Abstract
Background/Aims
Clinical trials of older adults are increasingly common, but risks of serious adverse events (SAE) may vary. We describe the incidence of SAE in two randomized trials, one community-based and one nursing home-based.
Methods
We performed a secondary data analysis from two randomized clinical trials at one academic health center and 21 nursing homes involving 200 sedentary community dwellers aged 70–89 years and 185 female nursing home residents aged 65 years or older. Interventions included structured physical activity to reduce mobility disability in the Lifestyle Interventions and Independence for Elders (LIFE) study and oral cranberry capsules to reduce bacteriuria plus pyuria in nursing home residents (CRANNY) trial. We measured SAE incidence per 100 person-years and incidence of protocol-related unanticipated SAE per 100 person-years in LIFE and CRANNY trials.
Results
Mean age and proportion of patients with dementia in LIFE and CRANNY trials were 79.3 years and 86.4 years and 0% and 78%, respectively. There were 179 total SAE in LIFE including 8 (4%) deaths, and 116 total SAE in CRANNY including 33 (28%) deaths. SAE incidence was 33.7 (95% CI 27.2, 41.8) events per 100 person-years in LIFE and 69.4 (95% CI 49.1, 98.1) events per 100 person-years in CRANNY. No protocol-related unanticipated SAE occurred in either trial.
Conclusions
The frequency and severity of SAE vary in older adults. While SAE are common in nursing home residents, protocol-related, unanticipated SAE are rare in nursing home residents and community dwellers. This finding can inform trial monitoring protocols.
Trial registration
ClinicalTrials.gov identifiers: NCT01072500 and NCT01691430.
Keywords: Adverse events, Clinical trials, Older adults
1. Introduction
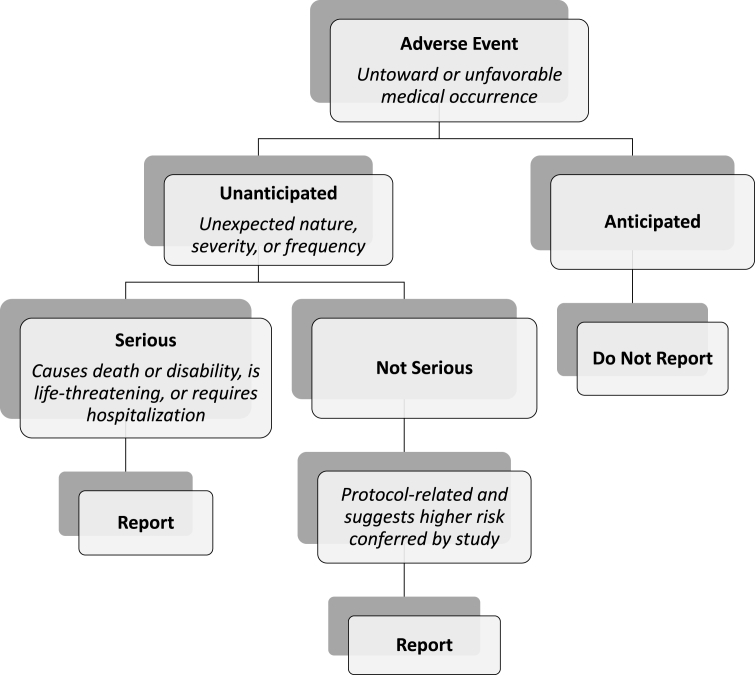
Diseases of aging require continued study with intervention trials to reduce disease severity and prevent disability. Inherent in all intervention trials is the need to monitor and report adverse events. The vast majority of adverse events are anticipated, and only unanticipated problems as defined by the U.S. Department of Health and Human Services that are protocol-related warrant reporting (Fig. 1) [1]. Current National Institute on Aging (NIA) guidelines on adverse event surveillance require documentation of all adverse events with expedited reporting (within 48 h of Principal Investigator notification) of all serious adverse events (SAE) to the Data and Safety Monitoring Board and NIA irrespective of protocol relationship [2].
Fig. 1.
Adapted from current adverse event reporting guidelines under Department of Health and Human Services Code of Federal Regulations Title 45 Part 46.
Resources required to meet NIA reporting guidelines may be prohibitive for intervention trials of older adults. Typically, personnel record adverse event data on paper forms including the nature and time of the event, associated hospitalizations, when the Principal Investigator was notified, and whether the event is ongoing or warrants reporting to external entities or study participants. This process may be labor-intensive for older adults that have differing susceptibilities to SAE. Specifically, nursing home residents may be high-risk for SAE by virtue of functional disabilities, grouped quarter living hazards (e.g., exposure to infectious disease outbreaks) and greater comorbidities compared to highly functional older community dwellers. Thus, high-risk nursing home residents are more likely than older community dwellers to meet SAE definitions during participation in intervention trials. However, data regarding SAE occurrence in low-risk intervention trials of older adults are lacking [[3], [4], [5]]. Quantifying SAE incidence in low-risk intervention trials of older adults may inform trial monitoring protocols and resource allocation for clinical personnel. For example, if expected SAE incidence is low, Principal Investigators may consider assigning less personnel time and effort towards SAE surveillance.
As investigators from two older adult clinical trials, one among a cohort of community dwellers (i.e., lifestyle interventions and independence for elders [LIFE] trial) and one among a cohort of nursing home residents (i.e., CRANberry capsules for prevention of urinary tract infection in Nursing home residents at Yale [CRANNY]), we are uniquely positioned to describe SAE using primary data from two distinct older adult populations. This study aimed to describe the incidence of SAE per participant-month of surveillance in LIFE (including only participants at the Yale site) and CRANNY and to describe the incidence of protocol-related, unanticipated SAE among participants in LIFE and CRANNY to inform resource allocation for SAE monitoring and reporting.
2. Methods
2.1. Participants
This study consisted of 200 participants enrolled in the LIFE trial at the Yale site, and 185 participants enrolled in the CRANNY trial. Only the Yale participants in LIFE were included to allow for comparable samples sizes and geographic distribution between both clinical trials. Participants in LIFE were sedentary older men and women with functional limitations randomized to a physical activity intervention or a successful aging health education intervention targeting prevention of major mobility disability. Participants in CRANNY were women nursing home residents age 65 or older who were randomized to cranberry capsules versus placebo capsules for reduction or prevention of bacteriuria plus pyuria. Further details of these participants have been reported elsewhere [6,7]. The Yale Human Investigation Committee approved this study.
2.2. Data collection
Baseline demographic and SAE data collected through the parent clinical trials have been reported previously [6,7]. In LIFE, SAE included death, a life-threatening event, persistent disability/incapacity, hospitalizations, and clinically significant laboratory and clinical test results. In CRANNY, because of the significant baseline frailty of the population, SAE included deaths and hospitalizations. NIA guidelines for adverse event monitoring and reporting were followed in both trials over the participant surveillance period [8].
Anticipated SAE were outlined in each IRB protocol. Unanticipated SAE included those that were unexpected, in terms of nature, severity, or frequency given (a) the research procedures described in the protocol-related documents (e.g., IRB protocol, informed consent document); and (b) the characteristics of the study population. Protocol-relatedness of the SAE was defined as a reasonable possibility that the incident, experience, or outcome may have been caused by the procedures involved in research. SAE categorization as anticipated and/or protocol-related was made by study personnel.
2.3. Statistical analysis
Means and standard deviations and counts and percentages are reported for characteristics of LIFE and CRANNY study participants. Tests of significance for differences between the two cohorts are not provided because of multiform distribution differences and lack of measurement standardization between the two study samples. Observed counts and rates are reported for SAE for both cohorts. Generalized linear models with Poisson distributions using natural logarithms for time at risk offsets were used to generate rates and 95% confidence intervals for events per 100 person-years of follow-up adjusted for overdispersion. Model results were exponentiated to return results to the original measurement scale. The “Rule of Three” method was used to generate upper bounds for confidence intervals of zero count results [9].
3. Results
The mean age of participants was 79.3 (±5.4) years in LIFE and 86.4 (±8.2) years in CRANNY (Table 1). LIFE excluded persons with dementia, and CRANNY was limited to women only. The percentage of study participants of white race was 90% in CRANNY. CRANNY participants also had frequent comorbidities consistent with expected estimates in an adult nursing home population. Consistent with community-dwelling populations, nearly one-third of LIFE participants had diabetes.
Table 1.
Characteristics of LIFE and CRANNY study participantsa.
| Characteristics | LIFE (n = 200) | CRANNY (n = 185) |
|---|---|---|
| Age (years) range (65–101), mean (SD) | 79.3 (5.4) | 86.4 (8.2) |
| Female, n (%) | 124 (62.0) | 185 (100.0) |
| White race, n (%) | 159 (79.5) | 167 (90.3) |
| Hispanic ethnicity, n (%) | 9 (4.5) | 6 (3.2) |
| Dementia,b n (%) | 0 (0.0) | 145 (78.4) |
| Number of disabled ADLs, mean (SD) | 1.3 (0.4) | 0.9 (1.6) |
| Number of in-common comorbidities, mean (SD) | 2.4 (1.4) | 3.2 (1.5) |
| Arrhythmia, n (%) | 54 (27.0) | 38 (20.5) |
| Cancer, n (%) | 51 (25.5) | 35 (18.9) |
| Chronic obstructive pulmonary disease, n (%) | 43 (21.5) | 41 (22.2) |
| Congestive heart failure, n (%) | 7 (3.5) | 57 (30.8) |
| Coronary artery disease, n (%) | 25 (12.5) | 60 (32.4) |
| Diabetes, n (%) | 63 (31.5) | 51 (27.6) |
| Hemiplegia, n (%) | 2 (1.0) | 9 (4.9) |
| Hypertension, n (%) | 144 (72.0) | 152 (82.2) |
| Liver disease, n (%) | 4 (2.0) | 4 (2.2) |
| Psychiatric disease, n (%) | 64 (32.0) | 125 (67.6) |
| Stroke, n (%) | 19 (9.5) | 26 (14.1) |
| Person-years in study, mean (SD) | 2.7 (0.4) | 0.9 (0.2) |
Study participants from LIFE study were restricted to Yale site.
Eligible participants in LIFE had no diagnosis of dementia or significant cognitive impairment on the Modified Mini-Mental State Examination.
Mortality and hospitalization rates in CRANNY and LIFE are shown (Table 2). Notably, all deaths in both studies were anticipated and unrelated to the study protocols. All hospitalizations in CRANNY and most hospitalizations (97%, 171/176) in LIFE were anticipated and protocol-unrelated. In LIFE, because the intervention involved physical activity, there were 5 hospitalizations that were anticipated and possibly related to the study protocol. These included four hospitalizations for elective knee replacement surgery and one hospitalization for deconditioning. In both trials, there were no unanticipated SAE.
Table 2.
Serious Adverse Event Ratesa in LIFE (at Yale) and CRANNY trials.
| Serious Adverse Events | LIFE (n = 200)b |
CRANNY (n = 185)c |
||
|---|---|---|---|---|
| Observed Counts | Rates (95% CI) | Observed Counts | Rates (95% CI) | |
| Anticipated/Unrelated | ||||
| Mortality | 8 | 1.5 (0.7, 3.2) | 33 | 19.7 (10.3, 37.9) |
| Hospitalization | 171 | 32.2 (26.1, 39.8) | 83 | 49.7 (34.8, 70.8) |
| Total SAE | 179 | 33.7 (27.2, 41.8) | 116 | 69.4 (49.1, 98.1) |
| Anticipated/Related | ||||
| Mortality | 0 | 0 | 0 | 0 |
| Hospitalization | 5 | 0.9 (0.3, 2.8) | 0 | 0.0 (0.0, 1.8)d |
| Total SAE | 5 | 0.9 (0.3, 2.8) | 0 | 0.0 (0.0, 1.8)d |
| Unanticipated/Unrelated | ||||
| Mortality | 0 | 0 | 0 | 0 |
| Hospitalization | 0 | 0 | 0 | 0 |
| Total SAE | 0 | 0 | 0 | 0 |
| Unanticipated/Related | ||||
| Mortality | 0 | 0 | 0 | 0 |
| Hospitalization | 0 | 0 | 0 | 0 |
| Total SAE | 0 | 0 | 0 | 0 |
Rates and 95% confidence intervals (CI) in events/100 person-years are generated from generalized linear models using Poisson distributions, natural logarithms of time at risk offset, and overdispersion adjustments.
Person-years in study was 530.7.
Person-years in study was 167.2.
The upper 95% CI for zero events were calculated using the “Rule of Three” method.
4. Discussion
Monitoring and reporting adverse events is inherent to the conduct and publication of randomized controlled trials. Data regarding the expected frequency of SAE are relevant to Principal Investigators when planning personnel time and effort required for adverse event surveillance, particularly in trials of older adults who have variable predisposition to adverse events. In this secondary analysis of two low-risk intervention trials in older adults, there were no unanticipated hospitalizations and deaths irrespective of protocol-relatedness. As expected, hospitalizations and deaths in the nursing home trial were frequent, but none of these SAE were protocol-related. Collectively, these data may inform personnel allocation for SAE surveillance.
Recent data highlight the cost and time-intensive nature of systemic reporting of all adverse events and SAE [10]. In the CRANNY trial, approximately 70% of two research nurses' time was devoted to overall adverse event surveillance, and on average 15 h per week per nurse was spent on assessing and recording SAE. Performing chart review, retrieving hospitalization records, and adjudicating records accounted for 15 h per week spent assessing SAE in CRANNY. This degree of personnel investment may be disproportionately high in studies with more limited resources and impede the conduct of intervention trials in older adults with multiple comorbidities. Based on our data, we propose that future studies consider quantifying the degree of personnel investment required according to observed versus expected SAE occurrence.
Our findings correspond with prior clinical trials of nursing home residents and older community dwellers. Prior exercise program and physical rehabilitation studies in nursing home residents have shown no adverse events [11,12]. Adverse events were also rare among nursing home residents participating in clinical trials of vitamin D, melatonin, and donepezil [[13], [14], [15], [16]]. Collectively, these data may guide budget development for monitoring adverse events in clinical trials of older adults.
Notably, all 116 SAE in CRANNY were reported to the NIA within 48 h of Principal Investigator notification. This finding is important because recent reports suggest SAE are frequently underreported or unreported in publications [17]. In one study of 300 clinical trials posted on clinicaltrials.gov, many SAE were omitted from publication [[18], [19], [20]]. We provide a benchmark for SAE incidence in both community dwellers with functional limitations and nursing home residents for investigators who are considering conducting intervention trials in older adults.
Our study has limitations. First, only one site from LIFE was examined. These results may not be representative of the broader LIFE study [6]. Nevertheless, these findings would not impact our conclusions and parallel literature highlighting variability in adverse event reporting and the need for standardization [21]. Second, CRANNY included exclusively women nursing home residents. It is unclear whether SAE incidence would be similar in men. Third, SAE criteria were more inclusive for LIFE versus CRANNY, and we did not account for lifestyle intervention intensity. Nevertheless, there were no unanticipated deaths and inpatient hospitalizations from LIFE, and thus our conclusions remain unchanged.
In summary, in two intervention trials of older adults, there were no unanticipated hospitalizations and deaths, and all deaths were unrelated to the study protocol. In nursing home residents and community dwellers, the incidence of SAE was concordant with the relative degree of underlying comorbidities. These data quantify and reaffirm the safety of low-risk intervention trials in older adults and may inform investigators allocate resources for personnel involved in SAE surveillance.
Declaration of conflicting interests
The Authors declare that there is no conflict of interest.
Funding sources
This work was supported by the National Institutes of Health, National Institute on Aging, R01 AG041153 (MJM), K07 AG030093 (VQ), and K07AG3587 (TG). The Lifestyle Interventions and Independence for Elders Study was funded by a National Institutes of Health/National Institute on Aging Cooperative Agreement #U01 AG22376 and a supplement from the National Heart, Lung and Blood Institute 3U01AG022376-05A2S, and sponsored in part by the Intramural Research Program, National Institute on Aging, NIH. The research is partially supported by the Claude D. Pepper Older Americans Independence Centers at the University of Florida (1 P30 AG028740), Wake Forest University (1 P30 AG21332), Tufts University (1P30AG031679), University of Pittsburgh (P30 AG024827), and Yale University (P30AG021342) and the NIH/NCRR CTSA at Stanford University (UL1 RR025744), Tufts University is also supported by the Boston Rehabilitation Outcomes Center (1R24HD065688-01A1).
Acknowledgements
All authors made substantial contributions to the study concept or design, helped draft the manuscript, offered critical revisions for important intellectual content, and approved the version to be published.
References
- 1.U.S. Department of Health & Human Services, Office for Human Research Protections . 2007. Unanticipated Problems Involving Risks & Adverse Events Guidance.http://www.hhs.gov/ohrp/policy/advevntguid.html [Google Scholar]
- 2.National Institute on Aging . 2017. Data and Safety Monitoring.https://www.nia.nih.gov/research/dgcg/clinical-research-study-investigators-toolbox/data-and-safety-monitoring [Google Scholar]
- 3.Kiel D.P., Magaziner J., Zimmerman S. Efficacy of a hip protector to prevent hip fracture in nursing home residents: the HIP PRO randomized controlled trial. J. Am. Med. Assoc. 2007;298:413–422. doi: 10.1001/jama.298.4.413. [DOI] [PubMed] [Google Scholar]
- 4.Fink H.A., Taylor B.C., Tacklind J.W. Treatment interventions in nursing home residents with urinary incontinence: a systematic review of randomized trials. Mayo Clin. Proc. 2008;83:1332–1343. doi: 10.1016/S0025-6196(11)60781-7. [DOI] [PubMed] [Google Scholar]
- 5.De Deyn P.P., Katz I.R., Brodaty H. Management of agitation, aggression, and psychosis associated with dementia: a pooled analysis including three randomized, placebo-controlled double-blind trials in nursing home residents treated with risperidone. Clin. Neurol. Neurosurg. 2005;107:497–508. doi: 10.1016/j.clineuro.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 6.Pahor M., Guralnick J.M., Ambrosius W.T. Effect of structured physical activity on prevention of major mobility disability in older adults: the LIFE Study Randomized Clinical Trial. J. Am. Med. Assoc. 2014;311:2387–2396. doi: 10.1001/jama.2014.5616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Juthani-Mehta M., Van Ness P.H., Bianco L. Effect of cranberry capsules on bacteriuria plus pyuria among older women in nursing homes: a randomized clinical trial. J. Am. Med. Assoc. 2016;316:1879–1887. doi: 10.1001/jama.2016.16141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.National Institute on Aging . 2017. Adverse Event and Serious Adverse Event Guidelines.https://www.nia.nih.gov/research/dgcg/clinical-research-study-investigators-toolbox/adverse-events [Google Scholar]
- 9.Hanley J.A., Lippman-Hand A. If nothing goes wrong, is everything all right? Interpreting zero numerators. J. Am. Med. Assoc. 1989;249:1743–1745. [PubMed] [Google Scholar]
- 10.Wallace S., Myles P.S., Zeps N. Serious adverse event monitoring in investigator-initiated clinical trials. Med. J. Aust. 2015;204:231–233. doi: 10.5694/mja15.01007. [DOI] [PubMed] [Google Scholar]
- 11.Rolland Y., Pillard F., Klapouszczak A. Exercise program for nursing home residents with Alzheimer's disease: a 1-year randomized controlled trial. J. Am. Geriatr. Soc. 2007;55:158–165. doi: 10.1111/j.1532-5415.2007.01035.x. [DOI] [PubMed] [Google Scholar]
- 12.Mulrow C.D., Gerety M.B., Kanten D. A randomized trial of physical rehabilitation for very frail nursing home residents. J. Am. Med. Assoc. 1994;271:519–524. [PubMed] [Google Scholar]
- 13.Broe K.E., Chen T.C., Weinberg J. A higher dose of vitamin D reduces the risk of falls in nursing home residents: a randomized, multiple dose study. J. Am. Geriatr. Soc. 2007;55:234–239. doi: 10.1111/j.1532-5415.2007.01048.x. [DOI] [PubMed] [Google Scholar]
- 14.Riemersma-van der Lek R.F., Swaab D.F., Twisk J. Effect of bright light and melatonin on cognitive and noncognitive function in elderly residents of group care facilities. J. Am. Med. Assoc. 2008;299:2642–2655. doi: 10.1001/jama.299.22.2642. [DOI] [PubMed] [Google Scholar]
- 15.Tariot P.N., Cummings J.L., Katz I.R. A randomized, double-blind, placebo-controlled study of the efficacy and safety of donepezil in patients with Alzheimer's disease in the nursing home setting. J. Am. Geriatr. Soc. 2001;49:1590–1599. [PubMed] [Google Scholar]
- 16.Raskin J., Wiltse C.G., Siegal A. Efficacy of duloxetine on cognition, depression, and pain in elderly patients with major depressive disorder: an 8-week, double-blind, placebo-controlled trial. Am. J. Psychiatr. 2007;164:900–909. doi: 10.1176/ajp.2007.164.6.900. [DOI] [PubMed] [Google Scholar]
- 17.Sivendran S., Latif A., McBride R.B. Adverse event reporting in cancer clinical trial publications. J. Clin. Oncol. 2014;32:83–89. doi: 10.1200/JCO.2013.52.2219. [DOI] [PubMed] [Google Scholar]
- 18.Tang E., Ravaud P., Riveros C. Comparison of serious adverse events posted at ClinicalTrials.gov and published in corresponding journal articles. BMC Med. 2015;13:189. doi: 10.1186/s12916-015-0430-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ionnidis J.A., Lau J. Completeness of safety reporting in randomized trials: an evaluation of 7 medical areas. J. Am. Med. Assoc. 2001;285:437–443. doi: 10.1001/jama.285.4.437. [DOI] [PubMed] [Google Scholar]
- 20.Hartung D.M., Zarin D.A., Guise G.M. Reporting discrepancies between the ClinicalTrial.gov results database and peer-reviewed publications. Ann. Intern. Med. 2014;160:477–483. doi: 10.7326/M13-0480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sivendran S., Galsky M.D. Adverse event reporting in oncology clinical trials – lost in translation? Expet Opin. Drug Saf. 2016;15:893–896. doi: 10.1080/14740338.2016.1175429. [DOI] [PubMed] [Google Scholar]