Abstract
OBJECTIVE
Our objective was to determine the association between muscle cross-sectional area and attenuation, as measured on routine CT scans, and mortality in older patients with hip fracture.
MATERIALS AND METHODS
A retrospective 10-year study of patients with hip fracture was conducted with the following inclusion criteria: age 65 years or older, first-time hip fracture treated with surgery, and CT of the chest, abdomen, or pelvis. This yielded 274 patients (70.4% women; mean [± SD] age, 81.3 ± 8.3 years). On each CT scan, two readers independently measured the size (cross-sectional area, indexed for patient height) and attenuation of the paravertebral muscle at T12 and the psoas muscle at L4. We then determined the association between overall mortality and the muscle size and muscle attenuation, while adjusting for demographic variables (age, sex, ethnicity, and body mass index), American Society of Anesthesiologists (ASA) classification, and Charlson comorbidity index (CCI).
RESULTS
The overall mortality rate increased from 28.3% at 1 year to 79.5% at 5 years. Mortality was associated with decreased thoracic muscle size (odds ratio [OR], 0.66; 95% CI, 0.49–0.87). This association persisted after adjusting for demographic variables (OR, 0.69; 95% CI, 0.50–0.95), the ASA classification (OR, 0.70; CI, 0.51–0.97), and the CCI (OR, 0.72; 95% CI, 0.52–1.00). Similarly, decreased survival was associated with decreased thoracic muscle attenuation after adjusting for all of these combinations of covariates (OR, 0.67–0.72; 95% CI, 0.49–0.99). Decreased lumbar muscle size and attenuation trended with decreased survival but did not reach statistical significance.
CONCLUSION
In older adults with hip fractures, CT findings of decreased thoracic paravertebral muscle size and attenuation are associated with decreased overall survival.
Keywords: CT, hip fracture, mortality, muscle, sarcopenia
Sarcopenia, which is broadly defined as significant loss of muscle mass and function [1], is recognized as a growing epidemic in rapidly aging populations worldwide [2]. The public health implications of sarcopenia include physical disability [3], falls [4], prolonged hospitalization [5], and the associated health care costs [6]. Various approaches to diagnosing sarcopenia have included functional muscle testing and imaging, including dual x-ray absorptiometry, ultrasound, MRI, and CT [1]. In nonorthopedic patients, CT measurements of muscle size and attenuation have been associated with postoperative complications and premature death [7–10].
The association between sarcopenia and decreased survival has been studied most extensively in patients with various forms of cancer, who may be uniquely affected by cancer cachexia and toxicity from chemo-therapy [1, 11]. However, little is known about any association between sarcopenia and overall survival in patients with osteoporotic hip fractures, even though the mortality rates among elderly patients with hip fractures are higher than those for many forms of cancer (e.g., breast cancer) [12]. After hip fracture, the 1-year mortality rates are generally 20–30% [13–15], but reportedly can range widely from 14% to 36% [16]. In patients with hip fracture, increased mortality has been associated with increased age, male sex, and increased number of comorbidities [15, 16].
Importantly, patient prognosis may influence surgical decision making. For example, for patients with a favorable life expectancy, treatment of displaced femoral neck fractures with total hip arthroplasty rather than hemiarthroplasty may be indicated, resulting in lower reoperation rates, better hip function, and better quality of life [8]. Unfortunately, there are currently no accepted objective CT findings that would help the orthopedic surgeon assess frailty and determine prognosis. Although physicians have traditionally assessed prognosis subjectively on the basis of their clinical experience, such an assessment may be subject to personal biases, may lack a scientifically proven method, and may have low reproducibility [9, 10]. Furthermore, risk stratification has become a major priority in medicine because of the growing emphasis on health care safety, quality, and cost effectiveness (e.g., pay-for-performance models of reimbursement) [17]. Mortality rates associated with hip fractures are now widely tracked as an important indicator of inpatient quality of care [18].
Approximately 82 million CT scans are performed annually in the United States alone [19]. For elderly patients after a ground-level fall, CT examinations are sometimes obtained to evaluate for radiographically occult fracture, assess a fracture for preoperative planning, or evaluate for other contemporaneous conditions that commonly occur with various medical comorbidities. Previous studies have validated CT measurement of muscle size as a biomarker for muscle atrophy [20] and muscle attenuation as a biomarker for muscle fatty infiltration [21], which could be useful in diagnosing sarcopenia. However, these CT measurements have not been used for patients with hip fracture to opportunistically evaluate for sarcopenia.
We hypothesized that CT findings of sarcopenia are associated with increased mortality in older patients with hip fracture. Our specific objectives were to determine whether muscle cross-sectional area and muscle attenuation are associated with clinical outcomes and whether these CT measurements are associated with mortality, beyond established risk factors such as patient demographics, the American Society of Anesthesiologists (ASA) classification, and the Charlson comorbidity index (CCI).
Materials and Methods
Patients and Study Design
After institutional review board approval with a waiver of informed consent, we performed a retrospective study at a large academic medical center by searching both the electronic medical record and the PACS for all patients who met the following three inclusion criteria: age 65 years or older with a low-energy injury (e.g., fall from standing position) between March 1, 2005, and February 28, 2015; first-time hip fracture treated with surgery by one of four board-certified orthopedic surgeons; and diagnostic CT examination of the chest, abdomen, or pelvis. This yielded 274 patients. (For 258 of the 274 patients, surgery was performed within 3 days, with surgery delayed for up to 1 week for the remaining 16 patients owing to comorbidities requiring medical optimization.) No cases met our predefined exclusion criteria of belonging to a vulnerable population (e.g., imprisoned) or exhibiting postoperative changes at the ROIs (e.g., metallic artifact from spinal instrumentation).
Using the electronic medical record entry at the time of hospitalization, three categories of medical data were collected: demographic characteristics (sex, age, self-identified ethnicity, body mass index [BMI; weight in kilograms divided by the square of height in meters], ASA physical status classification [22], and CCI score [23]), proximal femoral fracture location (neck, intertrochanteric, or subtrochanteric), and fracture treatment (internal fixation, hemiarthroplasty, or total hip arthroplasty).
The CCI is a measure of aggregate chronic disease burden that has been validated for predicting mortality as well as high health care costs [23]. The CCI is a weighted sum of numerous diagnoses (e.g., 1 point for congestive heart failure or diabetes without complications, 2 points for moderate or severe renal disease, or 3 points for moderate or severe liver disease). The CCI is used extensively in the medical literature for comorbidity adjustment.
Annual subject enrollment was as follows: year 1 (n = 16), year 2 (n = 17), year 3 (n = 20), year 4 (n = 17), year 5 (n = 33), year 6 (n = 30), year 7 (n = 30), year 8 (n = 28), year 9 (n = 26), year 10 (n = 48), and year 11 (n = 9). CT examinations were performed within 3 months of the hip fracture for 239 of 274 patients (87%), at 3–12 months for 23 (8%), and at more than 12 months for 12 (4%). The primary clinical indications for CT were to evaluate for fracture (157/274; 57%), vascular disorder (42/274; 15%), tumor (27/274; 10%), infection (24/274; 9%), or other conditions (e.g., abdominal pain) (24/274; 9%). For these patients, the primary diagnostic conclusions given in the CT reports were fracture (156/274; 57%), no acute derangement (63/274; 23%), active infection (18/274; 7%), active tumor (22/274; 8%), and vascular disorder (15/274; 6%). Scoliosis was incidentally observed in 41 of 274 patients (15%).
Mortality was determined using the National Death Index, which is a centralized database of death record information available from the National Center for Health Statistics at the U.S. Centers for Disease Control and Prevention. The National Death Index is updated annually, is considered a robust information source available to investigators (but not the general public), and is widely accepted as the reference standard for studies of mortality outcomes. In cross-checking National Death Index data with our hospital electronic medical records, we found that National Death Index data provided a more comprehensive accounting of mortality events, presumably because death often occurs outside the hospital.
CT Examination Protocol
Patients were scanned on one of five MDCT scanners (one 16-MDCT GE Healthcare scanner, one 64-MDCT GE Healthcare scanner, two 64-MDCT Siemens Healthcare scanners, and one 128-MDCT Siemens Healthcare scanner) that completed regular daily calibration using manufacturer-supplied phantoms to ensure consistency in attenuation measurements. Detailed cross-evaluation of contemporaneous scanner records showed only slight systematic variance between vendors for water (0 HU) and polymethyl methacrylate (120 HU) phantoms that bracket the normal attenuation of muscle. For water, the average values recorded were 3 HU for the GE Healthcare scanners versus 1 HU for the Siemens Healthcare scanners. For polymethyl methacrylate, the values recorded were 123 HU for the GE Healthcare scanners versus 124 HU for the Siemens Healthcare scanners. Of the 274 examinations, 263 were performed on GE Healthcare scanners. An audit of the American College of Radiology phantom results recorded for all CT scanners used in this study showed consistent close calibration (variability, < 5%). Scans were performed routinely at 120 kV with a reference effective tube current of 220–260 mA. Beam collimation ranged from 20 to 40 mm, with all ROI measurements made on 5-mm-thick axial images windowed for soft tissue and using standard reconstruction kernels (standard body filter for the GE Healthcare scanners and B40 for the Siemens Healthcare scanners). IV contrast material was administered in 184 of 274 cases (67%), which resulted in enhancement of approximately 11 HU in the portal phase. We conducted sensitivity analyses, where all statistical models were stratified by administration of contrast material, and saw no significant alterations in the results (data not shown).
CT Image Measurements
Measurements were made independently on every CT scan by two readers (one faculty radiologist with 3 years of practice after fellowship training in musculoskeletal radiology and one fellow in musculoskeletal radiology) who were blinded to clinical data. Measurements were made with the routine clinical PACS software (iSite version 3.6, Philips Healthcare) used by all physicians at our institution.
Muscle cross-sectional area and attenuation were measured on axial images by outlining a freehand ROI around the circumference of the paravertebral muscles at the T12 pedicle level (on CT scans of the chest and abdomen) (Fig. 1) and at the L4 pedicle level (on CT scans of the abdomen and pelvis) (Fig. 2), using a method similar to that reported elsewhere for liver transplant recipients [9] and patients with pancreatic adenocarcinoma [24]. This method involves measuring muscles bilaterally, which helps account for any muscle asymmetry associated with scoliosis. These measurements were used to calculate the following variables: thoracic muscle index is the sum of the left and right paravertebral muscle areas (centimeters squared) divided by patient height (meters squared), thoracic muscle density is the average attenuation of the left and right paravertebral muscles (measured in Hounsfield units), lumbar muscle index is the total psoas muscle area (centimeters squared) divided by patient height (meters squared), and lumbar muscle density is the average of left and right psoas muscle attenuation (measured in Hounsfield units).
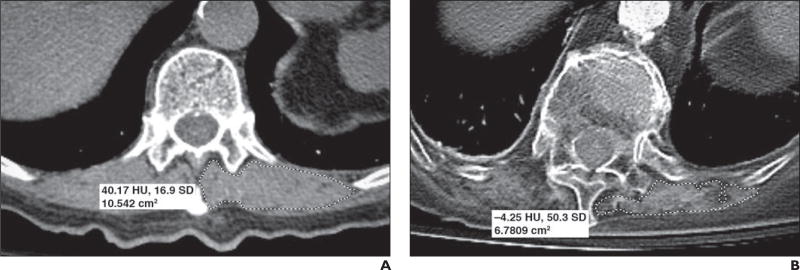
Fig. 1.
Two different 81-year-old women. Axial CT images outlining paravertebral muscles unilaterally were obtained at T12 level for both women.
A, For first woman, image shows cross-sectional area (dashed outline) measuring 10.54 cm2 and mean muscle attenuation measuring 40.17 HU (SD, 16.9 HU).
B, For second woman, image shows cross-sectional area (dashed outline) measuring 6.78 cm2 and mean muscle attenuation measuring −4.25 HU (SD, 50.3 HU). Although only left-sided measurements are shown, all study subjects had bilateral measurements that were then averaged.
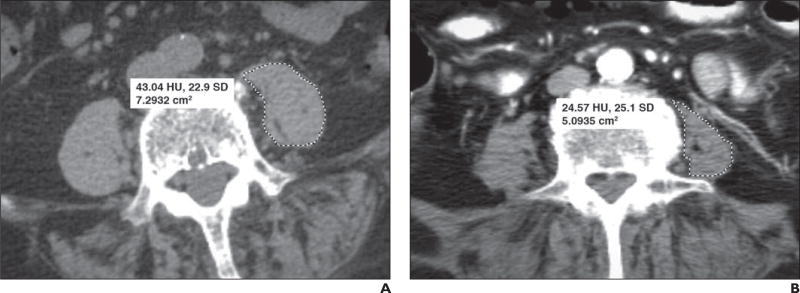
Fig. 2.
Two different 74-year-old women. Axial CT images outlining psoas muscles unilaterally were obtained at L4 level for both women.
A, For first woman, image shows cross-sectional area (dashed outline) measuring 7.29 cm2 and mean muscle attenuation measuring 43.04 HU (SD, 22.9 HU).
B, For second woman, image shows cross-sectional area (dashed outline) measuring 5.09 cm2 and mean muscle attenuation measuring 24.57 HU (SD, 25.1 HU). Although only left-sided measurements are shown, all study subjects had bilateral measurements that were then averaged.
Statistical Analysis
The measurements by the two readers were averaged for the purposes of the outcome analyses. Interreader reliability for thoracic muscle index, thoracic muscle density, lumbar muscle index, and lumbar muscle density were analyzed by calculating intraclass correlation coefficients and coefficients of variation.
Baseline clinical and demographic characteristics were summarized using descriptive measures, and comparisons of characteristics between the sexes were performed using t tests for continuous variables and chi-square tests for categoric variables. The association of patient mortality versus baseline muscle metrics was performed using logistic regression and Cox proportional hazards models fitting each of the four muscle metrics standardized by sex as linear predictors. Each association was presented unadjusted; adjusted for age, sex, ethnicity, and BMI (model 1); model 1 plus ASA risk classification score (model 2); and model 1 plus CCI score (model 3). Kaplan-Meier survival plots were produced using thoracic muscle index and thoracic muscle density stratified into standardized score categories: Z ≤ −1; Z > −1 and Z ≤ 1; and Z > 1. All analyses were conducted using SAS (version 9.4, SAS Institute), and statistical estimates were produced using 95% CIs. A p < 0.05 was set as the definition of statistical significance. A nonsignificant trend was defined as p between 0.05 and 0.10.
Results
Patient Characteristics
Of 274 patients, 193 (70.4%) were women. With respect to clinical features, women and men were similar in terms of age, ethnicity, BMI, ASA classification, CCI score, fracture location, and fracture treatment (Table 1). CT measurements of muscle attenuation (thoracic muscle density and lumbar muscle density) also were similar in women and men, but the muscle size metrics (thoracic muscle index and lumbar muscle index) were significantly lower in women than men (p < 0.0001).
TABLE 1.
Characteristics of Patients in This Study
| Characteristic | Men (n = 81) |
Women (n=193) |
All (n=274) |
P |
|---|---|---|---|---|
|
| ||||
| Demographic | ||||
| Age (y), mean ± SD | 80.5 ± 9.0 | 81.7 ± 8.0 | 81.3 ± 8.3 | 0.2587 |
| Race or ethnicity, no. (%) of patients | 0.7686 | |||
| White | 57 (70.4) | 129(66.8) | 186 (67.9) | |
| Hispanic | 7(8.6) | 12(6.2) | 19 (6.9) | |
| Black | 2(2.5) | 6 (3.1) | 8(2.9) | |
| Asian | 5(6.2) | 20 (10.4) | 25 (9.1) | |
| Other | 10 (12.3) | 26 (13.5) | 36 (13.1) | |
| Body mass indexa, mean ± SD | 24.6 ± 4.7 | 24.1 ± 5.6 | 24.3 ± 5.3 | 0.4584 |
| American Society of Anesthesiologists score (1–6), mean ± SD | 3.0 ± 0.6 | 3.1 ± 0.6 | 3.1 ± 0.6 | 0.4863 |
| Charlson comorbidity index, mean ± SD | 3.0 ± 2.2 | 2.5 ± 2.0 | 2.6 ± 2.1 | 0.0507 |
| Fracture location, no. (%) of patients | 0.5501 | |||
| Neck | 41 (50.6) | 84 (43.5) | 125 (45.6) | |
| Intertrochanteric | 35 (43.2) | 94 (48.7) | 129 (47.1) | |
| Subtrochanteric | 5(6.2) | 15 (7.8) | 20 (7.3) | |
| Fracture treatment, no. (%) of patients | 0.3881 | |||
| Total hip arthroplasty | 3(3.7) | 8 (4.1) | 11 (4.0) | |
| Hemiarthroplasty | 29 (35.8) | 53 (27.5) | 82 (29.9) | |
| Internal fixation | 49 (60.5) | 132 (68.4) | 181 (66.1) | |
| CT muscle metrics | ||||
| Thoracic muscle index (cm2/m2), mean ± SD | 8.6 ± 2.0 | 6.9 ± 2.1 | 7.4 ± 2.2 | < 0.0001b |
| Lumbar muscle index (cm2/m2), mean ± SD | 7.2 ± 1.9 | 5.4 ± 1.4 | 5.9 ± 1.7 | < 0.0001b |
| Thoracic muscle attenuation (HU), mean ± SD | 20.3 ± 7.1 | 18.1 ± 8.1 | 18.8 ± 7.9 | 0.0599 |
| Lumbar muscle attenuation (HU), mean ± SD | 21.6 ± 5.6 | 21.9 ± 6.5 | 21.8 ± 6.2 | 0.7990 |
| IV contrast material, no. (%) of patients | 55 (67.9) | 129 (66.8) | 184 (67.2) | 0.8644 |
Weight in kilograms divided by the square of height in meters.
p < 0.05.
Mortality
The overall mortality rate observed during this study was 55.1% (151/274 patients). This was not significantly different in men (55.6%; 45/81) compared with women (54.9%; 106/193) (p = 0.9234). Table 2 shows cumulative mortality rates at time points ranging from 1 week to 8 years. The mean survival time was 21.2 months (SD, 22.9 months), with a median survival time of 13.2 months (interquartile range, 3.1–31.8 months).
TABLE 2.
Cumulative Mortality Over Time in 274 Geriatric Patients With Hip Fracture
| Follow-Up Time | Cumulative No. of Deaths | Cumulative No. of Patients at Risk |
Cumulative Mortality Rate (%) |
|---|---|---|---|
|
| |||
| 1 Week | 6 | 266 | 2.2 |
| 1 Month | 18 | 245 | 6.7 |
| 3 Months | 45 | 206 | 17.2 |
| 6 Months | 58 | 181 | 22.7 |
| 1 Year | 70 | 142 | 28.3 |
| 2 Years | 94 | 96 | 41.8 |
| 3 Years | 117 | 59 | 56.9 |
| 4 Years | 133 | 34 | 68.4 |
| 5 Years | 144 | 20 | 79.5 |
| 6 Years | 146 | 12 | 82.1 |
| 7 Years | 146 | 9 | 82.1 |
| 8 Years | 151 | 3 | 92.6 |
Note—The cohort included 151 deaths and 123 censored patients. Cumulative mortality rates were estimated using the Kaplan-Meier product limit method.
Mortality and CT Muscle Metrics
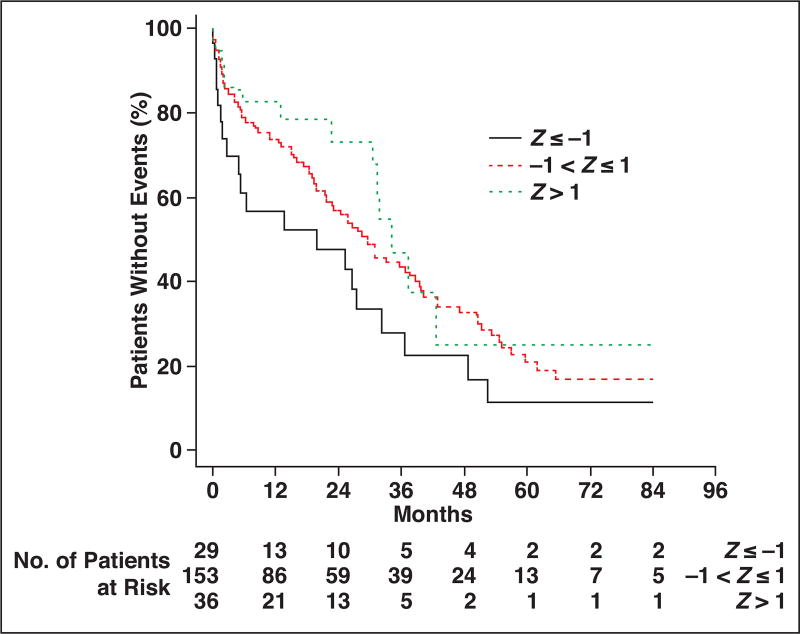
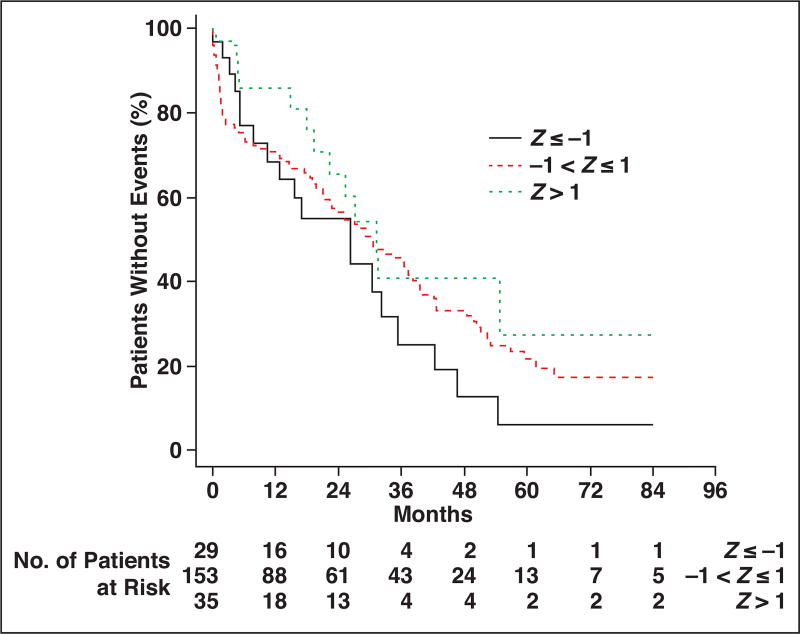
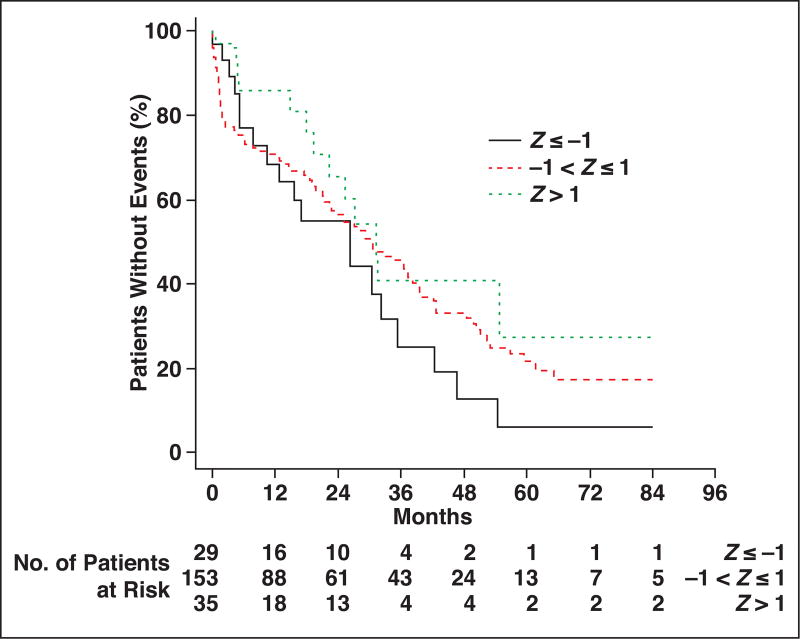
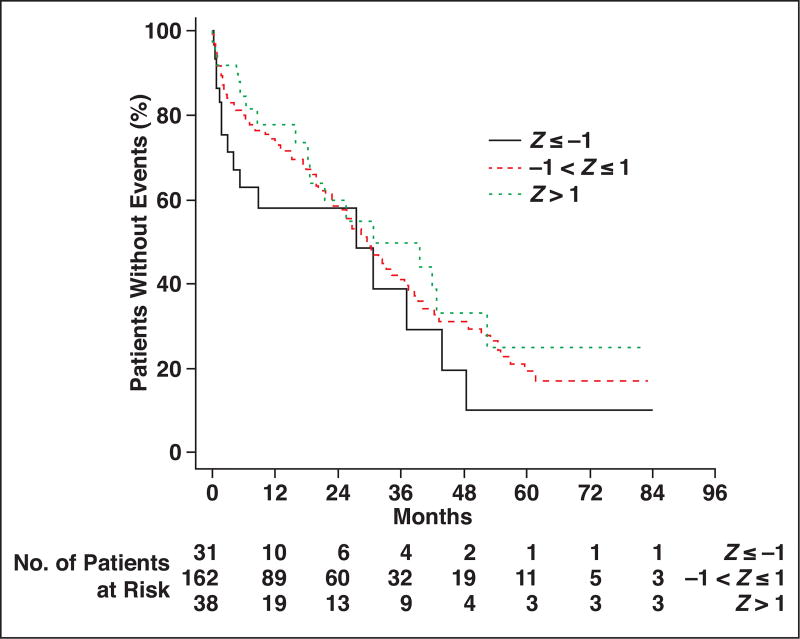
Kaplan-Meier survival analysis for thoracic muscle index is shown in Figure 3. Comparing patients at least 1 SD above and below the cohort median thoracic muscle index, the patients with more muscle (higher thoracic muscle index) had more favorable overall survival at each time point after hip fracture up through 7 years. Similarly, comparing patients with thoracic muscle density measurements less than 1 SD from the cohort median (indicative of fatty muscle) versus patients with thoracic muscle density measurements more than 1 SD from the median (indicative of denser muscle), the overall survival was more favorable for patients with denser muscle at each time point (Fig. 4). Similar survival analysis for lumbar muscle index and lumbar muscle density are shown in Figure 5 and Figure 6, respectively.
Fig. 3.
Kaplan-Meier plot for patients stratified by thoracic muscle index measurements. Patient survival is compared among three groups, ranging from at least 1 SD below cohort median (Z ≤ −1) to more than 1 SD above cohort median (Z > 1).
Fig. 4.
Kaplan-Meier plots for patients stratified by thoracic muscle attenuation measurements. Patient survival is compared among three groups, ranging from at least 1 SD below cohort median (Z ≤ −1) to more than 1 SD above cohort median (Z > 1).
Fig. 5.
Kaplan-Meier plots for patients stratified by lumbar muscle index measurements. Patient survival is compared among three groups, ranging from at least 1 SD below cohort median (Z ≤ −1) to more than 1 SD above cohort median (Z > 1).
Fig. 6.
Kaplan-Meier plots for patients stratified by lumbar muscle attenuation measurements. Patient survival is compared among three groups, ranging from at least 1 SD below cohort median (Z ≤ −1) to more than 1 SD above cohort median (Z > 1).
Table 3 shows the unadjusted and adjusted associations between baseline CT muscle metrics and mortality. The odds ratio (OR) for death was 0.66 (95% CI, 0.49–0.87) for each sex-specific SD increase in thoracic muscle index. For both men and women, a thoracic muscle index increase of 2.0 cm2/m2 was associated with the odds of death decreasing by 34% (unadjusted for comorbidities). Similarly, with each SD increase in thoracic muscle index, the odds of death was significantly decreased even after adjusting for age, sex, ethnicity, and BMI (model 1 OR, 0.69; 95% CI, 0.50–0.95). This association between the thoracic muscle index and decreased mortality also persisted after adjusting for the ASA classification (model 2 OR, 0.70; 95% CI, 0.51–0.97) and the CCI (model 3 OR, 0.72; 95% CI, 0.52–1.00).
TABLE 3.
Unadjusted and Adjusted Associations Between Baseline CT Muscle Metrics and Mortality
| Association, Model | Thoracic Muscle Index |
Thoracic Muscle Attenuation |
Lumbar Muscle Index |
Lumbar Muscle Attenuation |
|---|---|---|---|---|
|
| ||||
| Odds ratio (95% CI) | ||||
| Unadjusted | 0.66 (0.49–0.87) | 0.77 (0.58–1.01) | 0.74 (0.56–0.97) | 0.92 (0.71–1.20) |
| Model 1 | 0.69 (0.50–0.95) | 0.67 (0.49–0.91) | 0.98 (0.71–1.34) | 0.83 (0.63–1.10) |
| Model 2 | 0.70 (0.51–0.97) | 0.69 (0.50–0.94) | 0.94 (0.68–1.30) | 0.85 (0.64–1.12) |
| Model 3 | 0.72 (0.52–1.00) | 0.72 (0.53–0.99) | 1.00 (0.71–1.39) | 0.94 (0.70–1.26) |
| Hazard ratio (95% CI) | ||||
| Unadjusted | 0.83 (0.70–1.00) | 0.82 (0.68–0.98) | 0.93 (0.75–1.14) | 0.84 (0.69–1.02) |
| Model 1 | 0.82 (0.67–1.00) | 0.80 (0.65–0.97) | 1.03 (0.81–1.31) | 0.82 (0.67–0.99) |
| Model 2 | 0.85 (0.70–1.04) | 0.82 (0.67–1.00) | 1.01 (0.79–1.28) | 0.84 (0.69–1.01) |
| Model 3 | 0.86 (0.70–1.04) | 0.82 (0.67–1.00) | 1.01 (0.80–1.29) | 0.84 (0.69–1.02) |
Note—Odds ratios and hazard ratios are presented per sex-specific SD of each muscle metric. Model 1 adjustments were made for age, sex, ethnicity, and baseline body mass index. Model 2 was adjusted for all covariates in model 1 plus the American Society of Anesthesiologists classification. Model 3 was adjusted for all covariates in model 1 plus the Charlson comorbidity index.
The thoracic muscle density also was independently associated with the odds of death after adjusting for comorbidities. For example, for each sex-specific increase in the thoracic muscle density (8.1 HU for women and 7.1 HU for men), the odds of death decreased by 31%, after controlling for age, sex, ethnicity, BMI, and ASA classification.
Correlation Between CT Metrics
Correlations between the four different CT-based muscle metrics were analyzed. The muscle cross-sectional area indexed for patient height had a moderate correlation (0.44; p ≤ 0.0001) between the thoracic and lumbar spine. In comparing the attenuation of muscle in the thoracic and lumbar spine, there was also a moderate correlation (0.53; p ≤ 0.0001). There was a small, but significant, correlation between the thoracic paravertebral muscle size and attenuation metrics (0.16; p = 0.0170). There was no correlation between the psoas muscle size and attenuation metrics (0.02; p = 0.7579). Interreader reliability was very good for all muscle metrics using intraclass correlation coefficients: thoracic muscle index (0.85), thoracic muscle density (0.93), lumbar muscle index (0.90), and lumbar muscle density (0.84).
Discussion
Although sarcopenia commonly accompanies aging, disuse, and numerous diseases, the presence of muscle atrophy and fatty infiltration may be clinically occult [11]. The major findings in this study are that CT can be used to make muscle measurements (thoracic muscle index and thoracic muscle density) that are independently associated with future mortality in both men and women. These previously unused CT measurements therefore may have implications when planning surgery (e.g., deciding on pinning vs arthroplasty) and when determining expectations about outcomes with patients. To our knowledge, no prior studies have used CT to evaluate survival using muscle size and attenuation in elderly patients with hip fractures.
Patient Characteristics and Sarcopenia
The characteristics of patients in this study are similar to those in other studies of mortality in elderly patients with hip fracture with respect to sex [13–15], age [13–15], BMI [13, 14], ASA classification [13, 15], CCI score [15], fracture location [13, 15, 16], and fracture treatment [13]. We chose to use CT to evaluate the T12 and L4 levels because this technique has been validated as prognostically significant for evaluation of the body core musculature in nonorthopedic patients [9, 25, 26] and because these areas are commonly visualized on CT scans of the chest, abdomen, pelvis, and thoracolumbar spine. Although sarcopenia is considered an independent and potentially treatable risk factor for adverse outcomes [27], there is no universal agreement on diagnostic criteria [28]. Indeed, since the term was first introduced in 1989 [29], numerous different definitions for the diagnosis of sarcopenia have been proposed [30–32]. Not surprisingly, the frequency of sarcopenia varies widely, depending on the diagnostic criteria and patient population.
In elderly patients with hip fracture, the frequency of sarcopenia reportedly varies from 17% [33] to 71% [34] using bioelectrical impedance analysis and from 22% to 95% [35] using dual x-ray absorptiometry. Drawbacks of bioelectrical impedance analysis as a diagnostic technique include that it is not specific to muscle and that alterations in body water (overhydration or dehydration) can lead to substantial errors [36]. Although dual x-ray absorptiometry is widely used for evaluating whole-body lean mass, its use is not practical in patients with acute hip fracture. In contrast, CT has been validated for the measurement of muscle size and attenuation [21], is widely available [19], and may be performed for older patients presenting with hip fractures, often due to comorbid medical conditions. Although CT measurements of muscle size and attenuation have not been studied as risk factors for mortality in patients with hip fracture, lower psoas cross-sectional area measured by CT has been associated with loss of independence on discharge from the hospital (e.g., discharge to skilled-nursing facility or nursing home) [37].
Mortality and CT Muscle Metrics
The causes of death in older patients with hip fracture are known to be multifactorial [14, 38, 39]. Our study showed increased mortality rates in patients with decreased muscle size and increased fatty infiltration at the T12 level. At the L4 level, psoas muscle fatty infiltration also showed a trend in predicting mortality. This difference may be related to the posterior paravertebral muscles being composed primarily of type I (slow-twitch) fibers, which can undergo preferential atrophy with disuse, whereas the psoas muscle possesses a higher proportion of type II (fast-twitch) fibers.
Fatty infiltration in muscle can be quantified accurately by CT; an increase in lipid concentration of 1 g/100 mL causes decreased CT attenuation by approximately 1 HU [40]. Previous investigators have suggested a threshold of 30 HU or less for diagnosing fatty infiltration on CT after postprocessing data using specialized thresholding software that requires additional time and training [21]. For our measurements, we used the routine PACS viewing software available to all the health care providers at our hospital and found that muscle attenuation values were in line with those expected for older patients. In our study population, the mean (± SD) muscle attenuation measured in the thoracic and lumbar regions was 19 ± 8 HU and 22 ± 6 HU, respectively.
In addition to healthy muscle decreasing the risk of falls and fractures in elderly patients [4], muscle may be an important indicator of physiologic reserve (i.e., frailty), given that muscle is the principal reservoir for amino acids to maintain protein synthesis and is normally the largest tissue in the human body [41]. Healthy muscle also is thought to play an important role in decreasing the risk of premature mortality by promoting insulin sensitivity and has systemic effects as an endocrine organ (e.g., antiinflammatory roles) [41, 42]. In light of these observations, numerous targeted therapies for sarcopenia are under investigation, including nutritional supplementation (e.g., vitamin D and branched chain amino acids) [43–45], physical activity (e.g., resistance exercise) [43, 46], and pharmacologic treatment [47] (e.g., androgen therapy [48] and myostatin antibodies [49]).
The findings of this study must be interpreted in light of several limitations. First, no conclusion can be made about causality or generalizability to other clinical contexts from a retrospective investigation at a single institution. Second, we studied only patients with hip fracture who underwent CT, which may have resulted in some unknown selection bias. Our cohort is a convenience sample of patients with hip fracture that is intended to mirror the real-world workflow and utilization of CT examinations of the chest, abdomen, and pelvis. Our approach is supported by the available literature that indicates that muscle measurements at T12 and L4 are valid proxies for assessment of generalized muscle loss in patients with sarcopenia [1, 9, 10]. However, although body composition information on CT scans being obtained in our patients showed prognostic significance, we are not proposing routine CT scans for all patients with hip fracture. Third, owing to our use of routine PACS viewing software, no postprocessing of the CT data was performed. Without this additional thresholding step performed in other studies, our investigation may have been less sensitive to changes in muscle size and attenuation. This is a possible reason that psoas size metrics were not statistically significantly associated with survival. However, motivated by the desire to investigate a practical clinical tool, we used a method that did not rely on image postprocessing by trained personnel on a separate workstation. Fourth, although there are numerous variables that can reduce the accuracy of quantitative evaluation based on attenuation (e.g., reconstruction kernel, scatter correction algorithms, tube voltage, and patient girth), all CT scanners used in this study showed consistent close calibration (variability, < 5%), which compares favorably with the 4–5 HU variability reported for rigorously calibrated equipment [21]. Such asynchronous calibration enables the increasingly accepted opportunistic use of CT for measuring quantitative body composition phenotypes that may have diagnostic, prognostic, and therapeutic implications.
This study also has several strengths. First, in a relatively large pilot study of elderly patients with hip fractures, we have investigated both short- and long-term mortality outcomes confirmed using the National Death Index, which is the most complete and reliable measure for this outcome. Second, we used CT, a widely used technique that has been validated for quantifying muscle atrophy and fatty infiltration, and applied it to older patients with hip fracture. Most important, we established that muscle size and attenuation are independently associated with all-cause mortality in orthopedic patients, even after accounting for the influence of age, sex, BMI, ethnicity, ASA classification, and CCI score. All-cause mortality is a widely accepted endpoint, is commonly regarded as a better measure than disease-specific mortality, and was intentionally selected as the outcome measure for this study. In our cohort of elderly patients with hip fracture, the strength of association between sarcopenia and all-cause mortality is similar to that observed in previous studies of patients with cancer and liver disease [1, 25].
In conclusion, although core muscles are not routinely analyzed in patients with hip fractures, CT examinations include them on every scan. Using routine CT, simple quantitative measures of muscle—that is, size and attenuation—are feasible with routine PACS viewing software. We found that muscle atrophy and fatty infiltration at CT of older patients with hip fractures are significantly associated with mortality in both men and women.
Acknowledgments
K. M. Beavers is supported by Research Scientist Development Award K01 AG047921 from the National Institutes of Health.
We thank J. Anthony Seibert, for his expertise in CT physics and quality assurance, and Barton L. Wise, for reviewing the manuscript.
Footnotes
Based on a presentation at the Society of Skeletal Radiology 2016 annual meeting, New Orleans, LA.
WEB
This is a web exclusive article.
References
- 1.Boutin RD, Yao L, Canter RJ, Lenchik L. Sarcopenia: current concepts and imaging implications. AJR. 2015;205:W255–W266. doi: 10.2214/AJR.15.14635. [web] [DOI] [PubMed] [Google Scholar]
- 2.Bruyère O, Beaudart C, Locquet M, Buckinx F, Petermans J, Reginster JY. Sarcopenia as a public health problem. Eur Geriatr Med. 2016;7:272–275. [Google Scholar]
- 3.Tyrovolas S, Koyanagi A, Olaya B, et al. The role of muscle mass and body fat on disability among older adults: a cross-national analysis. Exp Gerontol. 2015;69:27–35. doi: 10.1016/j.exger.2015.06.002. [DOI] [PubMed] [Google Scholar]
- 4.Clynes MA, Edwards MH, Buehring B, Dennison EM, Binkley N, Cooper C. Definitions of sarcopenia: associations with previous falls and fracture in a population sample. Calcif Tissue Int. 2015;97:445–452. doi: 10.1007/s00223-015-0044-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sousa AS, Guerra RS, Fonseca I, Pichel F, Amaral TF. Sarcopenia and length of hospital stay. Eur J Clin Nutr. 2016;70:595–601. doi: 10.1038/ejcn.2015.207. [DOI] [PubMed] [Google Scholar]
- 6.Sheetz KH, Waits SA, Terjimanian MN, et al. Cost of major surgery in the sarcopenic patient. J Am Coll Surg. 2013;217:813–818. doi: 10.1016/j.jamcollsurg.2013.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Du Y, Karvellas CJ, Baracos V, Williams DC, Khadaroo RG Acute Care and Emergency Surgery (ACES) Group. Sarcopenia is a predictor of outcomes in very elderly patients undergoing emergency surgery. Surgery. 2014;156:521–527. doi: 10.1016/j.surg.2014.04.027. [DOI] [PubMed] [Google Scholar]
- 8.Hedbeck CJ, Enocson A, Lapidus G, et al. Comparison of bipolar hemiarthroplasty with total hip arthroplasty for displaced femoral neck fractures: a concise four-year follow-up of a randomized trial. J Bone Joint Surg Am. 2011;93:445–450. doi: 10.2106/JBJS.J.00474. [DOI] [PubMed] [Google Scholar]
- 9.Lee CS, Cron DC, Terjimanian MN, et al. Dorsal muscle group area and surgical outcomes in liver transplantation. Clin Transplant. 2014;28:1092–1098. doi: 10.1111/ctr.12422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Canvasser LD, Mazurek AA, Cron DC, et al. Paraspinous muscle as a predictor of surgical outcome. J Surg Res. 2014;192:76–81. doi: 10.1016/j.jss.2014.05.057. [DOI] [PubMed] [Google Scholar]
- 11.Prado CM, Cushen SJ, Orsso CE, Ryan AM. Sarcopenia and cachexia in the era of obesity: clinical and nutritional impact. Proc Nutr Soc. 2016;75:188–198. doi: 10.1017/S0029665115004279. [DOI] [PubMed] [Google Scholar]
- 12.Allemani C, Weir HK, Carreira H, et al. Global surveillance of cancer survival 1995–2009: analysis of individual data for 25,676,887 patients from 279 population-based registries in 67 countries (CONCORD-2) Lancet. 2015;385:977–1010. doi: 10.1016/S0140-6736(14)62038-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sathiyakumar V, Greenberg SE, Molina CS, Thakore RV, Obremskey WT, Sethi MK. Hip fractures are risky business: an analysis of the NSQIP data. Injury. 2015;46:703–708. doi: 10.1016/j.injury.2014.10.051. [DOI] [PubMed] [Google Scholar]
- 14.Klop C, Welsing PM, Cooper C, et al. Mortality in British hip fracture patients, 2000–2010: a population-based retrospective cohort study. Bone. 2014;66:171–177. doi: 10.1016/j.bone.2014.06.011. [DOI] [PubMed] [Google Scholar]
- 15.Bliemel C, Sielski R, Doering B, et al. Pre-fracture quality of life predicts 1-year survival in elderly patients with hip fracture-development of a new scoring system. Osteoporos Int. 2016;27:1979–1987. doi: 10.1007/s00198-015-3472-8. [DOI] [PubMed] [Google Scholar]
- 16.Zuckerman JD. Hip fracture. N Engl J Med. 1996;334:1519–1525. doi: 10.1056/NEJM199606063342307. [DOI] [PubMed] [Google Scholar]
- 17.Menendez ME, Neuhaus V, Ring D. Inpatient mortality after orthopaedic surgery. Int Orthop. 2015;39:1307–1314. doi: 10.1007/s00264-015-2702-1. [DOI] [PubMed] [Google Scholar]
- 18.Schilling PL, Bozic KJ. Development and validation of perioperative risk-adjustment models for hip fracture repair, total hip arthroplasty, and total knee arthroplasty. J Bone Joint Surg Am. 2016;98:e2. doi: 10.2106/JBJS.N.01330. [DOI] [PubMed] [Google Scholar]
- 19.IMV Medical Information Division. [Accessed February 15, 2017];2016 CT market outlook report. IMV website. http://www.imvinfo.com/index.aspx?sec=def&sub=dis&pag=dis&ItemID=200081. Published November 2016.
- 20.Mitsiopoulos N, Baumgartner RN, Heymsfield SB, Lyons W, Gallagher D, Ross R. Cadaver validation of skeletal muscle measurement by magnetic resonance imaging and computerized tomography. J Appl Physiol. 1998;85:115–122. doi: 10.1152/jappl.1998.85.1.115. [DOI] [PubMed] [Google Scholar]
- 21.Aubrey J, Esfandiari N, Baracos VE, et al. Measurement of skeletal muscle radiation attenuation and basis of its biological variation. Acta Physiol (Oxf) 2014;210:489–497. doi: 10.1111/apha.12224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sankar A, Johnson SR, Beattie WS, Tait G, Wijeysundera DN. Reliability of the American Society of Anesthesiologists physical status scale in clinical practice. Br J Anaesth. 2014;113:424–432. doi: 10.1093/bja/aeu100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Charlson M, Wells MT, Ullman R, King F, Shmukler C. The Charlson comorbidity index can be used prospectively to identify patients who will incur high future costs. PLoS One. 2014;9:e112479. doi: 10.1371/journal.pone.0112479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Joglekar S, Asghar A, Mott SL, et al. Sarcopenia is an independent predictor of complications following pancreatectomy for adenocarcinoma. J Surg Oncol. 2015;111:771–775. doi: 10.1002/jso.23862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van Vugt JL, Levolger S, de Bruin RW, van Rosmalen J, Metselaar HJ, IJzermans JN. Systematic review and meta-analysis of the impact of computed tomography assessed skeletal muscle mass on outcome in patients awaiting or undergoing liver transplantation. Am J Transplant. 2016;16:2277–2292. doi: 10.1111/ajt.13732. [DOI] [PubMed] [Google Scholar]
- 26.Englesbe MJ, Lee JS, He K, et al. Analytic morphomics, core muscle size, and surgical outcomes. Ann Surg. 2012;256:255–261. doi: 10.1097/SLA.0b013e31826028b1. [DOI] [PubMed] [Google Scholar]
- 27.Reginster JY, Cooper C, Rizzoli R, et al. Recommendations for the conduct of clinical trials for drugs to treat or prevent sarcopenia. Aging Clin Exp Res. 2016;28:47–58. doi: 10.1007/s40520-015-0517-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McLean RR, Kiel DP. Developing consensus criteria for sarcopenia: an update. J Bone Miner Res. 2015;30:588–592. doi: 10.1002/jbmr.2492. [DOI] [PubMed] [Google Scholar]
- 29.Rosenberg IH. Summary comments. Am J Clin Nutr. 1989;50:1231–1233. [Google Scholar]
- 30.Cruz-Jentoft AJ, Baeyens JP, Bauer JM, et al. Sarcopenia: European consensus on definition and diagnosis: report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39:412–423. doi: 10.1093/ageing/afq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fielding RA, Vellas B, Evans WJ, et al. Sarcopenia: an undiagnosed condition in older adults—current consensus definition; prevalence, etiology, and consequences. International Working Group on Sarcopenia. J Am Med Dir Assoc. 2011;12:249–256. doi: 10.1016/j.jamda.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Correa-de-Araujo R, Hadley E. Skeletal muscle function deficit: a new terminology to embrace the evolving concepts of sarcopenia and age-related muscle dysfunction. J Gerontol A Biol Sci Med Sci. 2014;69:591–594. doi: 10.1093/gerona/glt208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.González-Montalvo JI, Alarcón T, Gotor P, et al. Prevalence of sarcopenia in acute hip fracture patients and its influence on short-term clinical outcome. Geriatr Gerontol Int. 2016;16:1021–1027. doi: 10.1111/ggi.12590. [DOI] [PubMed] [Google Scholar]
- 34.Fiatarone Singh MA, Singh NA, Hansen RD, et al. Methodology and baseline characteristics for the Sarcopenia and Hip Fracture study: a 5-year prospective study. J Gerontol A Biol Sci Med Sci. 2009;64:568–574. doi: 10.1093/gerona/glp002. [DOI] [PubMed] [Google Scholar]
- 35.Di Monaco M, Castiglioni C, Vallero F, Di Monaco R, Tappero R. Sarcopenia is more prevalent in men than in women after hip fracture: a cross-sectional study of 591 inpatients. Arch Gerontol Geriatr. 2012;55:e48–e52. doi: 10.1016/j.archger.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 36.Camina Martín MA, de Mateo Silleras B, Redondo del Río MP. Body composition analysis in older adults with dementia: anthropometry and bioelectrical impedance analysis—a critical review. Eur J Clin Nutr. 2014;68:1228–1233. doi: 10.1038/ejcn.2014.168. [DOI] [PubMed] [Google Scholar]
- 37.Fairchild B, Webb TP, Xiang Q, Tarima S, Brasel KJ. Sarcopenia and frailty in elderly trauma patients. World J Surg. 2015;39:373–379. doi: 10.1007/s00268-014-2785-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Panula J, Pihlajamäki H, Mattila VM, et al. Mortality and cause of death in hip fracture patients aged 65 or older: a population-based study. BMC Musculoskelet Disord. 2011;12:105. doi: 10.1186/1471-2474-12-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Piirtola M, Vahlberg T, Löppönen M, Räihä I, Isoaho R, Kivelä SL. Fractures as predictors of excess mortality in the aged-a population-based study with a 12-year follow-up. Eur J Epidemiol. 2008;23:747–755. doi: 10.1007/s10654-008-9289-4. [DOI] [PubMed] [Google Scholar]
- 40.Goodpaster BH, Kelley DE, Thaete FL, He J, Ross R. Skeletal muscle attenuation determined by computed tomography is associated with skeletal muscle lipid content. J Appl Physiol. 2000;89:104–110. doi: 10.1152/jappl.2000.89.1.104. [DOI] [PubMed] [Google Scholar]
- 41.Karstoft K, Pedersen BK. Skeletal muscle as a gene regulatory endocrine organ. Curr Opin Clin Nutr Metab Care. 2016;19:270–275. doi: 10.1097/MCO.0000000000000283. [DOI] [PubMed] [Google Scholar]
- 42.Karsenty G, Olson EN. Bone and muscle endocrine functions: unexpected paradigms of interorgan communication. Cell. 2016;164:1248–1256. doi: 10.1016/j.cell.2016.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Denison HJ, Cooper C, Sayer AA, Robinson SM. Prevention and optimal management of sarcopenia: a review of combined exercise and nutrition interventions to improve muscle outcomes in older people. Clin Interv Aging. 2015;10:859–869. doi: 10.2147/CIA.S55842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Smith GI, Julliand S, Reeds DN, Sinacore DR, Klein S, Mittendorfer B. Fish oil-derived n-3 PUFA therapy increases muscle mass and function in healthy older adults. Am J Clin Nutr. 2015;102:115–122. doi: 10.3945/ajcn.114.105833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rizzoli R. Nutrition and sarcopenia. J Clin Densitom. 2015;18:483–487. doi: 10.1016/j.jocd.2015.04.014. [DOI] [PubMed] [Google Scholar]
- 46.Phu S, Boersma D, Duque G. Exercise and sarcopenia. J Clin Densitom. 2015;18:488–492. doi: 10.1016/j.jocd.2015.04.011. [DOI] [PubMed] [Google Scholar]
- 47.Morley JE. Pharmacologic options for the treatment of sarcopenia. Calcif Tissue Int. 2016;98:319–333. doi: 10.1007/s00223-015-0022-5. [DOI] [PubMed] [Google Scholar]
- 48.Baillargeon J, Deer RR, Kuo YF, Zhang D, Goodwin JS, Volpi E. Androgen therapy and rehospitalization in older men with testosterone deficiency. Mayo Clin Proc. 2016;91:587–595. doi: 10.1016/j.mayocp.2016.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Becker C, Lord SR, Studenski SA, et al. Myostatin antibody (LY2495655) in older weak fallers: a proof-of-concept, randomised, phase 2 trial. Lancet Diabetes Endocrinol. 2015;3:948–957. doi: 10.1016/S2213-8587(15)00298-3. [DOI] [PubMed] [Google Scholar]