Abstract
Light chain (AL) amyloidosis is a plasma cell neoplasm associated with insoluble fibril deposition from clonal immunoglobulin chains systemically. The disease is associated with high early mortality and morbidity owing to advanced organ deposition as well as lack of proven de-fibrillogenic therapies. Pre-clinical and retrospective clinical data suggests that doxycycline has benefit in AL amyloidosis. The ongoing DUAL study is a single center, open label, phase 2 study in which patients with AL amyloidosis who are undergoing clone-directed therapy for the underlying neoplasm with oral doxycycline given for 1 year to test the hypothesis that prolonged doxycycline use will be safe, feasible, and lead to reduced early mortality in systemic AL amyloidosis and hasten organ amyloid response. Clinical follow up visits will occur at monthly intervals for systemic AL patients and at 3 monthly intervals for localized AL patients. Blood tests will be collected during these time points for hematologic response assessment. Organ testing will be conducted at 3 monthly intervals and radiologic testing will be conducted at 6 monthly intervals. Research blood samples will be collected at baseline, 6 and 12 months. Other correlative studies include matrix metalloproteinases (MMP), tissue inhibitor of metalloproteinases (TIMP) testing and patient-reported outcomes.
Keywords: Phase 2 clinical trial, Doxycycline, Amyloidosis, Light chain, Chemotherapy
1. Introduction
The amyloidoses are a diverse group of protein misfolding diseases wherein proteins aggregate and form insoluble, fibrillar deposits (amyloid) in tissues [1]. Examples of amyloid diseases include Alzheimer's disease, hereditary transthyretrin-associated familial amyloid polyneuropathy, dialysis related amyloidosis, AA amyloidosis with chronic systemic inflammatory states and immunoglobulin-derived light chain (AL) amyloidosis. Human amyloid-associated illnesses pose a therapeutic challenge since amyloid is a long-lived protein and there are no approved drugs demonstrated to disrupt pre-formed amyloid.
Light chain amyloidosis is a malignant plasma cell disease characterized by the formation of amyloid from immunoglobulin light chains produced by clonal plasma cells [1]. The amyloid deposits into vital organs such as the heart, liver, kidney, and nerves resulting in multisystemic decline in function and culminating in death. Of the 31 currently known extracellular proteins resulting in amyloidosis in humans, AL amyloidosis is the most common in the developed world and the most rapidly fatal with a median survival of 6 months in advanced disease [1], [2]. Current therapies used in AL amyloidosis eradicate cells that produce AL amyloid protein but have no effect on pre-formed amyloid. This produces a hematologic response by clearing circulating immunoglobulin light chains which would have eventually been deposited into amyloid. Treatments range from high dose therapy with autologous stem cell transplantation in eligible patients [3], or anti-myeloma chemotherapies. Novel anti-myeloma agents have excellent anti-plasma cell efficacy in amyloidosis but are also associated with organ and tissue toxicity making their use in clinical practice challenging, particularly in advanced AL disease [4]. One of the critical unmet needs of AL amyloidosis therapy is early mortality in patients, particularly those with advanced stage amyloidosis; this has remained unchanged since the 1970s [5] despite the availability of more effective anti-myeloma chemotherapy that have clearly improved myeloma outcomes during the same time frame [6]. Thus, consideration of therapies that complement current cytotoxic anti-amyloid treatment by hastening organ response is required.
Doxycyline is a semisynthetic tetracycline developed for antibacterial use; an effect primarily mediated by binding to the bacterial ribosome and inhibiting protein synthesis. Separate from their anti-microbial effect, tetracyclines also possess the ability to inhibit members of the matrix metalloproteinase (MMP) family of endopeptidases. The MMPs are zinc-dependent proteases involved in a gamut of physiological and pathophysiological processes such as embryogenesis, tissue remodeling, inflammation and tumor invasion [7]. It is hypothesized that overproduction of MMPs can result in AL renal and cardiac damage [8], [9]. High levels of MMPs appear to correlate with diastolic dysfunction and clinical manifestations of AL cardiomyopathy [9]. Doxycycline-induced inhibition of MMPs appears to be beneficial in conditions associated with pathologic MMP-mediated proteolysis of the extracellular matrix, including cardiac remodeling, periodontitis, arthritis and cancer [10], [11], [12]. Owing to its lipophilicity, doxycycline also concentrates in organs at sites of injury including gums in gingivitis, brain in meningitis and the myocardium in infarcts [13]. The first report of the anti-amyloidogenic activity of doxycycline was suggested in a study of Alzheimer's disease [14]. Forloni et al., showed that co-incubation of tetracyclines with β 1–42 synthetic peptide, which is highly represented in Alzheimer amyloid deposits, resulted in a) marked reduction of amyloid fibril formation, b) inhibition of amyloid aggregation and c) de-fibrillogenic effect against pre-formed amyloid fibrils [14]. Cardoso et al. tested various tetracyclines and showed doxycycline to be the most effective of the family in disrupting transthyretrin amyloid fibrils after incubation [15]. This group also showed that the anti-amyloid effect is independent of the amyloid precursor protein [16]. Doxycycline also disrupted amyloid in animal models, including a familial amyloid polyneuropathy transgenic mouse model [17]. Further studies showed that tetracyclines also produced de-structuration of β2-microglobulin in dialysis related amyloidosis [18]. In AL amyloidosis, Ward, et al. showed that doxycycline can inhibit amyloid fibril aggregation and can destroy preformed amyloid in vitro and in a transgenic murine AL model [19].
Doxycycline is well-tolerated and safe, and is widely used in clinical practice for antibacterial prophylaxis, community acquired pneumonia and chronic obstructive pulmonary disease. It is also efficacious in unusual infections such as Lyme disease, cholera, syphilis, plague and malaria [13]. The side-effect profile of doxycycline is well-studied. Prolonged doxycycline administration at doses of 100–200 mg daily for 6–24 months is well tolerated [20], [21], [22]. Commonly described adverse events with doxycycline use are cutaneous photosensitivity and self-limiting non-specific gastrointestinal symptoms [21], [22]. Within clinical oncology practice, doxycycline is commonly used in patients with solid tumors and hematologic malignancies for anti-microbial prophylaxis in the setting of chemotherapy-induced neutropenia, particularly in patients with penicillin allergies with good tolerance [20], [23].
There are limited but positive data suggesting an anti-amyloid efficacy of doxycycline in humans. Montagna et al. treated 3 patients with severe painful arthropathy related to β2-microglobulin dialysis related amyloidosis with resolution of arthropathy in all three patients [24]. Kumar et al. reviewed a large cohort of AL patients who underwent stem cell transplantation for therapy, and received a year of doxycycline treatment for anti-bacterial prophylaxis. They found that among patients who had a hematologic response to stem cell transplantation, those patients on doxycycline prophylaxis had a higher survival rate than those on penicillin G prophylaxis [23]. This study also suggested the tolerability and safety of doxycycline administration in AL amyloidosis patients even in the critical post-transplant setting [23]. Doxycycline is currently being studied in patients with familial amyloid polyneuropathy and localized AL. We undertook this prospective clinical trial to study the effect of doxycycline in the treatment of AL amyloidosis patients.
2. Materials and methods
2.1. Overview of study design
This is a single center, prospective, open label, phase 2 clinical trial evaluating the safety and efficacy of oral doxycycline for 1 year in newly diagnosed AL patients treated additionally with the standard of care anti-AL therapy. The study is registered under ClinicalTrials.gov with the identifier NCT02207556.
2.2. Hypothesis and rationale
Organ response to anti-plasma cell directed therapy in AL lags behind hematologic response as chemotherapy has no effect on pre-formed amyloidDoxycycline has pleiotropic, de-fibrillogenic and inhibitory effects on amyloid fibrils shown to be beneficial in in vitro studies, murine models, and other preclinical studies. We hypothesize that doxycycline use will be safe and efficacious in improving organ response compared to standard of care therapy in AL amyloidosis.
2.3. Objectives
Primary objectives:
-
1.
Determine the efficacy of adjunctive doxycycline in addition to specific anti plasma cell therapy in patients with AL amyloidosis in improving amyloid organ response at 1 year.
-
2.
Assess the safety of doxycycline + anti-plasma cell chemotherapy regimen in AL amyloidosis.
Secondary Objectives:
-
1.
To assess rates of hematologic response.
-
2.
To assess rates of amyloid organ response at months 6 and 12.
-
3.
To assess rates of mortality at 1 month, 6 months and 1 year.
-
4.
To measure patient reported outcomes at baseline, 3, 6, 9 and 12 months.
-
5.
To evaluate MMP-2, MMP-7, MMP-8, MMP-9 and tissue inhibitor of metalloproteinases (TIMP) levels at baseline, 6 and 12 months.
2.4. Intervention
Oral doxycycline monohydrate 100 mg twice daily will be administered and continued for 1 year as long as there is no contraindication to take doxycycline.
2.5. Trial eligibility
Inclusion criteria:
-
1.
Biopsy-proven AL amyloidosis
-
2.
Patients aged 18 or above
-
3.
Measurable amyloid organ involvement of a vital organ. Localized amyloidosis will be eligible if the amyloid is radiologically measurable.
-
4.
Creatinine clearance of >25 mL/min
-
5.
Patients who have previously been taking doxycycline will be eligible as long as there is no contraindication to stay on doxycycline 100 mg BID for 1 year in the opinion of the treating physician.
-
6.
A negative pregnancy test will be required for all women of child bearing potential. Breast feeding is not permitted.
Exclusion criteria:
-
1.
Patients with severe malabsorption syndrome precluding absorption of oral agents
-
2.
Known intolerance or allergic reactions with doxycycline
-
3.
Previous chemotherapy for AL amyloidosis.
2.6. Correlative studies
2.6.1. Patient-reported outcomes (PROs)
There are no disease-relevant or validated PROs for AL amyloidosis. In this study, PROs will be obtained using Patient Reported Outcomes Measurement Information System (PROMIS). Three short forms namely PROMIS Global Health, PROMIS-29 and Fatigue-8 will be administered at baseline and monthly intervals. This trial will be the first to describe PROs in AL amyloidosis using PROMIS measures.
2.6.2. Biomarkers of doxycycline activity
Blood will be collected from patients at baseline, months 6 and 12. We will perform serum inflammatory markers known to be associated with doxycycline use [11] to identify possible biomarkers of amyloid response to doxycycline. We will obtain MMP-2, MMP-7, MMP-8, MMP-9 and TIMP-1 levels in all patients at baseline, months 6 and 12 of doxycycline use. To measure MMP activity, zymograms will be performed on sera from all the patients. Briefly, zymography is a simple sensitive and functional assay to analyze MMP activity. Proteins are separated by electrophoresis utilizing SDS-PAGE gels containing the according MMP substrate, MMP2 and 9 are gelatinases therefore the gel will contain gelatin. The gel for MMP7 will contain casein and for MMP8 collagen-I. The MMP activity will be measured by the degradation of the substrate and therefore the inability of Coomassie Blue to stain the gel at the molecular weight appropriate spot. The TIMP-1 expression will be measured by ELISA utilizing TIMP-1 ELISA kit (abcam #ab100651) [25], [26]. Briefly, ELISA is a test that uses antibodies and color change to identify an antigen in a liquid substrate measured with a microplate reader. These tests will be performed using techniques that have already been optimized and validated in human sera and myocardial interstitial fluid [27], [28], [29], [30], [31]. Descriptive statistics will be computed for all the correlative laboratory parameters. If a significant difference is seen in these biomarkers at 6 and 12 months, and organ response is seen (i.e. positive study), these results will be used to for further hypothesis generating questions, and phase III design.
2.7. Follow-up and study endpoints
Patients with systemic amyloidosis will be followed monthly, while patients with localized amyloidosis will be followed 3 monthly. Table 1, Table 2 show the clinical assessments that will be done during the course of the study.
Table 1.
Systemic AL amyloidosis.
| Baselinea | Months |
End of Treatment (within 30 days) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1a | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | |||
| Inclusion & Exclusion criteria | ✕ | |||||||||||||
| Informed consent | ✕ | |||||||||||||
| H & PEd | ✕ | ✕ | ✕ | ✕ | ✕ | ✕ | ✕ | ✕ | ✕ | ✕ | ✕ | ✕ | ✕ | ✕ |
| Vital signse eee | ✕ | ✕ | ✕ | ✕ | ✕ | ✕ | ✕ | ✕ | ✕ | ✕ | ✕ | ✕ | ✕ | ✕ |
| ECOG performance status | ✕ | ✕ | ✕ | ✕ | ✕ | ✕ | ||||||||
| NYHA class | ✕ | ✕k | ✕k | ✕k | ||||||||||
| Complete Blood Count with differential | ✕ | ✕ | ✕ | ✕ | ✕ | ✕ | ✕ | ✕ | ✕ | ✕ | ✕ | ✕ | ✕ | ✕ |
| Serum chemistries panelf | ✕ | ✕ | ✕ | ✕ | ✕ | ✕ | ✕ | ✕ | ✕ | ✕ | ✕ | ✕ | ✕ | ✕ |
| Amyloid subtypeb | ✕ | |||||||||||||
| β2 microglobulin | ✕ | |||||||||||||
| Myeloma screening panelg | ✕ | ✕ | ✕ | ✕ | ✕ | ✕ | ✕ | ✕ | ✕ | ✕ | ✕ | ✕ | ✕ | ✕ |
| Bone marrow aspirate/biopsy | ✕j | |||||||||||||
| Β-HCG serum pregnancy testh | ✕ | |||||||||||||
| Bone surveyn | ✕j | |||||||||||||
| 2D echocardiogram (IVS + LVEF) | ✕ | ✕k | ✕k,c | |||||||||||
| Troponin-T, NT-proBNP | ✕ | ✕k | ✕k | ✕k | ✕k | ✕k | ✕k | ✕k | ✕k | ✕k | ✕k | ✕k | ✕k | ✕k,c |
| Abdominal Imaging | ✕j | ✕l | ✕l,c | |||||||||||
| 24 h urinary protein with UPEP/immunofixation | ✕ | ✕m | ✕m | ✕m | ✕m,c | |||||||||
| Toxicity assessment | ✕ | ✕ | ✕ | ✕ | ✕ | ✕ | ✕ | ✕ | ✕ | ✕ | ✕ | ✕ | ||
| Research specimensi | ✕ | ✕ | ✕c | |||||||||||
| Patient-reported outcomeso | ✕ | ✕ | ✕ | ✕ | ✕ | ✕ | ✕ | ✕ | ✕ | ✕ | ✕ | ✕ | ✕ | ✕ |
H & PE = history and physical examination.
ECOG- Eastern Co-operative Oncology Group performance score.
NYHA- New York Heart Association Class.
2D echocardiogram- Two-dimensional transthoracic echocardiogram.
IVS- interventricular septal thickness.
LVEF- left ventricular ejection fraction.
Baseline and Month 1 can occur within 7 days without the need to repeat the Month 1 testing.
If a patient has had amyloid subtyping performed in the past, this need not be repeated again.
May be performed anytime during cycle 12 or within 30 days of the last dose of doxycycline.
History is only required at baseline.
Vital signs: blood pressure, pulse rate, respiratory rate and temperature.
Serum chemistries panel: electrolytes, BUN, ALT, AST, creatinine, bilirubin, alkaline phosphatase, LDH, albumin, uric acid. Electrolytes to include sodium, potassium, chloride, carbon dioxide, and calcium.
Myeloma screening panel includes serum protein electrophoresis, immunofixation electrophoresis, free light chain assay.
Females of reproductive potential only.
Peripheral blood specimens: 6 mL blood in heparin containing tube at indicated time points and send to the Tissue Bank at MCW after processing.
May be performed within 3 months of baseline.
Cardiac assessment will be followed only if baseline cardiac involvement.
Abdominal imaging will be repeated only if baseline liver involvement.
Urinary studies will be repeated only if baseline renal involvement.
Not required if ≤ 10% plasma cells in bone marrow.
PROMIS Global Health Scale, PROMIS-29, PROMIS Fatigue-8 short forms.
Table 2.
Localized AL.
| Baselinea | 1a | 4 | 7 | 10 | End of Treatment (within 30 days) | |
|---|---|---|---|---|---|---|
| Inclusion & Exclusion criteria | ✕ | |||||
| Informed consent | ✕ | |||||
| H & PEe | ✕ | ✕ | ✕ | ✕ | ✕ | ✕ |
| Vital signsff | ✕ | ✕ | ✕ | ✕ | ✕ | ✕ |
| ECOG performance status | ✕ | ✕ | ✕ | ✕ | ✕ | |
| Complete Blood Count with differential | ✕ | ✕ | ✕ | ✕ | ✕ | ✕ |
| Serum chemistries panelg | ✕ | ✕ | ✕ | ✕ | ✕ | ✕ |
| Amyloid subtyped | ✕ | |||||
| Beta2 microglobulin | ✕l | |||||
| Myeloma screening panelh | ✕ | ✕l | ✕l | ✕l | ✕l | ✕l |
| Β-HCG serum pregnancy testi | ✕ | |||||
| Troponin-T, NT-proBNP | ✕k | |||||
| 24 h urinary protein with UPEP/immunofixationm | ✕l,c | |||||
| Bone marrow aspirate/biopsy | ✕c | |||||
| 2D echocardiogram (IVS + LVEF) | ✕c | |||||
| Abdominal Imaging | ✕c | |||||
| Radiologic imagingk | ✕ | ✕ | ✕b | |||
| Toxicity assessment | ✕ | ✕ | ✕ | ✕ | ||
| Research specimensj | ✕ | ✕ | ✕b | |||
| Patient questionnaire | ✕ | ✕ | ✕ | ✕ | ✕ | ✕ |
H & PE = history and physical examination.
ECOG- Eastern Co-operative Oncology Group performance score.
Baseline and Month 1 can occur within 7 days without the need to repeat the Month 1 testing.
May be performed anytime during cycle 12 or within 30 days of the last dose of doxycycline.
Bone marrow evaluation, 2D echocardiogram, Abdominal Ultrasound and 24 h urine protein test may be held based on the treating physician's judgment.
If a patient has had amyloid subtyping performed in the past, this need not be repeated again.
History is only required at baseline.
Vital signs: blood pressure, pulse rate, respiratory rate and temperature.
Serum chemistries panel: electrolytes, BUN, ALT, AST, creatinine, bilirubin, alkaline phosphatase, LDH, albumin, uric acid. Electrolytes to include sodium, potassium, chloride, carbon dioxide, and calcium.
Myeloma screening panel includes serum protein electrophoresis, immunofixation electrophoresis, free light chain assay.
Females of reproductive potential only.
Peripheral blood specimens: draw 6 mL in green top (heparin containing) tube at indicated time points and send to the Tissue Bank at MCW after processing. Samples should be labeled with patient study number, date of collection, time point (baseline, 7 months or End of Treatment) and MCW/FH IRB PRO number.
Radiologic imaging: as clinically indicated.
Per MD discretion.
A 24 h urine protein may be replaced by a urine protein/creatinine ratio for measure of proteinuria.
2.8. Assessment of adherence
At every visit, patients will bring their previous doxycycline pill bottle, and an assessment will be made regarding how many pills were missed, if any. Further, the clinical research coordinator will also ask the patient if any pills were missed.
2.9. Statistical analysis plan
The study is a single-arm phase II trial evaluating the safety and efficacy of doxycycline used in adjunct to anti-amyloid treatment to improve organ response in patients with AL amyloidosis. The primary objective of this study is to evaluate the cumulative organ response at 1 year. The incidence of organ response to conventional chemotherapy at 1 year is approximately 20–25%. At the time of publication, the results of the study will be retrospectively compared against AL amyloidosis patients undergoing chemotherapy alone at our institution in the last 5 years. Matching will be conducted for stage, organ involvement and chemotherapy regimen.
Sample size determination: We will use an exact single-stage phase II design. We will consider the “experimental” regimen of doxycycline with conventional anti-amyloid treatment to be no more effective than conventional anti-amyloid treatment alone, the true probability of organ response at 1 year is no less than 25% (p0). We will assume that the new experimental regimen is worthy of further study of the true probability of organ response greater than 50% (p1). In statistical terms, we are testing the null hypothesis H0: p ≤ 0.25, versus the alternate H1: p ≥ 0.5, where p is probability of organ response. Our power analysis indicated that we will need 26 patients to have 80% power to detect the designed difference at 5% significance level. We will increase the sample size by 15%–30 in order to account for patients with advanced disease who may die prior to response. The probability of response will be calculated using the cumulative incidence curve to accommodate for competing risks. The 6 and 12 month cumulative incidence rates of response with 95% confidence intervals will be reported. Point-wise test will be considered to compare response rates of study patients to response rates under standard treatment using historical controls from published literature. In the final analysis, if 11 or more of the patients develop organ response we will reject the null hypothesis. We anticipate that a maximum of 30 patients will be accrued to the study. Descriptive statistics (i.e. means, standard deviations, 95% confidence intervals for continuous variables, and frequencies for discrete data) will be computed for all correlative laboratory parameters. Patients who receive <1 month of doxycycline therapy and withdraw from the study will be replaced.
Safety/stopping rules: Development of grade 3-4 adverse events deemed related to doxycycline in 6 or more patients, will mandate halting further patient accrual until review by the Data Safety and Monitoring Board.
3. Discussion
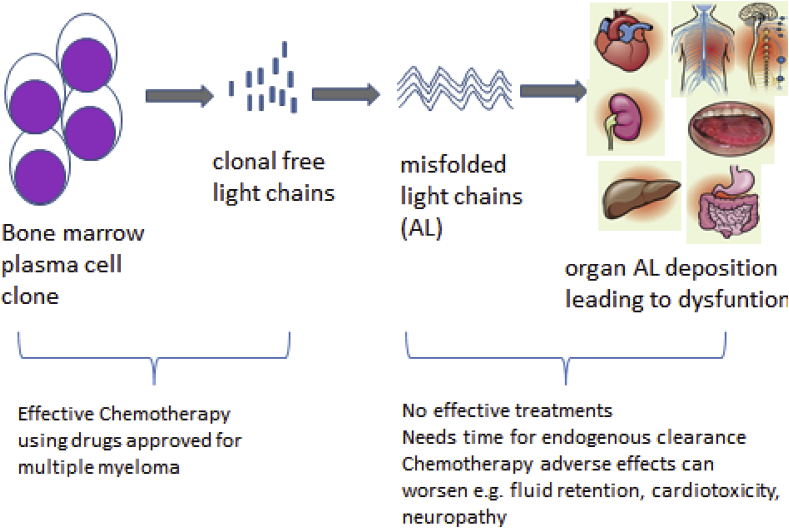
The current paradigm of AL amyloid treatment is focused upon eradicating the underlying malignant plasma cell clone with cytotoxic agents (Fig. 1). This produces a hematologic response by clearing circulating immunoglobulin light chains which would have eventually been deposited into amyloid. Treatments range from high dose therapy with autologous stem cell transplantation in eligible patients [3], or anti-myeloma chemotherapy regimens such as melphalan/dexamethasone [32]. Novel anti-myeloma chemotherapies such as thalidomide [33], [34], lenalidomide [35], [36], [37], [38], pomalidomide [39], and bortezomib [40], [41] have excellent anti-plasma cell efficacy in amyloidosis but are also associated with organ and tissue toxicity making their use in clinical practice challenging, particularly in advanced AL disease [4]. Treatment of AL amyloidosis thus remains suboptimal for patients, where clinically tenuous and frail patients with compromised vital organ function are treated with cytotoxic chemotherapy that often rapidly clears light chains from serum (i.e. hematologic response) but does not rapidly improve organ function. Many of these patients succumb to organ failure despite hematologic response. Patients with advanced stage cardiac amyloidosis may experience an initial worsening in cardiac biomarkers and even death with use of newer anti-myeloma drugs such as lenalidomide and bortezomib [4], [35], [40]. Patients with advanced stage amyloidosis have a 40% risk of mortality in the first year following diagnosis, a number that has not changed since the 1970s [5] despite the availability of more effective anti-myeloma chemotherapy that have clearly improved myeloma outcomes during the same time frame [6].
Fig. 1.
Pathophysiology and unmet needs in AL amyloidosis.
Thus, there is a clear need to identify treatments that do more than eradicate the malignant clone (the primary objective of anti-myeloma therapies) to improve the high early mortality that occurs even while on “effective” chemotherapy. The consideration of therapies that complement current cytotoxic anti-amyloid treatment by hastening organ response is required. Thus far, there are no approved amyloid fibril-directed therapies. There are some that are in development, including monoclonal antibodies [42], [43], [44]. The compelling preclinical data of doxycycline's potential to benefit amyloidosis support prospective investigation of testing the use of doxycycline for AL amyloidosis treatment. Our study aims to generate key safety and efficacy data of doxycycline administration to AL amyloidosis patients. Patients will potentially benefit from a novel mechanism of anti-amyloid effect in conjunction with anti-plasma cell chemotherapy with minimal additional risks and side-effects and, perhaps, even an added benefit of simultaneous anti-microbial activity. If successful, this study will be an example of successfully using a well-studied alternative to improve treatments of AL amyloidosis patients and potentially change clinical practice by providing access to a relatively safe and economical drug acting via a novel mechanism to enhance the limited anti-AL armamentarium.
3.1. Status of trial
Recruitment for this study has recently been concluded.
Acknowledgements
This study is funded by the American Cancer Society Institutional Research Grant # 86-004-26 (AD) and in part by the Research and Education Program Fund, a component of the Advancing a Healthier Wisconsin endowment at the Medical College of Wisconsin and by KL2TR001438 from the Clinical and Translational Science Award program of the National Center for Advancing Translational Sciences (AD). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
References
- 1.Sipe J.D., Benson M.D., Buxbaum J.N. Nomenclature 2014: amyloid fibril proteins and clinical classification of the amyloidosis. Amyloid. 2014;21:221–224. doi: 10.3109/13506129.2014.964858. [DOI] [PubMed] [Google Scholar]
- 2.Kyle R.A., Gertz M.A. Primary systemic amyloidosis: clinical and laboratory features in 474 cases. Semin. Hematol. 1995;32:45–59. [PubMed] [Google Scholar]
- 3.D'Souza A., Dispenzieri A., Wirk B. Improved outcomes after autologous hematopoietic cell transplantation for light chain amyloidosis: a center for international blood and marrow transplant research study. J. Clin. Oncol. 2015;33:3741–3749. doi: 10.1200/JCO.2015.62.4015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dispenzieri A., Gertz M.A., Buadi F. What do I need to know about immunoglobulin light chain (AL) amyloidosis? Blood Rev. 2012;26:137–154. doi: 10.1016/j.blre.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 5.Kumar S.K., Gertz M.A., Lacy M.Q. Recent improvements in survival in primary systemic amyloidosis and the importance of an early mortality risk score. Mayo Clin. Proc. 2011;86:12–18. doi: 10.4065/mcp.2010.0480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kumar S.K., Rajkumar S.V., Dispenzieri A. Improved survival in multiple myeloma and the impact of novel therapies. Blood. 2008;111:2516–2520. doi: 10.1182/blood-2007-10-116129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nagase H., Woessner J.F., Jr. Matrix metalloproteinases. J. Biol. Chem. 1999;274:21491–21494. doi: 10.1074/jbc.274.31.21491. [DOI] [PubMed] [Google Scholar]
- 8.Keeling J., Herrera G.A. Matrix metalloproteinases and mesangial remodeling in light chain-related glomerular damage. Kidney Int. 2005;68:1590–1603. doi: 10.1111/j.1523-1755.2005.00571.x. [DOI] [PubMed] [Google Scholar]
- 9.Biolo A., Ramamurthy S., Connors L.H. Matrix metalloproteinases and their tissue inhibitors in cardiac amyloidosis: relationship to structural, functional myocardial changes and to light chain amyloid deposition. Circ. Heart Fail. 2008;1:249–257. doi: 10.1161/CIRCHEARTFAILURE.108.788687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Golub L.M., Lee H.M., Ryan M.E. Tetracyclines inhibit connective tissue breakdown by multiple non-antimicrobial mechanisms. Adv. Dent. Res. 1998;12:12–26. doi: 10.1177/08959374980120010501. [DOI] [PubMed] [Google Scholar]
- 11.Kormi I., Alfakry H., Tervahartiala T. The effect of prolonged systemic doxycycline therapy on serum tissue degrading proteinases in coronary bypass patients: a randomized, double-masked, placebo-controlled clinical trial. Inflamm. Res. May 2014;63(5):329–334. doi: 10.1007/s00011-013-0704-2. [DOI] [PubMed] [Google Scholar]
- 12.Greenwald R.A., Moak S.A., Ramamurthy N.S. Tetracyclines suppress matrix metalloproteinase activity in adjuvant arthritis and in combination with flurbiprofen, ameliorate bone damage. J. Rheumatol. 1992;19:927–938. [PubMed] [Google Scholar]
- 13.Griffin M.O., Fricovsky E., Ceballos G. Tetracyclines: a pleitropic family of compounds with promising therapeutic properties. Review of the literature. Am. J. Physiol. Cell Physiol. 2010;299:C539–C548. doi: 10.1152/ajpcell.00047.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Forloni G., Colombo L., Girola L. Anti-amyloidogenic activity of tetracyclines: studies in vitro. FEBS Lett. 2001;487:404–407. doi: 10.1016/s0014-5793(00)02380-2. [DOI] [PubMed] [Google Scholar]
- 15.Cardoso I., Merlini G., Saraiva M.J. 4'-iodo-4'-deoxydoxorubicin and tetracyclines disrupt transthyretin amyloid fibrils in vitro producing noncytotoxic species: screening for TTR fibril disrupters. FASEB J. 2003;17:803–809. doi: 10.1096/fj.02-0764com. [DOI] [PubMed] [Google Scholar]
- 16.Cardoso I., Martins D., Ribeiro T. Synergy of combined doxycycline/TUDCA treatment in lowering Transthyretin deposition and associated biomarkers: studies in FAP mouse models. J. Transl. Med. 2010;8:74. doi: 10.1186/1479-5876-8-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cardoso I., Saraiva M.J. Doxycycline disrupts transthyretin amyloid: evidence from studies in a FAP transgenic mice model. FASEB J. 2006;20:234–239. doi: 10.1096/fj.05-4509com. [DOI] [PubMed] [Google Scholar]
- 18.Giorgetti S., Raimondi S., Pagano K. Effect of tetracyclines on the dynamics of formation and destructuration of beta2-microglobulin amyloid fibrils. J. Biol. Chem. 2011;286:2121–2131. doi: 10.1074/jbc.M110.178376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ward J.E., Ren R., Toraldo G. Doxycycline reduces fibril formation in a transgenic mouse model of AL amyloidosis. Blood. 2011;118:6610–6617. doi: 10.1182/blood-2011-04-351643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Innes H., Lim S.L., Hall A. Management of febrile neutropenia in solid tumours and lymphomas using the Multinational Association for Supportive Care in Cancer (MASCC) risk index: feasibility and safety in routine clinical practice. Support Care Cancer. 2008;16:485–491. doi: 10.1007/s00520-007-0334-8. [DOI] [PubMed] [Google Scholar]
- 21.Chang W.Y., Cane J.L., Kumaran M. A two year randomised placebo controlled trial of doxycycline for lymphangioleiomyomatosis. Eur. Respir. J. 2013 doi: 10.1183/09031936.00167413. [DOI] [PubMed] [Google Scholar]
- 22.Baxter B.T., Pearce W.H., Waltke E.A. Prolonged administration of doxycycline in patients with small asymptomatic abdominal aortic aneurysms: report of a prospective (Phase II) multicenter study. J. Vasc. Surg. 2002;36:1–12. doi: 10.1067/mva.2002.125018. [DOI] [PubMed] [Google Scholar]
- 23.Kumar S.K., Dispenzieri A., Lacy M.Q. Doxycycline used as post transplant antibacterial prophylaxis improves survival in patients with light chain amyloidosis undergoing autologous stem cell transplantation. ASH Annu. Meet. Abstr. 2012;120:3138. [Google Scholar]
- 24.Montagna G., Cazzulani B., Obici L. Benefit of doxycycline treatment on articular disability caused by dialysis related amyloidosis. Amyloid. 2013;20:173–178. doi: 10.3109/13506129.2013.803463. [DOI] [PubMed] [Google Scholar]
- 25.Xu H., Krolikowski J.G., Jones D.W. 4F decreases IRF5 expression and activation in hearts of tight skin mice. PLoS One. 2012;7:e52046. doi: 10.1371/journal.pone.0052046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu H., Zaidi M., Struve J. Abnormal fibrillin-1 expression and chronic oxidative stress mediate endothelial mesenchymal transition in a murine model of systemic sclerosis. Am. J. Physiol. Cell Physiol. 2011;300:C550–C556. doi: 10.1152/ajpcell.00123.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weihrauch D., Lohr N.L., Mraovic B. Chronic hyperglycemia attenuates coronary collateral development and impairs proliferative properties of myocardial interstitial fluid by production of angiostatin. Circulation. 2004;109:2343–2348. doi: 10.1161/01.CIR.0000129225.67353.1F. [DOI] [PubMed] [Google Scholar]
- 28.Snoek-van Beurden P.A., Von den Hoff J.W. Zymographic techniques for the analysis of matrix metalloproteinases and their inhibitors. Biotechniques. 2005;38:73–83. doi: 10.2144/05381RV01. [DOI] [PubMed] [Google Scholar]
- 29.Matsunaga T., Weihrauch D.W., Moniz M.C. Angiostatin inhibits coronary angiogenesis during impaired production of nitric oxide. Circulation. 2002;105:2185–2191. doi: 10.1161/01.cir.0000015856.84385.e9. [DOI] [PubMed] [Google Scholar]
- 30.Ludwig L.M., Tanaka K., Eells J.T. Preconditioning by isoflurane is mediated by reactive oxygen species generated from mitochondrial electron transport chain complex III. Anesth. Analg. 2004;99:1308–1315. doi: 10.1213/01.ANE.0000134804.09484.5D. table of contents. [DOI] [PubMed] [Google Scholar]
- 31.Gogly B., Groult N., Hornebeck W. Collagen zymography as a sensitive and specific technique for the determination of subpicogram levels of interstitial collagenase. Anal. Biochem. 1998;255:211–216. doi: 10.1006/abio.1997.2318. [DOI] [PubMed] [Google Scholar]
- 32.Palladini G., Perfetti V., Obici L. Association of melphalan and high-dose dexamethasone is effective and well tolerated in patients with AL (primary) amyloidosis who are ineligible for stem cell transplantation. Blood. 2004;103:2936–2938. doi: 10.1182/blood-2003-08-2788. [DOI] [PubMed] [Google Scholar]
- 33.Palladini G., Perfetti V., Perlini S. The combination of thalidomide and intermediate-dose dexamethasone is an effective but toxic treatment for patients with primary amyloidosis (AL) Blood. 2005;105:2949–2951. doi: 10.1182/blood-2004-08-3231. [DOI] [PubMed] [Google Scholar]
- 34.Dispenzieri A., Lacy M.Q., Rajkumar S.V. Poor tolerance to high doses of thalidomide in patients with primary systemic amyloidosis. Amyloid. 2003;10:257–261. doi: 10.3109/13506120309041743. [DOI] [PubMed] [Google Scholar]
- 35.Finsterer J., Hoftberger R., Stollberger C. Sudden death possibly related to lenalidomide given for cardiac and muscle AL amyloidosis secondary to light chain deposition disease. J. Oncol. Pharm. Pract. 2013;19:170–174. doi: 10.1177/1078155212443991. [DOI] [PubMed] [Google Scholar]
- 36.Dinner S., Witteles W., Afghahi A. Lenalidomide, melphalan and dexamethasone in a population of patients with immunoglobulin light chain amyloidosis with high rates of advanced cardiac involvement. Haematologica. 2013;98:1593–1599. doi: 10.3324/haematol.2013.084574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Palladini G., Russo P., Foli A. Salvage therapy with lenalidomide and dexamethasone in patients with advanced AL amyloidosis refractory to melphalan, bortezomib, and thalidomide. Ann. Hematol. 2012;91:89–92. doi: 10.1007/s00277-011-1244-x. [DOI] [PubMed] [Google Scholar]
- 38.Kumar S.K., Hayman S.R., Buadi F.K. Lenalidomide, cyclophosphamide, and dexamethasone (CRd) for light-chain amyloidosis: long-term results from a phase 2 trial. Blood. 2012;119:4860–4867. doi: 10.1182/blood-2012-01-407791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dispenzieri A., Buadi F., Laumann K. Activity of pomalidomide in patients with immunoglobulin light-chain amyloidosis. Blood. 2012;119:5397–5404. doi: 10.1182/blood-2012-02-413161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yamasaki S., Muta T., Higo T. Ventricular fibrillation after bortezomib therapy in a patient with systemic amyloidosis. Hematol. Rep. 2013;5:e12. doi: 10.4081/hr.2013.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dimopoulos M.A., Kastritis E. Bortezomib for AL amyloidosis: moving forward. Blood. 2011;118:827–828. doi: 10.1182/blood-2011-05-355115. [DOI] [PubMed] [Google Scholar]
- 42.Richards D.B., Cookson L.M., Berges A.C. Therapeutic clearance of amyloid by antibodies to serum amyloid P component. N. Engl. J. Med. 2015;373:1106–1114. doi: 10.1056/NEJMoa1504942. [DOI] [PubMed] [Google Scholar]
- 43.Gertz M.A., Landau H., Comenzo R.L. First-in-Human phase I/II study of NEOD001 in patients with light chain amyloidosis and persistent organ dysfunction. J. Clin. Oncol. 2016;34:1097–1103. doi: 10.1200/JCO.2015.63.6530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Edwards C.V., Gould J., Langer A.L. Interim analysis of the phase 1a/b study of chimeric fibril-reactive monoclonal antibody 11-1F4 in patients with AL amyloidosis. Amyloid. 2017;24:58–59. doi: 10.1080/13506129.2017.1292900. [DOI] [PubMed] [Google Scholar]