-response pathways in Arabidopsisare essential for resistance to distinct microbial pathogens. Proc Natl Acad Sci USA. 1998;95:15107–15111. doi: 10.1073/pnas.95.25.15107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomma BPHJ, Eggermont K, Tierens KFMJ, Broekaert WF. Requirement of functional Ethylene-Insensitive 2 gene for efficient resistance of Arabidopsis to infection by Botrytis cinerea. Plant Physiol. 1999;121:1093–1101. doi: 10.1104/pp.121.4.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tieman DM, Klee HJ. Differential expression of two novel members of the tomato ethylene receptor family. Plant Physiol. 1999;120:165–172. doi: 10.1104/pp.120.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tieman DM, Taylor MG, Ciardi JA, Klee HJ (2000) The tomato ethylene receptors NR and LeETR4 are negative regulators of ethylene response and exhibit functional compensation within a multigene family. Proc Natl Acad Sci USA (in press) [DOI] [PMC free article] [PubMed]
- Toppan A, Roby D, Esquerre-Tugaye M-T. Cell surfaces in plant-microorganism interactions: III. In vivo effect of ethylene on hydroxyproline-rich glycoprotein accumulation in the cell wall of diseased plants. Plant Physiol. 1982;70:82–86. doi: 10.1104/pp.70.1.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Kan JAL, Joosten MHAJ, Wagemakers CAM, van den Berg-Velthius GCM, de Wit PJGM. Differential accumulation of mRNAs encoding extracellular and in-tracellular PR proteins in tomato induced by virulent and avirulent races of Cladosporium fulvum. Plant Mol Biol. 1992;20:513–527. doi: 10.1007/BF00040610. [DOI] [PubMed] [Google Scholar]
- Wilkinson JQ, Lanahan MB, Yen H-C, Giovannoni JJ, Klee HJ. An ethylene-inducible component of signal transduction encoded by Never-ripe. Science. 1995;270:1807–1809. doi: 10.1126/science.270.5243.1807. [DOI] [PubMed] [Google Scholar]
- Wu GS, Short BJ, Lawrence EB, Levine EB, Fitzsimmons KC, Shah DM. Disease resistance conferred by expression of a gene encoding H2O2-generating glucose oxidase in transgenic potato plants. Plant Cell. 1995;7:1357–1368. doi: 10.1105/tpc.7.9.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Q, Maher EA, Maoud S, Dixon RA, Lamb CJ. Enhanced protection against fungal attack by constitutive coexpression of chitinase and glucanase genes in transgenic tobacco. Biotechnology. 1994;12:807–812. [Google Scholar]