Abstract
Background
Low enrollment rates are a threat to the external validity of clinical trials. The purpose of this study was to identify factors associated with lower enrollment rates in randomized controlled trials (RCTs) involving orthopedic procedures.
Methods
We performed a search in PubMed/MEDLINE for RCTs that involved any orthopedic surgical procedure, compared different intraoperative interventions, were published in English in a peer-reviewed journal between 2003 and 2014, and reported the numbers of both enrolled and eligible subjects. The primary outcome was the enrollment rate, defined as the number of enrolled subjects divided by the number of eligible subjects. We used a meta-regression to identify factors associated with lower enrollment rates.
Results
The combined estimate of enrollment rate across all 393 studies meeting inclusion criteria was 90% (95% CI: 89–92%). Trials in North America had significantly lower enrollment rates compared to trials in the rest of the world (80% vs. 92%, p < 0.0001). Trials comparing operative and non-operative treatments had significantly lower enrollment rates than trials comparing two different operative interventions (80% vs. 91%, p < 0.0001). Among trials comparing operative and non-operative interventions, there was a marked difference in enrollment rate by region: 49% in North America and 86% elsewhere (p < 0.0001).
Conclusions
RCTs investigating orthopedic procedures have variable enrollment rates depending on their location and the difference between the interventions being studied. North American trials that compare operative and non-operative interventions have the lowest enrollment rates. Investigators planning RCTs would be well advised to consider these data in planning recruitment efforts.
Keywords: Orthopedics, Randomized controlled trials, Recruitment
1. Introduction
Low enrollment rates can compromise clinical trials. The percentage of eligible individuals who consent to participate in a given randomized controlled trial (RCT) has been reported to be as low as 4% [1]. As a result, trials may be inconclusive or require additional time and funding to complete [2], [3]. In addition, even for adequately powered trials, low enrollment rates pose a threat to external validity [4].
As suggested by several recent trials that have helped shape treatment recommendations for common orthopedic procedures, RCTs are pivotal for investigating the efficacy of surgical procedures or their components [5], [6], [7]. It may be challenging to enroll subjects into RCTs that randomly allocate subjects to different types of procedures, since surgery is irrevocable [8], [9]. Previous studies have identified a number of reasons why an eligible individual may decline to participate in an RCT, such as a preference for one form of therapy over another, difficulty understanding the concept of an RCT, or discomfort with the idea of being randomized [1], [10], [11], [12], [13].
Investigators have examined the patient characteristics associated with whether an eligible patient enrolls or refuses to enroll in a particular trial. Factors associated with refusal to enroll have included older age, gender, marital status, language fluency, ethnicity, vocation, and socioeconomic status [10], [12], [14], [15], [16]. To our knowledge there has been no comprehensive study carried out at the trial (not subject) level that examines enrollment rates of trials and trial characteristics associated with enrollment rates.
In this study, we calculated the enrollment rates reported in publications of orthopedic RCTs over a 12-year period and assessed for associations between enrollment rate and various trial characteristics, including features of the interventions being studied, the investigators of the studies, and the publications in which the RCTs were reported. We hypothesized that trials in which the treatment arms offer similar patient experiences (e.g., two different screws for fracture fixation) would have higher enrollment, on average, than trials that randomized patients to very different experiences (e.g., surgical operation versus physical therapy). Accordingly, we hypothesized that trials comparing operative and non-operative interventions would have the lowest enrollment rates.
2. Materials and methods
1. Paper selection: Papers were included if they were written in English, were published in a peer-reviewed journal between January 2003 and December 2014, reported an RCT of living human subjects over the age of 18, and reported both the number of eligible subjects and the number of enrolled subjects. To be included, RCTs were required to have at least one arm that involved an orthopedic surgical procedure, and the arms were required to compare different intraoperative interventions. Reports of trials comparing preoperative or postoperative interventions, such as rehabilitation protocols, were not included.
Because we wished to focus on orthopedic aspects of management, we excluded studies that compared interventions involving only injections (e.g., corticosteroid vs. saline injections). Similarly, we excluded studies of different types of anesthetics. We included trials comparing intraoperative interventions such as tourniquets, drainage, and antimicrobials if they otherwise met criteria. Manuscripts that had been retracted were excluded. We selected a 12-year period in order to adequately power our findings and minimize the risk of secular trends—that is, major shifts in subject or investigator approaches to RCTs over time.
We used PubMed/MEDLINE to search for publications of orthopedic RCT results. The search was last performed on November 4, 2015. We used the following search term to identify 6727 papers for initial screening for inclusion in our study:
“(((((“2003/1”[Date - Publication]: “2014/12”[Date - Publication])) AND English[Language]) AND Randomized Controlled Trial[Publication Type]) AND (orthoped* OR orthopaed* OR arthroplast* OR arthroscop* OR meniscect* OR “cruciate ligament” OR “rotator cuff” OR laminect* OR “spinal fusion” OR “carpal tunnel release” OR “open reduction” OR “internal fixation” OR “external fixation” OR osteotom* OR “bone grafting” OR arthrodesis OR patellect* OR capsulot* OR synovect* OR syndesmot* OR “tendon repair” OR tenodesis OR “trigger finger release” OR fasciect* OR laminect* OR discect*)) AND (humans[MeSH Terms])”.
Papers were accessed through the library systems of Harvard University and two major academic hospitals (Brigham and Women's Hospital and Massachusetts General Hospital). We excluded papers if they were not available through these three library systems.
2. Abstraction of data: Two investigators (C.T.L. and H.J.R.) performed the screening of papers and data abstraction. They each independently screened the same initial set of 200 papers and abstracted data from papers that met inclusion criteria, and then met to resolve discrepancies and ensure a uniform approach to excluding and including papers. Thereafter, they divided all remaining papers for screening and data collection. Any papers raising uncertainty were set aside to be resolved by the team.
For each paper that met inclusion criteria, we performed a manual data extraction to obtain the following data elements. We extracted the number of subjects screened for the trial, the number of eligible subjects, and the number enrolled. We characterized the difference between study interventions; each study was categorized as either a comparison of operative and non-operative management or as one of various comparisons of operative techniques or strategies (as shown in Appendix 1). We extracted the orthopedic subspecialty area of the clinical problem addressed by the RCT, the number of months of follow-up, and whether there was inpatient follow-up only or outpatient follow-up. In terms of investigator-related data, we extracted the number of study sites for the trial, the nationality of the first author's primary institution (by region: USA/Canada, Europe, Asia/Middle East, Australia/New Zealand, Mexico/Central America/South America, other), reported external funding sources (public, foundation, and/or industry), and the number of months of recruitment. Lastly, we extracted the year of paper publication.
3. Characterization of included and excluded papers: We gathered data on our screening process by recording the total number of papers screened, the number of papers excluded for each inclusion/exclusion criterion, and the number of papers included in the study. Many papers were excluded based on more than one criterion, and if so they were categorized by the most salient exclusion criterion, with two exceptions. First, papers were only categorized as “not accessible” if they otherwise met criteria for inclusion. Second, among papers that met criteria for inclusion and whose full manuscripts were accessible, papers were excluded if they did not report the number of eligible subjects.
4. Statistical analysis: The primary outcome variable was enrollment rate, which was calculated as the number of enrolled subjects divided by the number of eligible subjects. The secondary outcome variable was screening yield, which was calculated as the number of enrolled subjects divided by the number of screened subjects. We employed a logistic random-effects model, which incorporates properties of the logistic and binomial distributions, to model the number enrolled and thus obtain an estimate for enrollment rate. The logistic random-effects model uses the exact binomial likelihood to estimate the within-study variability. Random-effects estimated by maximum likelihood were used to account for between-study variability. We used the model to calculate within-study variability, to calculate an overall combined estimate for enrollment rate and to evaluate the effect of study-level characteristics on enrollment rate [20], [21]. This allowed us to include studies with zero cells (i.e., 100% enrollment) without requiring an ad-hoc adjustment. In our model, enrollment rate and screening rate were the dependent variables, and all other variables gathered (as described in “Abstraction of data” above) were predictor variables: orthopedic subspecialty, degree of intervention difference, inpatient only vs. outpatient follow-up, duration of follow-up, single-center vs. multi-center, nationality of first author's institution, external funding source (if reported), duration of subject enrollment, and year of publication.
Study-level variables found to be significantly associated with enrollment rate differences in bivariate analysis were examined further for interactions. Given our hypothesis that studies with patient-relevant differences in intervention would have lower enrollment rates, we planned to perform interaction analyses between intervention difference and other significant predictors.
For each year of publication, we calculated the proportion of included papers (i.e., those meeting all inclusion criteria) to papers meeting all inclusion criteria except the reporting of number of eligible subjects. We termed this result the paper inclusion rate, as a proxy for the proportion of investigators reporting enrollment rates for their RCTs. We chose to examine this result rather than the proportion of included papers to all papers screened, because other RCT characteristics (e.g., the number of trials studying anesthetic use) could affect the proportion of included papers. We then assessed for any trends in paper inclusion rate by calculating a correlation coefficient between year and the paper inclusion rate.
5. General approach: Our study and the reporting thereof reflect PRISMA guidelines [22].
3. Results
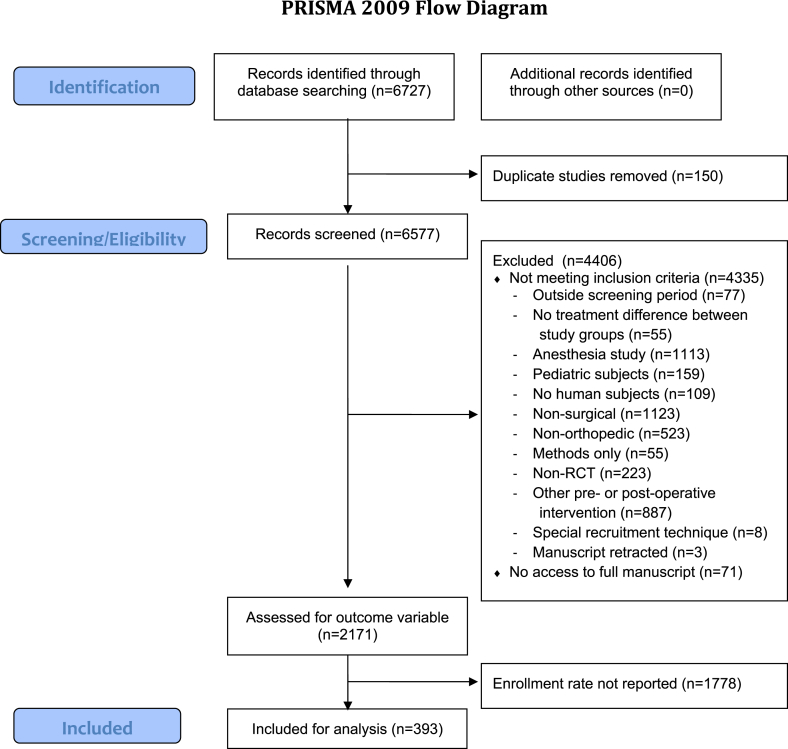
1. Descriptive results: 6727 papers were screened for inclusion (See Fig. 1). 150 papers (2.2%) were duplicate citations, and were discarded. 6184 (91.9%) were excluded for not meeting the inclusion and exclusion criteria: 1778 papers (26.4%) did not report the number of eligible subjects but met all other criteria, and 4406 (65.4%) failed to meet at least one of the other criteria. 393 papers (5.8%) met all inclusion criteria, including the reporting of both the numbers of eligible and enrolled subjects, and were thus included in this study. Characteristics of the included papers are shown in Appendix 1.
Fig. 1.
Paper inclusion flow chart.
The combined random-effects estimate for enrollment rate was 90.2% (95% CI: 88.9–91.5%).
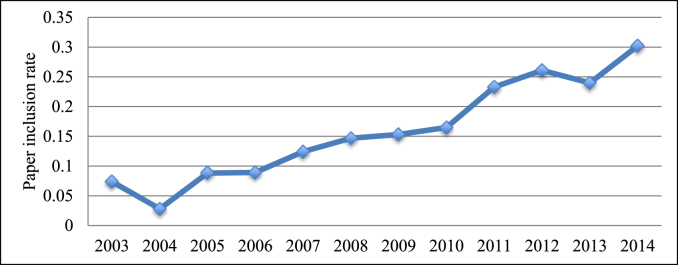
The paper inclusion rate, a proxy for the proportion of RCT investigators reporting the enrollment rate for their trials, increased steadily over the twelve-year study period (Pearson correlation coefficient r = 0.966, Fig. 2).
Fig. 2.
Proportion of included papers (i.e., meeting all inclusion criteria) to papers meeting all inclusion criteria except reporting of number of eligible subjects, by publication year (Pearson correlation coefficient r = 0.966, p < 0.0001).
2. Intervention-related factors: Studies in which subjects were randomized to either an operative or a non-operative intervention had significantly lower enrollment rates than studies in which subjects were randomized to one of two or more operative arms (80.0% vs. 91.3%, p < 0.0001, Table 1). We observed no significant difference in enrollment rate by follow-up type, orthopedic subspecialty, or duration of follow-up.
Table 1.
Estimate of trial enrollment rate (with 95% confidence interval) by degree of analyzed data.
| Characteristic | p-value | Enrollment rate (95% CI) |
|---|---|---|
| Degree of intervention difference | <0.0001 | |
| Operative vs. non-operative | 80.0% (74.0–86.0%) | |
| Other | 91.3% (90.1–92.5%) | |
| Orthopedic subspecialty | 0.2792 | |
| Trauma | 90.3% (87.3–93.3%) | |
| Sports | 88.9% (85.6–92.2%) | |
| Reconstructive | 91.4% (89.7–93.1%) | |
| Spine | 85.5% (80.0–91.0%) | |
| Foot and ankle | 89.6% (78.6–100%) | |
| Hand | 90.8% (84.6–97.0%) | |
| Other | 94.6% (85.7–100%) | |
| Inpatient only vs. outpatient follow-up | 0.0546 | |
| Inpatient only | 94.0% (90.9–97.1%) | |
| Outpatient | 89.9% (88.5–91.3%) | |
| Duration of follow-up | 0.3450 | |
| Nationality of first author's institution | <0.0001 | |
| USA and Canada | 79.5% (74.8–84.3%) | |
| Other | 92.0% (90.9–93.2%) | |
| Single-center vs. multi-center | 0.0003 | |
| Single-center | 91.4% (90.1–92.7%) | |
| Multi-center | 84.8% (80.8–88.8%) | |
| Public funding | 0.1655 | |
| No | 90.0% (87.9–92.1%) | |
| Yes | 84.1% (79.4–88.8%) | |
| Not reported | 92.0% (90.5–93.6%) | |
| Foundation funding | 0.2959 | |
| No | 88.5% (86.1–90.8%) | |
| Yes | 88.3% (84.5–92.1%) | |
| Not reported | 92.1% (90.5–93.7%) | |
| Industry funding | 0.2868 | |
| No | 89.0% (86.7–91.3%) | |
| Yes | 88.1% (84.6–91.7%) | |
| Not reported | 91.9% (90.3–93.6%) |
Investigator-related factors: Studies in which the first author was primarily affiliated with an institution in the United States or Canada, used as a proxy for studies performed in those countries, had significantly lower enrollment rates than studies in which the first author was primarily affiliated with an institution in another country (79.5% vs. 92.0%, p < 0.0001, Table 1). Studies that were performed at a single site had significantly higher enrollment rates than studies performed at multiple sites (91.4% vs. 84.8%, p = 0.0003, Table 1). We observed no significant difference in enrollment rate by funding source or duration of subject recruitment.
3. Publication-related factors: No significant difference in enrollment rate was observed by year of publication.
4. Interactions: After identifying nationality, intervention difference, and number of trial sites as having statistically significant effects on enrollment rate, we assessed for significant interactions between these variables. First, we evaluated the interaction between nationality and intervention difference to determine whether the effect of intervention difference on enrollment rate differed by location. We found a statistically significant interaction (p = 0.0272). For trials comparing operative and non-operative interventions, there was a marked difference in enrollment rates between studies in the US or Canada versus those in other countries (49.2% vs. 86.4%, p < 0.0001, Table 2). There was a smaller though still significant geographic difference in enrollment rates for trials comparing operative interventions, with lower rates in the US and Canada than in other countries (83.5% vs. 92.6%, p < 0.0001). In addition, the interaction between the number of sites and intervention difference was statistically significant (p = 0.0101). The difference in enrollment rates between single-site trials comparing operative and non-operative interventions and other single-site trials was not statistically significant (87.8% vs. 91.6%, p = 0.1309), whereas this difference was significant among multi-site trials (66.5% vs. 90.0%, p < 0.0001). Lastly the interaction between the number of sites and nationality was not statistically significant (p = 0.4763).
Table 2.
Table of estimate of trial enrollment rate (with 95% confidence interval) and number of trials by degree of intervention difference and author nationality.
| Nationality |
|||
|---|---|---|---|
| USA and Canada | Other | ||
| Degree of difference between interventions | Operative vs. non-operative | 49.2% (32.1–66.4%) | 86.4% (81.7–91.2%) |
| 13 trials | 41 trials | ||
| Other | 83.5% (79.3–87.8%) | 92.6% (91.5–93.7%) | |
| 70 trials | 269 trials | ||
5. Screening yield: Of the 393 included papers, 330 (84.0%) reported the number of patients screened for eligibility. The combined random-effects estimate for screening yield was 65.9% (95% CI: 62.3%–69.5%). Significant differences in screening yield were also observed between trials comparing operative and non-operative interventions and those comparing two or more operative interventions (46.0% vs. 69.0%, p < 0.0001), between studies performed by US or Canadian investigators and those performed by investigators elsewhere (46.0% vs. 70.2%, p < 0.0001), and between studies performed at a single site and those performed at multiple sites (69.4% vs. 51.1%, p < 0.0001).
4. Discussion
Low rates of patient enrollment in RCTs threaten their completion and their generalizability. Previous studies have described the characteristics of and reasons stated by subjects who refuse to enroll in clinical trials, but no study has quantitatively examined the association between enrollment rate and intervention-, investigator-, and publication-related factors. In this analysis of 393 orthopedic RCTs, enrollment rate varied significantly with the degree of difference between treatment arms, region of the world in which the study was conducted, and number of sites at which the study was conducted. Of note, the difference in enrollment rates between trials comparing non-operative and operative interventions and other trials varied according to region, with a much larger enrollment rate difference in North America.
As we hypothesized, patients were less likely to enroll in studies that compared an operative to a non-operative intervention than studies that compared two or more operative interventions, regardless of the country in which the trial was performed. This may be because patients have strong preferences surrounding the irrevocable nature of the decision to undergo surgery and are reluctant to leave this decision to chance [1], [9]. Alternatively, patients may have a strong preference to undergo a surgical procedure that is the standard of care. Our finding is consistent with a prior study of 114 publicly funded RCTs in the United Kingdom, which also found lower rates of patients recruited per month per trial site among trials comparing operative and non-operative interventions [19]. However, this study examined the absolute number of patients enrolled per month, and not actual enrollment rates. Our study is the first of which we are aware to examine the proportion of patients consenting to enrollment among those eligible. Furthermore, we similarly observed a significant difference in overall screening yield between these two trial types.
Multiple factors may underlie our finding that orthopedic RCTs in the US and Canada had lower enrollment rates than those across the rest of the world. North American patients may be less willing to consent to clinical trials, perhaps because they have stronger treatment preferences or feel more authorized than their counterparts in other countries to express these preferences. It is also possible that North American investigators provide a more detailed consent process or are more willing to offer the opportunity to refuse participation, whether due to cultural differences or differences in review board requirements. Given that the most striking geographic difference in enrollment rates was in trials comparing operative and non-operative interventions, we hypothesize that patient preferences and agency to act upon these preferences played a major role. North American patients may have been willing to consent to being randomized to one of two operative treatments with seemingly minor differences, whereas they may have exercised their right to decline participation in trials that required relinquishing their choice to undergo an operation at all. Although international differences in patient recruitment have been noted in prior studies [24], further investigation of these hypotheses is warranted.
Despite wide trial-to-trial variation in approaches to recruiting a screening population, our analyses found significant differences in screening yield among factors associated with enrollment rate differences, namely author nationality, intervention difference, and site number. Investigators conducting certain types of trials may anticipate lower screening yields and thus screen larger numbers of patients to adequately power their RCTs. Given that these factors were associated with both enrollment rate and screening yield, our interpretation is that nationality and intervention difference are relevant in patients' enrollment decisions, not only in the process of confirming eligibility. The effects on the enrollment decision are strong enough to have significant effects on the overall yield of subjects from screening.
In the past two decades, an increasing emphasis on full transparency of methodology has been standardized through the Consolidated Standards of Reporting Trials (CONSORT) statements, first published in 1996 and subsequently updated in 2001 and 2010 [25], [26], [27]. Among the items recommended by the CONSORT statement is a diagram of the recruitment process that includes the numbers of screened, eligible, and enrolled subjects. In this study, we noted that the proportion of papers excluded solely on the basis of not reporting the number of eligible subjects decreased over the 12-year period, which may be in response to implementation of the CONSORT statements.
The scope of this study was limited to orthopedic RCTs; thus, we caution against generalizing to other surgical specialties. Studying the factors that are associated with enrollment rate in RCTs in other specialties may help to generalize our findings and identify other key factors that influence enrollment rates. Another limitation of this study was that we did not report on subject-related characteristics, as demographic statistics about the population of eligible individuals who declined participation in a study are seldom included in papers. In addition, we did not adjust for multiple comparisons in our analysis of factors associated with enrollment rate. Lastly, we noted that multi-center trials that compared operative and non-operative interventions had lower enrollment rates than multi-center trials comparing operative interventions, whereas this difference was much less striking for single-center trials. We are not certain about the reason for this interaction. It is possible that multi-center trials involve more complex decisions for subjects (e.g., more invasive procedures), resulting in lower enrollment.
Our results show that the country in which a trial is performed may influence enrollment rate, especially for trials comparing operative and non-operative interventions. This finding has implications beyond orthopedics that warrant further investigation. Underlying the geographic differences in enrollment rates may be cultural and economic differences pertaining to the relationship between patients and medical professionals as well as incentives to conduct or enroll in trials. As lower enrollment rates may compromise external validity, we urge more research in this area to validate these findings and devise strategies to remedy them.
Source of funding
There was no external funding source for this study.
Appendix 1. Characteristics of included papers
| Paper characteristics | Number of papers (%) |
|---|---|
| Orthopedic subspecialty | |
| Trauma | 68 (17.3) |
| Sports | 75 (19.1) |
| Reconstructive | 184 (46.8) |
| Spine | 42 (10.7) |
| Oncology | 0 (0.0) |
| Foot and ankle | 6 (1.5) |
| Hand | 15 (3.8) |
| Other | 3 (0.8) |
| Degree of intervention difference | |
| Operative vs. non-operative | 54 (13.7) |
| Other | |
| Different procedures | 50 (12.7) |
| Different approaches or techniques, same procedure | 80 (20.4) |
| Same procedure with or without additional procedure | 32 (8.1) |
| Different materials, same procedure | 79 (20.1) |
| Different use of intraoperative technology, same procedure | 32 (8.1) |
| Other | 66 (16.8) |
| Inpatient only vs. outpatient follow-up | |
| Inpatient only | 30 (7.6) |
| Outpatient follow-up | 361 (91.9) |
| Not reported | 2 (0.5) |
| Duration of follow-up | |
| ≤6 months | 110 (28.0) |
| >6 months, up to 12 months | 115 (29.3) |
| >12 months, up to 24 months | 103 (26.2) |
| >24 months | 63 (16.0) |
| Not reported | 2 (0.5) |
| Single-center vs. multi-center | |
| Single-center | 309 (78.6) |
| Multi-center | 80 (20.4) |
| Not reported | 4 (1.0) |
| Nationality of first author's institution | |
| USA and Canada | 83 (21.1) |
| Europe | 204 (51.9) |
| Asia and Middle East | 75 (19.1) |
| Australia and New Zealand | 24 (6.1) |
| Mexico, Central America, and South America | 4 (1.0) |
| Other | 3 (0.8) |
| External funding source, if reported (Not mutually exclusive categories) | |
| Public funding | 64 (16.3) |
| Foundation funding | 61 (15.5) |
| Industry funding | 71 (18.1) |
| Duration of subject enrollment | |
| 0–12 months | 54 (13.7) |
| 13–24 months | 116 (29.5) |
| 25–48 months | 115 (29.3) |
| >48 months | 49 (12.5) |
| Not reported | 59 (15.0) |
| Year of publication | |
| 2003 | 7 (1.8) |
| 2004 | 3 (0.8) |
| 2005 | 12 (3.1) |
| 2006 | 14 (3.6) |
| 2007 | 18 (4.6) |
| 2008 | 27 (6.9) |
| 2009 | 30 (7.6) |
| 2010 | 31 (7.9) |
| 2011 | 50 (12.7) |
| 2012 | 67 (17.0) |
| 2013 | 57 (14.5) |
| 2014 | 77 (19.6) |
References
- 1.Sibai T., Carlisle H., Tornetta P., 3rd The darker side of randomized trials: recruitment challenges. J. Bone Joint Surg. Am. 2012;94(Suppl 1):49–55. doi: 10.2106/JBJS.L.00240. [DOI] [PubMed] [Google Scholar]
- 2.McDonald A.M., Knight R.C., Campbell M.K. What influences recruitment to randomised controlled trials? A review of trials funded by two UK funding agencies. Trials. 2006;7:9. doi: 10.1186/1745-6215-7-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haidich A.B., Ioannidis J.P. Patterns of patient enrollment in randomized controlled trials. J. Clin. Epidemiol. 2001;54:877–883. doi: 10.1016/s0895-4356(01)00353-5. [DOI] [PubMed] [Google Scholar]
- 4.Bower P., Brueton V., Gamble C. Interventions to improve recruitment and retention in clinical trials: a survey and workshop to assess current practice and future priorities. Trials. 2014;15:399. doi: 10.1186/1745-6215-15-399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Katz J.N., Brophy R.H., Chaisson C.E. Surgery versus physical therapy for a meniscal tear and osteoarthritis. N. Engl. J. Med. 2013;368:1675–1684. doi: 10.1056/NEJMoa1301408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moseley J.B., O'Malley K., Petersen N.J. A controlled trial of arthroscopic surgery for osteoarthritis of the knee. N. Engl. J. Med. 2002;347:81–88. doi: 10.1056/NEJMoa013259. [DOI] [PubMed] [Google Scholar]
- 7.Herrlin S., Hallander M., Wange P. Arthroscopic or conservative treatment of degenerative medial meniscal tears: a prospective randomised trial. Knee Surg. Sports Traumatol. Arthrosc. 2007;15:393–401. doi: 10.1007/s00167-006-0243-2. [DOI] [PubMed] [Google Scholar]
- 8.Horng S., Miller F.G. Is placebo surgery unethical? N. Engl. J. Med. 2002;347:137–139. doi: 10.1056/NEJMsb021025. [DOI] [PubMed] [Google Scholar]
- 9.Leighton P., Lonsdale A.J., Tildsley J. The willingness of patients presenting with advanced glaucoma to participate in a trial comparing primary medical vs primary surgical treatment. Eye (Lond) 2012;26:300–306. doi: 10.1038/eye.2011.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abraham N.S., Young J.M., Solomon M.J. A systematic review of reasons for nonentry of eligible patients into surgical randomized controlled trials. Surgery. 2006;139:469–483. doi: 10.1016/j.surg.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 11.Ross S., Grant A., Counsell C. Barriers to participation in randomised controlled trials: a systematic review. J. Clin. Epidemiol. 1999;52:1143–1156. doi: 10.1016/s0895-4356(99)00141-9. [DOI] [PubMed] [Google Scholar]
- 12.Wright J.R., Whelan T.J., Schiff S. Why cancer patients enter randomized clinical trials: exploring the factors that influence their decision. J. Clin. Oncol. 2004;22:4312–4318. doi: 10.1200/JCO.2004.01.187. [DOI] [PubMed] [Google Scholar]
- 13.Featherstone K., Donovan J.L. “Why don't they just tell me straight, why allocate it?” the struggle to make sense of participating in a randomised controlled trial. Soc. Sci. Med. 2002;55:709–719. doi: 10.1016/s0277-9536(01)00197-6. [DOI] [PubMed] [Google Scholar]
- 14.Creel A.H., Losina E., Mandl L.A. An assessment of willingness to participate in a randomized trial of arthroscopic knee surgery in patients with osteoarthritis. Contemp. Clin. Trials. 2005;26:169–178. doi: 10.1016/j.cct.2004.12.010. [DOI] [PubMed] [Google Scholar]
- 15.Myles P.S., Fletcher H.E., Cairo S. Randomized trial of informed consent and recruitment for clinical trials in the immediate preoperative period. Anesthesiology. 1999;91:969–978. doi: 10.1097/00000542-199910000-00016. [DOI] [PubMed] [Google Scholar]
- 16.Ganz P.A., Land S.R., Antonio C. Cancer survivorship research: the challenge of recruiting adult long term cancer survivors from a cooperative clinical trials group. J. Cancer Surviv. 2009;3:137–147. doi: 10.1007/s11764-009-0093-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cook J.A., Ramsay C.R., Norrie J. Recruitment to publicly funded trials–are surgical trials really different? Contemp. Clin. Trials. 2008;29:631–634. doi: 10.1016/j.cct.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hamza T.H., van Houwelingen H.C., Stijnen T. The binomial distribution of meta-analysis was preferred to model within-study variability. J. Clin. Epidemiol. 2008;61:41–51. doi: 10.1016/j.jclinepi.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 21.Chang B.H., Waternaux C., Lipsitz S. Meta-analysis of binary data: which within study variance estimate to use? Stat. Med. 2001;20:1947–1956. doi: 10.1002/sim.823. [DOI] [PubMed] [Google Scholar]
- 22.Moher D., Liberati A., Tetzlaff J. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Butler J., Subacius H., Vaduganathan M. Relationship between clinical trial site enrollment with participant characteristics, protocol completion, and outcomes: insights from the EVEREST (Efficacy of Vasopressin Antagonism in Heart Failure: outcome Study with Tolvaptan) trial. J. Am. Coll. Cardiol. 2013;61:571–579. doi: 10.1016/j.jacc.2012.10.025. [DOI] [PubMed] [Google Scholar]
- 25.Begg C., Cho M., Eastwood S. Improving the quality of reporting of randomized controlled trials. The CONSORT statement. JAMA. 1996;276:637–639. doi: 10.1001/jama.276.8.637. [DOI] [PubMed] [Google Scholar]
- 26.Moher D., Schulz K.F., Altman D.G. The CONSORT statement: revised recommendations for improving the quality of reports of parallel-group randomized trials. Ann. Intern Med. 2001;134:657–662. doi: 10.7326/0003-4819-134-8-200104170-00011. [DOI] [PubMed] [Google Scholar]
- 27.Schulz K.F., Altman D.G., Moher D. CONSORT 2010 statement: updated guidelines for reporting parallel group randomized trials. Ann. Intern Med. 2010;152:726–732. doi: 10.7326/0003-4819-152-11-201006010-00232. [DOI] [PubMed] [Google Scholar]