Abstract
Introduction
Crotoxin has a broad antitumor activity but has shown frequent neurotoxic toxicity. To induce tolerance and limit this toxicity, we propose a new design with intra-patient dose escalation.
Methods
A new Dose Limiting Toxicity definition was used. The concept of Target Ceiling Dose was introduced.
Results
Dose Limiting Toxicity was the inability to dose escalate twice. Target Ceiling Dose was the highest planned dose to be administered to a patient and could change for patients along time. Recommended Dose was defined similarly as in a (3 + 3) conventional design.
Conclusion
This innovant design was used and the clinical trial is now closed for inclusions. Results will be presented later.
Keywords: Clinical trial, Phase 1, Intra-patient dose escalation, Cancer
1. Introduction
Crotoxin is secreted in the venom of some South American rattlesnakes. Crotoxin has a significant and broad spectrum antitumor activity in vitro and in vivo. Its affinity for tumor cells is thought to be related to the target Crocalbine, a transmembrane protein overexpressed in many tumor cells, notably lung cancer, central nervous system tumors and melanoma [1]. Attachment to the Crocalbine receptor released Crotactine where it has a disruptive effect on the cell membrane, causing a liberation of arachidonic acid that indirectly activates caspases responsible for apoptosis.
The sensitivity of tumor cells to Crotoxin was associated with the expression of the epidermal growth factor receptor, a membrane receptor associated with a malignant cell phenotype [2], [3].
The Crotoxin has already been tested in clinical trial, in 23 patients with treatment resistant cancers. It was administered intramuscularly. The maximum tolerated dose was determined to 0.21 mg/m2. The main toxicity was grade 1 or 2 neuromuscular effects, presenting in 78% of patients (n = 18/23) (diplopia, strabismus, nystagmus and eyelid ptosis), that was reversible. One case of grade 3 anaphylaxis was observed (4%). Biologically, increases in liver transaminases and creatinine clearance of grade 1–2 levels were reported in 56% of patients (13/23) [4].
In terms of efficacy, 17% of patients (4/23) had a decrease in tumor burden or partial responses using imagery in addition to an improvement in pain levels and quality of life.
Intramuscular administration was responsible for local immune reactions with erythema, itching or pain, which could last from 24 h to three weeks. These effects subsided over several weeks. Intravenous administration of Crotoxin could improve the local tolerance.
Moreover, the administration of gradually increasing doses of Crotoxin could induce tolerance to its neurotoxic effects without losing antitumor activity. The administration of potentially lethal doses was possible if dose escalation was implemented. The administration of doses 20 to 35 times the original 50% lethal dosage (LD50) to mice made tolerant to Crotoxin was achieved without toxicity [5].
To explore these two hypotheses, we designed a clinical trial with intravenous administration of Crotoxin and intra-patient dose escalation. The purpose of this article is to present the design of this Phase I trial. The clinical results will be published elsewhere.
2. Method
A Phase I unicentric clinical trial was set up at the Georges Pompidou European Hospital, Paris, France. This trial was performed in patients with stage IV cancer.
The primary endpoint was toxicity, measured by Dose-Limiting Toxicity (DLT) of Crotoxin administered intravenously using an intra-patient dose escalation. Determination of Recommended Dose (RD) was planned.
The secondary objective was the anti-tumor efficacy, evaluated according to radiological RECIST criteria 1.0.
The need for an intra-patient dose escalation with a product inducing tolerance is not common in cancer and required the creation of two innovations: a new definition of DLT and for a new design for the RD, using the innovating concept of the Target Ceiling Dose (TCD), knowing that each patient was included at the lowest dose level.
3. Results
3.1. Study design
The proposed dosing schedule and duration was based on the pre-clinical toxicity studies [6], [7] and the previous clinical experience outlined in human studies [8], [9]. The dosing increments described for the Cura et al. study were 0.03, 0.06, 0.12, and 0.18 mg/m2. The current trial protocol proposed doses of 0.04–0.32 mg/m2 (0.0012–0.01 mg/kg), which were 12.5 and 1.5 fold, below the NOEL dose in mice [10]. No toxicities were reported in the Cura et al. (2002) Phase I study at doses up to 0.12 mg/m2 [4].
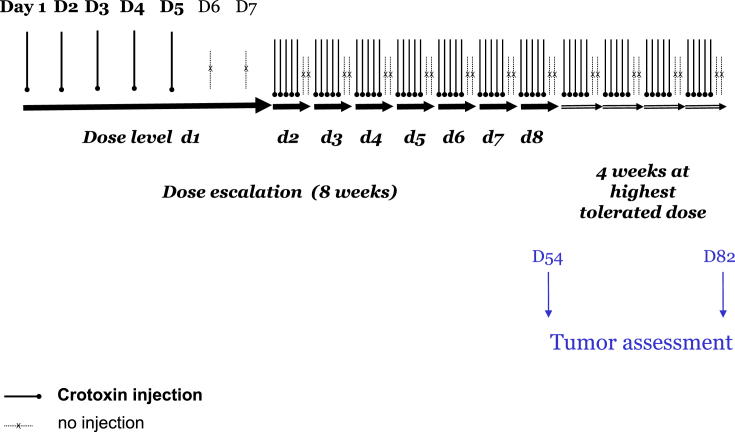
Each dose was initially administered for 5 consecutive days with 2 days break over the week-end (Table 1). Five days period was chosen because of the short half-life of Crotoxin (24 h after the injection (approximately 5 half-life) 97% of the product was eliminated) and because of the lack of cumulative toxicity. Crotoxin was administered daily by intravenous administration over a 2-hour period by saline drip. Patients received increasing doses over the course of 40 treatment days (8 dose levels). Intra-patient dose escalation was mandatory. Table 1 presents the dose escalation schema. Highest dose level was 0.32 mg/m2.
Table 1.
COHORT I: dose escalation levels for CROTOXIN.
| Dose Level | CROTOXIN Daily Dose | Days (starting Monday), no treatment on weekends | Duration (days) |
|---|---|---|---|
| d1 | 0.04 mg/m2/day | 1–5 | 5 |
| d2 | 0.08 mg/m2/day | 8–12 | 5 |
| d3 | 0.12 mg/m2/day | 15–19 | 5 |
| d4 | 0.16 mg/m2/day | 22–26 | 5 |
| d5 | 0.20 mg/m2/day | 29–33 | 5 |
| d6 | 0.24 mg/m2/day | 36–40 | 5 |
| d7 | 0.28 mg/m2/day | 43–47 | 5 |
| d8 | 0.32 mg/m2/day | 50–54 | 5 |
The patient was offered to continue on the highest tolerated dose for another 4 weeks, subject to clinical assessments from the treating physician.
Tumor assessment was performed to assess potential efficacy of the tested compound after the 8 weeks of the dose escalation (day 54) and after the optional extra 4 weeks (day 82) (see Fig. 1).
Fig. 1.
Study design.
3.2. Definition of dose-limiting toxicity
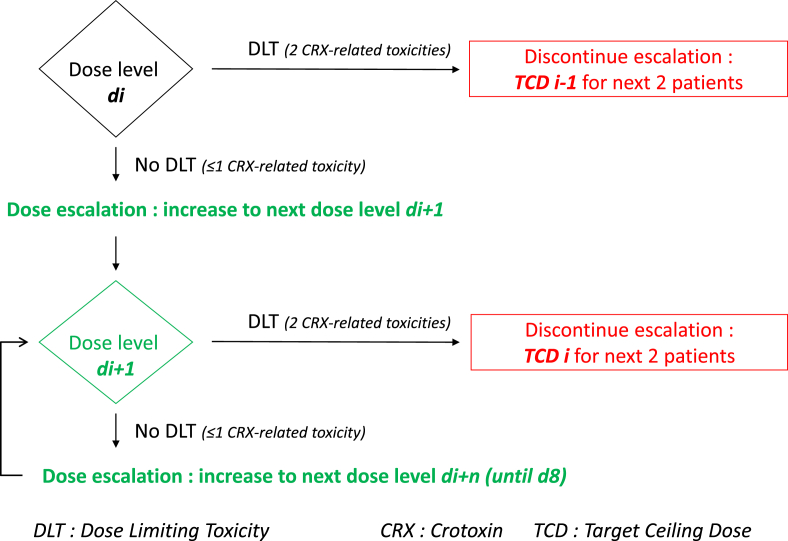
Definition of Dose-Limiting Toxicity (DLT) was described as inability to dose escalate twice in one patient. Inability was defined by the occurrence of grade 2 or 3 ocular/visual (palpebral ptosis, nystagmus, diplopia) or anxiety or hypertension events related to Crotoxin and not recovered within 24 h at a given dose.
If the patient presented a drug-related inability to dose escalate at a dose level di, he received previous dose level (di-1) for another cycle (5 days) upon which 2nd dose escalation was attempted at the di dose. If he presented a new drug-related inability to dose escalate after 2nd dose escalation attempt, the escalation was abandoned for this patient and the dose to which escalation was made (di-1) was assessed as DLT for this patient. It defined the highest planned dose to be administered to the next patients also called Target Ceiling Dose (TCDi-1). We allowed dose re-escalation because of the pharmacological properties of Crotoxin. First, because neurological tolerance was demonstrated: we hypothesized that a longer time of exposition at a lower dose might diminish the neurotoxicity at a more elevated dose. Second, because the half-life of Crotoxin is short, around 5 h, we supposed that even in case of a first occurrence of neurotoxicity, a second occurrence would be rapidly reversible (see Fig. 2).
Fig. 2.
Intra-patient dose escalation and Dose Limiting Toxicity.
3.3. Definition of the target ceiling dose and of the recommended dose
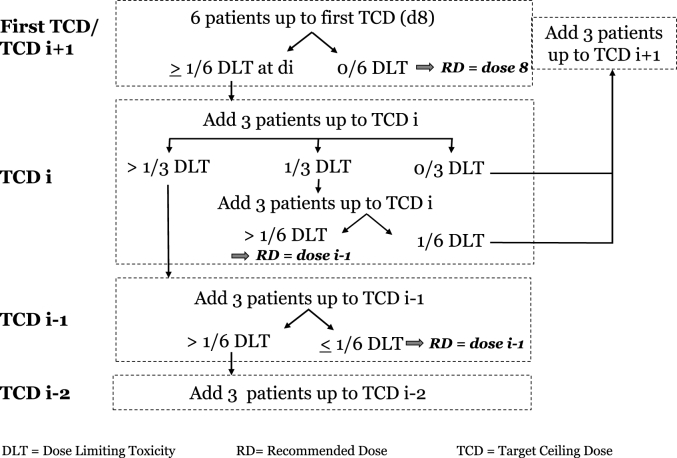
Target Ceiling Dose (TCD) was the highest theoretical planned dose to be administered to a patient. At the beginning of the current study, the first TCD was d8: 0.32 mg/m2/day.
Each time a DLT is observed in a patient at a dose d below the theoretical planned TCD, then this dose d becomes a new TCD for the next patients. The first time a DLT was observed at a dose di lower than the first TCD then two more patients were included up to di, which became the second TCD (or TCDi). If no DLT was observed at the second TCD for the two additional patients, then 3 more patients were included up to di + 1. If one or more DLT were observed at the second TCD, then a third TCD (TCD i-1) was determined (the dose just below the second TCD) and the same procedure was followed. If no more DLT was observed for the second TCD, then dose escalation continued following the rules above, with a fourth TCD (TCDi+1) i.e. the dose just above the second TCD (see Fig. 3).
Fig. 3.
Dose escalation strategy for cohort.
Recommended Dose (RD) was defined as the highest dose where a maximum of no DLT in 3 patients or one DLT in 6 patients was reported, as in a (3 + 3) conventional trial.
4. Conclusion
Neuromuscular toxicity of Crotoxin is observed in up to 75% of patients but can be limited with induction of tolerance, based on an intra-patient dose escalation. An innovative design was created: DLT was the inability to dose escalate twice, TCD was the highest planned dose to be administered to a patient and could change for patients along time and RD was defined similarly as in a (3 + 3) conventional design. Other designs with intra-patients dose escalation exist. They are not frequently used (less than 1% among phase 1 designs) [11]. One should think about an innovative mathematical modelling method such as an adapted Continual Reassessment Method, focusing on determination of dose levels and determination of duration of each dose administration.
Following our design, the clinical trial is now closed for inclusion and the results will be presented later.
References
- 1.Calvo A., Xiao N., Kang J., Best C.J., Leiva I., Emmert-Buck M.R. Alterations in gene expression profiles during prostate cancer progression: functional correlations to tumorigenicity and down-regulation of selenoprotein-P in mouse and human tumors. Cancer Res. 2002 Sep 15;62(18):5325–5335. [PubMed] [Google Scholar]
- 2.Hirai M., Gamou S., Minoshima S., Shimizu N. Two independent mechanisms for escaping epidermal growth factor-mediated growth inhibition in epidermal growth factor receptor-hyperproducing human tumor cells. J. Cell Biol. 1988 Aug;107(2):791–799. doi: 10.1083/jcb.107.2.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nishikawa K., Rotbein J., Vijjeswarapu D., Owen-Schaub L., Rosenblum M.G., Donato N.J. Positive and negative selection for tumor necrosis factor responsiveness reveals an inhibitory role for EGF receptor in TNF-induced antiproliferation. Lymphokine Cytokine Res. 1994 Feb;13(1):37–45. [PubMed] [Google Scholar]
- 4.Cura J.E., Blanzaco D.P., Brisson C., Cura M.A., Cabrol R., Larrateguy L. Phase I and pharmacokinetics study of crotoxin (cytotoxic PLA(2), NSC-624244) in patients with advanced cancer. Clin. Cancer Res. 2002 Apr;8(4):1033–1041. [PubMed] [Google Scholar]
- 5.Okamoto M., Viskatis L.J., de la Roza G., Vidal J.C. Induction of tolerance to crotoxin in mice. J. Pharmacol. Exp. Ther. 1993 Apr;265(1):41–46. [PubMed] [Google Scholar]
- 6.Bon C., Bouchier C., Choumet V., Faure G., Jiang M.S., Lambezat M.P. Crotoxin, half-century of investigations on a phospholipase A2 neurotoxin. Acta Physiol. Pharmacol. Latinoam. 1989;39(4):439–448. [PubMed] [Google Scholar]
- 7.Corin R.E., Viskatis L.J., Vidal J.C., Etcheverry M.A. Cytotoxicity of crotoxin on murine erythroleukemia cells in vitro. Investig. New Drugs. 1993 Feb;11(1):11–15. doi: 10.1007/BF00873905. [DOI] [PubMed] [Google Scholar]
- 8.Costa L.A., Miles H.A., Diez R.A., Araujo C.E., Coni Molina C.M., Cervellino J.C. Phase I study of VRCTC-310, a purified phospholipase A2 purified from snake venom, in patients with refractory cancer: safety and pharmacokinetic data. Anticancer Drugs. 1997 Oct;8(9):829–834. doi: 10.1097/00001813-199710000-00003. [DOI] [PubMed] [Google Scholar]
- 9.Costa L.A., Miles H., Araujo C.E., Gonzalez S., Villarrubia V.G. Tumor regression of advanced carcinomas following intra- and/or peri-tumoral inoculation with VRCTC-310 in humans: preliminary report of two cases. Immunopharmacol. Immunotoxicol. 1998 Feb;20(1):15–25. doi: 10.3109/08923979809034806. [DOI] [PubMed] [Google Scholar]
- 10.Habermann E., Walsch P., Breithaupt H. Biochemistry and pharmacology of the cortoxin complex. II. Possible interrelationships between toxicity and organ distribution of phospholipase A, crotapotin and their combination. Naunyn Schmiedeb. Arch. Pharmacol. 1972;273(4):313–330. doi: 10.1007/BF00499666. [DOI] [PubMed] [Google Scholar]
- 11.Le Tourneau C., Lee J.J., Siu L.L. Dose escalation methods in phase I cancer clinical trials. J. Natl. Cancer Inst. 2009 May 20;101(10):708–720. doi: 10.1093/jnci/djp079. [DOI] [PMC free article] [PubMed] [Google Scholar]