Abstract
A population specific understanding of barriers and facilitators to participation in clinical trials could improve recruitment of elderly and minority populations. We investigated how prior exposure to clinical trials and incentives were associated with likelihood of participation in a vaccine clinical trial through a questionnaire administered to 200 elderly patients in an academic general internal medicine clinic. Wilcoxon signed rank sum test compared likelihood of participation with and without monetary incentives. Logistic regression evaluated characteristics associated with intent to participate in an influenza vaccine trial, adjusted for age, gender, language, and education history. When asked about likelihood of participation if there was monetary compensation, there was a 12.2% absolute increase in those reporting that they would not participate, with a significant difference in the distribution of likelihood before and after mentioning a monetary incentive (Wilcoxon signed rank test, p = 0.001). Those with previous knowledge of clinical trials (54.4%) were more likely to report they would participate vs. those without prior knowledge (OR 2.5, 95% CI [1.2, 5.2]). The study highlights the importance of pre-testing recruitment materials and incentives in key group populations prior to implementing clinical trials.
Keywords: Geriatrics, Clinical trials, Research design, Disparities
1. Introduction
The elderly and people from racial and ethnic minority groups are underrepresented in clinical research [1], [2], [3]. With growing rates of these populations in the United States and disproportionate disease burden among them [4], their recruitment and retention in clinical research is important to inform patient-centered care. The National Institutes of Health, the Federal Drug Administration, and the Centers for Medicare and Medicaid Services among other organizations have all implemented initiatives to increase participant diversity in clinical research [5], [6], [7], [8]. However, ongoing barriers to participation include fear and mistrust, lack of knowledge about clinical research, and absence of cultural and linguistic adaptation of recruitment materials and strategies to key groups [9], [10]. A better understanding of barriers to and facilitators of clinical trial participation in vaccine trials could improve recruitment in elderly, minority populations [11], [12].
We hypothesized that 1) those with prior knowledge of clinical trials, prior participation in clinical trials, and prior receipt of the influenza vaccine would have higher likelihood of participation and 2) incentives would increase likelihood of participation.
2. Methods
We conducted a cross-sectional study of a convenience sample of patients in the waiting area of the Associates in Internal Medicine (AIM) practice in 2016. AIM is a joint resident physician and faculty group practice, part of NewYork-Presbyterian/Columbia University Medical Center, which provides primary care to an underserved New York City community. The study was approved by the Institutional Review Board at Columbia University and individual verbal consent was obtained. The study was conducted in part to assess the feasibility of conducting an influenza vaccine clinical trial in this specific population.
Eligible patients were adults aged ≥65 years who receive care in the AIM practice. Nearly half the patients in the practice are covered by Medicare (most with Medicaid), another 30% are covered by Medicaid only, and 10% are uninsured. Patients are primarily from racial and ethnic minority groups.
2.1. Questionnaire
A research assistant fluent in Spanish and English, masked to the study hypotheses, administered a questionnaire which included demographic information and self-reported receipt of the influenza vaccine in the previous year. Participants were read a description of vaccine clinical trials:
“Clinical research trials are very important in developing vaccines that can help keep people healthy and save lives. Clinical trials are studies conducted either before a vaccine is made available or sometimes after it is already licensed to see how well it works and if there are any common side effects.”
Then they were asked about previously hearing about clinical trials (yes/no), previous participation in a clinical trial (yes/no). This was followed by a series of questions assessing likelihood of participation in different scenarios: 1) in an influenza vaccine clinical trial for patients 65 years and older, 2) in scenario #1 with $80 monetary incentive, 3) in scenario #2 with the additional requirement of blood tests, 4) in scenario #3 with additional $50 monetary incentive and reimbursement of taxi fare. Response categories included: would not do it, unlikely, not sure, likely, and would do it. If a participant responded they would not do it in scenario 2 or 3 they were not asked about subsequent scenarios.
2.2. Statistical analysis
Wilcoxon signed rank sum test compared paired data from likelihood of participation between the different clinical trial scenarios. Logistic regression evaluated characteristics associated with likelihood of participation in influenza vaccine clinical trial scenario #1, adjusted for age, gender, preferred language, and highest level of education. For the logistic regression, the outcome of likelihood of participation was categorized as likely (would do it or likely) versus unlikely (not sure, unlikely, or would not do it). Statistical analyses were conducted with SAS statistical software (version 9.4; SAS Institute Inc., Cary, NC).
3. Results
Of the 227 eligible patients approached, 200 (88%) agreed to participate in the study; patient characteristics are shown in Table 1. After hearing a description of a vaccine clinical trial, approximately one half (54.4%) reported previously hearing about clinical trials, 17.6% reported previous participation in a clinical trial, and 75.0% reported receipt of the influenza vaccine in the past year.
Table 1.
Participant characteristics.
| Patient Characteristics | (N = 200) |
|---|---|
| Age in years, mean (SD) | 74 (SD 6.8) |
| Gender | |
| Female, n (%) | 146 (73.0) |
| Preferred language | |
| Spanish, n (%) | 186 (93.0) |
| English, n (%) | 14 (7.0) |
| Hispanic, n (%) | 186 (93.0) |
| Race | |
| Black/African American, n (%) | 11 (5.5) |
| White, n (%) | 32 (16.0) |
| Multiracial, n (%) | 30 (15.0) |
| Other,*n (%) | 123 (61.5) |
| Education | |
| Less than high school, n (%) | 132 (66.0) |
| High school/GED, n (%) | 40 (20.0) |
| Trade or vocational school, n (%) | 5 (2.5) |
| Some college, n (%) | 21 (10.5) |
| Prior knowledge of clinical trials, n (%) | 105 (54.4) |
| Prior participation in a clinical trial, n (%) | 34 (17.6) |
| Influenza vaccine in last year, n (%) | 150 (75.0) |
∗ Free response for “Other race” primarily included Latino/a, Dominican, Hispanic.
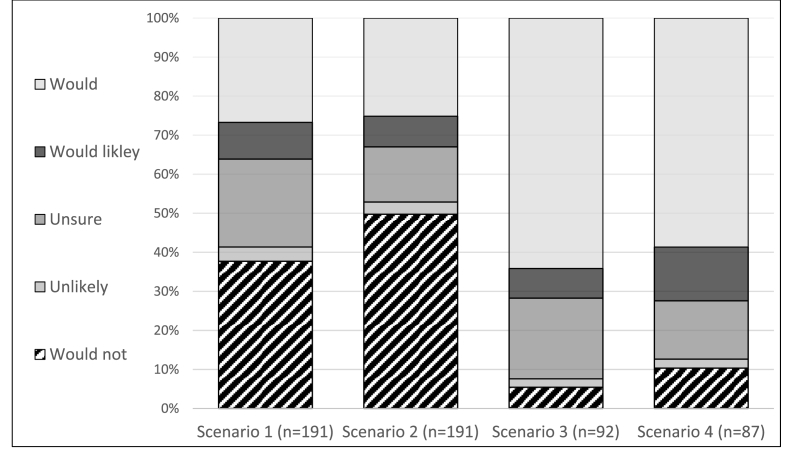
Likelihood of participation was compared between the initial description of the influenza vaccine clinical trial and the subsequent scenarios involving monetary incentives and additional laboratory requirements (Fig. 1). When comparing likelihood of participation between scenario 1 and scenario 2, there was 12.2% absolute increase in participants stating they would not participate if there was a monetary incentive (Wilcoxon signed rank test, p = 0.001). Among the 92 participants who were asked about both scenario 2 and 3, there was no significant difference in the distribution of likelihood of participation with the addition of blood test requirements (Wilcoxon signed rank test, p = 0.48). Among the 87 participants who were asked about both scenario 3 and 4, there was a significant shift towards less likelihood of participation with the addition of extra monetary incentive and travel reimbursement (Wilcoxon signed rank test, p = 0.002). The trend towards less likelihood of participation with incentives was seen among participants with prior participation in clinical trials and those with prior receipt of the influenza vaccine.
Fig. 1.
Likelihood of participation in an influenza vaccine clinical trial for adults ≥65 years (Scenario 1), with addition of monetary incentive (Scenario 2), with additional requirement of blood draw (Scenario 3), with additional monetary incentive and reimbursement of travel (Scenario 4). Participants who answered would not for Scenario 2 or 3 were not asked subsequent questions.
Participants who reported prior knowledge of clinical trials (OR 2.46, 95% CI [1.18, 5.15]) and prior participation in clinical trials (OR 2.95, 95% CI [1.19, 7.29]) had higher odds of intent to participate compared to those without prior knowledge and participation after adjusting for age, language, gender and education history (Table 2). Those who had received the influenza vaccine in the last year (OR 2.55, 95% CI [1.17, 5.59]) also higher adjusted odds of intent to participate compared to those who had not received the vaccine.
Table 2.
Participant characteristics associated with intention to participate in an influenza vaccine clinical trial for patients ≥65 years.
| Variables | Likely to participate |
Unlikely to participate |
Likely versus unlikely to participate (n = 191) |
|---|---|---|---|
| N (%) | N (%) | Adjusted OR (95% CI) | |
| Age, year | 1.0 (0.9, 1.0) | ||
| Gender | |||
| Female | 51 (36.2) | 90 (63.8) | 1.2 (0.6, 2.5) |
| Male | 18 (36.0) | 32 (64.0) | -- |
| Language | |||
| Spanish | 67 (37.4) | 112 (62.6) | 0.33 (0.1, 1.7) |
| English | 2 (16.7) | 10 (83.3) | -- |
| Education* | |||
| High school or less | 60 (36.1) | 106 (63.9) | -- |
| More than high school | 9 (36.0) | 16 (64.0) | 0.7 (0.3, 1.9) |
| Prior clinical trial experience | |||
| No prior knowledge, no prior participation | 21 (25.0) | 63 (75.0) | -- |
| Prior knowledge, no prior participation | 30 (42.9) | 40 (57.1) | 2.5 (1.2, 5.2) |
| Prior knowledge and prior participation | 16 (47.1) | 18 (52.9) | 3.0 (1.2, 7.3) |
| Influenza vaccine in last year | |||
| Yes | 58 (40.6) | 85 (59.4) | 2.6 (1.2, 5.6) |
| No | 11 (22.9) | 37 (77.1) | -- |
* More than high school includes trade/vocational school or college; high school or less includes GED or less than high school.
Statistically significant estimates in bold text.
4. Discussion
In this study of elderly, primarily Hispanic patients, likelihood of participation in a clinical trial was associated with prior knowledge of and/or participation in clinical trials and recent exposure to the proposed intervention, the influenza vaccine. Previous studies have shown that when aware or offered participation in clinical research, minorities including people of Hispanic ethnicity, enroll at the same rate as non-Hispanic whites [13], [14]. This suggests that further educational outreach may be part of a successful recruitment strategy.
A high rate of likelihood of participation in our population may be reflective of using research staff that spoke Spanish and could relate to our participants. Cultural congruence, racial matching of study staff, and ethnically targeted statements have been found to improve recruitment of minority groups [10], [12], [15], [16]. Our research assistant noted that after asking the study questions, nine participants stated they would participate, but emphasized that it was not for the money. Altruism, such as advancing medical knowledge or helping others, has been shown to be an important motivator for clinical trial participation among both Hispanic and healthy volunteer populations [16], [17]. Thus, highlighting altruistic motivations may be an important recruitment strategy.
Among healthy volunteers, a main motivator for participation in clinical trials is financial benefit [17]. Paradoxically, we found that monetary incentives reduced likelihood of participation. This response may have been seen because the juxtaposition of scenarios with and without incentives may increase fear of mistreatment or experimentation, a frequently cited barrier to participation [10]. One previous study demonstrated that potential clinical trial participants associated increasing payment amounts with increased perception of risk [18]. Unsurprisingly, evaluation of risk has been associated with willingness to participate in clinical trials [17]. Additional barriers to participation in elderly, minority populations include access to medical care, complexity of the informed consent process, co-morbidities, and legal status [10], [16], [19]. In our study population, those who previously received the influenza vaccine and those with prior participation in clinical trials had higher likelihood of participation, suggesting that familiarity with the exposures may mitigate common barriers.
Study limitations include the cross-sectional design and convenience sample. However, a high proportion of those approached completed the survey. The order of hypothetical scenarios with and without monetary incentives may influence participants' response due to social desirability or default bias. Future studies investigating the role of incentives in elderly and minority participant recruitment could randomize the order of the incentive scenarios or randomize participants to hear only one of the scenarios in order to better isolate the relationship between incentive and likelihood of participation in a clinical trial. Additionally, we do not know how self-report of likelihood of participation in a clinical trial relates to actual recruitment. Another potential limitation is the ability to extrapolate findings to non-influenza vaccine clinical research.
In conclusion, increased likelihood of participation in clinical trials for those with prior knowledge suggests increased public information on clinical research could improve recruitment in this population. Offering incentives unexpectedly reduced participants' likelihood of participation, warranting further investigation. The study highlights the importance of pre-testing recruitment materials and incentives in key group populations prior to implementing a clinical trial.
Acknowledgements
We thank our research assistant, Angela Barrett. S. Rikin was supported by Health Resources and Services Administration, Grant Number T0BHP293020100.
Contributor Information
Sharon Rikin, Email: sr3243@columbia.edu.
Steven Shea, Email: ss35@columbia.edu.
Philip LaRussa, Email: psl1@columbia.edu.
Melissa Stockwell, Email: mss2112@columbia.edu.
References
- 1.Ford J.G., Howerton M.W., Lai G.Y. Barriers to recruiting underrepresented populations to cancer clinical trials: a systematic review. Cancer. 2008;112(2):228–242. doi: 10.1002/cncr.23157. [DOI] [PubMed] [Google Scholar]
- 2.Eshera N., Itana H., Zhang L. Demographics of clinical trials participants in pivotal clinical trials for new molecular entity drugs and biologics approved by FDA from 2010 to 2012. Am. J. Ther. 2015:22. doi: 10.1097/MJT.0000000000000177. [DOI] [PubMed] [Google Scholar]
- 3.Heiat A., Gross C.P., Krumholz H.M. Representation of the elderly, women, and minorities in heart failure clinical trials. Arch. Intern Med. 2002:162. doi: 10.1001/archinte.162.15.1682. [DOI] [PubMed] [Google Scholar]
- 4.National Healthcare Quality and Disparities Report and 5th Anniversary Update on the National Quality Strategy. Content Last Reviewed May 2016. Agency for Healthcare Research and Quality; Rockville, MD: 2015. http://www.ahrq.gov/research/findings/nhqrdr/nhqdr15/index.html [Google Scholar]
- 5.UyBico S.J., Pavel S., Gross C.P. Recruiting vulnerable populations into research: a systematic review of recruitment interventions. J. Gen. Intern Med. 2007;22(6):852–863. doi: 10.1007/s11606-007-0126-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.National Institutes of Health . 2001. NIH Policy and Guidelines on the Inclusion of Women and Minorities as Subjects in Clinical Research.https://grants.nih.gov/grants/funding/women_min/guidelines_amended_10_2001.htm (Accessed 1 Febraury 2017) [Google Scholar]
- 7.U.S. Food and Drug Administration . U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER), Center for Biologics Evaluation and Research (CBER), Center for Devices and Radiologic Health (CDRH), Office of the Commissioner (OC); Washington, DC: September 2005. Guidance for Industry: Collection of Race and Ethnicity Data in Clinical Trials.http://www.fda.gov/downloads/RegulatoryInformation/Guidances/ucm126396.pdf (Accessed 6 April 2016) [Google Scholar]
- 8.Centers for Medicare and Medicaid Services . 2013. Medicare Clinical Trial Policies.https://www.cms.gov/Medicare/Coverage/ClinicalTrialPolicies/index.html?redirect=/ClinicalTrialPolicies (Accessed 1 Febraury 2017) [Google Scholar]
- 9.Hussain-Gambles M., Atkin K., Leese B. Why ethnic minority groups are under-represented in clinical trials: a review of the literature. Health Soc. Care Community. 2004;12(5):382–388. doi: 10.1111/j.1365-2524.2004.00507.x. [DOI] [PubMed] [Google Scholar]
- 10.George S., Duran N., Norris K. A systematic review of barriers and facilitators to minority research participation among African Americans, Latinos, Asian Americans, and Pacific Islanders. Am. J. Public Health. 2014;104(2):e16–31. doi: 10.2105/AJPH.2013.301706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Taras H.L., Kalichman M.W., Schulteis G. Soliciting views of various communities on health research: a prelude to engagement in specific research projects. Health Expect. 2015;18(6):2753–2763. doi: 10.1111/hex.12249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nicholson L.M., Schwirian P.M., Groner J.A. Recruitment and retention strategies in clinical studies with low-income and minority populations: progress from 2004–2014. Contemp. Clin. Trials. 2015:45. doi: 10.1016/j.cct.2015.07.008. Part A:34-40. [DOI] [PubMed] [Google Scholar]
- 13.Wallington S.F., Luta G., Noone A.-M. Assessing the awareness of and willingness to participate in cancer clinical trials among immigrant latinos. J. Community Health. 2012;37(2):335–343. doi: 10.1007/s10900-011-9450-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ellington L., Wahab S., Sahami Martin S. Factors that influence Spanish- and English-speaking participants' decision to enroll in cancer randomized clinical trials. Psychooncology. 2006;15(4):273–284. doi: 10.1002/pon.943. [DOI] [PubMed] [Google Scholar]
- 15.Brown S.D., Lee K., Schoffman D.E. Minority recruitment into clinical trials: experimental findings and practical implications. Contemp. Clin. Trials. 2012;33(4):620–623. doi: 10.1016/j.cct.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garza M.A., Quinn S.C., Li Y. The influence of race and ethnicity on becoming a human subject: factors associated with participation in research. Contemp. Clin. Trials Comm. 2017;7:57–63. doi: 10.1016/j.conctc.2017.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stunkel L., Grady C. More than the money: a review of the literature examining healthy volunteer motivations. Contemp. Clin. Trials. 2011;32(3):342–352. doi: 10.1016/j.cct.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cryder C.E., John London A., Volpp K.G., Loewenstein G. Informative inducement: study payment as a signal of risk. Soc. Sci. Med. 2010;70(3):455–464. doi: 10.1016/j.socscimed.2009.10.047. [DOI] [PubMed] [Google Scholar]
- 19.Ridda I., MacIntyre C.R., Lindley R.I., Tan T.C. Difficulties in recruiting older people in clinical trials: an examination of barriers and solutions. Vaccine. 2010;28(4):901–906. doi: 10.1016/j.vaccine.2009.10.081. [DOI] [PubMed] [Google Scholar]