Abstract
Introduction
Home fortification powders containing iron and other micronutrients have been recommended by World Health Organisation to prevent iron deficiency anaemia in areas of high prevalence. There is evidence, however, that home fortification at this iron dose may cause gastrointestinal adverse events including diarrhoea. Providing a low dose of highly absorbable iron (3 mg iron as NaFeEDTA) may be safer because the decreased amount of iron in the gut lumen can possibly reduce the burden of these adverse effects whilst resulting in similar or higher amounts of absorbed iron.
Objective
To show non-inferiority of home fortification with 3 mg iron as NaFeEDTA compared with 12.5 mg iron as encapsulated ferrous fumarate, with haemoglobin response as the primary outcome.
Design
338 Kenyan children aged 12–36 months will be randomly allocated to daily home fortification with either: a) 3 mg iron as NaFeEDTA (experimental treatment), b) 12.5 mg iron as encapsulated ferrous fumarate (reference), or c) placebo. At baseline, after 30 days of intervention and within 100 days post-intervention, blood samples will be assessed for primary outcome (haemoglobin concentration), iron status markers, Plasmodium parasitaemia and inflammation markers. Urine and stool samples will be assessed for hepcidin concentrations and inflammation, respectively. Adherence will be assessed by self-reporting, sachet counts and by an electronic monitoring device.
Conclusion
If daily home fortification with a low dose of iron (3 mg NaFeEDTA) has similar or superior efficacy to a high dose (12.5 mg ferrous fumarate) then it would be the preferred choice for treatment of iron deficiency anaemia in children.
Keywords: Adherence, Anaemia, Child, Preschool, Dietary supplements, Iron, Non-inferiority, Fortification
1. Introduction
Home fortification aims at supplementing local diets by adding micronutrient powders to semi-solid, ready-prepared foods (http://www.hftag.org/). The World Health Organisation (WHO) recommends daily universal home fortification with iron for children aged 6–23 months in populations where the prevalence of anaemia in children under 5 years of age is ≥ 20% [1]. Prevalence values within this range indicate a moderate-to-severe public health problem, which is the situation in virtually all developing countries [2].
The WHO-recommended iron dose for home fortification (10–12.5 mg iron as ferrous salt for children aged 6–23 months, [1]) corresponds to the dose that was previously established for iron supplementation in this age range [3]. There is evidence from randomised controlled trials among young children in low-income countries to suggest that home fortification with iron-containing micronutrients may cause an excess burden of diarrhoea, and increased numbers of potentially pathogenic enterobacteria, with a concurrent increase in gut inflammation [4]. Other gastrointestinal adverse effects of oral iron supplementation, such as epigastric discomfort, nausea and constipation, are common, are dose-dependent and are likely to reduce adherence [5].
Compared to the conventional daily dose (12.5 mg as ferrous salt), home fortification or supplementation with a low dose of highly absorbable iron (3 mg iron as NaFeEDTA) may result in similar or higher amounts of absorbed iron [6], [7] but the decreased amount of iron in the gut lumen can possibly reduce the burden of adverse gastrointestinal effects.
There is substantial evidence that iron interventions in young children can also increase rates of malaria and possibly respiratory disease [8], [9], [10], [11]. Because adverse events associated with such systemic diseases are likely to depend on the absorbed amount of iron, the risks may be similar when comparing a daily dose of 3 mg iron as NaFeEDTA and 12.5 mg as ferrous salt. WHO has recommended that iron interventions should be implemented in conjunction with measures to control malaria [1].
We aimed to show non-inferiority of home fortification with 3 mg iron as NaFeEDTA compared with 12.5 mg iron as encapsulated ferrous fumarate in young Kenyan children protected for 3–4 weeks against malaria by chemoprevention.
2. Study methodology
2.1. Study site
The study will be conducted from January–December 2014 in the administrative units of Kanyawegi, Osiri and Ojolla in Kisumu-West District, a rural area at an altitude below 1,300 m, adjacent to Lake Victoria, Kenya. This area covers 395 square kilometres with a population of approximately 12,000 people, of whom 20% are children aged below five years. The majority of the population consists of subsistence farming families but inadequate and unreliable rainfall patterns have immensely affected agricultural activities in the area [12]. The local diet is mainly based on maize and vegetables. Animal foods, which are rich sources of iron, are rarely consumed and often sold in the urban markets to boost income. Malaria transmission is perennial and stable [13], with most infections being due to Plasmodium falciparum [14]. The prevalence of P. falciparum infection in children aged 1–4 years has been reported to range between 39% and 63% [15]. The area is endemic for Schistosoma mansoni, with a prevalence of infection in infants of 14% [16]. Hookworm and Trichuris trichiura infections are also common in young children [17]. Co-infection of hookworm, T. trichiura and P. falciparum has been associated with low haemoglobin concentrations in pre-school children [18].
2.2. Study design
This study concerns a randomised, double-blind, non-inferiority trial comparing daily home fortification for 30 days with 3 mg iron as NaFeEDTA (investigational intervention), 12.5 mg iron as encapsulated ferrous fumarate (reference) and placebo. We conceived it as an explanatory trial to evaluate the efficacy of daily home iron fortification under maximal compliance.
2.3. Sample size determination
Sample size calculations are based on procedures for non-inferiority trials as recommended by USA Food and Drug Administration [19], [20].
-
1.
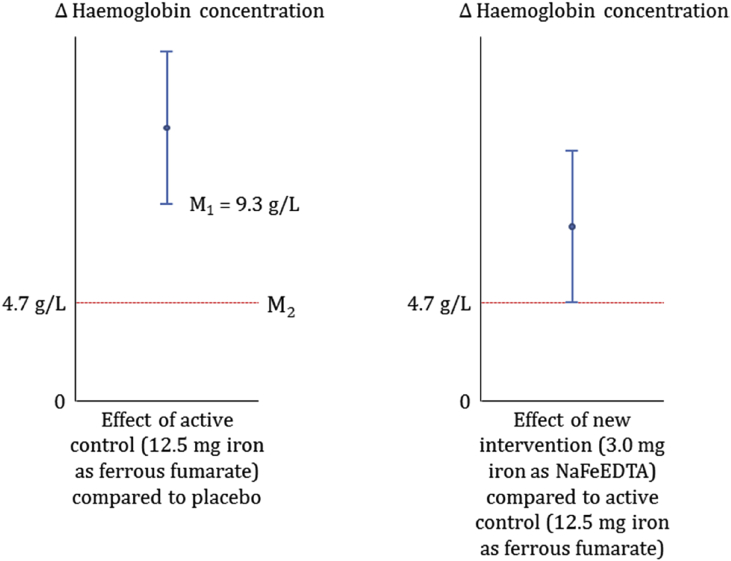
Based on a meta-analysis [21] we estimated the expected effect of 12.5 mg iron as ferrous fumarate on haemoglobin concentration relative to placebo. The lower limit of the 95% CI thus obtained (9.3 g/L) was used as M1, the minimum anticipated effect of 12.5 mg iron as ferrous fumarate (Fig. 1; left panel).
-
2.
Next, we set M2 as the margin specified to preserve 50% of the anticipated minimum effect of 12.5 mg ferrous fumarate. This margin (haemoglobin concentration of 4.7 g/L) can be interpreted as the largest loss of effect compared to 12.5 mg ferrous fumarate (inferiority) that would be acceptable, and is below an effect for 5 g/L iron as NaFeEDTA that we considered to be of minimum importance for public health.
-
3.
We set the sample size at 339 children (estimating 113 children per intervention group) so that the lower limit of the 95% CI around the difference in haemoglobin concentration between the two iron formulations (i.e. 12.5 mg ferrous fumarate and 3.0 mg iron as NaFeEDTA) would lie above M2 (Fig. 1; right panel).
Fig. 1.
Theoretical framework for sample size determination. The treatment effects are shown as 95% CIs around the estimates (shown by the blue lines). The estimated margin values are shown by the dotted red lines. Left panel: M1 is the lower bound of 95% Cl, estimated at 9.3 g/L and being the smallest effect of the 12.5 mg iron as ferrous fumarate (reference intervention) versus placebo. M2 – is the non-inferiority margin estimated as a 50% reduction of the minimum anticipated value effect (9.3%g/L) of reference intervention which corresponds to 4.7 g/L; Right panel: Success will be estimated as the difference in haemoglobin concentration between 12.5 mg ferrous fumarate and 3.0 mg iron as NaFeEDTA should lie above M2, a conservative effect considered to be of minimum public health relevance. Using 113 children per group, the trial had 90% probability to detect superiority of the investigational arm over placebo and 95% probability showing non-inferiority relative to reference intervention given the following assumptions: effect of 5 g/L; equal group SDs of 10 g/L; 2-sided α = 0.05; maximally 5% of children will drop out of the iron group, no ‘drop-in’ will occur of children crossing over from the placebo group to the iron group.
2.4. Recruitment
The research assistants will hold meetings with local authorities, community health workers and parents to inform them about study aims and procedures. The community health workers will compile a list of parents with children aged 1–3 years residing within the three administrative units, and invite parents to bring these children for screening to the research clinic, where they will be asked to sign an informed consent form (Appendices 1, 2).
At the screening visit, research assistants will collect vital data and information on household characteristics: a) date of birth as recorded in the birth certificate or health card held by the mother or, if not available, from records of the Expanded Program of Immunization held by local clinics; b) anthropometric data that include weight measured to the nearest 100 g using a Salter scale (UNICEF, catalogue 0145555, Copenhagen, Denmark) that is calibrated daily using a 5 kg weight. During measurement, the child will wear neither clothes nor shoes; standing height (children ≥24 months or ≥ 85 cm) or recumbent length (children ≤24 months or ≤ 85 cm) will be measured within 0.1 cm using wooden measuring boards (UNICEF, catalogue 0114500); and mid-upper arm circumference, a marker of wasting, using a measuring tape (UNICEF, catalogue 145600) within 0.1 cm.
Medical staff will conduct medical examinations and collect the following data: a) a parent-reported 48-h history of illness including fever, diarrhoea, vomiting or breathing distress; b) parent-reported history of signs of major systemic disorders; c) parent-reported use of specific medicines (antiretroviral drugs, rifampicin, carbamazepine, phenytoin or phenobarbital); c) parent-reported drug allergies, or 30-day history of using drugs (antimalarials, benzimidazoles, praziquantel) that might interfere with the study treatment protocol.
Clinical officers will ask parents to bring children for re-screening two weeks later if the child has a 48 h history of antimalarial drug use, or has received treatment for malaria. Children with axillary temperature ≥37.5 °C plus demonstrated blood infection (rapid dipstick tests positive for malaria) or minor illnesses will be treated immediately and also asked to return after two weeks for re-screening.
Phlebotomists will collect venous blood (4 mL) in tubes containing Li-heparin. We will determine haemoglobin concentration (HemoCue 301, Ängelholm, Sweden) and zinc protoporphyrin:haem ratio (AVIV, model 206D, Lakewood NJ, USA) in whole blood as a marker of iron-deficient erythropoiesis, each in triplicate. We will assay Plasmodium antigenaemia by rapid tests (see section ‘Laboratory analyses’ below). We will transfer aliquots of whole blood (125 μL) to DNA collection cards (FTA Mini Card, catalogue WB120055, Little Chalfont, UK) for storage at ambient temperature and subsequent detection by PCR of Plasmodium infection; and we will prepare thick and thin blood smears to allow for detection and counting of Plasmodium parasites.
An aliquot (1.0 mL) of blood will be centrifuged (600 × g, 10 min). Plasma (500 μL) will be transferred to a microtube, centrifuged (2000–3000 × g, 15 min), transferred to a cryovial, and stored immediately in liquid nitrogen (−196 °C). The erythrocyte sediment (500 μL) will be washed and centrifuged (600 × g, 8 min) three times with isotonic phosphate-buffered saline (Medicago, Uppsala, Sweden; catalogue 09-9400-100) to allow measurement in triplicate of the erythrocyte zinc protoporphyrin:haem ratio. Measurement of zinc protoporphyrin:haem ratio in washed erythrocytes is considered a more valid measurement when compared to whole blood because the washing process removes substances dissolved in the plasma such as bilirubin that fluorescence in the same wavelength range as ZPP [22]. An aliquot of washed erythrocytes will be transferred to a cryovial for storage and subsequent measurement of folate concentration, and to a cryotube prefilled with 0.9% saline solution and a lysing agent (Celite, Sigma-Aldrich, catalogue 525235, St. Louis, MO) for subsequent acid extraction and measurement of metal-free protoporphyrin.
The remainder of the blood (2.75 mL) will be centrifuged (2000–3000 × g; >15 min); aliquots of plasma will be stored in liquid nitrogen for subsequent measurement of iron markers (concentrations of ferritin, soluble transferrin receptor) and inflammation indicators (concentrations of C-reactive protein, α-1-acid glycoprotein). 125 μL buffy coat will be pipetted on DNA collection cards (FTA Mini Card), allowed to dry, stored and sealed in multi-barrier pouches containing 1 g desiccant for subsequent genotyping for host polymorphisms associated with susceptibility to malaria.
Research assistants will collect urine samples at the research clinic using 100 mL paediatric collection bags (Changzhou Huankang, Changzhou, Jiangsu, China). Prior to urine collection, we will clean and dry the area around the vulva or penis using disinfectant baby wipes or soap and water, apply the urine bags, and re-apply the child's diapers or pants. Parents will be asked to check regularly to ensure that the child does not remove the urine bag, and to inspect if the child has produced urine. Urine will be drained into a sterile 125 mL container. Samples (2 mL) will be stored immediately in liquid nitrogen for subsequent assessment of Schistosoma ova and hepcidin concentrations.
Research assistants will collect faecal samples at the research clinic on an aluminium sheet placed either inside a child's potty or directly onto the floor. Stool that is mixed with urine will be discarded. If a child is unable to produce stool, the parent will be asked to bring him/her again and retry on the subsequent 3 days until the stool is produced. A scoop (10 mL) will be transferred using a plastic spatula into a sterile disposable container that is placed immediately into a cool box and taken to the laboratory, where aliquots (2 mL) will be stored in liquid nitrogen for subsequent measurement of calprotectin concentration as an indicator of intestinal inflammation, and to assess for intestinal infections.
2.5. Premedication
Pre-medication will be administered to every eligible child during screening visit. We will give a therapeutic course of dihydroartemisinin-piperaquine (Sigma-tau, Rome, Italy; 40 mg dihydroartemisinin/320 mg piperaquine, administered as a daily dose for 3 consecutive days of ½ tablet and 1 tablet for children in weight ranges 7–12.99 kg and 13–24 kg, respectively) with the aim to protect children against malaria in the subsequent intervention period.
To protect against severe anaemia during the intervention period, we will administer two antihelminth drugs at the research clinic as per WHO recommendations [23]. Albendazole (Indoco Remedies, Mumbai, India) will be administered for 3 days at a daily dose of 200 mg or 400 mg for children aged 12–24 months and >24–36 months, respectively. Praziquantel (Cosmos, Nairobi, Kenya; 600 mg tablets) will be administered as a single target dose of 40 mg per kg body weight (<10 kg: ½tablet; 10–12 kg: ¾ tablet; >13 kg: 1 tablet). The clinical officer will administer praziquantel and the first dose of albendazole and dihydroartemisinin-piperaquine at the research clinic, and instruct parents to administer the remaining doses at home. Albendazole and dihydroartemisinin-piperaquine will be given 1 h after consumption of food, while praziquantel will be administered after child has consumed a cup of uji (maize gruel, a common food locally given to young children) or after lunch to avoid adverse effects (e.g. nausea, vomiting, and abdominal pain). The clinical officer will crush and mix the tablets with clean drinking water and observe that the child swallowed them. If a child vomits the medicine within a period of 10 min, a repeat dose will be given immediately. The clinical officer will inform parents about the reasons why their child should complete the remaining two drug doses. Parents will be requested to observe any possible adverse reactions and report immediately to the field research workers. Parents will also be asked not to give their children foods based on maize or sorghum flour 2–3-h before returning to the research clinic on the scheduled return date.
2.6. Eligibility criteria
We will include children if aged 12–36 months; resident in the study area and whose parents intended to stay in the area in the subsequent nine months; parental consent form signed by both parents; not acutely sick or febrile (axillary temperature ≥37 °C) at the time of recruitment; absence of reported or suspected major systemic disorder (e.g. HIV infection, sickle cell disease); no use of antiretroviral drugs against HIV, rifampicin, carbamazepine, phenytoin or phenobarbital; no twin sibling. Children will be excluded if: haemoglobin concentration <70 g/L; severely wasted (weight-for-height z-score <‒3 SD); known allergy to dihydroartemisinin-piperaquine, benzimidazoles or praziquantel; parent-reported history of using antihelminthic drugs in the 1-month period before the screening date; not at risk of malaria (e.g. children who received chemoprophylaxis against malaria because of HIV infection or sickle cell disease); after three days parent-reports child has not completed the 2nd and 3rd doses of dihydroartemisinin-piperaquine and benzimidazoles; has adverse effects associated with pre-medication; has fever (axillary temperature ≥37 °C); presents with any other illness.
2.7. Randomisation
To achieve group balance in size and baseline haemoglobin concentration, randomisation will be based on a stratified block design. A person not involved in the field work will create the randomisation scheme by assigning three treatment groups in a sequence of random permuted blocks of sizes 6 or 9 within two strata defined by baseline haemoglobin concentration class (<100 g/L and ≥100 g/L), using tables with random numbers and random permuted blocks. Using this scheme, two other persons not involved in the field work will produce a set of labels with a child identification number that includes a letter for stratum (A or B) and a consecutive allocation number as indicated by the randomisation scheme. These labels will be stuck on a) sealed opaque envelopes each containing a paper slip with the word ‘iron’ or ‘placebo’; and b) plastic bottles, each containing 30 sachets of one of the three types of micronutrient powders (see ‘Interventions’, below). The bottles will then be arranged in boxes according to stratum and sequential number as indicated in the randomisation scheme and handed over, together with the sealed envelopes, to the field team. All research staff (including trial coordinator) will not be allowed to open the envelopes until the end of the 30-day intervention period.
On the randomisation day visit, the trial coordinator will assign children successively to the next available allocation number within the appropriate stratum (indicated by haemoglobin concentration measured at the screening visit). This process will continue until the target sample size has been attained.
2.8. Interventions
We will use three types of micronutrient powders manufactured specifically for this trial by DSM Nutrition Products (Johannesburg, South Africa) and that contain 1 g sachets with either 3 mg iron as NaFeEDTA, 12.5 mg iron as encapsulated ferrous fumarate or without iron (placebo). The encapsulate consists of a thin coat of soy lipid. All powder types will contain thirteen micronutrients other than iron (Table 1), as recommended by the Home Fortification Technical Advisory Group except for folic acid, which we will omit because of our concerns that it may be utilized by Plasmodium parasites and increase the failure risk of anti-folate drugs, and because there is no evidence that folate deficiency is a public health problem among children in developing countries [24]. At the randomisation visit, research assistants will instruct parents on the use of the fortificants, give them a supply of 30 sachets in a plastic bottle randomly assigned for each child by the trial coordinator, and ask them to daily add the contents of one sachet per child to semi-solid, ready-prepared foods for a period of 30 days. The assistants will also show them how to mix the content of the sachet (the first dose) with uji.
Table 1.
Formulation of micronutrient powders.
| Micronutrient | Content |
|---|---|
| Vitamin A, μg RE | 300 |
| Vitamin D, μg | 5 |
| Vitamin E, mg | 5 |
| Vitamin C, mg | 30 |
| Thiamin (vitamin B1), mg | 0.5 |
| Riboflavin (vitamin B2), mg | 0.5 |
| Niacin (vitamin B3),mg | 6 |
| Vitamin B6 (pyridoxine), mg | 0.5 |
| Vitamin B12 (cobalamine), μg | 0.9 |
| Iron | |
| EITHER iron as encapsulated ferrous fumarate, mg | 12.5 |
| OR iron as NaFeEDTA, mg | 3 |
| OR no iron (placebo) | 0 |
| Zinc, mg | 5 |
| Copper, mg | 0.56 |
| Selenium, μg | 17 |
| Iodine, μg | 90 |
2.9. Blinding
Each type of micronutrient powders will be packed in identical plain white foiled sachets except for the batch number. Parents will receive 30 sachets for each child in a white plastic bottle that contains no other marker except the label with stratum, allocation number, name, start date and return date. The three types of micronutrient powders do not have apparent differences in taste, texture or colour of uji. Research assistants will observe consumption of each cup of uji during the administration of the first dose of treatment at the research clinic. Researchers, outcome assessors and parents will remain fully blinded to allocation and intervention until the 30-day intervention period has been completed. At that time, the trial coordinator will open the sealed envelopes to determine whether a child had received iron or placebo. Because the information in the envelope will not reveal the type of iron group, research assistants will be partially de-blinded; full de-blinding will be done after the statistical analysis plan has been completed and after crude intervention effects have been analysed without identification of the iron interventions.
2.10. Adherence monitoring
Adherence to intervention will be primarily monitored using an electronic monitoring and time-recording device (MEMS 6 TrackCap 45 mm without LCD display; http://www.mwvaardex.com/) that will be given for the duration of the study to parents of participating children. This battery-operated device consists of a cap that fits the bottle containing the micronutrient sachets, with a built-in microprocessor that records and stores date and time of all closings. Adherence assessment using these devices is considered the reference standard [25], [26] and superior to medication counts and self-reported adherence, which are commonly used methods that tend to result in over-estimates [27], [28]. Each bottle will be labelled with a child's identification number, serial number of the cap, name of child, start date and end date for ease of identification and tracking. Except for the trial coordinator and one field supervisor, neither parents nor research assistants will be informed about the function of the electronic device. Instead they will be informed that the MEMS cap is essential for maintaining the moisture content and good hygienic conditions of the micronutrient powder. Parents will be thoroughly instructed to close the bottle after each opening, and will be shown how to use the storage bottle with the MEMS cap. In addition, parents will be requested to keep empty sachets in a zip-lock plastic bag marked with the child's name and identification number. These bags will be collected at the end of the study to allow adherence assessment by sachet count.
Parents will be taught how to fill out self-reporting forms written in their local language (Dholuo), and requested to daily record (by a tick) when the fortificant-containing food is given (morning, mid-morning, lunch, mid-afternoon or evening) during the 30-day intervention period. Lastly, parents will be instructed about the importance of immediately reporting any sickness or adverse reactions experienced by the child during the 30 days, and the date of reporting back to the research clinic.
2.11. Assessment of non-transferrin bound iron (NTBI)
Three hours after administering the first dose of home fortificants with uji, we will collect capillary blood (400–500 μL) by finger puncture in tubes without anticoagulant (Becton Dickinson, Breda, The Netherlands), using vinyl gloves to avoid contamination with trace elements, and avoiding finger squeezing. After clotting (30 min), serum will be transferred to a microtube, centrifuged (6000–15,000 × g, 10 min), and aliquots (300 μL) transferred to cryovials and stored in liquid nitrogen for subsequent NTBI analysis.
2.12. Follow-up during 30-day intervention period
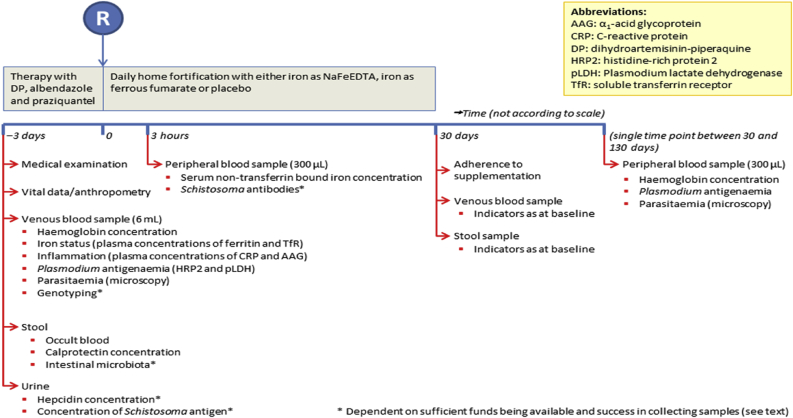
Fig. 2 provides an overview of data and samples that will be collected in the course of the trial. Field workers will conduct weekly pre-announced home visits to check if the child is still in the study area, if parents are following protocol when administering the fortificants, and if parents are filling out forms and storing empty sachets. During these visits, field workers will discuss problems or clarify procedures, but they will not give parents instructions additionally to those given during the randomisation visit. All observations and problems experienced by parents will be recorded in a form and submitted to the field supervisor at the end of each day. Sick children will be referred to the research clinic. Clinicians and laboratory technicians will be available 24 h per day. Children with severe illness or serious adverse events will be referred to a nearby referral hospital (Kisumu town), and taken either by project vehicle or by local transport with refund of transport fees. Parents who withdraw children from the intervention will be asked for reasons and for permission to keep and analyse data and samples already collected.
Fig. 2.
Data collection timelines.
2.13. Survey at 30 days of intervention
Parents will be asked to bring their children to the research clinic at 30 days post-randomisation. During this visit, clinicians will perform a medical examination and research assistants and phlebotomists will collect anthropometric data and samples (blood, urine and stool) as described for screening. Parents will be asked to return the plastic bottles with the MEMS cap, empty sachets and the self-reporting form. We will count the number of sachets and download information from the electronic device onto a computer. In addition, we will administer a standardised questionnaire to collect information on possible factors affecting adherence. Once all data and samples are collected, the trial coordinator will open the sealed brown envelope to determine child's intervention group (iron or placebo).
2.14. Post-intervention period
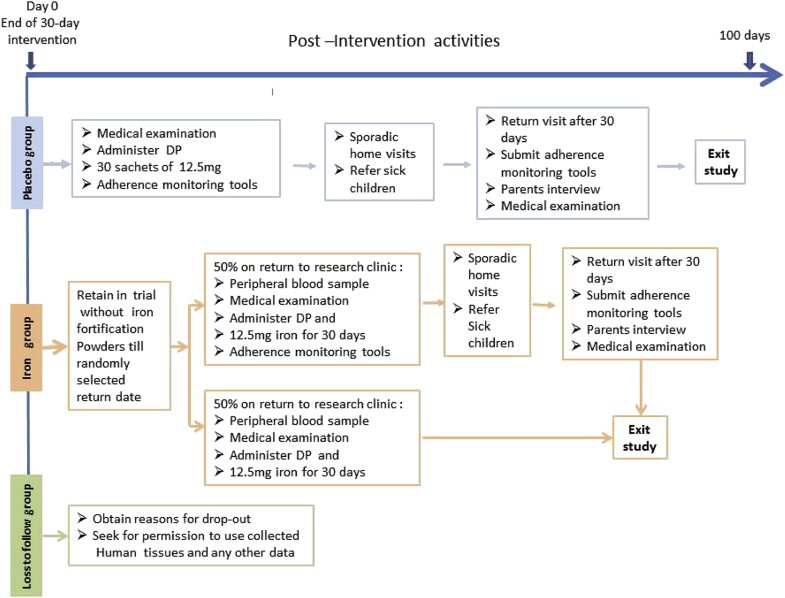
Children who received placebo will be retained in the study to observe adherence to home fortification during another 30-day period in the absence of regular monitoring visits by research assistants. Thus they will be given a 3-day therapeutic course of dihydroartemisinin-piperaquine and 30 sachets of 12.5 mg encapsulated ferrous fumarate in a bottle with the MEMS cap and again receive self-reporting forms, and instructions for use. Research assistants will conduct sporadic but pre-announced visits to their homes (one visit per child and additional visits as needed for a child with adverse events) to observe if the child is still resident and follows protocol and to check for sickness or adverse reactions. At the end of the 30-day post-intervention period, parents will be asked to returned to the clinic to submit the bottle with the MEMS cap, self-reporting forms and empty sachets. Children will be medically examined, treated for incident illness as appropriate, and exit the study.
Children allocated to the iron group will not be given fortification powders but instead will be retained in the study to monitor the population decline in haemoglobin concentration over time in a 100-day follow-up period. During this period, haemoglobin concentrations are expected to decline exponentially (i.e. at a rate that is proportional to its current value), up to a point when it would be theoretically desirable to retreat the group with a new cycle of therapeutic course of antimalarial drugs with iron fortification. We aim to estimate the time point when ≥10% of children has developed severe anaemia haemoglobin concentration <70 g/L; [29], taking into account our wish to restrict phlebotomies during the post-intervention period (for ethical reasons) to a single occasion per child. Thus we will phlebotomise each child on a single, randomly selected day in the 100-day follow-up period. We will use pre-programmed MS Excel software to randomly select a date of their return visit within a 100-day period. The date of this return visit will be concealed in the MS excel program until after the 30-day intervention return visit. Once the date is randomly calculated by the software, parents will be asked to take each child home and return on the randomly selected date. Parents will be requested to report immediately any sickness or adverse reactions experienced by the child during the post intervention period.
On the return visit, a laboratory assistant will collect capillary blood by finger puncture to measure haemoglobin concentrations in duplicate from a single drop, and to store DNA on a FTA Mini Card for subsequent assessment by PCR assay of Plasmodium parasites. Immediately following phlebotomy, half of these children will be withdrawn from further study (for ethical reasons) and will be given a therapeutic course of dihydroartemisinin-piperaquine and a supply of sachets for daily home fortification with 12.5 mg iron as encapsulated ferrous fumarate stored in silver blister pockets for another 30 days. The other half will be given a therapeutic course of dihydroartemisinin-piperaquine and a supply of sachets for daily home fortification with 12.5 mg iron as encapsulated ferrous fumarate in bottles with MEMS cap and a reporting format to determine the effect of interrupted fortification on adherence. These children will be requested to visit the research clinic after 30 days, the parents will be interviewed and informed on the three adherence tools processed similar to the end of 30-day intervention period. A summary of the flow of activities for the post intervention period is presented in Fig. 3.
Fig. 3.
Post intervention flow of activities.
2.15. Laboratory analyses
We will use two rapid tests (AccessBio, Somerset, NJ; CareStart G0151 and G0171) to detect P. falciparum-specific histidine-rich protein 2, P. falciparum-specific lactate dehydrogenase (LDH), and LDH specific for human Plasmodium spp. other than P. falciparum. The pLDH-based test was used to detect current infection [30], [31], [32]. We will use two commercially available tests (Hemoccult SENSA, Clindia Benelux, Almere, The Netherlands, catalogue no. 20000702; FOB advanced+, Ulti Med, Roeselare, Belgium, catalogue 010A210-20) to detect the presence of faecal occult blood, following assay instructions given by the manufacturers. Faecal occult blood will be interpreted as evidence of intestinal bleeding due to gastrointestinal helminths. Iron markers (plasma concentrations of ferritin, soluble transferrin receptor, transferrin), inflammation markers (plasma concentrations of C-reactive protein [CRP] and α1-acid glycoprotein), albumin and vitamin B12 will be measured on an Abbott Architect C16000 and i2000 SR analyser as per manufacturer's instructions.
2.16. Study outcomes
We will use the following outcome definitions: anaemia: haemoglobin concentration <110 g/L; mild, moderate and severe anaemia: haemoglobin concentration 100–109 g/L, 70–99 g/L and <70 g/L, respectively [29]; iron status: deficient (plasma ferritin concentration < 12 μg/L), replete (plasma ferritin concentration ≥ 12 μg/L in the absence of inflammation) or uncertain (plasma ferritin concentration ≥ 12 μg/L in the presence of inflammation) [33]; iron deficiency anaemia: concurrent anaemia and iron deficiency; inflammation: plasma concentrations of C-reactive protein and/or α1-acid glycoprotein of >5 mg/L [34] and >1.0 g/L [35], respectively; Plasmodium infection: presence or absence of parasites as indicated by histidine-rich protein-2, lactate dehydrogenase specific for P. falciparum or human Plasmodium spp. other than P. falciparum (i.e. P. vivax, P. malariae or P. ovale); high, medium and low Plasmodium parasite density: parasitaemia ≥10,000/μL, 1000–9999/μL and <1000/μL, respectively. We will define high adherence (≥80%, 24 days or more) and low adherence (<80%, 23 days or less) of scheduled fortification powders, as indicated by the MEMS device. This threshold is arbitrary, but is often used in published studies on medication adherence [28], [36], [37], [38], [39].
2.17. Statistical analysis
A statistical analysis plan will be finalised after data collection but before breaking the randomisation code.
Anthropometric indices will be calculated using WHO Anthro software vs.3.2.2 (World Health Organisation, Geneva, Switzerland). Data analysis will be done using SPSS 21 (IBM, Armonk, NY), CIA 2.2.0 (http://www.som.soton.ac.uk/research/sites/cia/), R software version 3.2.0 (www.r-project.org), and PowerView vs.3.5.2 (AARDEX Group ltd, Sion Switzerland; to analyse electronic adherence data). Since this is perceived to be an explanatory trial and as per recommendations by the European Medicine Agency for non-inferiority trials [40], we will pursue the primary (non-inferiority) objective by comparing results obtained by both intention-to-treat analysis and per protocol analysis, without formal adjustment for multiplicity (further details in discussion section).
Proportions and group means will be compared by conventional methods and expressed as absolute differences with corresponding 95% CIs; and with log-transformations as appropriate. We will estimate effects when possible; P-values, where reported, will be 2-sided. For primary analysis, we will estimate the difference in haemoglobin concentrations at the end of the 30-day fortification period between groups of children allocated to different iron formulations. Non-inferiority will be rejected if the difference between groups is less than the non-inferiority margin of 4.7 g/L.
2.18. Data and safety monitoring
We will appoint a trial monitor and an independent data and safety monitoring committee to review un-blinded data for safety purposes, monitor the progress of the trial, and to assess whether there were any safety issues that should be brought to participants' attention. No interim analyses will be conducted.
2.19. Ethical clearance
Ethical clearance has been obtained from London School of Hygiene and Tropical Medicine Ethical Committee, UK (6503) and the Kenyatta National Hospital/University of Nairobi Ethical Review Committee, Kenya (KNH-ERC/A/402). The trial is registered with ClinicalTrials.gov (NCT02073149).
3. Discussion
3.1. Duration of the intervention
We selected a relatively short 30-day intervention with iron in the expectation that premedication with dihydroartemisinin-piperaquine will prevent malaria during this period, with a long-term view that the protection afforded by repeated chemoprevention with this combination drug would allow time windows for safe administration of short courses of iron intervention. In a recent study among preschool children in Burkina Faso, two cycles of chemoprevention with dihydroartemisinin-piperaquine, administered at the same target dose as in our study, resulted in a protection against malaria that persisted at a high level for 3–4 weeks and decreased rapidly thereafter, indicating that protection lasts at most 3–4 weeks [41]. In an earlier placebo-controlled randomised trial among Kenyan children aged 2–36 months, it was shown with smaller sample size (79 iron; 76 placebo) than the present study that weekly supplementation with 6 mg elemental iron as ferrous fumarate per kg bodyweight improved haemoglobin concentration at 4 weeks after the start of intervention [42].
3.2. Justification for use of a placebo
The use of placebo in non-inferiority trials is controversial. Opponents have argued that: a) the use of placebos as controls is unethical and mostly disregard the interest of patients [43]; b) placebo group are unnecessary where there is proof of the effect of the existing treatment and as such any new treatment should be tested against the existing treatment [44] and c) its use in trials should decline as medical knowledge increases [45].
Inclusion of a third arm (placebo) in this trial adheres to the guidelines for non-inferiority trials as stipulated by the European Medicine Agency [40] and the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use [46]. We perceive a placebo arm to be ethical in the presence of an active control because in our study area there is no national policy for preventive, community-based supplementation or home fortification with iron in children and yet the children under five year are at a greater risk of iron deficiency anaemia. Because there is an on-going uncertainty about the safety of iron interventions in children living in malaria-endemic areas, our trial represents the only chance for eligible children to receive fortificants of iron with micronutrients for the iron arms and fortificants of micronutrients for the placebo arm with malaria chemoprevention. Thus prohibition of the trial on ethical grounds would be against the interest of eligible children and their guardians.
Use of a placebo is necessary in our explanatory trial because we aim to demonstrate that the experimental treatment (3 mg iron as NaFeEDTA) is non-inferior to the active control (12.5 mg iron as encapsulated ferrous fumarate). The demonstration of non-inferiority in a trial with only two arms can have two meanings only: both interventions are equally effective, or both interventions are equally ineffective against placebo. Furthermore, a placebo matches the comparative treatments in all ways except for the therapeutic components, and therefore the use of a standard treatment alone may not necessarily control for the same set of non-specific factors as a placebo [47]. Overall, the placebo group is useful for a) demonstration of superiority of home fortification with 3 mg iron as NaFeEDTA over placebo (proof of efficacy); b) demonstration of superiority of the reference (12.5 mg iron as encapsulated ferrous fumarate) over placebo (proof of assay sensitivity) c) demonstration that home fortification with 3 mg iron as NaFeEDTA retains most of the efficacy of the reference over placebo (proof of non-inferiority) because failure for a test drug to demonstrate effectiveness does not necessarily mean it is not efficacious.
Increased medical knowledge has mistakenly been used to justify dropping the use of placebo in trials; conversely, increased medical knowledge has subsequently propell the production of new treatments. Thus dropping the use of placebos in a trial limits the determination of efficacy and safety of the new treatment [48] consequently denying physicians' opportunities to apply treatment options when needed.
3.3. Adjusting for multiplicity
It has been suggested that multiplicity adjustments may be necessary in non-inferiority tests especially in studies with multiple objectives [49]. European Medicine Agency regulatory guidelines [40] clearly state that when interpreting a non-inferiority trial for a potentially superior outcome there is no need to do multiplicity adjustment because a statistical significance test must be done to reject the non-inferiority. In line with these regulatory guidelines for non-inferiority trials we will not adjust for multiplicity for various reasons; first, our study has only one pre-defined primary variable (haemoglobin concentration at the end of the 30-day intervention period) that will be used to demonstrate the treatment effect. Second, as outlined in the preceding section, all three comparisons of treatment effects must show statistical significance of the haemoglobin concentration. We will therefore conduct multiple regression analysis to investigate evidence for group differences in the intervention effects and to determine the extent of bias due to irregularities between end points, because we believe that results from multiple comparisons should be mutually reinforcing, not mutually debasing [50] and hence no need for multiplicity adjustments. Third, any absence of treatment effect differences will be interpreted by confidence interval in the context of the set threshold for haemoglobin concentration (at a pre-set margin of 4.7 g/L) and all reported p-values will be 2-sided. The use of confidence intervals and statistical tests are of an exploratory nature and therefore no justification for a claim is anticipated. In addition, any multiple secondary endpoints analysis will provide supportive evidence related to our primary objective (proof of efficacy) and therefore no confirmatory conclusions are necessary. Fourth, non-inferiority will be rejected if the haemoglobin concentration differences between groups are less than the already set margin of 4.7 g/L and the results show statistical significance; thus the rest of the secondary outcomes will be considered supportive [51], [52]; as such formal adjustments for the type 1 error will be considered irrelevant.
4. Funding and role of the funding agencies
The trial is funded by Sight and Life, a non-profit humanitarian initiative established by DSM Chemicals, Heerlen, The Netherlands. DSM Nutrition Products (Johannesburg, South Africa) manufactured the supplements with micronutrient powders. The International Nutrition Group of the Medical Research Council supported ET through a personal grant. Micronutrients other than iron will be included in the home fortificants at the request of Sight and Life; the funder will have no further role in study design, data collection and analysis, preparation of the manuscript, or decision to publish.
Acknowledgments
We acknowledge all the institutions and their respective staff who collaborate with us on this study: MRC-ING, The Gambia; Wageningen University, The Netherlands; Maseno University, Kenya; Kenyatta Hospital Ethical Committee, Kenya, Amphia Hospital, Breda, The Netherlands,; Meander Medical Centre, Amersfoort, The Netherlands; Pharmacy and Poison Board of Kenya.
Footnotes
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.conctc.2017.04.007.
Appendix 1. Information brochure
We would like to invite you to take part in a research study. Before you decide you need to understand why the research is being done and what it would involve for you. Please take time to read the following information carefully. Talk to others about the study if you wish. Ask us if there is anything that is not clear or if you would like more information. Take time to decide whether or not you wish to take part.
What is the purpose of the study?
We want to conduct a study to compare home fortification with two iron formulations. Many young children in Kenya have anaemia, a disorder that is characterized by blood with a light red colour, instead of a healthy dark red. The red pigment in blood is necessary to transport oxygen from inspired air to muscle. Children with anaemia often feel weak or tired, and may have difficulty learning. To prevent anaemia in children, medical doctors often prescribe supplements that contain 12.5 mg iron in a specific form (ferrous salts). A new form of iron has recently become available that can probably be given at lower doses, because the body is better able to absorb this type of iron.
In our study, we will divide young children into three groups and give each group a different treatment. Group 1 will receive the form of iron that has been used so far (12.5 mg iron as ferrous fumarate), Group 2 will receive the new form of iron (3 mg iron as sodium iron EDTA), and Group 3 will receive no iron. The results will be compared to determine if the new type of iron (3 mg iron as sodium iron EDTA) can prevent anaemia, and to determine if it is equally as good as the form of iron that has been used so far (12.5 mg iron as ferrous fumarate).
We will provide both forms of iron in sachets (little bags). Each day, the mother should empty and mix the contents of a single sachet into ready-prepared uji or some other type of food, before giving it to the child. This should be repeated every day for a period of 30 days. The child will be followed for some time afterwards.
Although iron is good to prevent anaemia, there are some concerns that it may increase the risk of malaria. For this reason, we will treat each child with a special medicine against malaria (dihydroartemisinin-piperaquine) at the start of the study, before the first dose of iron is provided. This medicine will protect the child against malaria for the time period in which the child will receive iron. In addition, all children will be dewormed at the start using two drugs (albendazole and praziquantel).
Do you have to participate?
We have asked you to take part because your child is within the age range suitable for our study. In total, we want to study 324 children. You can let your child join the study at your own free will, and withdraw your child at any moment, with or without giving reasons. . If you decide not to participate, or to withdraw from the study, this will not affect the normal care you receive in clinics or hospital elsewhere.
Read this information sheet and listen to our explanation of the study. We will then ask you to sign a consent form to show you have agreed to take part.
What will happen to you if you take part?
Screening visit: Our staff will invite you and your child to our research clinic to tell you about the aims and procedures of the study. If you agree for your child to participate, we will ask you to sign their fingerprint on a consent form. To decide whether your child can participate in the study, we will then ask you questions about your child, we will carry out a medical examination, and we will then collect samples of blood (6 mL, a volume equal to one table spoon) by arm prick, urine and stool from the child. This will take at least several hours. We will ask you to stay until if the child has produced stool. If necessary we will invite to and your child again the next day to try again. After sample collection, your child will be administered medicines (albendazole, praziquantel against worms, and dihydroartemisinin-piperaquine against malaria). You will be asked to bring your child again to the research clinic 3 days later.
Randomisation visit: At this visit, we will use the information collected so far to decide if your child can take part in the rest of the study. Participating children will receive sachets with powder. These sachets will be contained in a special dispensing bottle that you will receive with instructions for use. For one-third of children, these sachets will contain 12.5 mg iron as ferrous fumarate, one-third will contain 3 mg iron as sodium iron EDTA, and one-third contains no iron. For all three groups, the sachets also contain a mixture of other vitamins and minerals that are important for health. The allocation to group will be decided by chance (randomly). All sachets will look identical; we will not know which supplements contain iron until after the study. The first sachet will be given with food at the research dispensary. From 2 h before this point until 3 h afterwards, children will be allowed to drink but can only eat foods selected by the project team. We will then collect another blood sample (about 5–6 drops) by finger prick. From then on, community volunteers will daily supervise the supplementation in or close to your homestead.
During the 30-day intervention period: In the next 30 days, you should add and mix the contents of a single sachet to uji or any other food given to the child. Community volunteers will visit your home at least once a week to answer questions that you may have about the study. Children who become sick during this 30-day period will be referred to receive routine care by the regular health services. You may decide to withdraw your child at any point from the study. You may refuse to give reasons for your refusal, or to give permission for future collection of samples.
End-of-intervention survey: At the end of the 30-day period, we will collect the dispensing bottle and ask you some questions. We will again collect samples of blood (6 mL) by arm prick, stool and urine, using the same procedures as earlier. For each child, we will then break the randomisation code. Those who received placebo will be given antimalarial medicines (dihydroartemisinin-piperaquine) and 30-day supply of sachets with iron (12.5 mg iron as ferrous fumarate).
Follow-up after the 30-day intervention period: For children who received placebo, field staff will collect the dispensing bottle with the electronic device at the end of this 30-day period. Children who received iron will continue to be followed for a maximum of 100 days. In this period, we will collect a single sample of blood (5–6 drops, by finger prick). The time point for this collection will be decided by chance: for some children it may be as early as 1 week after home fortification was stopped; for others, it may be at the end of the 100-day period. Immediately following blood collection, children will be withdrawn from further study and will receive antimalarial medicines (dihydroartemisinin-piperaquine) and 30-day supply of sachets with iron (12.5 mg iron as ferrous fumarate). Field staff will also collect the dispensing bottle with the electronic device at this time.
To summarise, we will collect four blood samples from each child:
-
•
Randomisation visit: 1 sample of 6 mL (a volume equal to one table spoon), to be collected by arm prick;
-
•
3 h later: 1 sample of 5–6 drops, to be collected by finger prick;
-
•
At the end of the 30-day period: 1 sample of 6 mL, to be collected by arm prick;
-
•
After the 30-day intervention period: 1 sample of 5–6 drops, to be collected by finger prick.
We will store part of the blood samples in frozen condition, so that we can subsequently conduct tests to assess the success of the interventions. We may also check for hereditary factors that affect malaria and anaemia. Some of these tests may have to be done abroad.
Confidentiality: Results of this study will be shared with the public in a form of academic publication or presentation. The purpose of this publication or presentation is to create awareness and promote understanding of safely and efficiently treating anaemia in malaria endemic areas.
We will keep any information about your child confidential. Readers of the publication based on this research will not know that you gave this information. All personal information will be stored securely. This means that whenever we write or talk about anything we have been told, we never use your real name. The only information that we may have to pass on is if your child is at risk of serious harm.
Benefits and compensation: You will not receive financial benefits for participating in this study. If you need to stay more than 4 h, we will give you a small financial compensation to account for the lost hours.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.WHO Guideline: Use of Multiple Micronutrient Powders for Point-of-use Fortification of Foods Consumed by Infants and Young Children Aged 6–23 Months and Children Aged 2–12 Years. World Health Organization; Geneva, Switzerland: 2016. http://apps.who.int/iris/bitstream/10665/252540/1/9789241549943-eng.pdf?ua=1 Available at: (Accessed 6 February 2017) [PubMed] [Google Scholar]
- 2.De Benoist B., McLean E., Egli I., Cogswell M., editors. Worldwide Prevalence of Anaemia 1993-2005: WHO Global Database on Anaemia. World Health Organization; Geneva, Switzerland: 2008. http://apps.who.int/iris/bitstream/10665/43894/1/9789241596657_eng.pdf Available at: (Accessed 6 February 2017) [Google Scholar]
- 3.Nestel P., Alnwick D., for the International Nutritional Anaemia Consultative Group (INACG) ILSI Human Nutrition Institute; Washington DC, USA: 1997. Iron/multi-micronutrient Supplements for Young Children: Summary and Conclusions of a Consultation Held at UNICEF, Copenhagen, August 19–20, 1996.http://ilsirf.org/wp-content/uploads/sites/5/2016/04/INACG_Iron_Multi-Micronutrient_Supplement-for-Young-Children.pdf Available at: (Accessed 6 February 2017) [Google Scholar]
- 4.Paganini D., Uyoga M.A., Zimmermann M.B. Iron fortification of foods for infants and children in low-income countries: effects on the gut microbiome, gut inflammation, and diarrhea. Nutrients. 2016;8(8):494. doi: 10.3390/nu8080494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.WHO, UNICEF, UNU . World Health Organization; Geneva: 2001. Iron Deficiency Anaemia: Assessment, Prevention, and Control. A Guide for Programme Managers. Document Reference WHO/NHD/01.3.http://apps.who.int/iris/bitstream/10665/66914/1/WHO_NHD_01.3.pdf?ua=1 Available at: (Accessed 6 February 2017) [Google Scholar]
- 6.Troesch B., Egli I., Zeder C., Hurrell R.F., de Pee S., Zimmermann M.B. Optimization of a phytase-containing micronutrient powder with low amounts of highly bioavailable iron for in-home fortification of complementary foods. Am. J. Clin. Nutr. 2009;89(2):539–544. doi: 10.3945/ajcn.2008.27026. [DOI] [PubMed] [Google Scholar]
- 7.Verhoef H., Veenemans J. Safety of iron-fortified foods in malaria-endemic areas. Am. J. Clin. Nutr. 2009;89(6):1949–1950. doi: 10.3945/ajcn.2009.27785. [DOI] [PubMed] [Google Scholar]
- 8.Soofi S., Cousens S., Iqbal S.P., Akhund T., Khan J., Ahmed I., Zaidi A.K. Effect of provision of daily zinc and iron with several micronutrients on growth and morbidity among young children in Pakistan: a cluster-randomised trial. Lancet. 2013;382(9886):29–40. doi: 10.1016/S0140-6736(13)60437-7. [DOI] [PubMed] [Google Scholar]
- 9.World Health Organization Secretariat on behalf of the participants of the Consultation Conclusions and recommendations of the WHO Consultation on prevention and control of iron deficiency in infants and young children in malaria-endemic areas. Food Nutr. Bull. 2007;28(4 Suppl):S621–S627. doi: 10.1177/15648265070284s414. [DOI] [PubMed] [Google Scholar]
- 10.Veenemans J., Milligan P., Prentice A.M., Schouten L.R., Inja N., van der Heijden A.C. Effect of supplementation with zinc and other micronutrients on malaria in Tanzanian children: a randomised trial. PLoS Med. 2011;8(11):e1001125. doi: 10.1371/journal.pmed.1001125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goheen M.M., Wegmüller R., Bah A., Darboe B., Danso E., Affara M. Anemia offers stronger protection than sickle cell trait against the erythrocytic stage of falciparum malaria and this protection is reversed by iron supplementation. EBioMedicine. 2016;14:123–130. doi: 10.1016/j.ebiom.2016.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Government of Kenya (GoK). Kisumu district strategic plan 2005-2010 for implementation of the national population policy for sustainable development.
- 13.Government of Kenya (GoK), Ministry of Public Health and Sanitation, Malaria indicator survey (KMIS) report 2010. Available at: https://dhsprogram.com/pubs/pdf/MIS7/MIS7.pdf (Accessed 6 February 2017).
- 14.Mwangi M.N., Roth J.M., Smit M.R., Trijsburg L., Mwangi A.M., Demir A.Y. Effect of daily antenatal iron supplementation on Plasmodium infection in Kenyan women: a randomized clinical trial. JAMA. 2015;314(10):1009–1020. doi: 10.1001/jama.2015.9496. [DOI] [PubMed] [Google Scholar]
- 15.Munyekenye O.G., Githeko A.K., Zhou G., Mushinzimana E., Minakawa N., Yan G. Plasmodium falciparum spatial analysis, western Kenya highlands. Emerg. Infect. Dis. 2005;11(10):1571–1577. doi: 10.3201/eid1110.050106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Verani J.R., Abudho B., Montgomery S.P., Mwinzi P.N., Shane H.L., Butler S.E. Schistosomiasis among young children in Usoma, Kenya. Am. J. Trop. Med. Hyg. 2011;84(5):787–791. doi: 10.4269/ajtmh.2011.10-0685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brooker S., Peshu N., Warn P., Mosobo M., Guyatt H.L., Marsh K. The epidemiology of hookworm infection and its contribution to anaemia among pre-school children on the Kenya coast. Trans. R. Soc. Trop. Med. Hyg. 1999;93(3):240–246. doi: 10.1016/s0035-9203(99)90007-x. [DOI] [PubMed] [Google Scholar]
- 18.Albonico M., Allen H., Chitsulo L., Engels D., Gabrielli A.F., Savioli L. Controlling soil-transmitted helminthiasis in pre-school-age children through preventive chemotherapy. PLoS Negl. Trop. Dis. 2008;2(3):e126. doi: 10.1371/journal.pntd.0000126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.D'Agostino R.B., Massaro J.M., Sullivan L.M. Non-inferiority trials: design concepts and issues – the encounters of academic consultants in statistics. Stat. Med. 2003;22(2):169–186. doi: 10.1002/sim.1425. [DOI] [PubMed] [Google Scholar]
- 20.Schumi J., Wittes J.T. Through the looking glass: understanding non-inferiority. Trials. 2011;12:106. doi: 10.1186/1745-6215-12-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Neuberger A., Okebe J., Yahav D., Paul M. Oral iron supplements for children in malaria-endemic areas. Cochrane Database Syst. Rev. 2016;2 doi: 10.1002/14651858.CD006589.pub4. CD006589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hastka J., Lasserre J., Schwarzbeck A., Strauch M., Hehlmann R. Washing erythrocytes to remove interferents in measurements of zinc protoporphyrin by front-face hematofluorometry. Clin. Chem. 1992;38(11):2184–2189. [PubMed] [Google Scholar]
- 23.Preventive chemotherapy in human helminthiasis. Coordinated Use of Antihelminthic Drugs in Control Interventions: a Manual for Health Professionals and Programme Managers. World Health Organization; Geneva, Switzerland: 2006. http://whqlibdoc.who.int/publications/2006/9241547103_eng.pdf Available at: (Accessed 6 February 2017) [Google Scholar]
- 24.Verhoef H., Veenemans J., Mwangi M.N., Prentice A.M. Safety and benefits of interventions to increase folate status in malaria-endemic areas. Br. J. Haematol. 2017 doi: 10.1111/bjh.14618. http://onlinelibrary.wiley.com/doi/10.1111/bjh.14618/full [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cramer J.A., Mattson R.H., Prevey M.L., Scheyer R.D., Ouellette V.L. How often is medication taken as prescribed? A novel assessment technique. JAMA. 1989;261(22):3273–3277. [PubMed] [Google Scholar]
- 26.Vrijens B., Urquhart J. Patient adherence to prescribed antimicrobial drug dosing regimens. J. Antimicrob. Chemother. 2005;55(5):616–627. doi: 10.1093/jac/dki066. [DOI] [PubMed] [Google Scholar]
- 27.Olivieri N.F., Matsui D., Hermann C., Koren G. Compliance assessed by the medication event monitoring system. Arch. Dis. Child. 1991;66(12):1399–1402. doi: 10.1136/adc.66.12.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grosset K.A., Bone I., Reid J.L., Grosset D. Measuring therapy adherence in Parkinson's disease: a comparison of methods. J. Neurol. Neurosurg. Psychiatry. 2006;77(2):249–251. doi: 10.1136/jnnp.2005.064709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.WHO . World Health Organization; Geneva, Switzerland: 2011. Haemoglobin Concentrations for the Diagnosis of Anaemia and Assessment of Severity. Vitamin and Mineral Nutrition Information System. Document Reference WHO/NMH/NHD/MNM/11.1.http://www.who.int/entity/vmnis/indicators/haemoglobin.pdf Available: (Accessed 6 February 2017) [Google Scholar]
- 30.Makler M.T., Palmer C.J., Ager A.L. A review of practical techniques for the diagnosis of malaria. Ann. Trop. Med. Parasitol. 1998;92(4):419–433. doi: 10.1080/00034989859401. [DOI] [PubMed] [Google Scholar]
- 31.Piper R., Lebras J., Wentworth L., Hunt-Cooke A., Houzé S., Chiodini P. Immunocapture diagnostic assays for malaria using Plasmodium lactate dehydrogenase (pLDH) Am. J. Trop. Med. Hyg. 1999;60(1):109–118. doi: 10.4269/ajtmh.1999.60.109. [DOI] [PubMed] [Google Scholar]
- 32.Moody A. Rapid diagnostic tests for malaria parasites. Clin. Microbiol. Rev. 2002;15(1):66–78. doi: 10.1128/CMR.15.1.66-78.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Serum Ferritin Concentrations for the Assessment of Iron Status and Iron Deficiency in Populations. Vitamin and Mineral Nutrition Information System. World Health Organization; Geneva: 2011. http://apps.who.int/iris/bitstream/10665/85843/1/WHO_NMH_NHD_MNM_11.2_eng.pdf?ua=1 (WHO/NMH/NHD/MNM/11.2) (Accessed on 6 February 2017) [Google Scholar]
- 34.Abraham K., Muller C., Gruters A., Wahn U., Schweigert F.J. Minimal inflammation, acute phase response and avoidance of misclassification of vitamin A and iron status in infants—importance of a high-sensitivity C-reactive protein (CRP) assay. Int. J. Vitam. Nutr. Res. 2003;73(6):423–430. doi: 10.1024/0300-9831.73.6.423. [DOI] [PubMed] [Google Scholar]
- 35.Ayoya M.A., Spiekermann-Brouwer G.M., Stoltzfus R.J., Nemeth E., Habicht J.P., Ganz T. Alpha 1-acid glycoprotein, hepcidin, C-reactive protein, and serum ferritin are correlated in anemic schoolchildren with Schistosoma haematobium. Am. J. Clin. Nutr. 2010;91(6):1784–1790. doi: 10.3945/ajcn.2010.29353. [DOI] [PubMed] [Google Scholar]
- 36.Wang Y., Kong M.C., Ko Y. Comparison of three medication adherence measures in patients taking warfarin. J. Thromb. Thrombolysis. 2013;36(4):416–421. doi: 10.1007/s11239-013-0872-5. [DOI] [PubMed] [Google Scholar]
- 37.Knafl G.J., Schoenthaler A., Ogedegbe G. Secondary analysis of electronically monitored medication adherence data for a cohort of hypertensive African-Americans. Patient Pref. Adherence. 2012;6:207–219. doi: 10.2147/PPA.S30582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shalansky S.J., Levy A.R., Ignaszewski A.P. Self-reported Morisky score for identifying nonadherence with cardiovascular medications. Ann. Pharmacother. 2004;38(9):1363–1368. doi: 10.1345/aph.1E071. [DOI] [PubMed] [Google Scholar]
- 39.Ho P.M., Bryson C.L., Rumsfeld J.S. Medication adherence: its importance in cardiovascular outcomes. Circulation. 2009;119(23):3028–3035. doi: 10.1161/CIRCULATIONAHA.108.768986. [DOI] [PubMed] [Google Scholar]
- 40.Committee for Proprietary Medicinal Products (CPMP) European Medicine Agency; London, UK: 2002. Points to Consider on Multiplicity Issues in Clinical Trials. CPMP/EWP/908/99.http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003640.pdf Available at: (Accessed 6 February 2017) [Google Scholar]
- 41.Zongo I., Milligan P., Compaore Y.D., Some A.F., Greenwood B., Tarning J., Rosenthal P.J., Sutherland C., Nosten F., Ouedraogoa J.-B. Randomized noninferiority trial of dihydroartemisinin-piperaquine compared with sulfadoxine-pyrimethamine plus amodiaquine for seasonal malaria chemoprevention in Burkina Faso. Antimicrob. Agents Chemother. 2015;59(8):4387–4396. doi: 10.1128/AAC.04923-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Verhoef H., West C.E., Nzyuko S.M., de Vogel S., van der Valk R., Wanga M.A., Kuijsten A., Veenemans J., Kok F.J. Intermittent administration of iron and sulfadoxine-pyrimethamine to control anaemia in Kenyan children: a randomised controlled trial. Lancet. 2002;360(9337):908–914. doi: 10.1016/S0140-6736(02)11027-0. [DOI] [PubMed] [Google Scholar]
- 43.Rothman K.J., Michels K.B. The continuing unethical use of placebo controls. N. Engl. J. Med. 1994;331(6):394–398. doi: 10.1056/NEJM199408113310611. [DOI] [PubMed] [Google Scholar]
- 44.Stang A., Hense H.W., Jöckel K.H., Turner E.H., Tramèr M.R. Is it always unethical to use a placebo in a clinical trial? PLoS Med. 2005;2(3):e72. doi: 10.1371/journal.pmed.0020072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rothman K.J. Placebo mania: as medical knowledge accumulates, the number of placebo trials should fall. BMJ. 1996;313:3–4. doi: 10.1136/bmj.313.7048.3a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.ICH Harmonised tripartite guideline . International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) 2000. Choice of control group and related issues in clinical trials E10.http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E10/Step4/E10_Guideline.pdf Available at: (Accessed 6 February 2017) [Google Scholar]
- 47.McQuay H., Moore A. Placebo mania: placebo are essential when extent and variability of placebo response are unknown. BMJ. 1996;313:1008. doi: 10.1136/bmj.313.7063.1008a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Senn S. Ethical considerations concerning treatment allocation in drug development trials. Stat. Methods Med. Res. 2002;11(5):403–411. doi: 10.1191/0962280202sm299ra. [DOI] [PubMed] [Google Scholar]
- 49.Dmitrienko A, Wiens B. Branching tests in clinical trials with multiple objectives. Available at: http://www.amstat.org/meetings/fdaworkshop/presentations/2005/G5_Dmitrienko_Multiplicity.pdf (accessed 6 February 2017).
- 50.Schulz K.F., Grimes D.A. Multiplicity in randomised trials I: endpoints and treatments. Lancet. 2005;365(9470):1591–1595. doi: 10.1016/S0140-6736(05)66461-6. [DOI] [PubMed] [Google Scholar]
- 51.Altman D.G., Bland J.M. Absence of evidence is no evidence of absence. BMJ. 1995;311(7003):485. doi: 10.1136/bmj.311.7003.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sterne J.A., Smith Davey G. Sifting the evidence—what's wrong with significance tests? BMJ. 2001;322(7280):226–231. doi: 10.1136/bmj.322.7280.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.