Abstract
Introduction
Radio-frequency ablation (RFA) is a promising minimal-invasive treatment option for early liver cancer, however monitoring or predicting the size of the resulting tissue necrosis during the RFA-procedure is a challenging task, potentially resulting in a significant rate of under- or over treatments. Currently there is no reliable lesion size prediction method commercially available.
Objectives
ClinicIMPPACT is designed as multicenter-, prospective-, non-randomized clinical trial to evaluate the accuracy and efficiency of innovative planning and simulation software. 60 patients with early liver cancer will be included at four European clinical institutions and treated with the same RFA system. The preinterventional imaging datasets will be used for computational planning of the RFA treatment. All ablations will be simulated simultaneously to the actual RFA procedure, using the software environment developed in this project. The primary outcome measure is the comparison of the simulated ablation zones with the true lesions shown in follow-up imaging after one month, to assess accuracy of the lesion prediction.
Discussion
This unique multicenter clinical trial aims at the clinical integration of a dedicated software solution to accurately predict lesion size and shape after radiofrequency ablation of liver tumors. Accelerated and optimized workflow integration, and real-time intraoperative image processing, as well as inclusion of patient specific information, e.g. organ perfusion and registration of the real RFA needle position might make the introduced software a powerful tool for interventional radiologists to optimize patient outcomes.
Keywords: RFA, Liver, Lesion prediction, Segmentation, Perfusion CT
Abbreviations: BF, Blood flow; BV, Blood volume; CT, Computed tomography; EU, European Union; HCC, Hepatocellular carcinoma; IMPPACT, Intervention Modelling, Planning and Proof for Ablation Cancer Treatment; RFA, Radio frequency ablation; US, Ultrasound
1. Introduction
Liver cancer has several treatment options, while each approach comes along with specific limitations. For hepatocellular cancer (HCC) chemotherapy often has unsatisfactory results [1], whereas surgery (resection or transplant) can be curative, but is not always suitable especially for older patients with existing comorbid conditions, e.g. liver cirrhosis [2]. Therefore adequate patient selection is of paramount importance [3], [4]. Radiofrequency ablation (RFA) has become the first treatment choice for early-stage HCC in cirrhotic livers [5]. Also, for early metastatic liver cancer in inoperable patients, RFA is considered a viable alternative with comparable overall survival [6]. Percutaneous RFA offers minimal invasiveness due to precise image guidance and reliable needle placement. Ultrasound (US) or computed tomography (CT) guided RFA is the most commonly used ablation technique worldwide.
Although RFA requires a relatively low technical effort compared to, e.g., cryo- or lasertherapy, one cannot currently monitor therapy in real time, which can cause significant discrepancies between the expected and real sizes of ablation zones. This potentially results in an over- (up to 9% of major complications) [7], or under treatment (up to 40% local recurrence) [7], [8].
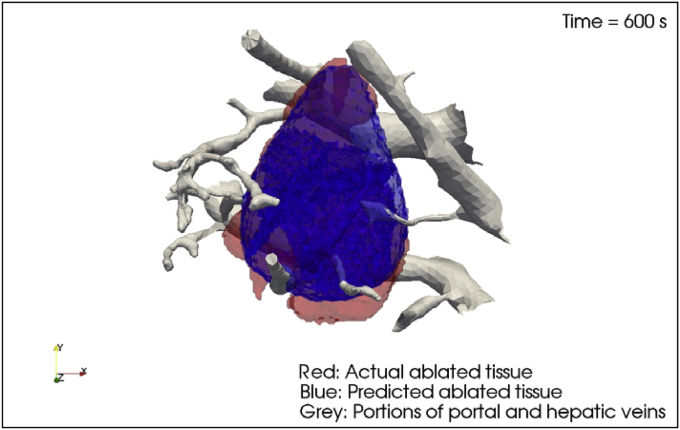
The ClinicIMPPACT proposal is based on the results of the IMPPACT project, in which we created a model for facilitating accurate RFA treatments [9], [10]. The required software was developed during IMPPACT and basically provides a simulator for radiologists to plan, review and optimize procedures. With IMPPACT, extensive experiments were performed on cells and animals to create a heat dependent cellular death model, which was implemented into the simulation algorithm. The histological workups of the RFA-induced lesions in porcine liver were used to calibrate the preliminary RFA model. Subsequently, eight lesions were selected from a database of accurately documented clinical procedures, and the software was used to retrospectively simulate interventions and predict lesion shapes (Fig. 1).
Fig. 1.
Actual segmented versus simulated lesion shape. IMPPACT Project, Grant Number: 223 877; FP7.
The predicted volumes were then compared to the real thermal lesions visualized and segmented in contrast-enhanced CT one month after ablation. These comparisons showed that simulated and real lesion volumes matched acceptably, after taking virtual tissue perfusion values into account. Individual perfusion parameters are essential for an accurate lesion prediction because intrahepatic tumor/vessel proximity can cause unwanted heat transfer during radiofrequency ablations. Some lesion shapes were mismatched, possibly due to inaccuracies in segmenting non standardized radiological images [11].
Our hope is, that the treatment of early liver cancer with RFA could be improved using a validated software solution to estimate lesion size and identify possible complications in advance. Self-evident, such a solution has to be adapted to real-time clinical requirements.
The first goal of ClinicIMPPACT is to refine an existing simulation tool, driven by a user-friendly, ergonomically optimized graphical user interface, to support the complex requirements of clinicians performing radiofrequency ablations of liver tumors. Therefore, the working steps of this European joint research project and its partners are to accelerate simulation speed, optimize the graphical user interface, enable patient specific needle registration, and integrate patients' individual perfusion values into the software calculations. The second aim of this trial is to ensure an accurate validation and to produce reliable predictions during the interventional procedure.
2. The ClinicIMPPACT study protocol
2.1. Study management
The ClinicIMPPACT project is a multicenter, non-randomized prospective clinical trial funded by the Seventh Framework Program of the European Union (grant number 610886). The original proposal was designed and conducted by the Fraunhofer Institute for Applied Informational Technologies (FIT) in St. Augustin (Germany) in collaboration with medical and technical partners, which are listed below. The medical aspect of the study is shared between four university hospitals located in Turku (Finland), Graz (Austria), Nijmegen (Netherlands), and Leipzig (Germany). The local study center of the University of Leipzig (ZKS, Center for Clinical Studies) is responsible for monitoring patient safety and adherence to good clinical practice. Each medical unit consists of a project leader (experienced interventional radiologist) and one or two assistants (e.g. resident radiologists). Technical partners, who provide the software development and data implementation, are located at Graz (Austria), Helsinki (Finland), and Dundalk (Ireland). Approvals were granted by the institutional ethics committees and the German Federal Office for Radiation Protection (Bundesamt für Strahlenschutz, BfS, only necessary for Leipzig, Germany). The study has been registered at clinical trial databases (http://drks-neu.uniklinik-freiburg.de/drks_web/, and https://clinicaltrials.gov).
2.2. Study population eligibility
All participating subjects are patients (both men and women) with primary (hepatocellular carcinoma, HCC) or secondary (e.g. colorectal metastasis) malignant liver tumors who are referred for RFA treatment after interdisciplinary consent of the local tumor board (consisting of radiologists, oncologists and hepatobiliary surgeons), according to AASDL guidelines for HCC (http://www.aasdl.org) and interdisciplinary agreement for secondary liver tumors regarding operability, patient safety and compliance. All patients must also fulfill the standard eligibility criteria for undergoing preinterventional diagnostic such as contrast enhanced multiphase CT of the abdomen and perfusion CT of the liver. Table 1 shows the complete list of criteria.
Table 1.
Overall inclusion and exclusion criteria for the ClinicIMPPACT Trial (for both imaging and RFA).
| Inclusion criteria |
|
|
|
|
|
|
| Exclusion criteria |
|
|
|
|
|
|
2.3. Trial design and enrollment
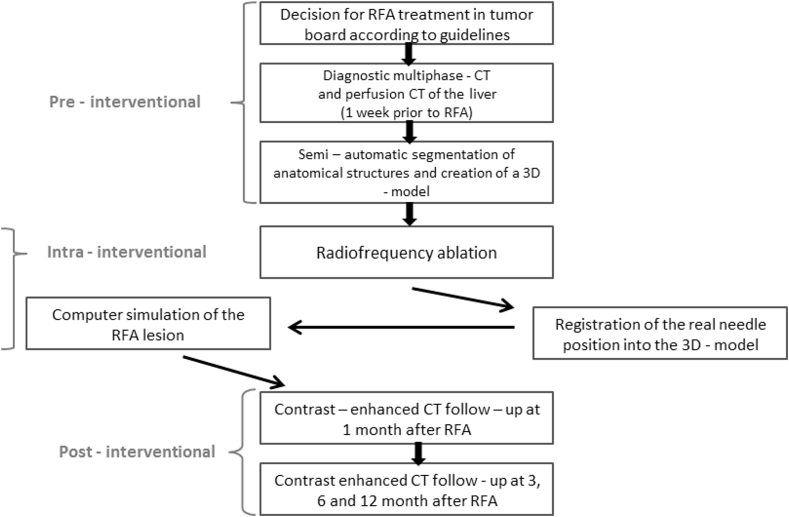
The trial flow chart and the visit schedule are given in Fig. 2 and Table 2, respectively. Each trial participant (15 per clinical site, 60 patients in total) requires a pre-existing primary or secondary tumor of the liver, preferably shown in two independent imaging modalities, alternatively histologically proven. Decisions on optimal therapies will be conducted through each local tumor board, which will evaluate options such as surgical resection, liver transplantation, adjuvant or neoadjuvant chemotherapy or image guided procedures such as RFA.
Fig. 2.
Flow chart of the ClinicIMPPACT Trial.
Table 2.
Visit schedule for ClinicIMPPACT Trial subjects.
| Follow-up | ||||||
|---|---|---|---|---|---|---|
| Treatment/Procedure | Visit 1(baseline) | Visit 2(RFA) | Visit 3(1 mo)a | Visit 4(3 mo)a | Visit 5(6 mo)a | Visit 6(12 mo)a |
| Informed consent | ● | |||||
| Inclusion criteria | ● | |||||
| Exclusion criteria | ● | |||||
| Demographic data | ● | |||||
| Physical examinationb | ● | ● | ||||
| Anamnesis | ● | ● | ||||
| Concomitant diseases | ● | ● | ||||
| CT (Soc) | ● | ● | ● | ● | ● | |
| Perfusion CT (Nsoc) | ● | |||||
| Complications | ● | ● | ● | ● | ● | |
| Concomitant medication | ● | ● | ||||
| Occurrence of tumor | ● (Soc) | ● (Soc) | ● (Soc) | ● (Soc) | ||
Nsoc = Not standard of care Soc = Standard of care.
Months after RFA.
Physical examination includes height, weight, blood parameters, Karnofsky – score, ECOG - status.
2.4. Pre-interventional diagnostic imaging
Preinterventional diagnostic imaging is substantial for a successful therapy of liver tumors [12], [13]. In this study, diagnostic imaging contains contrast enhanced multiphase abdominal CT and an additional perfusion scan of the liver. Even though MR imaging [14] is a promising and well established examination tool for liver disease, CT images are necessary for quantitative CT perfusion measurement, planning and segmentation prior to RFA and for image guidance as part of the intervention in this study.
Multiphase diagnostic CT imaging will be performed as part of the clinical routine comprising the parameters shown in Table 3. Patients should fast at least 4 h prior to the examination. Oral contrast agent will not be used. Rather, intravenous contrast agent will be injected at a flow rate of 3 ml/s (contrast agent ≥300mlg/ml) and CT scans will be conducted in the arterial, portal venous and venous contrasting phase. Total contrast volume will depend on body weight. All images will be acquired during breathold in expiration, with elevated arms, if possible.
Table 3.
Scan parameters for diagnostic CT imaging.
| Parameters | Native scan | Arterial phase | Portal venous phase | Venous phase |
|---|---|---|---|---|
| Coverage Area | Liver | Liver | Liver to Symphysis | Liver |
| Delay (s) | – | 15 | 45 | 60 |
| Collimation (mm) | (256) × 0.625 | (256) × 0.625 | (256) × 0.625 | (256) × 0.625 |
| Rotation time (s) | 0.75 | 0.75 | 0.75 | 0.75 |
| mAs | 180 | 220 | 250 | 250 |
| kV | 120 | 120 | 120 | 120 |
| Reconstruction (mm) | 2 | 2 | 2 | 2 |
| Increment (mm) | 0.5 | 0.5 | 0.5 | 0.5 |
2.4.1. Perfusion CT of the liver
It is known that perfusion is effectively a source of tissue cooling and may substantially affect heat transfer and ultimately the size of the induced thermal lesion [15]. The double blood supply of the liver can differ significantly between individuals, e.g. patients with liver cirrhosis show a higher flow in the hepatic artery while the portal venous flow is strongly reduced which leads to a lower overall perfusion [16], [17], compared to healthy liver tissue. Dynamic CT measurements after contrast administration (Perfusion CT, PCT) can be used to quantify tissue perfusion in the liver [17].
The IMPPACT project (Grant No. 223877, completed in February 2012) has shown that the inclusion of patient-specific perfusion values in the heat transfer calculation can substantially improve the accuracy of the simulated lesion size [18].
A dedicated imaging protocol (Table 4) had to be developed to meet a low radiation exposure with adequate image quality. Some of the key examination factors were:
-
(a)
The use of a peripheral venous catheter (preferably 18G) to enable a high injection rate (at least 6 ml/s);
-
(b)
A quick contrast injection (8–10 s bolus)
-
(c)
A small volume of contrast agent 40 ml followed by a 30-ml saline chaser;
-
(d)
Data acquisition under shallow breathing to improve patient comfort and reduce liver motion.
Table 4.
Parameters for perfusion CT imaging.
| Parameters | Native | Contrast-enhanced phase |
|---|---|---|
| Coverage area | Liver | Liver |
| Number of cycles | – | 30 |
| Interval between cycles (sec) | – | 1.5 |
| Collimation (mm) | (256) × 0.625 | – |
| kVp | 80 | 80 |
| mAs | 120 | 100 |
| Pitch | 0.933 | 0 |
| Slice thickness (2 mm) | 2 | 2.5 |
2.4.2. CT perfusion analysis
Over the past years, a number of different techniques, both in research as well as commercial tools, have been used to quantify liver perfusion with CT [17], [19]. Quantitative parameters such as arterial liver perfusion (ALP), portal venous perfusion (PVP), blood flow (BF), blood volume (BV), or capillary permeability can be derived from the time course of the contrast related signal increase depending on the specific mathematical method used, e.g. deconvolution [17] or maximum slope [20]. Results from different methods however, are not necessarily interchangeable as has recently been suggested in a study that analysed the same raw data [19].
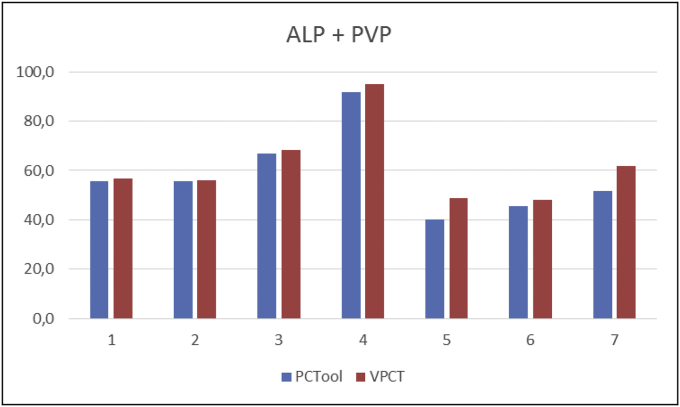
The four clinical partners of the ClinicIMPPACT project use CT scanners from three different manufactures (Siemens, Philips and Toshiba). Development of a common software tool for standardized CT data analysis was therefore essential [18]. Arterial and portal venous perfusion, both measured in ml/min/100 ml, were identified as the most important perfusion parameters related to the microcirculation in non-tumorous parenchyma. The maximum slope (MS) method was chosen because of several specific advantages, in particular its robustness, independence from model assumptions and efficient use of data over the first 40 s only.
The performance of the PCT tool was tested on seven perfusion data sets by comparing its results with those from a commercial maximum-slope analysis tool (VPCT, Siemens Healthcare, Erlangen, Germany). Regions of interest (ROI) were visually placed at the same positions with both tools. The comparison showed good absolute agreement of total liver perfusion between both software tools (Fig. 3). The PCT tool was therefore used for all further analyses within this study [18].
Fig. 3.
Comparison of total perfusion (ALP + PVP, in ml/min/100 ml) in normal liver parenchyma (average of three ROIs) in seven patients between custom made (PCTool) and commercial analysis software (VPCT, Siemens Healthcare).
2.5. Radiofrequency ablation
2.5.1. Real-time RFA prediction
Requires fast extraction and alignment of the heat sources during its execution. Since the 3D model of the liver is built offline from the preoperative CT data, the heat source (i.e., the needle) must be registered with this precomputed model during the RFA procedure.
For this project, we will rely on image based registration. Some algorithms are available to register interventional and preoperative liver data, using either rigid [22] or non-rigid transformations [23], [24]. The accuracy of rigid needle registration [25] tends to be higher than the accuracy limit of 5.0 mm agreed up on by the project medical partners. Thus, the project designed a novel registration method that offers both fast computing (<2 min) and accuracy (average absolute error < 5.0 mm).
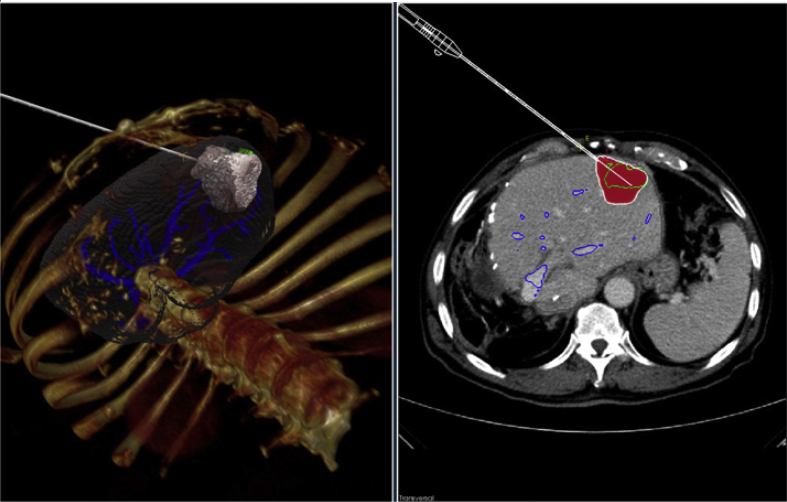
Real-time simulation of RFA cancer treatment requires fast solvers to predict heat transfer and cell death evolution, which can be nonlinear due to temperature dependencies, and therefore computationally expensive. In the IMPPACT project a prototype for RFA simulation [26] was developed, that fulfills the basic requirements of computer assisted intervention planning (screenshot: Fig. 4), and provides state of the art visualization for RFA. Other tools and prototypes use either simplified abstractions of the heating areas [27], [28] or heat distribution simulations which do not perform in accordance with our requirements regarding precision [29], [30]. The earlier IMPPACT tool derived from the initial project was the first to consider cell death instead of simple heat approximations for its simulation. However, this tool was unable to perform real-time simulations, actual planning of a treatment could take several hours. Needle positions, which may vary during the real intervention from the planned procedure and changes in the patients' anatomical conditions, cannot be treated correctly with the currently available tools.
Fig. 4.
Prototype for a clinical interface that was developed during the initial IMPPACT project. The needle position and RFA protocol can be selected to provide a patient-specific simulation of the predicted cell death. This simulation usually took several hours, which made its clinical use impractical.
The complete refinement of the presented RFA simulation tool has been currently finalized. Different data representations have to be combined and processed upon the start of the clinical study. Processing such different data modalities in a single algorithm is called “multivariate data processing.” ClinicIMPPACT will therefore improve the field of multivariate data processing with real-time measures to visualize combined (structured) clinical CT data, (unstructured) simulation data and surface based segmentation data in a single first step. This can be achieved by dedicated methods, using a modern graphics processing unit (GPU) [9], [31], [32].
The aim is to study the optimal balance between uncertainty awareness and multivariate data representation in an initial study, and refine our methods during the clinical phase of this project. Thereby the current state of medical uncertainty visualization will be enhanced and the conveyance of additional information for already complex base visualizations will be improved.
2.5.2. Clinical RFA intervention
The clinical protocol for RFA includes a sequence of steps and procedures, i.e. patient data acquisition, data analysis, intervention procedures, etc. (Table 5), carried out by an experiences IR at all four clinical sites. The RFA software tools are designed to support IRs at each step of the RFA, and will be installed locally on a server in the intervention room. As the software is optimized to suit the standard clinical RFA procedure, no changes are necessary to the clinical workflow. Every procedure will be documented by a specific checklist/case report form that covers the patients' details, size and number of lesions, etc.
Table 5.
Clinical RFA algorithm.
| Step | Clinical protocol |
|---|---|
| 1 | Interventional native planning CT for tumor localization and access planning |
| 2 | CT-guided needle insertion |
| 3 | Control CT scan in end-expiratory apnea to visualize deployed needle |
| 4 | Registration of the real needle position into the 3D model |
| 5 | Computer simulation of the RFA lesion |
| 6 | Selection of the appropriate therapy protocol by the IR and start of RFA independent of the simulation results |
| 7 | Immediate acquisition of control CT with CE in end-expiratory apnea |
| I. Lesion not adequately ablated (repeat from step 4 in case of needle replacement) | |
| II. Adequate ablation (move on to step 8) | |
| 8 | Removal of probe (while ablating track); termination of anesthesia |
First, a non-enhanced CT scan with the patient under full anesthesia will be performed to localize tumors and plan the optimal needle access. As the IR inserts the needle under CT guidance, in-room visualization of the 3D model begins. Once the IR is satisfied with the needle position, the hooks are completely deployed and a non-enhanced CT scan (breathing being temporarily held in expiration) is performed. The IR will confirm that the deployed needle is in the proper treatment position on CT scans; CT data is then transferred to the external PC for fast needle registration, which semi-automatically registers the needle into the 3D model [10].
The next step is the simultaneous start of the RFA and the computer simulation of the lesion, following a standard or a modified ablation protocol (Table 6). Optionally, after the intervention, a control CT with contrast agent is performed in breath-hold technique. In case the lesion is not sufficiently treated, needle adjustment and additional ablation will be performed under CT-guidance. If the ablation result is sufficient, the needle is removed by ablating its track and the treatment is finished.
Table 6.
Ablation protocol for 5-cm ablation (RITA Medical, Boston Scientific).
| Deploy to | Target temp | Power | Timer | Heating duration |
|---|---|---|---|---|
| 2 cm | 105 °C | 150 W | 15 min | Until target temp. is reached, then deploy to 3 cm |
| 3 cm | 105 °C | 150 W | 14.5 min | Until target temp. is reached, then deploy to 4 cm |
| 4 cm | 105 °C | 150 W | 14 min | 7 min after target temp (i.e., after hearing the beep, wait 7 min), then deploy to 5 cm |
| 5 cm | 105 °C | 150 W | 7 min | 7 min after target temp (i.e., after hearing the beep wait 7 min), then deploy cool down |
2.6. Follow up imaging
Follow up imaging will be performed 1, 3, 6, and 12 months after RFA via multiphase CT (non-enhanced, arterial, portal venous, venous) for HCC lesions or by monophasic CT (portal venous) for, e.g., (colorectal) metastases in expiration. All CT images, whether pre-, peri- or postinterventional (i.e. follow up) will be evaluated by radiologists with more than 10 years of experience in abdominal imaging, at each participating medical site.
2.7. Stopping rules
Patients may withdraw their consent to participate at any time without giving reasons. However, these patients should be asked for their reasons for premature termination after being informed that he/she does not need to do so. Information as to when and why a patient was registered and when he/she withdrew consent must be retained in the documentation. Patients are to be informed that if they revoke consent the stored data may be used further, as the data may be necessary to (a) assess effects of the software being tested; (b) guarantee that the patients' personal interests are not adversely affected; and (c) comply with the requirement to provide complete authorization documentation.
Furthermore, the trial can be terminated prematurely by the coordinating investigator in the event of (a) serious adverse events; (b) changes in the risk/benefit considerations, e.g., as a result of unexpected adverse events; (c) new insights from other trials; or (d) an insufficient recruitment rate. The final decision regarding the premature termination of the trial will be made by the coordinating investigator or his/her authorized representative.
Data captured in the clinical trial, which cannot be used for trial analyses due to a patients' premature trial termination may be used for clinical decisions (such as staging), if this is beneficial to the patients' treatment. Premature trial terminations must be documented, as precisely as possible, with the date and (if possible) reasons and circumstances and submitted to the data management and the coordinating investigator as soon as possible.
As follow up care is based on the guidelines for liver carcinoma treatment and is not part of this trial, premature terminations of follow ups can be neglected. A potential reason for premature termination of follow up might the need for a patient to travel long distances to the trial site. In such cases, we will attempt to obtain the CT data from the patients' treating radiology department (pending the patients' consent).
2.8. Objectives
The primary objective of this study is the comparison of the size and shape of the real ablation zone one month after a RFA treatment of liver tumors with the simulation results of the ClinicIMPPACT software. This information will help evaluate the accuracy of the prediction.
The secondary objectives of this study are the evaluation of the workflow steps and the clinical feasibility of the ClinicIMPPACT procedure. We will also analyze if there is a potential benefit for the patient, i.e. if the simulation result would have been known by the treating IR, would it have influenced the treatment protocol? By design the IRs were not allowed to take the simulation results into consideration during the intervention.
2.9. Statistical analysis
This trial aims at the collection of pilot clinical data, not to verify a specific hypothesis. Hence it is not possible to determine a sample size in the traditional sense by specifying a concrete alternative hypothesis and requiring a certain power. For the primary endpoint, we will compare lesions, on a level of volume- and surface deviation, visualized by routine CT one month after an ablation with their simulated counterparts to define the accuracy of the method itself. The coinciding volumes of the real RFA lesion and the simulated one will be determined by counting the number of matching voxels, i.e. voxels of simulation and recorded data, sharing the space coordinates, and dividing by the sum of the voxels of simulated and real lesions.
Normally distributed data will be described with means and standard deviations and skewed variables will be described with medians and interquartile ranges. Paired t-tests will used to assess differences between simulated and real ablation lesion volumes. The contributions of factors such as cirrhosis, ablation duration, tissue perfusion, and tumor size to volume and surface deviations will be investigated in separate multivariate linear regression models. Effect sizes of multivariate linear regression models will be given as mean ± standard deviation and [95% confidence limits].
To define the accuracy of the simulation as a parameter, we introduce the following categories:
Based on the ratio of segmented to simulated lesion volume the results are categorized as follows:
-
a)
-
Iseg/sim <60% (much smaller)
-
II60% ≤ seg/sim <80% (smaller)
-
III≤80% seg/sim ≤120% (comparable)
-
IV120% < seg/sim ≤140% (larger)
-
Vseg/sim >140% (much larger)
-
I
-
b)If the simulated lesion is comparable with the real lesion based on a), we further investigate the accuracy based on the following two metrics namely, sensitivity (SN) and average absolute error. We introduce the rating level between 1 and 5 for the simulated lesion based on two metrics as explained below. Simulation rating based on average absolute error:
-
1If average absolute error >5
-
2If 4 < average absolute error ≤5
-
3If 3.5 < average absolute error ≤4
-
4If 3 < average absolute error ≤3.5
-
5If average absolute error ≤3
-
1
Here, the average absolute error is computed by trilinear interpolation from the distance level set at the center of the face and weighted over the area of the triangle face, per-vertex metrics have equal weight. Sensitivity is defined as the ratio between common volume of the segmented and simulated lesions to the segmented lesion volume.
Simulation rating (based on volume deviation):
-
1
If SN ≤ 50
-
2
If 50 < SN ≤ 60
-
3
If 60 < SN ≤ 70
-
4
If 70 < SN ≤ 80
-
5
If SN > 80
-
c)We take the average of the rating level obtained from the average absolute error and sensitivity. If the average rating level is:
-
I< 3, then real and simulated lesion strongly differ.
-
II≥ 3, then real and simulation do not differ strongly.
-
I
Further details on lesion comparison are described elsewhere [9].
Secondary endpoints are.
-
a)Efficiency of workflow steps
-
IProof of the procedures feasibility
-
•Is the procedure stable enough for clinical routine (yes/no)?
-
•
-
IIDuration of the simulation
-
•Would it have been short enough to be feasible in clinical practice (yes/no)?
-
•
-
I
-
b)Would the treatment protocol have been influenced by the simulation results if they would have been known prior to the treatment.
-
IInfluence Yes/No
-
•If yes, is there an expectable potential benefit for the patient due to a modification of the treatment protocol?
-
•
-
IIDoes the follow up (3, 6, 12 months) imaging support these assumptions regarding local tumor control
-
•The tumor is completely treated with sufficient safety margins; healthy tissue has been largely spared by the ablation and there is no loco regional recurrence visible in the follow up imaging (= loco regional recurrence free survival).
-
•The tumor (including safety margins) is completely treated, but lots of healthy tissue has been damaged with the risk of serious complications.
-
•The tumor is treated incompletely or there is a visible recurrent tumor in the follow up examination.
-
•
-
I
3. Discussion
We introduced the first prospective multicenter clinical trial for the evaluation of a software application, developed to predict RFA induced hepatic tissue necrosis in patients with early liver cancer [9], [10]. After developing a prototype in the European Union - funded IMPPACT project we carried out comprehensive improvement of the usability (e.g. performance, system stability, and ergonomics), integration of patient specific perfusion data, and adaption to the clinical workflow. The aim of the proposed prospective multicenter phase 2b exploration clinical trial (ClinicIMPPACT, according IDEAL criteria – 36) is to determine the accuracy of the lesion prediction and the effectiveness of the software tool to assist interventional radiologist in clinical routine - radiofrequency ablation of liver tumors.
RFA nowadays serves as a common first- and second line treatment for several malignancies in different organ systems, including liver, kidney, lung, and bone. Successful treatment of neoplastic lesions is defined by complete tumor destruction, which can be evaluated by follow up imaging (mainly Ultrasound, MRI, and CT, usually performed 1 month after treatment). Monitoring the size of tissue necrosis during the RFA-procedure however is a challenging task and there is currently no reliable method commercially available. Thus, there is a significant rate of under- and overtreatment after RFA using the standard protocols recommended by the manufactures. Overtreatment potentially compromises sensitive surrounding structures and increases the complication rate. Under treatment leads to incomplete tumor destruction and subsequently to tumor recurrence. Hence, regularly the interventionist modifies the standard treatment protocol according to his/her experience. It can be assumed, that this might be the reason, why patient outcomes are strongly dependent on the experience of the interventionist [33]. Despite new developments in depiction and prediction of the ablation zone remains difficult. To overcome this specific problem, accurate simulation of the RFA lesion before ablation will potentially improve treatment outcome and will elevate the learning curve for less experienced interventionists.
To better understand the complex mechanism of heat induced tissue necrosis, extensive cell and animal experiments were performed in the previous project: IMPPACT, The 7th Framework Program for Research of the European Union. These results were used to establish a cell death equation and to engineer a dedicated algorithm to compute the tissue necrosis in a patient specific computer model [34]. During IMPPACT, mismatches between simulated and real lesions were seen for some lesion shapes, which could be explained by errors in the segmentation of radiological images. Data processing took about 5 h, and was therefore not feasible for intraprocedural clinical use. Therefore, a clinical adaptation of the model required several improvements related to predictive power, ergonomics, computational performance and the seamless integration into the clinical workflow. Several individual factors are affected by inner body structures (blood flow, liver tissue properties, tumor type and perfusion, proximity to vessels and liver surface, etc.) that substantially affect the size and configuration of the ablated lesion. None of these factors are taken into account in the available manufacturers' ablation protocols. We believe that based on the results of the ClinicIMPPACT project the available simulation tool could be optimized for clinical use and might potentially predict the size and shape of a RFA treatment precisely. Using a dedicated RFA protocol and including patient specific data (e.g. liver perfusion quantification data) will further improve the simulation outcomes.
Compared to the first prototype, the software has been significantly improved in regards to processing speed, ergonomical use, and it now adequately respects the clinical workflow. Therefore, the highly complex simulation algorithm of the previous project was optimized and accelerated with a newly developed numerical solver to fulfill clinical time restrictions.
Preliminary validations with patient data from clinical routine in the four medical institutions participating in the projects show that the graphical user interface is “physician friendly” and runs stable (9,10). The calculation times to prepare the patients 3D model are feasible for the users. There are several reports about software tools assisting thermal ablation of liver tumors. Some of them support treatment planning and placing of the probe [35], [36]. Others allows simulation of the thermal necrosis and cooling effects due to vessels with numerical models and fast approximations [37].
Schumann et al. report an evaluation of the suitability of these methods in a retrospective clinical study [30]. For a precise simulation of the ablation zone after RFA multiple criteria have to be considered. Unfortunately, in clinical routine several of these parameters, e.g. the precise localization of the probe and the full ablation protocol or individual perfusion values of the liver are not documented. Due to this fact, a retrospective evaluation is always prone to error. To the best of the author's knowledge there is no validation of accuracy and efficiency of software for prediction of RFA-induced necrosis in the liver in a prospective clinical trial. The primary endpoint of the proposed phase 2a clinical trial will be quantification of the accuracy of the method. Secondary endpoints will investigate the functionality of the application in various clinical workflow environments. One potential drawback of the study is the lack of a randomized control group, which represents an imposed restriction from the institutional review board. We will address this issue by the analysis of a matched, retrospective control group, e.g. comparing intervention durations.
Once reliable accuracy of the prediction and functionality of the overall system is assured CE-marking will be requested. The clinically validated and certified tool will potentially help to gain wider acceptance by the radiology community and might be implemented into future routine RFA of liver tumors. Further randomized clinical trials are needed to test whether the use of this tool improves patient outcome.
Finally, an adaption of this tool could be evaluated for the minimal invasive treatments of cancers in other organs with different techniques (e.g. cryoablation and microwave ablation).
In summary, this first of its kind multicenter clinical trial aims at the clinical integration of a dedicated software solution to accurately predict lesion size and shape after radiofrequency ablation of liver tumors. As mispredictions of true ablation zones can lead to increased tumor recurrence due to under treatment or complications due to overtreatment this tool will be of great interest to the interventional radiologist community.
Conflict of interest
The authors declare no conflict of interests.
Acknowledgments
The research leading to these results has received funding from the European Community's Seventh Framework Program under grant agreement no. 610886, project ClinicIMPPACT and grant agreement no. 600641, project GoSmart. Furthermore we would like to thank and name all associated investigators: Martin van Amerongen, Jan Egger, Jukka Ilari, Philipp Stiegler, Horst Portugaller, Dieter Schmalstieg, Mark Dokter, Phil Weir, Nikita Garnov, Tuomas Alhonnoro, Miko Lilja and Bianca Schmerböck.
References
- 1.Hsu C.-Y., Shen Y.-C., Yu C.-W., Hsu C., Hu F.-C., Hsu C.-H. Dynamic contrast-enhanced magnetic resonance imaging biomarkers predict survival and response in hepatocellular carcinoma patients treated with sorafenib and metronomic tegafur/uracil. J. Hepatol. 2011;55(4):858–865. doi: 10.1016/j.jhep.2011.01.032. Oct. [DOI] [PubMed] [Google Scholar]
- 2.Bruix J., Sherman M. Practice guidelines committee, American association for the study of liver diseases. Manage. Hepatocell. Carcinoma. Hepatol. Balt. Md. 2005;42(5):1208–1236. doi: 10.1002/hep.20933. Nov. [DOI] [PubMed] [Google Scholar]
- 3.Hung A.K., Guy J. Hepatocellular carcinoma in the elderly: meta-analysis and systematic literature review. World J. Gastroenterol. 2015 Nov 14;21(42):12197–12210. doi: 10.3748/wjg.v21.i42.12197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Attwa M.H., El-Etreby S.A. Guide for diagnosis and treatment of hepatocellular carcinoma. World J. Hepatol. 2015 Jun 28;7(12):1632–1651. doi: 10.4254/wjh.v7.i12.1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thandassery R.B., Goenka U., Goenka M.K. Role of local ablative therapy for hepatocellular carcinoma. J. Clin. Exp. Hepatol. 2014;4(Suppl 3):S104–S111. doi: 10.1016/j.jceh.2014.03.046. Aug. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crocetti L., de Baere T., Lencioni R. Quality improvement guidelines for radiofrequency ablation of liver tumours. Cardiovasc. Interv. Radiol. 2010 Feb;33(1):11–17. doi: 10.1007/s00270-009-9736-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wong S.L., Mangu P.B., Choti M.A., Crocenzi T.S., Dodd G.D., Dorfman G.S. American Society of Clinical Oncology 2009 clinical evidence review on radiofrequency ablation of hepatic metastases from colorectal cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2010;28(3):493–508. doi: 10.1200/JCO.2009.23.4450. Jan 20. [DOI] [PubMed] [Google Scholar]
- 8.Fukuhara T., Aikata H., Hyogo H., Honda Y., Morio K., Morio R. Efficacy of radiofrequency ablation for initial recurrent hepatocellular carcinoma after curative treatment: comparison with primary cases. Eur. J. Radiol. 2015;84(8):1540–1545. doi: 10.1016/j.ejrad.2015.04.020. Aug. [DOI] [PubMed] [Google Scholar]
- 9.Mariappan P., Weir P., Flanagan R., Voglreiter P., Alhonnoro T., Pollari M. GPU-based RFA simulation for minimally invasive cancer treatment of liver tumours. Int. J. Comput. Assist. Radiol. Surg. 2017;12(1):59–68. doi: 10.1007/s11548-016-1469-1. Jan. [DOI] [PubMed] [Google Scholar]
- 10.Voglreiter P., Mariappan P., Alhonnoro T., Busse H., Weir P., Pollari M., Flanagan R., Hofmann M., D, Seider, Brandmaier P., van Amerongen M.J., Rautio R., Jenniskens S., Blanco Sequeiros R., Portugaller R.H., Stiegler P., Futterer J., Schmalstieg D., Kolesnik M., Moche M. RFA guardian: comprehensive simulation of the clinical workflow for patient specific planning, guidance and validation of RFA treatment of liver tumors. Int. J. Comput. Assist. Radiol. Surg. 2016;11(Suppl. 1):1–286. June. [Google Scholar]
- 11.Moche M., Seider D., Flanagan R., Bost C., Payne S.J., Meng, O'Neill D.P. Oral presentation at RSNA; Chicago, U.S.A: 2011. The Development of a Simulation Tool for Clinical Use in the Radio-frequency Ablation (RFA) of Liver Cancer. December. [Google Scholar]
- 12.Choi J.-Y., Lee J.-M., Sirlin C.B. CT and MR imaging diagnosis and staging of hepatocellular carcinoma: part II. Extracellular agents, hepatobiliary agents, and ancillary imaging features. Radiology. 2014;273(1):30–50. doi: 10.1148/radiol.14132362. Oct. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ronot M., Vilgrain V. Hepatocellular carcinoma: diagnostic criteria by imaging techniques. Best. Pract. Res. Clin. Gastroenterol. 2014;28(5):795–812. doi: 10.1016/j.bpg.2014.08.005. Oct. [DOI] [PubMed] [Google Scholar]
- 14.Chen L., Zhang L., Liang M., Bao J., Zhang J., Xia Y. Magnetic resonance imaging with gadoxetic acid disodium for the detection of hepatocellular carcinoma: a meta-analysis of 18 studies. Acad. Radiol. 2014 Dec;21(12):1603–1613. doi: 10.1016/j.acra.2014.08.003. [DOI] [PubMed] [Google Scholar]
- 15.Lu X., Kikuchi H., Hirooka K., Isobe Y., Watanabe H., Kobayashi Y. Conf Proc Annu Int Conf IEEE Eng Med Biol Soc IEEE Eng Med Biol Soc Annu Conf. 2015. Method for estimating the temperature distribution associated with the vessel cooling effect in radio frequency ablation; pp. 4836–4839. 2015 Aug. [DOI] [PubMed] [Google Scholar]
- 16.Wu D., Tan M., Zhou M., Sun H., Ji Y., Chen L. Liver computed tomographic perfusion in the assessment of microvascular invasion in patients with small hepatocellular carcinoma. Invest. Radiol. 2015;50(4):188–194. doi: 10.1097/RLI.0000000000000098. Apr. [DOI] [PubMed] [Google Scholar]
- 17.Kim S.H., Kamaya A., Willmann J.K. CT perfusion of the liver: principles and applications in oncology. Radiology. 2014;272(2):322–344. doi: 10.1148/radiol.14130091. Aug. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Amerongen M.J., Garnov N., Jenniskens S.F.M., Alhonnoro T., Pollari M. Proceedings of the European Congress of Radiology. 2016. Liver CT perfusion values for colorectal liver metastases – a protocol for future use in radiofrequency simulation software. Vienna, Austria; C-1661. [Google Scholar]
- 19.Mazzei M.A., Squitieri N.C., Sani E., Guerrini S., Imbriaco G., Di Lucia D. Differences in perfusion CT parameter values with commercial software upgrades: a preliminary report about algorithm consistency and stability. Acta Radiol. Stockh. Swed. 1987;54(7):805–811. doi: 10.1177/0284185113484643. 2013 Sep. [DOI] [PubMed] [Google Scholar]
- 20.Burton K.R., Dhanoa D., Aviv R.I., Moody A.R., Kapral M.K., Laupacis A. Perfusion CT for selecting patients with acute ischemic stroke for intravenous thrombolytic therapy. Radiology. 2015;274(1):103–114. doi: 10.1148/radiol.14140728. Jan. [DOI] [PubMed] [Google Scholar]
- 22.Carrillo A., Duerk J.L., Lewin J.S., Wilson D.L. Semiautomatic 3-D image registration as applied to interventional MRI liver cancer treatment. IEEE Trans. Med. Imaging. 2000;19(3):175–185. doi: 10.1109/42.845176. Mar. [DOI] [PubMed] [Google Scholar]
- 23.Archip N., Tatli S., Morrison P., Jolesz F., Warfield S.K., Silverman S. Non-rigid registration of pre-procedural MR images with intra-procedural unenhanced CT images for improved targeting of tumors during liver radiofrequency ablations. Med. Image Comput. Comput-Assist Interv. MICCAI Int. Conf. Med. Image Comput. Comput-Assist Interv. 2007;10(Pt 2):969–977. doi: 10.1007/978-3-540-75759-7_117. [DOI] [PubMed] [Google Scholar]
- 24.Elhawary H., Oguro S., Tuncali K., Morrison P.R., Shyn P.B., Tatli S. Intra-operative multimodal non-rigid registration of the liver for navigated tumor ablation. Med. Image Comput. Comput-Assist Interv. MICCAI Int. Conf. Med. Image Comput. Comput-Assist Interv. 2009;12(Pt 1):837–844. doi: 10.1007/978-3-642-04268-3_103. [DOI] [PubMed] [Google Scholar]
- 25.Wilson D.L., Carrillo A., Zheng L., Genc A., Duerk J.L., Lewin J.S. Evaluation of 3D image registration as applied to MR-guided thermal treatment of liver cancer. J. Magn. Reson Imaging JMRI. 1998;8(1):77–84. doi: 10.1002/jmri.1880080117. Feb. [DOI] [PubMed] [Google Scholar]
- 26.Kerbl Bernhard. Springer Berl Heidelb; 2012. Intervention Planning of Hepatocellular Carcinoma Radio-frequency Ablations. [Google Scholar]
- 27.Butz Torsten. Springer Berl Heidelb; 2000. Pre-and Intra-operative Planning and Simulation of Percutaneous Tumor Ablation. [Google Scholar]
- 28.Villard C., Soler L., Gangi A. Radiofrequency ablation of hepatic tumors: simulation, planning, and contribution of virtual reality and haptics. Comput. Methods Biomech. Biomed. Engin. 2005 Aug;8(4):215–227. doi: 10.1080/10255840500289988. [DOI] [PubMed] [Google Scholar]
- 29.Rieder C., Kröger T., Schumann C., Hahn H.K. GPU-based real-time approximation of the ablation zone for radiofrequency ablation. IEEE Trans. Vis. Comput. Graph. 2011;17(12):1812–1821. doi: 10.1109/TVCG.2011.207. Dec. [DOI] [PubMed] [Google Scholar]
- 30.Schumann C., Rieder C., Haase S., Teichert K., Süss P., Isfort P. Interactive multi-criteria planning for radiofrequency ablation. Int. J. Comput. Assist. Radiol. Surg. 2015;10(6):879–889. doi: 10.1007/s11548-015-1201-6. Jun. [DOI] [PubMed] [Google Scholar]
- 31.Rieder Christian. A shader Framework for rapid prototyping of GPU-based volume rendering. Comput. Graph. Forum. 2011;30(3) Blackwell Publishing Ltd. [Google Scholar]
- 32.Steinberger, Markus, et al. Softshell: Dynamic Scheduling on GPUs.
- 33.Hildebrand P., Leibecke T., Kleemann M., Mirow L., Birth M., Bruch H.P. Influence of operator experience in radiofrequency ablation of malignant liver tumours on treatment outcome. Eur. J. Surg. Oncol. J. Eur. Soc. Surg. Oncol. Br. Assoc. Surg. Oncol. 2006;32(4):430–434. doi: 10.1016/j.ejso.2006.01.006. May. [DOI] [PubMed] [Google Scholar]
- 34.Payne S., Flanagan R., Pollari M., Alhonnoro T., Bost C., O'Neill D. Image-based multi-scale modelling and validation of radio-frequency ablation in liver tumours. Philos. Trans. A Math. Phys. Eng. Sci. 2011;369(1954):4233–4254. doi: 10.1098/rsta.2011.0240. Nov 13. [DOI] [PubMed] [Google Scholar]
- 35.McCreedy E.S., Cheng R., Hemler P.F., Viswanathan A., Wood B.J., McAuliffe M.J. Radio frequency ablation registration, segmentation, and fusion tool. IEEE Trans. Inf. Technol. Biomed. Publ. IEEE Eng. Med. Biol. Soc. 2006;10(3):490–496. doi: 10.1109/titb.2006.872076. Jul. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Seitel A., Engel M., Sommer C.M., Radeleff B.A., Essert-Villard C., Baegert C. Computer-assisted trajectory planning for percutaneous needle insertions. Med. Phys. 2011;38(6):3246–3259. doi: 10.1118/1.3590374. Jun. [DOI] [PubMed] [Google Scholar]
- 37.Kröger T., Pätz T., Altrogge I., Schenk A., Lehmann K.S., Frericks B.B. Fast estimation of the vascular cooling in RFA based on numerical simulation. Open Biomed. Eng. J. 2010;4:16–26. doi: 10.2174/1874120701004020016. Feb 4. [DOI] [PMC free article] [PubMed] [Google Scholar]