Abstract
Objective
This study compared the additional effect of rinsing with a fluoride-free and alcohol-free 0.075% cetylpyridinium chloride (CPC) mouthwash to brushing alone on dental plaque, gingival inflammation, and supragingival plaque bacteria.
Methods
Adult subjects [n = 68] completed a washout period prior to baseline evaluations that evaluated gingival inflammation, gingival bleeding, dental plaque, and pocket probing depths along with microbiological analysis of supragingival plaque for bacteria. Subjects were randomized to two treatment groups: brush with fluoride toothpaste and rinse with the CPC mouthwash (test) or brush with fluoride toothpaste only (control), twice daily for the next four weeks. Subjects abstained from oral hygiene for twelve-hours prior to two-week and four-week post-treatment microbiological analysis of supragingival plaque for bacteria. Clinical assessments for gingival inflammation, gingival bleeding, dental plaque, and pocket probing depths were conducted at the four-week post-treatment visit.
Results
Compared to baseline, bacteria of dental plaque in the test group were reduced by 61.1% and 83.0% at the two-week and four-week evaluations, respectively (p < 0.05). Compared to baseline, bacteria of supragingival plaque in the control group were reduced by 2.3% at either post-treatment evaluations (p < 0.05). Additionally, dental plaque bacteria in the test was 69.8% and 86.8% lower than the control at the two-week and four-week evaluations (p < 0.05), respectively. After four-weeks, the test group showed 14.3% less gingivitis, 11.2% less dental plaque, 7.5% less gingival bleeding compared to the control group (p < 0.05).
Conclusions
Oral hygiene comprising toothbrushing and rinsing with a mouthwash containing 0.075% cetylpyridinium chloride demonstrated greater reductions of dental plaque bacteria, improving gingival health, and eliminating supragingival plaque than toothbrushing alone.
Keywords: Dental plaque, Bacteria, Mouthwash, Hygiene, Gingivitis
Clinical relevance
Scientific rationale for study: Poor oral hygiene resulting in microbial accumulations of dental plaque has been associated with common conditions such as gingivitis. This study utilized a unique study design to examine improvements in oral hygiene provided by a regimen comprising toothbrushing and rinsing with a cetylpyridinium chloride mouthrinse than toothbrushing alone. Study evaluated effects on oral bacteria and on supragingival plaque and gingivitis over the study duration.
Principal findings: A regimen comprising toothbrushing and rinsing with a cetylpyridinium chloride mouthrinse demonstrated significantly greater reductions in oral bacteria and clinical outcomes than toothbrushing alone. Additionally, the regimen demonstrated progressive improvements in evaluated outcomes over the study period.
Practical implications: Oral hygiene comprising toothbrushing and rinsing with a mouthwash containing 0.075% cetylpyridinium chloride demonstrated greater reductions of dental plaque bacteria, improving gingival health, and eliminating supragingival plaque than toothbrushing alone.
1. Introduction
The most common oral health regimen in Western nations is brushing with fluoride-containing toothpaste to remove dental plaque and prevent dental caries [1], [2], [3], [4], [5]. Many people complement this practice with additional methods of hygiene [1], [2], [3]. Cleaning interproximally with floss or a brush, removing detritus from the tongue with a brush or a scraper, using an oral irrigator, and/or rinsing with mouthwash can all help reduce plaque, manage halitosis, and avoid tooth decay [1], [2], [3].
While the efficacy of certain aspects of oral hygiene, such as toothbrushing, has been well established, there are less data on the virtue of other facets of dental self-care. For example, there is strong evidence to show that daily brushing with a dentifrice containing fluoride prevents more dental caries than using a non-fluoride toothpaste [5]. Studies also affirm that toothbrushing combined with flossing may reduce dental plaque, with concomitant reductions in gingivitis, more than brushing alone [1], [2], [3], [6]. There is also data to support the idea that children who use a fluoride mouthrinse in addition to brushing their teeth with a fluoride toothpaste have fewer dental caries than children who use fluoride toothpaste alone [7], [8]. In addition, a review of five previously conducted clinical trials found that subjects who only brushed their teeth showed less improvement in halitosis than those who brushed in addition to using mouthrinses containing chlorhexidine, cetylpyridinium chloride, or chlorine dioxide and zinc [9].
In the present study, we hypothesized that patients who brushed with fluoride-containing toothpaste in addition to rinsing with a fluoride- and alcohol-free mouthwash containing 0.075% cetylpyridinium chloride (CPC) would show greater reduction in dental plaque organisms than those who used fluoride toothpaste alone.
2. Materials and Methods
This was a double-blind, two-treatment, parallel design, randomized controlled clinical trial conducted at a single site. At the screening appointment conducted at the School of Dental Medicine, University of Buffalo, male and female volunteers between 18 and 70 years of age completed an informed consent form, a health screening form, and a demographic questionnaire. They were then evaluated by a dentist for oral soft and hard tissue health and underwent whole-mouth evaluations assessing six sites per tooth for gingival inflammation (Loe-Silness Index) [10], gingival bleeding, [11] and dental plaque [12]. Subjects who met the following criteria were eligible for participation in the study: (1) good general health, (2) ability to read, understand, and sign the informed consent form; (3) willingness to comply with study procedures and sampling schedules, (4) at least 20 uncrowned permanent natural teeth, (5) gingival index ≥ 1.010, and (6) plaque index ≥ 1.512. Subjects were excluded from the study if they (1) had a history of significant adverse effects caused by oral hygiene products, (2) had allergies to personal care products or their ingredients, (3) had gross dental caries or extensively restored facial or lingual tooth surfaces, (4) had fixed or removable orthodontic appliances, (5) had removable partial dentures, (6) had a history of, or current, diabetes mellitus, renal disease, heart disease, alcoholism, recreational drug use or other serious medical conditions or transmissible infectious diseases (such as hepatitis or AIDS); (7) required antibiotics prior to dental treatment, (8) used antibiotics, anti-inflammatory drugs, or anticoagulants in the prior month; (9) had significant oral pathology (including, but not limited to, gingival enlargement, severe gingivitis, moderate to severe periodontitis including ≥ one periodontal pocket > 5 mm); (10) had participated in a clinical study involving oral care products in the prior month; (11) reported currently being pregnant or breast-feeding, or (12) had lip or tongue piercings.
The details of the study were explained to each eligible subject, who were given the opportunity to ask for any needed clarification. They acknowledged their consent and their willingness to comply with study procedures and sampling schedules by signing the informed consent form. The clinical protocol and consent forms were reviewed and approved by the University at Buffalo Health Sciences Institutional Review Board with the study conducted at the School of Dental Medicine, University at Buffalo.
Subjects were instructed to refrain from oral hygiene for 12 h and from food, drink or smoking for at least 4 h before the baseline, two-week and four-week examinations. At the baseline and four-week examinations, the subjects underwent whole-mouth evaluations at six sites for clinical parameters. Clinical evaluations included assessments for gingival inflammation (Loe-Silness Index) [10], gingival bleeding, [11] and dental plaque [12] and full-mouth pocket probing depths using a University of Michigan probe [13]. Supragingival dental plaque was collected at the baseline, two-week, and four-week examinations for microbiological analysis of bacteria. At each examination, plaque samples for microbiological analyses were randomly collected from either the upper right or left quadrants.
After the baseline examination and dental plaque sampling conducted by a dental professional, the subjects were randomly assigned to test or control groups using a computer-generated assignment sequence by a study co-ordinator. Subjects assigned to the test group were instructed to brush twice daily (morning and evening) with a commercially available regular fluoride toothpaste (Colgate Dental Cream, Colgate Great Regular Flavor, Colgate-Palmolive Company New York, NY) and soft-bristled toothbrush (Colgate Extra Clean, Colgate-Palmolive Company New York, NY). After brushing, subjects were instructed to rinse for 30 s with 20 ml of a fluoride- and alcohol-free mouthwash containing 0.075% CPC (Colgate Total, Colgate-Palmolive Company New York, NY). Subjects assigned to the control group were instructed to brush twice daily (morning and evening) with a commercially available regular fluoride toothpaste (Colgate Dental Cream, Colgate Great Regular Flavor, Colgate-Palmolive Company New York, NY). All products were overwrapped, coded and supplied by Colgate-Palmolive Company, New York, NY with subjects and dental examiners blinded to treatment assignment. Subject recruitment commenced in March 2013 and the study completed in July 2013.
Supragingival dental plaque samples obtained at the baseline, two-week and four-week examinations were dispersed by sonication and then serially diluted by tenfold in phosphate-buffered saline. Undiluted samples and sample dilutions 101 to 104 were distributed in duplicate (Spiral Systems Autoplate 4000 Spiral Plater) on enriched trypticase soy agar containing 5% sheep blood (ETSA). The inoculated media were incubated at 37 °C for 5–7 days under anaerobic conditions. Viable counts for each sample was recorded as mean colony-forming units (CFU)/ml from duplicate cultures from dilutions demonstrating at least 20 colony-forming units. Results from viable counts were log transformed (log10) for analysis.
3. Statistical analysis
Sample size calculations were based on unpublished historical data that a sample size of 30 subjects would detect a difference of 0.6 Log CFU/ml in viable plaque bacteria between treatments at 80% probability assuming a standard deviation of 0.7 Log CFU/ml for bacteria (unpublished data). Viable counts from the bacterial cultures were calculated as the mean colony-forming units (CFU)/ml of duplicate cultures from dilutions demonstrating at least 20 colony-forming units. The viable counts were log transformed (log10) for analysis. Mean scores were computed for gingival index, bleeding index and plaque index scores at the baseline and four-week whole mouth examinations. Demographic results between the two treatment groups were compared by a chi-square analysis. Clinical and microbiological data were analyzed using analysis of variance (ANOVA), paired t-tests, and analysis of covariance (ANCOVA). All statistical tests of hypotheses were two-sided and employed a level of significance of α = 0.05. Analyses were conducted with Minitab (Minitab Inc., State College, PA).
4. Results
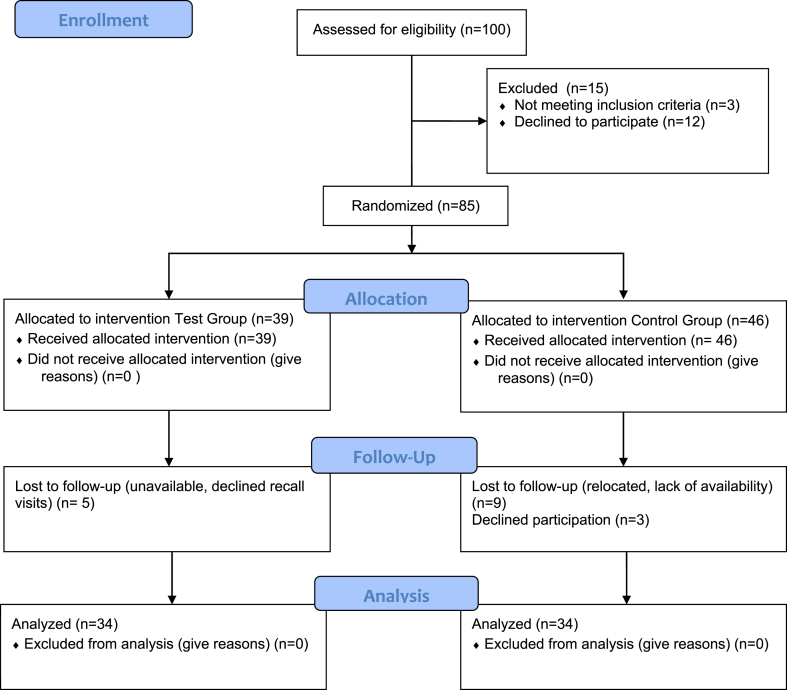
The demographic data for the study population screened and enrolled are presented as a CONSORT diagram (Fig. 1). There were 34 subjects in both the test and control groups (Table 1) who completed the entire study. Statistical analyses indicate no significant (p > 0.05) differences between test or control groups at baseline for any of the clinical or microbiological assessments (Table 1). There were no observed or reported adverse events on the oral soft or hard tissues observed by the clinical examiner or reported by the subjects during the study.
Fig. 1.
CONSORT Flow diagram.
Table 1.
Subject demographics.
| Treatment group | Number of Subjects |
Ageb |
Race | |||
|---|---|---|---|---|---|---|
| Male | Female | Totala | Mean | Range | ||
| Test Group | 17 | 17 | 34 | 43.4 | 24–70 | Caucasian = 32 Asian = 1 African American = 1 |
| Control Group | 11 | 23 | 34 | 40.3 | 20–64 | Caucasian = 29 Asian = 3 African American = 2 |
Total number of subjects in each treatment group. No significant differences between treatment groups for subject gender by a chi-square test (p > 0.05).
Age of subjects in treatment group. No significant differences between treatment groups for subject age by two-sample t-test (p > 0.05).
Table 2 presents a summary of the mean number of cultivable oral bacteria (log CFU/ml) collected twelve (12) hours after two and four weeks of product use. Percent reductions were calculated via the formula (1-10diff), where “diff” is equal to the difference between treatment groups or time points. After two weeks of use and 12 h after oral hygiene, the percent reductions in the number of cultivable supragingival plaque bacteria compared to baseline was statistically significant (p < 0.05) for both the test and the control groups. Compared to baseline, the mean number of cultivable oral bacteria at two weeks (log CFU/ml) was 5.57 for the test group for a 61.1% reduction and 6.09 for the control group for a 2.3% reduction. After two weeks of product use, subjects in the test group exhibited a statistically significant (p < 0.05) 69.8% reduction in mean number of cultivable oral bacteria compared to the control group.
Table 2.
Microbiological results from samples collected over the study period.
| Evaluation | Treatment |
Between treatment comparisons (% Differences) | |
|---|---|---|---|
| Control group (Mean ± SD)a | Test group (Mean ± SD)a | ||
| Baseline | 6.10 ± 0.66 | 5.98 ± 0.61 | – |
| 2 weeks | 6.09 ± 0.67 | 5.57 ± 0.65∗ | 69.8%b |
| 4 weeks | 6.08 ± 0.66 | 5.21 ± 0.54∗ | 86.8%b |
*Significant differences between treatment groups by ANCOVA (p < 0.05).
Viable bacteria recovered (Unadjusted mean ± standard deviation).
Differences between the treatment groups at each evaluation expressed as a percentage.
At the four week evaluation conducted 12 h after oral hygiene, the percent reduction in the number of cultivable supragingival plaque bacteria was statistically significant (p < 0.05) for both test and control groups compared to baseline (Table 2). The mean number of cultivable oral bacteria (log CFU/ml) were 5.21 for the test group for an 83% reduction compared to baseline and 6.09 for the control group for a 2.3% reduction compared to baseline. After four weeks of product use, subjects in the test group exhibited a statistically significant (p < 0.05) reduction of 86.8% in mean number of cultivable oral bacteria compared to the control group.
A summary of the clinical assessments–gingival index, bleeding index, plaque index—observed after four weeks of product use is shown in Table 3. A negative percentage indicates an increase in the index scores.
Table 3.
Summary of the Clinical Evaluations conducted at Baseline and at the Four Weeks Post-Treatment Evaluations.
| Parameter | Treatment | Baseline Scores |
After 4 Weeks of Use |
Within-Treatment Comparison |
Between-Treatments Comparison |
|
|---|---|---|---|---|---|---|
| Mean ± s.da | Mean ± s.da | % Reductionb | % Differencec | sigd | ||
| Gingival Index | Test group | 1.22 ± 0.19 | 0.96 ± 0.23 | 21.3%∗ | 14.3% | p < 0.05 |
| Control Group | 1.18 ± 0.14 | 1.12 ± 0.13 | 5.1%∗ | |||
| Plaque Index | Test group | 1.96 ± 0.24 | 1.74 ± 0.28 | 11.2%∗ | 11.2% | p < 0.05 |
| Control Group | 1.86 ± 0.33 | 1.96 ± 0.34 | −5.4% | |||
| Bleeding Index | Test group | 0.51 ± 0.18 | 0.37 ± 0.14 | 27.5%∗ | 7.5% | p < 0.05 |
| Control Group | 0.39 ± 0.18 | 0.40 ± 0.19 | −2.6% | |||
| Control Group | 1.91 ± 0.19 | 1.89 ± 0.18 | 1.0% | |||
* Significant differences comparing baseline and final examinations by a paired t-test (p < 0.05).
Unadjusted mean ± s.d.
Percent reduction comparing final mean score to the baseline mean score.
Difference between the final mean score expressed as a percentage of the final mean score for the control group. A positive value indicates a reduction in index scores for the test group compared to the control group.
Significance of ANCOVA comparison of baseline-adjusted means.
The mean four-week gingival index scores were 0.96 for subjects in the test group and 1.12 for subjects in the control group. The mean percent reductions from baseline were 21.3% for subjects in the test group and 5.1% for subjects in the control group. Both the test and control groups demonstrated statistically significant reductions from their corresponding baselines (p < 0.05). Compared to subjects in the control group, subjects in the test group exhibited a statistically significant (p < 0.05) reduction of 14.3% in gingival index scores after four weeks of product use.
The mean four-week bleeding index scores for subjects in the test and control groups were 0.37 and 0.40 respectively. Mean percent reduction from baseline was 27.5% for subjects in the test group and a mean percent increase of 2.6% from baseline for subjects in the control group. Compared to baseline, reductions in the test group were statistically significant (p < 0.05), while there was no change in the control group. Compared to subjects in the control group, subjects in the test group exhibited a statistically significant (p < 0.05) reduction of 7.5% in bleeding index scores.
The mean four-week plaque index scores were 1.74 for subjects in the test group and 1.96 for subjects in the control group. The mean percent reduction from baseline was 11.2% for subjects in the test group. There was a mean percent increase of 5.4% in plaque index for subjects in the control group. Compared to baseline, reductions in the test group were statistically significant (p < 0.05), while there was no change in the control group. Compared to subjects in the control group, subjects in the test group exhibited a statistically significant (p < 0.05) reduction of 11.2% in plaque index scores after four weeks of product use.
At baseline, mean whole mouth probing depths in the control group was 1.91 mm (range 1.52–1.62) and 1.95 mm (range 1.52–2.92 mm) in the test group respectively. The mean four-week whole mouth probing depths were 1.77 mm (range 1.51–2.32) for subjects in the test group and 1.89 mm (range 1.52–1.41) for subjects in the control group. The mean reductions from baseline were 0.18 mm for subjects in the test group and 0.02 mm for subjects in the control group. Reductions from baseline in mean whole-mouth probing depths were statistically significant (p < 0.05) in the test group, but not in the control group. Compared to subjects in the control group, subjects in the test group exhibited a statistically significant (p < 0.05) reduction of 0.12 mm in pocket depth index scores after four weeks of product use.
5. Discussion
This double-blind clinical study compared the 12-h antibacterial effect of brushing with a commercially-available fluoride toothpaste and rinsing with a commercially available fluoride- and alcohol-free 0.075% cetylpyridinium chloride mouthwash to brushing alone. Microbiological changes were measured after two weeks and four weeks of product use while clinical efficacy measured as gingival inflammation, gingival bleeding, dental plaque, and pocket depth was measured after four weeks of product use.
We have previously demonstrated the antimicrobial activity of cetylpyridinium chloride mouthrinses in in vitro and ex vivo studies [14]. Those studies examined the in vitro antimicrobial effects of commercially available 0.05% cetylpyridinium chloride mouthrinse on strains of oral bacteria found in health, dental caries, gingivitis, halitosis and periodontitis as well as non-oral microorganisms and yeasts sometimes found in the oral cavity. Oral strains of Actinomyces, Campylobacter, Moraxella, Veillonella, periodontal pathogens including Aggregatibacter actinomycetemcomitans, P. gingivalis, P. intermedia and P. nigrescens, Candida albicans and non-oral species including Bacillus cereus and Staphylococcus aureus were inhibited by cetylpyridinium chloride mouthrinse. Ex vivo antimicrobial testing of human supragingival dental plaque samples in that study–reflecting a spectrum of cultivable dental plaque microorganisms—showed a greater than 90% reduction in the number of supragingival plaque bacteria cultivable on media containing 0.05% cetylpyridinium chloride. The negative control exhibited significantly less antimicrobial activity. Those studies, therefore, provide a biological basis for the present clinical study that demonstrates the efficacy of a fluoride- and alcohol-free cetylpyridinium chloride mouthrinse in reducing supragingival plaque and plaque-associated gingivitis.
Results in the present study are consistent with the reductions in dental plaque and gingivitis noted in previous research that also included the use of a CPC mouthrinse for oral hygiene. Allen et al. [15] reported that subjects using 0.05% cetylpyridinium chloride mouthrinses had more significant reductions in clinical evaluations for supragingival dental plaque than subjects using a fluoride rinse. The mean percent reduction in anaerobic bacteria for subjects using cetylpyridinium chloride mouthrinse was 57.9% after fourteen days of use compared to subjects using the fluoride mouthrinse. Hernandez-Cott et al. [16] examined subjects using a 0.05% cetylpyridinium chloride mouthrinse compared to subjects using a mouthrinse without cetylpyridinium chloride and found that, after seven days of product use and 12 h after rinsing, the 0.05% cetylpyridinium chloride mouthrinse group exhibited statistically significant greater reductions in whole-mouth plaque, interproximal plaque and in plaque index scores. Lotufo et al. [17] reported that after seven days of product use and 12 h after rinsing, mean plaque levels for subjects who brushed their teeth for 1 min, rinsed their mouths with water after brushing, and then rinsed for 1 min with 15 ml of a 0.05% cetylpyridinium chloride mouthrinse twice daily were statistically significantly lower (29.3%, p < 0.05) than the control group, who followed the same protocol but used a mouthrinse without 0.05% cetylpyridinium chloride.
A recent six-month study of the efficacy of a 0.07% cetylpyridinium chloride mouthrinse [18] reports similar findings as the present study regarding reductions in the number of anaerobic plaque bacteria. In that study, subjects were instructed to brush three times a day followed by rinsing with a 0.07% cetylpyridinium chloride mouthrinse or vehicle control without cetylpyridinium chloride. Three- and six-month evaluations were performed two to 3 h after toothbrushing and rinsing. In the cetylpyridinium chloride mouthrinse group, the number of anaerobic bacteria in pooled supragingival plaque and unstimulated saliva samples was reduced two to three times (p < 0.05) after six months (1.5 × 108 CFU/ml) compared to baseline (4.7 × 108 CFU/ml), but the number of anaerobic bacteria was not reduced in the control group after six months (3.0 × 108 CFU/ml at baseline vs 1.6 × 108 CFU/ml after six months). There were also significant reductions in clinical measures of dental plaque but not in clinical measures of gingival bleeding.
Independent use of a cetylpyridinium chloride mouthrinse can have significant benefits for overall health. Jeffcoat et al. [19] found that pregnant women with periodontal disease who rinsed twice daily for 30 s after regular toothbrushing with 20 mL of an alcohol-free antimicrobial mouthrinse containing 0.07% cetylpyridinium chloride had a lower incidence of preterm low birthweight compared to control subjects who were instructed to rinse with water. Subjects in the 0.07% cetylpyridinium chloride mouthrinse group had babies with significantly higher gestational age and birthweight compared to the control group.
This study recruited adults of either gender from the general population who were from the local community but were not seeking any dental or medical care. Subjects with gingivitis and a complement of natural teeth who qualified for other study criteria were enrolled but study population did not include those with periodontal disease or other oral conditions. Enrolled subjects were randomly assigned to a test group and provided instructions for use of assigned treatments with all of these steps conducted using standardized procedures. Subjects were instructed to perform oral hygiene with provided treatments twice daily and were reminded of these instructions at intervals over the study duration. To reduce the influences of other oral hygiene formulations or procedures, subjects were instructed to refrain from interdental cleaning, flossing or other commercial formulations unassociated with the study. Additionally, this study sought to reduce the influences of other subject features such as systemic diseases, prescription medications, pregnancy or any other chronic conditions by excluding individuals who reported any of these indications during their screening visit. These above steps and subject compliance reminders over the study period were designed to examine the effects of these treatments on community dwelling adults.
In summary, the results from this double-blind clinical study demonstrate that after two weeks and four weeks of subjects' twice-daily brushing with a commercially-available regular fluoride toothpaste and rinsing with a commercially available fluoride-free and alcohol-free 0.075% cetylpyridinium chloride mouthwash, samples taken 12 h after oral hygiene had significantly greater reductions in the number of oral bacteria compared to those who only brushed twice a day with a commercially-available regular fluoride toothpaste. In addition, after four weeks of twice-daily brushing with a commercially-available regular fluoride toothpaste and rinsing with a commercially available fluoride-free and alcohol-free 0.075% cetylpyridinium chloride mouthwash, subjects' samples taken 12 h after oral hygiene had significantly greater reductions in gingivitis, dental plaque, gingival bleeding and probing depth compared to subjects who only brushed twice a day with a commercially available regular fluoride toothpaste.
Sources of funding
This study was supported by Colgate-Palmolive Company.
Conflict of interest
Prem K. Sreenivasan is an employee of Colgate-Palmolive Company.
References
- 1.Wilder R.S., Bray K.S. Improving periodontal outcomes: merging clinical and behavioral science. Periodontology 2000. 2016;71(1):65–81. doi: 10.1111/prd.12125. [DOI] [PubMed] [Google Scholar]
- 2.Claydon N.C. Current concepts in toothbrushing and interdental cleaning. Periodontology 2000. 2008;48:10–22. doi: 10.1111/j.1600-0757.2008.00273.x. [DOI] [PubMed] [Google Scholar]
- 3.Sgan-Cohen H.D. Oral hygiene: past history and future recommendations. Int. J. Dent. Hyg. 2005;3(2):54–58. doi: 10.1111/j.1601-5037.2005.00114.x. [DOI] [PubMed] [Google Scholar]
- 4.Westfelt E. Rationale of mechanical plaque control. J. Clin. Periodontol. 1996;23(3 Pt 2):263–267. doi: 10.1111/j.1600-051x.1996.tb02086.x. [DOI] [PubMed] [Google Scholar]
- 5.Twetman S., Axelsson S., Dahlgren H. Caries-preventive effect of fluoride toothpaste: a systematic review. Acta Odontol. Scand. 2003;61(6):347–355. doi: 10.1080/00016350310007590. [DOI] [PubMed] [Google Scholar]
- 6.Sambunjak D., Nickerson J.W., Poklepovic T. Flossing for the management of periodontal diseases and dental caries in adults. Cochrane Database Syst. Rev. 2011;12 doi: 10.1002/14651858.CD008829.pub2. [DOI] [PubMed] [Google Scholar]
- 7.Marinho V.C.C., Higgins J.P.T., Logan S., Sheiham A. Fluoride mouthrinses for preventing dental caries in children and adolescents. Cochrane Database Syst. Rev. 2003;3 doi: 10.1002/14651858.CD002284. [DOI] [PubMed] [Google Scholar]
- 8.Marinho V.C.C., Higgins J.P.T., Sheiham A., Logan S. Combinations of topical fluoride (toothpastes, mouthrinses, gels, varnishes) versus single topical fluoride for preventing dental caries in children and adolescents. Cochrane Database Syst. Rev. 2004;1 doi: 10.1002/14651858.CD002781.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fedorowicz Z., Aljufairi H., Nasser M., Outhouse T.L., Pedrazzi V. Mouthrinses for the treatment of halitosis. Cochrane Database Syst. Rev. 2008;4 doi: 10.1002/14651858.CD006701.pub2. [DOI] [PubMed] [Google Scholar]
- 10.Fischman S.L. Clinical index systems used to assess the efficacy of mouthrinses on plaque and gingivitis. J. Clin. Periodontol. 1988;15:506–510. doi: 10.1111/j.1600-051x.1988.tb01022.x. [DOI] [PubMed] [Google Scholar]
- 11.Muhlemann H.R., Son S. Gingival sulcus bleeding-a leading symptom in initial gingivitis. Helvetica Odontol. Acta. 1971;15:107–113. [PubMed] [Google Scholar]
- 12.Turesky S., Gilmore N.D., Glickman I. Reduced plaque formation by chloromethyl analogue of victamine C. J. Periodontol. 1970;41:41–43. doi: 10.1902/jop.1970.41.41.41. [DOI] [PubMed] [Google Scholar]
- 13.Hefti A.F. Periodontal probing. Crit. Rev. Oral Biol. Med. 1997;8:336–356. doi: 10.1177/10454411970080030601. [DOI] [PubMed] [Google Scholar]
- 14.Sreenivasan P.K., Haraszthy V.I., Zambon J.J. Antimicrobial efficacy of 0·05% cetylpyridinium chloride mouthrinses. Lett. Appl. Microbiol. 2013;56:14–20. doi: 10.1111/lam.12008. [DOI] [PubMed] [Google Scholar]
- 15.Allen D.R., Davies R., Bradshaw B. Efficacy of a mouthrinse containing 0.05% cetylpyridinium chloride for the control of plaque and gingivitis: a 6-month clinical study in adults. Compend Contin. Educ. Dent. 1998;19(2 Suppl.):20–26. [PubMed] [Google Scholar]
- 16.Hernandez-Cott P.L., Elias Boneta A., Stewart B., DeVizio W., Proskin H.M. Clinical investigation of the efficacy of a commercial mouthrinse containing 0.05% cetylpyridinium chloride in reducing dental plaque. J. Clin. Dent. 2009;20(2):39–44. [PubMed] [Google Scholar]
- 17.Lotufo R., Calil C.M., Feng H.S., Sekiguchi R.T., Stewart B., DeVizio W., Proskin H.M. Clinical investigation of the efficacy of a commercial mouthrinse containing 0.05% cetylpyridinium chloride in preventing dental plaque. J. Clin. Dent. 2009;20(2):50–54. [PubMed] [Google Scholar]
- 18.Van Leeuwen M., Rosema N., Versteeg P. Long-term efficacy of a 0.07% cetylpyridinium chloride mouth rinse in relation to plaque and gingivitis: a 6-month randomized, vehicle-controlled clinical trial. Int. J. Dent. Hyg. 2014 Jul 15 doi: 10.1111/idh.12082. [DOI] [PubMed] [Google Scholar]
- 19.Jeffcoat M., Parry S., Gerlach R.W., Doyle M.J. Use of alcohol-free antimicrobial mouth rinse is associated with decreased incidence of preterm birth in a high-risk population. Am. J. Obstet. Gynecol. 2011;205(4) doi: 10.1016/j.ajog.2011.07.016. 382.e1-6. [DOI] [PubMed] [Google Scholar]