Abstract
Obesity and its associated metabolic dysregulation are established risk factors for many cancers. However, the biologic mechanisms underlying this relationship remain incompletely understood. Given the rising rates of both obesity and cancer worldwide, and the challenges for many people to lose excess adipose tissue, a systematic approach to identify potential molecular and metabolic targets is needed to develop effective mechanism-based strategies for the prevention and control of obesity-driven cancer. Epidemiological, clinical, and preclinical data suggest that within the growth-promoting, pro-inflammatory microenvironment accompanying obesity, crosstalk between adipose tissue (comprised of adipocytes, macrophages and other cells) and cancer-prone cells may occur via obesity-associated hormones, cytokines, and other mediators that have been linked to increased cancer risk and/or progression. We report here a systematic review on the direct “crosstalk” between adipose tissue and carcinomas in humans. We identified 4,641 articles with n=20 human clinical studies which are summarized as: (a) breast (n=7), (b) colorectal (n=4), (c) esophageal (n=2), (d) esophageal/colorectal (n=1), (e) endometrial (n=1), (f) prostate (n=4), and (g) ear-nose-throat (ENT) cancer (n=1). Findings from these clinical studies reinforce preclinical data and suggest organ-dependent crosstalk between adipose tissue and carcinomas via VEGF, IL-6, TNF-alpha and other mechanisms. Moreover, visceral white adipose tissue (VAT) plays a more central role as it is more bio-energetically active and is associated with a more pro-cancer secretome than subcutaneous adipose tissue (SAT). Efforts to eavesdrop and ultimately interfere with this cancer-enhancing crosstalk may lead to new targets and strategies for decreasing the burden of obesity-related cancers.
Keywords: Obesity, adipose tissue, inflammation, cytokines, cancer
INTRODUCTION
Obesity is a major global health challenge and is expected to further increase substantially over the next several decades (1). In the United States, 38% of adults are obese, defined as having a body mass index (BMI) >30 kg/m2, and nearly 8% are extremely obese, with a BMI >40 kg/m2 (2). A recent summary by the International Agency for Research on Cancer (IARC) reinforced obesity as a risk factor of many cancer types, including colorectal, postmenopausal breast, liver, endometrial, esophageal, kidney (renal cell), gastric, gall bladder, pancreatic, ovarian, thyroid, and multiple myeloma (3).
With cross-sectional studies investigating tumors in overweight or obese cancer patients, new knowledge can be gained on adipose-associated factors that drive tumor development and growth. The interactions between an evolving tumor and its microenvironment are known to involve a complex interplay among multiple cells, local and systemic secreted mediators and other components (4,5). In particular, emerging evidence suggests that non-cancer cell types in the tumor microenvironment, such as adipocytes and macrophages, interact to enhance inflammation, reprogram cancer cell metabolism, and affect processes involved in invasion, metastasis, and immune clearance, all of which can support tumor progression and impact patient outcome (6).
Adipose tissue classification
Adipose tissue can be classified into three different types: white (WAT), brown (BAT) and beige adipose tissue, whose presence differs with development, species, and anatomical location (7). While BAT and beige adipose tissue have been associated with thermoregulation, WAT is considered the key site for energy storage in the form of triacylglycerides (7). WAT can be further divided into distinct body compartments, which have differential impact on disease risk (8). Visceral adipose tissue (VAT) and subcutaneous adipose tissue (SAT) are characterized by differences in cellular structure, molecular composition, and secretome, each of which may be altered by the degree of adiposity itself (8). VAT is generally considered to be bioenergetically more active and responsive to substrates of the electron transport chain than SAT due to a higher concentration of mitochondria. However, BAT has even higher mitochondrial density than VAT, so the differences in metabolic activity between VAT and SAT can be influenced by their brown or beige adipocyte content. VAT adipocytes are also more lipolytically active than SAT adipocytes and thus, contribute more to plasma free fatty acid levels, particularly in obese individuals. In addition, while pro-and anti-inflammatory mediators and immune cells (e.g. Tregs, TH2, eosinophils, ILC2s) maintain immune balance in the lean (healthy) state of adipose tissue (6), increased WAT mass accelerates chronic inflammation through at least three mechanisms: altered generation of secreted inflammatory factors, increased tissue inflammation (immune cell infiltration and formation of crown-like structures by macrophages surrounding dead or dying adipocytes), and adipose tissue remodeling (9). Consequently, the evidence has shown a stronger correlation between WAT and cancer risk compared to BAT and beige adipose tissue (10). Visceral fat area has also been found to be a predictive factor of poor survival and treatment outcomes for different cancer types, such as colon, esophageal, and renal cancers (11–14).
Adipose tissue-induced inflammation
Inflammation, a hallmark of cancer (4), has been linked to obesity and cancer in both epidemiological and preclinical research (15). Evidence from preclinical in vitro and in vivo studies is emerging that obesity-associated adipose-derived factors, including the cytokines interleukin (IL)-6, IL-8, monocyte chemotactic protein 1 (MCP-1), and tumor necrosis factor alpha (TNF-α), as well as infiltrating inflammation-inducing cells (e.g. macrophages) can influence cellular metabolism and promote cancer (see Figure 1) (16–22). This expanded, inflamed adipose tissue appears to increase cancer risk more prominently than obesity itself (6) and may stimulate the hallmark events involved in the development and progression of cancer (23) including cellular transformation; cell survival and proliferation; invasion; angiogenesis; and metastasis (24) (see Figure 1).
Figure 1.
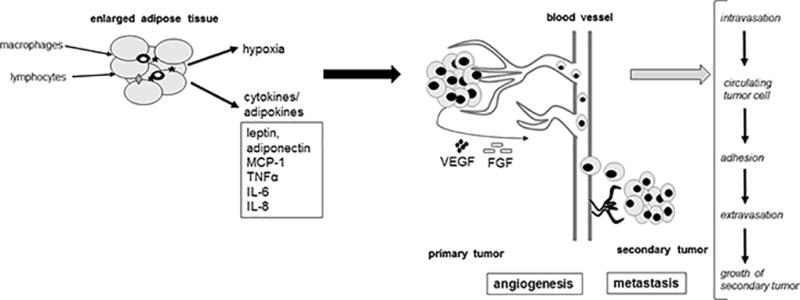
Adipocyte-secreted cytokines (e.g., leptin, adiponectin, MCP-1, TNFα, IL-6, IL-8) and adipocyte-induced conditions (e.g. hypoxia) and their impact on the main hallmarks of cancer development: proliferation, angiogenesis, metastasis.
O’Flanagan et al has demonstrated that obesity enhances the development and metastatic spread of orthotopically transplanted metM-Wntlung cells, a triple negative breast cancer (TNBC) cell line that metastasizes to the lung (25). Pascual et al also showed that diet-induced obesity increases the metastatic potential of several types of cancer cells, including oral squamous cell carcinoma, melanoma, luminal breast cancer, bladder cancer and small cell lung carcinoma, in a CD36-dependent manner (26). Muller’s group found a metabolic symbiosis between tumor-associated adipocytes and cancer cells involving transfer of triglyceride to the cancer cells, resulting in increased availability of free fatty acids for β-oxidation and enhanced metastatic potential (27). They also established that components of the adipocyte secretome, particularly IL-6, are able to stimulate the invasive capacity of breast cancer cells, independent of any effect on proliferation (28,29).
IL-6 regulates the inflammatory process by inducing the production of acute phase proteins and other inflammatory molecules (e.g. C-reactive protein (CRP), prostaglandins, and fibrinogen), the recruitment of CD3+ T lymphocytes and the proliferation of B-lymphocytes. Recent publications have demonstrated crosstalk between adipose tissue and breast as well as colon cancer cells through IL-6 (16–19,30). For example, Walter et al. have reported that IL-6 is secreted by adipose stromal cells (ASC) and promotes migration and invasion in breast cancer cells (30). A variety of signaling pathways have been investigated to elucidate how IL-6 induces cell proliferation. In colorectal cancer cell lines, for example, IL-6 triggers the phosphorylation of ERK, p38 (MAPK), MEK1/2, JAK2 and STAT3 – signaling molecules which control cell metabolism and proliferation (18). Obesity-associated systemic IL-6 also promotes ASC aromatase expression via direct effects and stimulation of breast cancer cell cyclooxygenase 2 (COX2) expression and prostaglandin E2 (PGE2) production (31). The subsequent elevation in estradiol levels promotes estrogen receptor positive breast cancer cell growth (17).
IL-8 shows chemotactic attributes and is particularly involved in the recruitment of leukocytes. Adipocytes in cancer stroma upregulate the expression of IL-8, which exerts its effects via the PI3K, JAK/STAT3, ERK and MAPK signaling pathways, resulting in cell proliferation, survival, angiogenesis and invasion (20).
MCP-1, also known as chemokine (CC motif) ligand (CCL2), plays a key role in the recruitment and accumulation of proinflammatory macrophages in both adipose and tumor tissues (16). Studies demonstrated elevated adipose tissue MCP-1 in obese mice compared with lean controls, indicating MCP-1 is an important factor for enhanced macrophage recruitment into obesity-associated adipose tissue (32). Consistent with this observation, obesity-induced MCP-1 (and IL1-β) expression in mammary fat depots leads to increased macrophage recruitment (16).
TNF-α is a cytokine mainly secreted by macrophages, including those in the adipose tissue. In breast cancer cells lines, TNF-α can have either growth promoting or inhibiting properties depending on cell type (19). Reports also suggest that TNF-α contributes to cancer cell proliferation via MAPK and PI3K/AKT signaling pathways (19).
Tumor infiltrating adipose stromal cells (ASC)
In addition to the pro-tumor para- or endocrine effects of inflammatory factors from dysfunctional adipose tissue, several studies have shown that ASC from the adipose actually infiltrate cancer lesions and contribute to a tumor-promoting microenvironment via paracrine and contact-dependent effects. Furthermore, the recruitment of ASC to tumors is enhanced by obesity. Kolonin and colleagues have published several pioneering studies in this area, first demonstrating that GFP-labeled ASC are recruited to tumors, but not other organs (33). They then found that obesity in mice is associated with increased tumor-infiltrating ASC that are traceable to an adipose tissue origin and that these ASC promote tumor growth in multiple cancer models by facilitating tumor vascularization (11–12). ASC migrate to tumors in response to tumor production of CXCL1 and CXCL8 (34). The application of these pre-clinical findings to human disease was evidenced by data indicating that obese prostate cancer patients have increased tumor CXCL1 expression, circulating ASC, and tumor-infiltrating ASC (34) and has been further demonstrated by studies indicating an increase in circulating mesenchymal stromal cells (which include ASC) in obese disease-free donors (35) and colorectal cancer patients (36) as well as an increase in circulating ASC in obese breast cancer survivors (37).
Others have similarly found that ASC play a key role in cancer progression via additional mechanisms, including the promotion of metastasis and alterations in extracellular matrix mechanics (38,39). In addition, obesity has been shown to promote a pro-fibrotic ASC phenotype, leading to a more fibrillar and stiffer extracellular matrix that enhances mammary tumor growth and the tumorigenic potential of pre-malignant human breast epithelial cells (40). These mechanisms may act in parallel to ASC’s effects on tumor vascularization, suggesting that the obesity-associated elevation in tumor ASC may play a significant role in obesity-induced tumor progression via multiple mechanisms.
Adipokines and hormones
Leptin is a peptide hormone mainly produced within adipose tissue. Beside its neuroendocrine function controlling food intake, leptin can impact a wide range of biological activities including angiogenesis, bone formation and modulation of immune responses (41,42). The intensity of its production and secretion by adipocytes depends on the body energy status and is highly increased in obese individuals; the resulting leptin resistance causes hyperphagia and increases adipose tissue volume (43).
Obesity-associated hyperleptinemia also promotes chronic low-grade inflammation by stimulating the production of IL-1, IL-6, IL-12 and TNF-α (43,44). In a murine model of preneoplastic (Apc Min/+; IMCE) colon epithelial cells, leptin treatment induced the production of IL-6 (18). Consequently, leptin seems to be an important initiating mediator in the pro-inflammatory cascade in adipose tissue (45). Multiple studies have reported that leptin-induced cell signaling cascades are associated with an enhanced risk for different types of cancer such as colorectal, hepatocellular, renal, breast, ovarian, endometrial and prostate (46).
Adiponectin, another major adipokine, antagonizes the oncogenic actions of leptin in several tissues. Also secreted by adipocytes, but at reduced levels in the obese versus non-obese state, adiponectin regulates the effects of insulin on adipocytes and attenuates inflammation (6,47). There is in vitro evidence of a protective effect of adiponectin on the development of cancer by inhibiting proliferation and metastasis (48–52). Particularly in breast cancer cells, it has been shown that adiponectin signaling results in antiproliferative responses (48–50). The peptide hormone stimulates AMPK and PPARα signaling and inhibits MAP-kinases pathways via two types of receptors (AdipoR1 and R2) (49,52).
Increased levels of the steroid hormone estradiol have been associated with breast and gynecologic cancers, such as cervical, endometrial and ovarian cancer. The conversion of androgen to estradiol by aromatase (a key step in estradiol synthesis) in adipose tissue is the major source of circulating estradiol in postmenopausal women (53). Estradiol’s effects are mediated by ER’s transcription factor activity as well as its stimulation of PI3K/Akt and MAPK signaling pathways (54). Several in vitro studies have demonstrated that obesity-associated factors (e.g. leptin, IL-6, TNF-α) increase the expression and activation of aromatase and estrogen receptors in ASCs and cancer cells (e.g. breast and endometrial) (55–60).
IGF-1 is a growth factor with similar molecular structure and signaling pathways to insulin and is mutagenic in many cancer cell lines (21,61). For example, crosstalk between leptin and IGF-1 has been reported to induce invasion and migration of breast cancer cells (21). Findings from fatless A-Zip/F1 transgenic mice, which lack white adipose tissue and have alipotrophic diabetes, suggest that leptin and adiponectin may be less critical to tumorigenesis when insulin and/or IGF-1 are elevated. Tumor growth in these mice following topical application of a carcinogen or crossbreeding with a transgenic model of breast cancer was enhanced despite the total lack of adipose tissue and associated adipokines (62).
The present report systematically reviews the evidence regarding crosstalk between the adipose tissue (as an entity, comprised of adipocytes, macrophages and others cells) and carcinomas. We characterize the dimensions of this crosstalk in the context of mechanisms highlighted above. In contrast to prior reviews, we describe the direct interactions that occur between tumor cells and adipose tissue compartments in multiple cancer types, where adipose tissue can be adjacent to the tumor or part of the peritumoral microenvironment. Our focus on cross-sectional studies investigating the adipose-tumor crosstalk provides insight into direct adipose-stroma-associated factors that drive tumor development and growth.
METHODS
We conducted a systematic literature search in PubMed/Medline covering publications from January 1946 to March 2017 with the goal to identify literature characterizing crosstalk between adipose tissue and carcinomas.
Two researchers (CH and MD) independently performed two searches with the following search terms: 1) (adipose OR fat OR obese) AND (tissue OR cell) AND (cancer OR tumor) AND (crosstalk OR microenvironment OR paracrine OR milieu OR interaction), and 2) adipose tissue in cancer patients. The queries resulted in 4,641 paper publications.
At the identification stage, abstracts were read, and the articles were selected according to the following inclusion criteria: English language, prospective human clinical studies, adults (>18 years), and solid tumor types (in addition to “cancer” overall, we searched specifically for e.g., breast, gastrointestinal, reproductive, melanoma and renal cancer).
At the screening stage, articles were screened based on the following criteria: crosstalk (e.g. paracrine influence, adipocytes as cancer microenvironment) between adipose tissue and carcinoma in cancer patients. Studies investigating solely the systematic effects of secreted products of either adipose tissue or carcinoma (e.g. inflammation markers, adipokines, hormones measured in plasma or serum) were not included. Because the diverse publications on this topic could not be identified with simple search terms, we used the above described broad search strategy. The primary reasons for exclusion were (1) no cancer patients, (2) animal study, (3) intervention study, (4) review.
Finally, n=20 primary research publications of human clinical studies were found to be directly relevant as describing adipose tissue/tumor interactions and are summarized in Table 1: (a) breast cancer (n=7), (b) colorectal cancer (n=4), (c) esophageal cancer (n=2), (d) esophageal and colorectal cancer (n=1), (e) endometrial (n=1), (f) prostate cancer (n=4), and (g) ear-nose-throat (ENT) cancer (n=1). Disagreements relating to data extraction were discussed between authors and resolved. The overall process is outlined in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses Protocols (PRISMA) flow diagram (Figure 2) (63).
Table 1.
Human clinical/ epidemiologic studies
| Author (year, journal) |
Study population/ tissue type | Focus | Results |
|---|---|---|---|
| Human | |||
| Breast cancer | |||
| Mullooly et al (2017, Breast Cancer Res) |
|
|
|
| Koru-Sengul et al (2016, Brest Cancer Res Treat) |
|
|
|
| Jung et al (2015, Tumour Biol.) |
|
|
|
| Iyengar et al (2015, Clin Cancer Res.) |
|
|
|
| Iyengar et al (2015, Cancer Prev Res (Phila)) |
|
|
|
| Savolainen-Peltonen et al (2014, J Clin Endocrinol metab.) |
|
|
|
| Morris et al (2011, Cancer Prev Res(Phila)) |
|
|
|
| Colorectal cancer | |||
| Liesenfeld et al (2015, Am J Clin Nutr.) |
|
|
|
| Amor et al (2015, Int J Colorectal Dis.) |
|
|
|
| Notarnicola et al (2012, Lipids.) |
|
|
|
| Catalan et al (2011, J Nutr Biochem.) |
|
|
|
| Esophageal cancer | |||
| Trevellin et al (2015, Oncotarget.) |
|
|
|
| Lysaght et al (2011, Br J Surg.) |
|
|
|
| Colorectal and Esophageal cancer | |||
| Lysaght et al (2011, Br J Surg.) |
|
|
|
| Endometrial cancer | |||
| Modesitt et al (2012 Int J Gynaecol Cancer) |
|
|
|
| Prostate cancer | |||
| Zhang et al (2016, Cytokine) |
|
|
|
| Venkatasubramanian et al (2014, Prostate) |
|
|
|
| Ribeiro et al (2012, BMC Med) |
|
|
|
| Finley et al (2009, J Urology) |
|
|
|
| Ear-nose-throat (ENT) cancer | |||
| Iyengar et al (2016, Cancer) |
|
|
|
Abbreviation: WAT, white adipose tissue; VAT, visceral adipose tissue; SAT, subcutaneous adipose tissue; BMI, body mass index (kg/m2); ERα, estrogen alpha receptor; Ob-R, leptin receptor; Lcn2, lipocalin 2, PLA2G10, phospholipase A2 G10; PTGD/PTGS2S, prostaglandin synthesis related enzymes; PDGFRα, platelet-derived growth factor receptor alpha; NF-kB, nuclear factor kappa B ; FAPα, fibroblast-activation protein alpha; LPL; lipoprotein lipase; FAS, fatty acid synthase; CLS, crown-like-structures, FERKO, fat-specific ERα knock-out mouse
Figure 2.
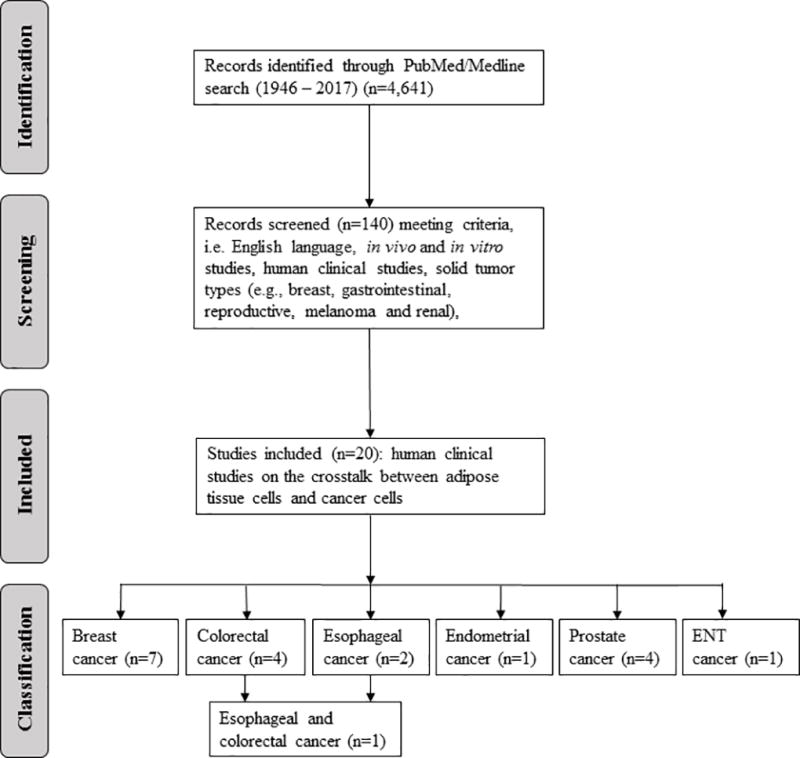
Preferred Reporting Items for Systematic Reviews and Meta Analyses Protocols (PRISMA) flow diagram (63).
RESULTS
1. Adipose tissue in cancer – human clinical studies
N=20 studies have been identified that were conducted in patients with different cancer types (breast, colorectal, esophageal, endometrial, prostate, and ENT) to investigate the crosstalk between patients’ adipose tissue and carcinomas (see Table 1) (8,64–75).
The majority of human studies have been conducted in breast cancer patients (n=7) (66,68,70,75–78). Other studies have been implemented in cancers of the gastrointestinal tract (colorectal cancer (n=4) (8,64,71,73), esophageal cancer (n=2) (65,79), combined (colorectal and esophageal) (n=1) (80)), reproductive cancer (endometrial (n=1) (72), prostate (n=4) (69,74,81,82)), or ENT tract (tongue (n=1) (83)).
1.1. Breast cancer
The tumor stroma - consisting of immune cells, fibroblasts, extracellular matrix and other cells - may have profound tumor-promoting effects and plays an important role when investigating the tumor microenvironment. Of interest, while the risk for breast cancer is increased for obese postmenopausal women, premenopausal obese women have a lower incidence of breast cancer (84). Adipocytes comprise about 90% of the normal breast tissue and thus, the question of adipocytes contributing directly to tumor progression as part of the tumor stroma has been addressed in several studies (66,68,70,75,76). Breast cancer stroma can be classified into adipose stroma cancer (>50% of cells are adipocytes), and fibrous stroma cancer (100% of cells are fibroblasts) or a combination of both stroma types (<100% of cells are fibroblasts, <50% are adipocytes) (76).
The most recent study collected benign breast tissue of n=83 postmenopausal women who were recently diagnosed with invasive breast cancer (75). Mullooly et al focused on the association between crown-like structures and steroid hormones in breast adipose tissue (75). In about 36% of the tissue samples crown-like structures were detected, and the frequency was increased in obese individuals (75). Women with a high ratio of estrone:androstendione were more likely to exhibit crown-like structures; individual hormone levels or tumor characteristics were not associated (75).
Another study investigated the association between the densities of tumor-associated macrophages with or without crown-like structures with patient survival (78). Further, the authors assessed whether there are differences between the racial/ethnicity groups (Caucasian, black, non-black Latinas). Densities of tumor-associated macrophages in black breast cancer patients were the highest (mean=142.21 cells/mm2) compared to Caucasian (62.72 cells/mm2) and non-black Latinas (110.16 cells/mm2) (78). Caucasian patients presented with a significantly lower density of tumor-associated macrophages than the other ethnicities (p<0.0001) (78). Tumor-associated macrophages detected in the tissue of black patients showed a higher proliferation activity of tumor-associated macrophages and survival rates for black patients were lower than for other ethnicities (78).
In a large study, n=939 breast cancer cases were classified depending on the adipose tissue and fibroblast content within the tumor stroma into adipose stroma type and fibrous stroma type (76). The differences between these two cancer types were investigated regarding the breast cancer subtypes, molecular tumor characteristics and the patients’ outcome (76). Cases with cancer of ‘adipose stroma type’ showed higher expression of cancer-associated and fibroblast-related proteins (e.g. fibroblast activation protein α (FAPα), prolyl 4-hydroxylase) compared to cases with ‘fibrous stroma type’. For example, FAPα, which is reported to be involved in extracellular matrix-modulation and tumor cell invasion, was higher expressed in stroma (p<0.001) and tumor (p<0.001) cells in ‘adipose stroma type’ patients (76). Furthermore, among cases of ‘adipose stroma type’, high tumor expression of prolyl 4-hydroxylase was associated with longer disease-free survival (p=0.03) (76). However, stromal expression of prolyl 4-hydroxylase was associated with shorter disease-free (p=0.005) and overall (p<0.001) survival (76). Taken together, the results show that breast cancer of ‘adipose stroma type’ present a distinct expression profile that may lead to an increase in cancer growth, invasion and metastasis compared to the ‘fibrous stroma type’.
Recently, Iyengar et al focused on breast WAT-induced inflammation which was defined by the presence of crown-like structures within WAT (66). Using biospecimens from two cohorts (cohort 1, prospective (n=100), cohort 2, retrospective (n=127)), they reported that 52 of 100 (52%) and 52 of 127 (41%) patients had breast WAT inflammation, respectively (66). Cohort 1 patients with WAT inflammation experienced increased levels of insulin, glucose, leptin, CRP, and IL-6 and lower high-density lipoprotein cholesterol and adiponectin (p<0.05). In cohort 2 WAT-induced inflammation correlated with hyperlipidemia, hypertension and diabetes (p<0.05). In both cohorts, WAT inflammation was associated with reduced recurrence-free survival suggesting that WAT inflammation may, at least in part, explain the relationship between metabolic syndrome and worse breast cancer prognosis (66).
The same study presented results on the association between breast WAT inflammation and menopausal status or BMI in n=237 breast cancer patients (77). They reported a significant association between breast tissue-associated crown-like structures (CLS-B) or the crown-like structures’ density (CLS-B/cm2) (indicating WAT inflammation) with menopausal status (p=0.008 and p<0.001) and BMI (both p<0.001) (77). Furthermore, the average size of adipocytes was correlated with crown-like structures in breast tissue (p<0.001) (77).
In a small study, Savolainen-Peltonen et al examined the concentrations of estrone, estradiol, and estradiol fatty acyl ester and the mRNA expression of estrogen-converting enzymes in subcutaneous breast adipose tissue (70). Samples were collected from postmenopausal women either with ER-positive breast cancer (n=14) or undergoing breast reduction mammoplasty (controls) (n=14) (70). The concentration of estradiol in breast subcutaneous adipose tissue was reduced in women with cancer compared to controls (p=0.002) (70). Expression of 17β-hydroxysteroid dehydrogenase type 12 was also lower in the adipose tissue of breast cancer patients compared to controls (p=0.018) (70). This suggests that estradiol metabolism may be differentially regulated in the adipose tissue of women with breast cancer.
Confirming their initial results of links between adiposity and inflammation in a mouse model (85), Morris et al showed in breast tissue of n=30 breast cancer patients that the severity of breast inflammation, defined as the crow-like structures of the breast index (CLS-B) (number of breast WAT slides with evidence of crown-like structures/ number of breast WAT slides examined), correlated with BMI (p<0.001) and adipocyte size (p=0.01) (68). In addition, increased NF-kB binding activity and elevated aromatase expression and enzyme activity were found in the inflamed breast tissue of overweight and obese women (68).
In summary, the current evidence on the local interaction between breast WAT and breast cancer cells supports the hypothesis that adipose tissue inflammation, defined by presence of crown-like structures, is a key player in cancer growth and progression. However, the involvement of steroid hormones as drivers of tumor progression represents an additional important aspect of the adipose-cancer link, particularly in hormone-dependent cancers, such as breast or endometrial cancer (17,86–88).
1.2. Colorectal cancer
Four studies were identified that have investigated the crosstalk between adipose tissue and colorectal cancer (8,64,71). Nested in the international cohort study ColoCare, Liesenfeld et al used a multi-omic approach to investigate differences between VAT compared with SAT in patients with colorectal cancer (8). They comprehensively assessed differences in metabolic, lipidomic, and transcriptomic profiles in paired human VAT and SAT samples and their association with colorectal cancer tumor stage (8). Mass spectrometry was used to measure 1,065 metabolites in adipose tissue and 1,810 metabolites in serum of n=59 patients, and parallel genome-wide gene expression data were used to perform integrated analyses of candidate metabolites (8). Compared with SAT, VAT was characterized by elevated markers of inflammatory lipid metabolism, phospholipases (PLA2G10), free arachidonic acid, and prostaglandin synthesis-related enzymes (PTGD/PTGS2S) (8). Several lipids showed a linear association with increasing tumor stage (not significant after correction for multiple testing) (8).
In the same year, Amor et al collected visceral adipose tissue of n=18 colorectal cancer patients and n=18 health controls (73). Participants were classified into four groups: i) obese with cancer, ii) lean with cancer, iii) obese without cancer, and iv) lean without cancer (73). Tissue samples were divided into peritumoral and non-tumoral fat. Their results showed that the secretion activity of peritumoral adipose tissue in cancer patients was higher compared to the control groups and non-tumoral tissue samples (73). Tissue samples from obese cancer patients had also an increased rate of secretion compared to lean patients (73).
Another study examined regional differences (peritumoral and distant from neoplasia) in the expression of lipogenic enzymes (e.g. lipoprotein lipase and fatty acid synthase) and their influence on events which sustain colorectal cancer growth (64). The evaluation of n=32 adipose tissue samples of colorectal cancer patients (adjacent [not defined] and distant [about 10 cm]) to neoplasia showed that lipoprotein lipase, as well as fatty acid synthase were less expressed in adipose tissue adjacent to the tumor compared to adipose tissue distant from the cancer (64). The results underline the influence of cancer cells on environmental adipose tissue’s lipid metabolism, demonstrating a cancer-induced impairment in the formation and lipid storing capacity of adjacent adipose tissue in patients with colorectal cancer (64).
In a small case-control study, differences in VAT gene expression of proinflammatory and angiogenesis-related factors between 11 colorectal cancer patients and 18 healthy individuals were assessed (71). Gene expression of lipocalin-2, osteopontin, chitinase-3 like-1, TNF-α, HIF1A and VEGFA was significantly elevated in VAT of colorectal cancer patients. These results suggest that inflammatory factors in VAT of colorectal cancer patients are elevated and may accelerate cancer development or progression (71).
In summary, the inflammatory features of the adipose tissue environment embedding the colon and rectum seem to play a crucial role when investigating the tumor-promoting effects of adipose tissue. Only one study considered the reciprocal influence of the tumor on the surrounding adipose tissue. Focusing on only lipogenic enzymes, this study illustrates that the adipose tissue – tumor interaction needs to be considered in both directions.
1.3. Esophageal cancer
A recent study reported that esophageal peritumoral adipose tissue and its secretion of tumor-promoting factors are directly correlated with increased tumor growth (65). Studying the morphological, histological, and molecular characteristics of peritumoral and omental adipose tissue in esophageal cancer patients (n=60), the study was designed to investigate whether adipose depot-specific differences affect tumor behavior (65). Only in peritumoral adipose tissue, increased adipocyte size was directly associated with leptin expression, angiogenesis (CD31), and lymph angiogenesis (podoplanin) (65). Thus, peritumoral adipose tissue may directly accelerate the progression of esophageal cancer by secreting paracrine factors; the adipokine leptin seems to be a key player in this crosstalk (65).
Lysaght et al performed flow cytometry to assess the activation of T cells and cytokine production in VAT (omental adipose tissue) of n=35 esophageal cancer patients (79). A large number of lymphocytes was observed in the omentum (79). Both CD4(+) and CD8(+) T cells showed significantly increased expression of the T cell activation markers CD69 (p< 0·001) and CD107a (CD8(+) T cells: p<0·01) compared with blood, as well as reduced CD62L expression (p<0.05). Similarly, higher proportions of CD45RO(+) T cells compared with CD45RA(+) T cells were present. Interferon γ was significantly elevated in VAT, compared to blood and subcutaneous adipose tissue (p<0.01) (79). Overall, this study confirmed that VAT is a major source of activated proinflammatory lymphocytes, which may help fuel chronic inflammation (79).
Although the number of studies in esophageal cancer are limited, they highlight two important aspects. First the theme of inflammatory mechanisms as a key player in the adipose-cancer link is also prominent in this cancer type; second, the fascinating work by Travellin et al (65) highlights the role of peritumoral adipocytes as a carcinogenic driver in esophageal cancer and possibly beyond.
1.4. Colorectal and esophageal cancer (combined)
In 2011, one study investigated the differences in cytokine and adipokine expression of VAT and SAT from normal-weight and centrally obese gastrointestinal cancer patients (including colorectal and esophageal cancer) and their effects on colorectal and esophageal cancer cell lines (80). They observed a higher IL-6, VEGF and LEP gene expression in VAT compared to SAT and a higher IL-6 and VEGF protein secretion from VAT into conditioned media compared to SAT (80). Adipose-tissue conditioned media from centrally obese patients induced significantly more proliferation in both esophageal and colorectal cancer cell lines, compared to adipose-tissue conditioned media from non-obese patients. Greater proliferation of cancer cell lines was observed after culture with VAT-conditioned media, compared to SAT-conditioned media. (80). This study illustrates the elevated expression of inflammatory and angiogenesis-related factors in VAT compared to SAT of cancer patients and translates it directly back to impact gastrointestinal cancer cell lines. In particular the link via VEGF highlights a potential mechanism whereby VAT from centrally obese patients may drive carcinogenic progression (80).
1.5 Endometrial cancer
To identify obesity-related endometrial cancer genes via microarray analysis in endometrial and adipose tissues, Modesitt et al collected endometrial tissue, VAT and SAT in n=8 (n=4 with endometrial cancer, n=4 without endometrial cancer) individuals undergoing hysterectomy (72). The authors noted no differences in hormone/metabolite levels between groups (72). Gene set enrichment analysis contrasting patients with and without endometrial cancer showed that endometrial, VAT and SAT displayed 40, 47, and 38 alternatively regulated gene set pathways, respectively (72). Eighteen pathways were regulated in opposite directions between VAT and SAT (72).
The results from this pilot study suggest that SAT and VAT have opposite patterns of gene expression in obese patients with and without endometrial cancer and may provide new potential targets for cancer treatment and prevention for obese women. However, considering the small sample size of this first study, more research is needed.
1.6. Prostate cancer
While visceral obesity has been associated with worse prognosis for prostate cancer patients, peri-prostatic adipose tissue may lead to an increase in the aggressive growth of this disease (69,74,81,82). A recent study analyzed the expression of IL-6, leptin and adiponectin in peri-prostatic adipose tissues specimens from n=30 prostate patients and n=10 non-cancer controls (82). IL-6, leptin and adiponectin gene expression was higher in the samples from prostate cancer patients compared to controls (p<0.001) (82). Further, IL-6 expression in adipose tissue was associated with increased aggressiveness of prostate cancer (p=0.001) (82).
One study collected peri-prostatic and subcutaneous adipose tissue in n=40 prostate cancer patients (81). In culture with either prostate cancer cells or endothelial cells, the peri-prostatic adipose tissue showed higher proliferative effects compared to SAT (81). Furthermore, this result was more significant in samples of obese patients (BMI≥30 kg/m2) compared to overweight (25–30 kg/m2) or lean (<25 kg/m2) patients (81).
Ribeiro et al (69) conducted a study with n=18 peri-prostatic adipose tissue samples, which were categorized into three groups based on post-surgical diagnosis and pathological analysis. Differentially expressed genes in the peri-prostatic tissue were investigated by microarrays (69). In the tissues of obese and overweight individuals an increased expression of genes were observed that are involved in adipogenic, proliferative and mild immunoinflammatory processes (e.g. LEP, ANGPT1) (69). In patients with prostate cancer the expression profile of peri-prostatic adipose tissue was consistent with hypercellularity and reduced immunosurveillance (69). The authors concluded that their findings are consistent with peri-prostatic adipose tissue among obese individuals providing a favorable environment for prostate cancer progression (69).
In 2009, another study collected peri-prostatic adipose tissue samples from n=7 patients undergoing surgery treatment (74). Analyzing the cytokine expression in the tissue samples, their results showed a 375 times higher expression of IL-6 in the per-prostatic adipose tissue compared to the patients serum sample (74). Further, the phosphorylation cell signaling of STAT3 in peri-prostatic adipose tissue was greater with high grade tumors (74).
All studies of prostate cancer collected peri-prostatic adipose tissue and suggest that altered adipose tissue metabolism in obese individuals forms a more auspicious microenvironment for prostate cancer development. An increase in cell proliferation and expression of proliferative genes, as well as IL-6 has been detected in expanded peri-prostatic adipose tissue of obese individuals. However, the results need to be confirmed in studies with larger sample sizes.
1.7. Ear-nose-throat (ENT) cancer
To assess the association between WAT inflammation of the tongue environmental adipose tissue and squamous cell carcinoma (SCC) of the tongue, Iyengar et al. analyzed n=125 tissue samples from oral cancer patients (83). The presence of dead or dying adipocytes surrounded by macrophages forming crown-like structures was defined as WAT inflammation (83). Thirty-nine percent of the patients presented WAT inflammation, and this was statistically significant associated with BMI, increased tumor thickness, and vascular invasion (p<0.05) (83). The cancer-specific survival rate was with 59% (95% CI, 46–76%) lower for patients presenting WAT inflammation compared to patients without (82%; 95% CI, 72–92%) (83). In n=70 patients with early stage SCC (without lymph node involvement, no indication for adjuvant therapy) tongue WAT inflammation was significant associated with both decreased cancer-specific and overall survival (p<0.05) (83).
The results of this study suggest that inflammation of neck and tongue WAT plays an important role in the development and growth of oral cancers. However, considering that this is the first study of this cancer type, further investigations are needed to confirm the results.
CONCLUSIONS
Obesity is an established risk and progression factor for many cancers (e.g. breast, colorectal, esophageal), but the underlying mechanisms are incompletely understood. A potential crosstalk between adipose tissue and carcinomas may contribute importantly to the observed associations between obesity and increased cancer risk and/or progression.
In vitro and in vivo studies have shown that adipose tissue is enriched for hormones, cytokines and other mediators (e.g., leptin, adiponectin). This milieu has already been characterized as a growth-promoting and pro-inflammatory microenvironment. More recent investigations have used a set of multi-omic techniques, including transcriptomics and metabolomics, yielding novel signals, as well as integrated effects on pathways (10). They also demonstrated clear distinctions of the adipose tissue type (visceral vs. subcutaneous) by these –omic characterizations.
Here, results of n=20 human clinical studies indicate that a) there is a direct/specific crosstalk between adipose tissue and carcinomas, likely with different mechanisms and different directions depending on the organ system; b) white adipose tissue (WAT) is more important than other adipose pools in this pattern of cellular communication; and c) visceral adipose tissue (VAT) plays a central role as it is metabolically more active than subcutaneous adipose tissue (SAT). In addition, peritumoral adipose tissue that is directly present in the organ may provide an imminently present microenvironment that fosters carcinogenic progression. Whether the mediators and intensity of crosstalk between adipose tissue and cancer is affected by the tissue distance is an important but currently unanswered question.
Despite the intriguing results of studies presented within this systematic review, we still miss the complete picture that elucidates the mechanisms underlying the adipose-tumor crosstalk. In addition to the limitation of small sample sizes in most studies, other aspects that are relevant in inflammatory processes were often not reported or assessed. Inflammation can be modified by several environmental factors, such as age, smoking, medication use and diet. Even though adding another layer of complexity, such variability in the investigated study populations should be considered. Furthermore, as detailed above, distinct adipose tissue compartments have different metabolic capabilities. These potentially generate adipose tissue-specific forces that affect the tumor, locally or systemically. However, only a limited number of studies assessed or has the means to assess the distribution of adipose tissue compartments in individuals. Rather than relying on body mass index, the amount of adipose tissue in a patient and its distribution would be much more informative to investigate the effect of adipose tissues on carcinogenesis to identify means of specific intervention.
Consequently, there is a clear need for larger and more comprehensive investigations of the adipose tissue as a central player in explaining the obesity-cancer link. Overall, data of the human clinical studies, as well as in vitro and in vivo studies, suggest that efforts to eavesdrop and ultimately interfere with this cancer-enhancing crosstalk between adipose tissue and carcinomas may lead to new targets and strategies for decreasing the burden of obesity-related cancers.
Acknowledgments
The authors thank Christy Warby and Jennifer Ose for critical review of the article.
Financial support:
C. Himbert is supported by the Stiftung LebensBlicke and Claussen-Simon-Stiftung, Germany.
M. Delphan is supported by Ministry of Science, Research and Technology, Iran.
C. Himbert, M. Delphan, and C. Ulrich are supported by the Huntsman Cancer Foundation and C.M. Ulrich also by R01 CA189184 and U01 CA206110.
S. Hursting is supported by R35 CA197627 from the National Cancer Institute.
L.W. Bowers is supported by R25 CA057726.
Footnotes
Conflicts of interest: None
References
- 1.American Geriatrics Society Workgroup on Vitamin DSfOA. Recommendations abstracted from the American Geriatrics Society Consensus Statement on vitamin D for Prevention of Falls and Their Consequences. Journal of the American Geriatrics Society. 2014;62(1):147–52. doi: 10.1111/jgs.12631. [DOI] [PubMed] [Google Scholar]
- 2.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011–2012. Jama. 2014;311(8):806–14. doi: 10.1001/jama.2014.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lauby-Secretan B, Scoccianti C, Loomis D, Grosse Y, Bianchini F, Straif K, et al. Body Fatness and Cancer--Viewpoint of the IARC Working Group. The New England journal of medicine. 2016;375(8):794–8. doi: 10.1056/NEJMsr1606602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–74. doi: 10.1016/j.cell.2011.02.013. S0092-8674(11)00127-9 [pii] [DOI] [PubMed] [Google Scholar]
- 5.Chen F, Zhuang X, Lin L, Yu P, Wang Y, Shi Y, et al. New horizons in tumor microenvironment biology: challenges and opportunities. BMC medicine. 2015;13:45. doi: 10.1186/s12916-015-0278-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deng T, Lyon CJ, Bergin S, Caligiuri MA, Hsueh WA. Obesity, Inflammation, and Cancer. Annual review of pathology. 2016;11:421–49. doi: 10.1146/annurev-pathol-012615-044359. [DOI] [PubMed] [Google Scholar]
- 7.Saely CH, Geiger K, Drexel H. Brown versus white adipose tissue: a mini-review. Gerontology. 2012;58(1):15–23. doi: 10.1159/000321319. [DOI] [PubMed] [Google Scholar]
- 8.Liesenfeld DB, Grapov D, Fahrmann JF, Salou M, Scherer D, Toth R, et al. Metabolomics and transcriptomics identify pathway differences between visceral and subcutaneous adipose tissue in colorectal cancer patients: the ColoCare study. The American journal of clinical nutrition. 2015;102(2):433–43. doi: 10.3945/ajcn.114.103804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ghigliotti G, Barisione C, Garibaldi S, Fabbi P, Brunelli C, Spallarossa P, et al. Adipose tissue immune response: novel triggers and consequences for chronic inflammatory conditions. Inflammation. 2014;37(4):1337–53. doi: 10.1007/s10753-014-9914-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Argolo DF, Iyengar NM, Hudis CA. Obesity and Cancer: Concepts and Challenges. Indian journal of surgical oncology. 2015;6(4):390–8. doi: 10.1007/s13193-015-0483-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ladoire S, Bonnetain F, Gauthier M, Zanetta S, Petit JM, Guiu S, et al. Visceral fat area as a new independent predictive factor of survival in patients with metastatic renal cell carcinoma treated with antiangiogenic agents. The oncologist. 2011;16(1):71–81. doi: 10.1634/theoncologist.2010-0227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guiu B, Petit JM, Bonnetain F, Ladoire S, Guiu S, Cercueil JP, et al. Visceral fat area is an independent predictive biomarker of outcome after first-line bevacizumab-based treatment in metastatic colorectal cancer. Gut. 2010;59(3):341–7. doi: 10.1136/gut.2009.188946. [DOI] [PubMed] [Google Scholar]
- 13.Shin DY, Kim A, Byun BH, Moon H, Kim S, Ko YJ, et al. Visceral adipose tissue is prognostic for survival of diffuse large B cell lymphoma treated with frontline R-CHOP. Annals of hematology. 2016;95(3):409–16. doi: 10.1007/s00277-015-2571-0. [DOI] [PubMed] [Google Scholar]
- 14.Massl R, van Blankenstein M, Jeurnink S, Hermans JJ, de Haan MC, Stoker J, et al. Visceral adipose tissue: the link with esophageal adenocarcinoma. Scandinavian journal of gastroenterology. 2014;49(4):449–57. doi: 10.3109/00365521.2013.873818. [DOI] [PubMed] [Google Scholar]
- 15.Iyengar NM, Gucalp A, Dannenberg AJ, Hudis CA. Obesity and Cancer Mechanisms: Tumor Microenvironment and Inflammation. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2016;34(35):4270–6. doi: 10.1200/JCO.2016.67.4283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arendt LM, McCready J, Keller PJ, Baker DD, Naber SP, Seewaldt V, et al. Obesity promotes breast cancer by CCL2-mediated macrophage recruitment and angiogenesis. Cancer research. 2013;73(19):6080–93. doi: 10.1158/0008-5472.can-13-0926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bowers LW, Brenner AJ, Hursting SD, Tekmal RR, deGraffenried LA. Obesity-associated systemic interleukin-6 promotes pre-adipocyte aromatase expression via increased breast cancer cell prostaglandin E2 production. Breast cancer research and treatment. 2015;149(1):49–57. doi: 10.1007/s10549-014-3223-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fenton JI, Hursting SD, Perkins SN, Hord NG. Interleukin-6 production induced by leptin treatment promotes cell proliferation in an Apc (Min/+) colon epithelial cell line. Carcinogenesis. 2006;27(7):1507–15. doi: 10.1093/carcin/bgl018. [DOI] [PubMed] [Google Scholar]
- 19.Weichhaus M, Broom I, Bermano G. The molecular contribution of TNF-alpha in the link between obesity and breast cancer. Oncology reports. 2011;25(2):477–83. doi: 10.3892/or.2010.1099. [DOI] [PubMed] [Google Scholar]
- 20.Welte G, Alt E, Devarajan E, Krishnappa S, Jotzu C, Song YH. Interleukin-8 derived from local tissue-resident stromal cells promotes tumor cell invasion. Molecular carcinogenesis. 2012;51(11):861–8. doi: 10.1002/mc.20854. [DOI] [PubMed] [Google Scholar]
- 21.Saxena NK, Taliaferro-Smith L, Knight BB, Merlin D, Anania FA, O'Regan RM, et al. Bidirectional crosstalk between leptin and insulin-like growth factor-I signaling promotes invasion and migration of breast cancer cells via transactivation of epidermal growth factor receptor. Cancer research. 2008;68(23):9712–22. doi: 10.1158/0008-5472.can-08-1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.D'Esposito V, Liguoro D, Ambrosio MR, Collina F, Cantile M, Spinelli R, et al. Adipose microenvironment promotes triple negative breast cancer cell invasiveness and dissemination by producing CCL5. Oncotarget. 2016;7(17):24495–509. doi: 10.18632/oncotarget.8336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McNelis JC, Olefsky JM. Macrophages, immunity, and metabolic disease. Immunity. 2014;41(1):36–48. doi: 10.1016/j.immuni.2014.05.010. [DOI] [PubMed] [Google Scholar]
- 24.Guffey CR, Fan D, Singh UP, Murphy EA. Linking obesity to colorectal cancer: recent insights into plausible biological mechanisms. Current opinion in clinical nutrition and metabolic care. 2013;16(5):595–600. doi: 10.1097/MCO.0b013e328362d10b. [DOI] [PubMed] [Google Scholar]
- 25.O'Flanagan CH, Rossi ES, McDonell SB, Chen X, Tsai YH, Parker JS, et al. Metabolic reprogramming underlies metastatic potential in an obesity-responsive murine model of metastatic triple negative breast cancer. NPJ Breast Cancer. doi: 10.1038/s41523-017-0027-5. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pascual G, Avgustinova A, Mejetta S, Martin M, Castellanos A, Attolini CS, et al. Targeting metastasis-initiating cells through the fatty acid receptor CD36. Nature. 2017;541(7635):41–5. doi: 10.1038/nature20791. [DOI] [PubMed] [Google Scholar]
- 27.Wang YY, Attane C, Milhas D, Dirat B, Dauvillier S, Guerard A, et al. Mammary adipocytes stimulate breast cancer invasion through metabolic remodeling of tumor cells. JCI Insight. 2017;2(4):e87489. doi: 10.1172/jci.insight.87489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dirat B, Bochet L, Dabek M, Daviaud D, Dauvillier S, Majed B, et al. Cancer-associated adipocytes exhibit an activated phenotype and contribute to breast cancer invasion. Cancer research. 2011;71(7):2455–65. doi: 10.1158/0008-5472.CAN-10-3323. [DOI] [PubMed] [Google Scholar]
- 29.Dirat B, Bochet L, Escourrou G, Valet P, Muller C. Unraveling the obesity and breast cancer links: a role for cancer-associated adipocytes? Endocr Dev. 2010;19:45–52. doi: 10.1159/000316896. [DOI] [PubMed] [Google Scholar]
- 30.Walter M, Liang S, Ghosh S, Hornsby PJ, Li R. Interleukin 6 secreted from adipose stromal cells promotes migration and invasion of breast cancer cells. Oncogene. 2009;28(30):2745–55. doi: 10.1038/onc.2009.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhao Y, Nichols JE, Bulun SE, Mendelson CR, Simpson ER. Aromatase P450 gene expression in human adipose tissue. Role of a Jak/STAT pathway in regulation of the adipose-specific promoter. The Journal of biological chemistry. 1995;270(27):16449–57. doi: 10.1074/jbc.270.27.16449. [DOI] [PubMed] [Google Scholar]
- 32.Sartipy P, Loskutoff DJ. Monocyte chemoattractant protein 1 in obesity and insulin resistance. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(12):7265–70. doi: 10.1073/pnas.1133870100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang Y, Daquinag A, Traktuev DO, Amaya-Manzanares F, Simmons PJ, March KL, et al. White adipose tissue cells are recruited by experimental tumors and promote cancer progression in mouse models. Cancer research. 2009;69(12):5259–66. doi: 10.1158/0008-5472.CAN-08-3444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang T, Tseng C, Zhang Y, Sirin O, Corn PG, Li-Ning-Tapia EM, et al. CXCL1 mediates obesity-associated adipose stromal cell trafficking and function in the tumour microenvironment. Nature communications. 2016;7:11674. doi: 10.1038/ncomms11674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bellows CF, Zhang Y, Simmons PJ, Khalsa AS, Kolonin MG. Influence of BMI on level of circulating progenitor cells. Obesity (Silver Spring, Md) 2011;19(8):1722–6. doi: 10.1038/oby.2010.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bellows CF, Zhang Y, Chen J, Frazier ML, Kolonin MG. Circulation of progenitor cells in obese and lean colorectal cancer patients. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2011;20(11):2461–8. doi: 10.1158/1055-9965.EPI-11-0556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ghosh S, Hughes D, Parma DL, Ramirez A, Li R. Association of obesity and circulating adipose stromal cells among breast cancer survivors. Molecular biology reports. 2014;41(5):2907–16. doi: 10.1007/s11033-014-3146-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Orecchioni S, Gregato G, Martin-Padura I, Reggiani F, Braidotti P, Mancuso P, et al. Complementary populations of human adipose CD34+ progenitor cells promote growth, angiogenesis, and metastasis of breast cancer. Cancer research. 2013;73(19):5880–91. doi: 10.1158/0008-5472.CAN-13-0821. [DOI] [PubMed] [Google Scholar]
- 39.Rowan BG, Gimble JM, Sheng M, Anbalagan M, Jones RK, Frazier TP, et al. Human adipose tissue-derived stromal/stem cells promote migration and early metastasis of triple negative breast cancer xenografts. PloS one. 2014;9(2):e89595. doi: 10.1371/journal.pone.0089595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Seo BR, Bhardwaj P, Choi S, Gonzalez J, Andresen Eguiluz RC, Wang K, et al. Obesity-dependent changes in interstitial ECM mechanics promote breast tumorigenesis. Science translational medicine. 2015;7(301):301ra130. doi: 10.1126/scitranslmed.3010467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stefanou N, Papanikolaou V, Furukawa Y, Nakamura Y, Tsezou A. Leptin as a critical regulator of hepatocellular carcinoma development through modulation of human telomerase reverse transcriptase. BMC cancer. 2010;10:442. doi: 10.1186/1471-2407-10-442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Meyers JA, Liu AY, McTiernan A, Wener MH, Wood B, Weigle DS, et al. Serum leptin concentrations and markers of immune function in overweight or obese postmenopausal women. The Journal of endocrinology. 2008;199(1):51–60. doi: 10.1677/joe-07-0569. [DOI] [PubMed] [Google Scholar]
- 43.Strong AL, Burow ME, Gimble JM, Bunnell BA. Concise review: The obesity cancer paradigm: exploration of the interactions and crosstalk with adipose stem cells. Stem cells (Dayton, Ohio) 2015;33(2):318–26. doi: 10.1002/stem.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Carbone F, La Rocca C, Matarese G. Immunological functions of leptin and adiponectin. Biochimie. 2012;94(10):2082–8. doi: 10.1016/j.biochi.2012.05.018. [DOI] [PubMed] [Google Scholar]
- 45.Deng T, Lyon CJ, Minze LJ, Lin J, Zou J, Liu JZ, et al. Class II major histocompatibility complex plays an essential role in obesity-induced adipose inflammation. Cell metabolism. 2013;17(3):411–22. doi: 10.1016/j.cmet.2013.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lipsey CC, Harbuzariu A, Daley-Brown D, Gonzalez-Perez RR. Oncogenic role of leptin and Notch interleukin-1 leptin crosstalk outcome in cancer. World journal of methodology. 2016;6(1):43–55. doi: 10.5662/wjm.v6.i1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nagaraju GP, Rajitha B, Aliya S, Kotipatruni RP, Madanraj AS, Hammond A, et al. The role of adiponectin in obesity-associated female-specific carcinogenesis. Cytokine & growth factor reviews. 2016 doi: 10.1016/j.cytogfr.2016.03.014. [DOI] [PubMed] [Google Scholar]
- 48.Arditi JD, Venihaki M, Karalis KP, Chrousos GP. Antiproliferative effect of adiponectin on MCF7 breast cancer cells: a potential hormonal link between obesity and cancer. Hormone and metabolic research = Hormon- und Stoffwechselforschung = Hormones et metabolisme. 2007;39(1):9–13. doi: 10.1055/s-2007-956518. [DOI] [PubMed] [Google Scholar]
- 49.Dieudonne MN, Bussiere M, Dos Santos E, Leneveu MC, Giudicelli Y, Pecquery R. Adiponectin mediates antiproliferative and apoptotic responses in human MCF7 breast cancer cells. Biochemical and biophysical research communications. 2006;345(1):271–9. doi: 10.1016/j.bbrc.2006.04.076. [DOI] [PubMed] [Google Scholar]
- 50.Dos Santos E, Benaitreau D, Dieudonne MN, Leneveu MC, Serazin V, Giudicelli Y, et al. Adiponectin mediates an antiproliferative response in human MDA-MB 231 breast cancer cells. Oncology reports. 2008;20(4):971–7. [PubMed] [Google Scholar]
- 51.Saxena NK, Sharma D. Metastasis suppression by adiponectin: LKB1 rises up to the challenge. Cell adhesion & migration. 2010;4(3):358–62. doi: 10.4161/cam.4.3.11541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kleinmann N, Duivenvoorden WC, Hopmans SN, Beatty LK, Qiao S, Gallino D, et al. Underactivation of the adiponectin-adiponectin receptor 1 axis in clear cell renal cell carcinoma: implications for progression. Clinical & experimental metastasis. 2014;31(2):169–83. doi: 10.1007/s10585-013-9618-1. [DOI] [PubMed] [Google Scholar]
- 53.Cui J, Shen Y, Li R. Estrogen synthesis and signaling pathways during aging: from periphery to brain. Trends in molecular medicine. 2013;19(3):197–209. doi: 10.1016/j.molmed.2012.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rajapaksa G, Thomas C, Gustafsson JA. Estrogen signaling and unfolded protein response in breast cancer. The Journal of steroid biochemistry and molecular biology. 2016 doi: 10.1016/j.jsbmb.2016.03.036. [DOI] [PubMed] [Google Scholar]
- 55.Catalano S, Marsico S, Giordano C, Mauro L, Rizza P, Panno ML, et al. Leptin enhances, via AP-1, expression of aromatase in the MCF-7 cell line. The Journal of biological chemistry. 2003;278(31):28668–76. doi: 10.1074/jbc.M301695200. [DOI] [PubMed] [Google Scholar]
- 56.Fusco R, Galgani M, Procaccini C, Franco R, Pirozzi G, Fucci L, et al. Cellular and molecular crosstalk between leptin receptor and estrogen receptor-{alpha} in breast cancer: molecular basis for a novel therapeutic setting. Endocrine-related cancer. 2010;17(2):373–82. doi: 10.1677/erc-09-0340. [DOI] [PubMed] [Google Scholar]
- 57.Liu L, Wang L, Zheng J, Tang G. Leptin promotes human endometrial carcinoma cell proliferation by enhancing aromatase (P450arom) expression and estradiol formation. European journal of obstetrics, gynecology, and reproductive biology. 2013;170(1):198–201. doi: 10.1016/j.ejogrb.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 58.Bowers LW, Cavazos DA, Maximo IX, Brenner AJ, Hursting SD, deGraffenried LA. Obesity enhances nongenomic estrogen receptor crosstalk with the PI3K/Akt and MAPK pathways to promote in vitro measures of breast cancer progression. Breast cancer research : BCR. 2013;15(4):R59. doi: 10.1186/bcr3453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Brown KA, McInnes KJ, Hunger NI, Oakhill JS, Steinberg GR, Simpson ER. Subcellular localization of cyclic AMP-responsive element binding protein-regulated transcription coactivator 2 provides a link between obesity and breast cancer in postmenopausal women. Cancer research. 2009;69(13):5392–9. doi: 10.1158/0008-5472.can-09-0108. [DOI] [PubMed] [Google Scholar]
- 60.Irahara N, Miyoshi Y, Taguchi T, Tamaki Y, Noguchi S. Quantitative analysis of aromatase mRNA expression derived from various promoters (I.4, I.3, PII and I.7) and its association with expression of TNF-alpha, IL-6 and COX-2 mRNAs in human breast cancer. International journal of cancer Journal international du cancer. 2006;118(8):1915–21. doi: 10.1002/ijc.21562. [DOI] [PubMed] [Google Scholar]
- 61.Hursting SD, Lavigne JA, Berrigan D, Perkins SN, Barrett JC. Calorie restriction, aging, and cancer prevention: mechanisms of action and applicability to humans. Annual review of medicine. 2003;54:131–52. doi: 10.1146/annurev.med.54.101601.152156. [DOI] [PubMed] [Google Scholar]
- 62.Nunez NP, Oh WJ, Rozenberg J, Perella C, Anver M, Barrett JC, et al. Accelerated tumor formation in a fatless mouse with type 2 diabetes and inflammation. Cancer research. 2006;66(10):5469–76. doi: 10.1158/0008-5472.can-05-4102. [DOI] [PubMed] [Google Scholar]
- 63.Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Systematic reviews. 2015;4:1. doi: 10.1186/2046-4053-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Notarnicola M, Miccolis A, Tutino V, Lorusso D, Caruso MG. Low levels of lipogenic enzymes in peritumoral adipose tissue of colorectal cancer patients. Lipids. 2012;47(1):59–63. doi: 10.1007/s11745-011-3630-5. [DOI] [PubMed] [Google Scholar]
- 65.Trevellin E, Scarpa M, Carraro A, Lunardi F, Kotsafti A, Porzionato A, et al. Esophageal adenocarcinoma and obesity: peritumoral adipose tissue plays a role in lymph node invasion. Oncotarget. 2015;6(13):11203–15. doi: 10.18632/oncotarget.3587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Iyengar NM, Zhou XK, Gucalp A, Morris PG, Howe LR, Giri DD, et al. Systemic Correlates of White Adipose Tissue Inflammation in Early-Stage Breast Cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. 2015 doi: 10.1158/1078-0432.ccr-15-2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Taguchi A, Rho JH, Yan Q, Zhang Y, Zhao Y, Xu H, et al. MAPRE1 as a plasma biomarker for early-stage colorectal cancer and adenomas. Cancer prevention research (Philadelphia, Pa) 2015;8(11):1112–9. doi: 10.1158/1940-6207.capr-15-0077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Morris PG, Hudis CA, Giri D, Morrow M, Falcone DJ, Zhou XK, et al. Inflammation and increased aromatase expression occur in the breast tissue of obese women with breast cancer. Cancer prevention research (Philadelphia, Pa) 2011;4(7):1021–9. doi: 10.1158/1940-6207.CAPR-11-0110. 1940-6207.CAPR-11-0110 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ribeiro R, Monteiro C, Catalan V, Hu P, Cunha V, Rodriguez A, et al. Obesity and prostate cancer: gene expression signature of human periprostatic adipose tissue. BMC medicine. 2012;10:108. doi: 10.1186/1741-7015-10-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Savolainen-Peltonen H, Vihma V, Leidenius M, Wang F, Turpeinen U, Hamalainen E, et al. Breast adipose tissue estrogen metabolism in postmenopausal women with or without breast cancer. The Journal of clinical endocrinology and metabolism. 2014;99(12):E2661–7. doi: 10.1210/jc.2014-2550. [DOI] [PubMed] [Google Scholar]
- 71.Catalan V, Gomez-Ambrosi J, Rodriguez A, Ramirez B, Silva C, Rotellar F, et al. Up-regulation of the novel proinflammatory adipokines lipocalin-2, chitinase-3 like-1 and osteopontin as well as angiogenic-related factors in visceral adipose tissue of patients with colon cancer. The Journal of nutritional biochemistry. 2011;22(7):634–41. doi: 10.1016/j.jnutbio.2010.04.015. [DOI] [PubMed] [Google Scholar]
- 72.Modesitt SC, Hsu JY, Chowbina SR, Lawrence RT, Hoehn KL. Not all fat is equal: differential gene expression and potential therapeutic targets in subcutaneous adipose, visceral adipose, and endometrium of obese women with and without endometrial cancer. International journal of gynecological cancer : official journal of the International Gynecological Cancer Society. 2012;22(5):732–41. doi: 10.1097/IGC.0b013e3182510496. [DOI] [PubMed] [Google Scholar]
- 73.Amor S, Iglesias-de la Cruz MC, Ferrero E, Garcia-Villar O, Barrios V, Fernandez N, et al. Peritumoral adipose tissue as a source of inflammatory and angiogenic factors in colorectal cancer. International journal of colorectal disease. 2016;31(2):365–75. doi: 10.1007/s00384-015-2420-6. [DOI] [PubMed] [Google Scholar]
- 74.Finley DS, Calvert VS, Inokuchi J, Lau A, Narula N, Petricoin EF, et al. Periprostatic adipose tissue as a modulator of prostate cancer aggressiveness. The Journal of urology. 2009;182(4):1621–7. doi: 10.1016/j.juro.2009.06.015. [DOI] [PubMed] [Google Scholar]
- 75.Mullooly M, Yang HP, Falk RT, Nyante SJ, Cora R, Pfeiffer RM, et al. Relationship between crown-like structures and sex-steroid hormones in breast adipose tissue and serum among postmenopausal breast cancer patients. Breast cancer research : BCR. 2017;19(1):8. doi: 10.1186/s13058-016-0791-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jung YY, Lee YK, Koo JS. Expression of cancer-associated fibroblast-related proteins in adipose stroma of breast cancer. Tumour biology : the journal of the International Society for Oncodevelopmental Biology and Medicine. 2015;36(11):8685–95. doi: 10.1007/s13277-015-3594-9. [DOI] [PubMed] [Google Scholar]
- 77.Iyengar NM, Morris PG, Zhou XK, Gucalp A, Giri D, Harbus MD, et al. Menopause is a determinant of breast adipose inflammation. Cancer prevention research (Philadelphia, Pa) 2015;8(5):349–58. doi: 10.1158/1940-6207.capr-14-0243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Koru-Sengul T, Santander AM, Miao F, Sanchez LG, Jorda M, Gluck S, et al. Breast cancers from black women exhibit higher numbers of immunosuppressive macrophages with proliferative activity and of crown-like structures associated with lower survival compared to non-black Latinas and Caucasians. Breast cancer research and treatment. 2016;158(1):113–26. doi: 10.1007/s10549-016-3847-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lysaght J, Allott EH, Donohoe CL, Howard JM, Pidgeon GP, Reynolds JV. T lymphocyte activation in visceral adipose tissue of patients with oesophageal adenocarcinoma. The British journal of surgery. 2011;98(7):964–74. doi: 10.1002/bjs.7498. [DOI] [PubMed] [Google Scholar]
- 80.Lysaght J, van der Stok EP, Allott EH, Casey R, Donohoe CL, Howard JM, et al. Pro-inflammatory and tumour proliferative properties of excess visceral adipose tissue. Cancer letters. 2011;312(1):62–72. doi: 10.1016/j.canlet.2011.07.034. [DOI] [PubMed] [Google Scholar]
- 81.Venkatasubramanian PN, Brendler CB, Plunkett BA, Crawford SE, Fitchev PS, Morgan G, et al. Periprostatic adipose tissue from obese prostate cancer patients promotes tumor and endothelial cell proliferation: a functional and MR imaging pilot study. The Prostate. 2014;74(3):326–35. doi: 10.1002/pros.22756. [DOI] [PubMed] [Google Scholar]
- 82.Zhang Q, Sun LJ, Yang ZG, Zhang GM, Huo RC. Influence of adipocytokines in periprostatic adipose tissue on prostate cancer aggressiveness. Cytokine. 2016;85:148–56. doi: 10.1016/j.cyto.2016.06.019. [DOI] [PubMed] [Google Scholar]
- 83.Iyengar NM, Ghossein RA, Morris LG, Zhou XK, Kochhar A, Morris PG, et al. White adipose tissue inflammation and cancer-specific survival in patients with squamous cell carcinoma of the oral tongue. Cancer. 2016;122(24):3794–802. doi: 10.1002/cncr.30251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bhaskaran K, Douglas I, Forbes H, dos-Santos-Silva I, Leon DA, Smeeth L. Body-mass index and risk of 22 specific cancers: a population-based cohort study of 5.24 million UK adults. Lancet. 2014;384(9945):755–65. doi: 10.1016/s0140-6736(14)60892-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Subbaramaiah K, Howe LR, Bhardwaj P, Du B, Gravaghi C, Yantiss RK, et al. Obesity is associated with inflammation and elevated aromatase expression in the mouse mammary gland. Cancer prevention research (Philadelphia, Pa) 2011;4(3):329–46. doi: 10.1158/1940-6207.capr-10-0381. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 86.Wang X, Docanto MM, Sasano H, Lo C, Simpson ER, Brown KA. Prostaglandin E2 inhibits p53 in human breast adipose stromal cells: a novel mechanism for the regulation of aromatase in obesity and breast cancer. Cancer research. 2015;75(4):645–55. doi: 10.1158/0008-5472.can-14-2164. [DOI] [PubMed] [Google Scholar]
- 87.Zhao H, Zhou L, Shangguan AJ, Bulun SE. Aromatase expression and regulation in breast and endometrial cancer. Journal of molecular endocrinology. 2016;57(1):R19–33. doi: 10.1530/jme-15-0310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wang X, Simpson ER, Brown KA. Aromatase overexpression in dysfunctional adipose tissue links obesity to postmenopausal breast cancer. The Journal of steroid biochemistry and molecular biology. 2015;153:35–44. doi: 10.1016/j.jsbmb.2015.07.008. [DOI] [PubMed] [Google Scholar]
- 89.Iyengar NM, Zhou XK, Gucalp A, Morris PG, Howe LR, Giri DD, et al. Systemic Correlates of White Adipose Tissue Inflammation in Early-Stage Breast Cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. 2016;22(9):2283–9. doi: 10.1158/1078-0432.ccr-15-2239. [DOI] [PMC free article] [PubMed] [Google Scholar]


