Abstract
Ovarian cancer is a silent killer and, due to late diagnosis, the primary cause of death amongst gynecological cancers, killing approximately 376 women annually in Denmark. The discovery of a specific and sensitive biomarker for ovarian cancer could improve early diagnosis, but also treatment, by predicting which patients will benefit from specific treatment strategies. The Mermaid III project is consisting of 3 parts including “Early detection, screening and long-term survival,” “Biomarkers and/or prognostic markers” and “The infection theory.” The present paper gives an overview of the part regarding biomarkers and/or prognostic markers, with a focus on rationale and design.
The study described has 3 major branches: microRNAs, epigenetics and Next Generation Sequencing. Tissue and blood from ovarian cancer patients, already enrolled in the prospective ongoing pelvic mass cohort, will be examined. Relevant microRNAs and DNA methylation patterns will be investigated using array technology. Patient exomes will be fully sequenced, and identified genetic variations will be validated with Next Generation Sequencing. In all cases, data will be correlated with clinical information on the patient, in order to identify possible biomarkers.
A thorough investigation of biomarkers in ovarian cancer, including large numbers of different markers, has never been done before. Besides from improving diagnosis and treatment, other outcomes could be markers for screening, knowledge of the molecular aspects of cancer and the discovery of new drugs. Moreover, biomarkers are a prerequisite for the development of precision medicine. This study will attack the ovarian cancer problem from several angles, thereby increasing the chance of successfully contributing to saving lives.
Keywords: Ovarian cancer, Diagnostic/prognostic biomarkers, MicroRNA, Epigenetics, Next Generation Sequencing
Abbreviations: OC, Ovarian cancer; FIGO, International Federation of Gynecology and Obstetrics; CA125, Cancer Antigen 125; RMI, Risk of Malignancy Index; DGCD, Danish Gynecologic Cancer Database; FFPE, Formalin fixed and paraffin embedded; O.C.T., Optimal cutting temperature; miRNAs, MicroRNAs; OS, Overall survival; PFS, Progression free survival; MALOVA, MALignant OVArian cancer study; NGS, Next Generation Sequencing; PARP, poly(adenosine diphosphate [ADP]-ribose) polymerase; HE4, Human Epididymis Protein 4; ROMA, Risk of Ovarian Malignancy Algorithm; CPH-I, Copenhagen Index; UKCTOCS, UK Collaborative Trial of OC Screening; ROCA, Risk of Ovarian Cancer Algorithm
1. Introduction
Ovarian cancer (OC) is the 5th most common cause of cancer death for women, with a 5 year survival rate of only 52% in Denmark [1], [2]. In 90% of OC cases the tumor is of epithelial origin, of which there are 5 histological subtypes; serous, endometrioid, clear cell, mucinous and undifferentiated. Serous carcinoma is counting nearly 70% of epithelial OC tumors [3], [4].
A primary cause for the high death rate of OC is that early OC symptoms are unspecific and more than 60% of the patients are diagnosed at a late stage of disease (stage III/IV, International Federation of Gynecology and Obstetrics, FIGO) [1], [2], [5], [6]. At late stages the 5 year survival is 15–29% whereas 88% of women diagnosed at stage I survive more than 5 years [2]. If OC is suspected in a Danish patient, examination includes measurements of Cancer Antigen 125 (CA125) serum levels and ultrasound scanning (Fig. 1). Risk of Malignancy Index (RMI) is calculated based on these tests and the menopausal state of the patient [4], [7]. However, the specificity and sensitivity of CA125 as a cancer marker is unsatisfactory [8], [9], [10], [11]. A biological marker with high sensitivity and specificity, which can detect early stages of OC, could therefore improve survival. Biomarkers which have potential to be used in screening programs are of highest priority. A screening strategy partly depending on CA125 was recently investigated, but with unsatisfying results [11].
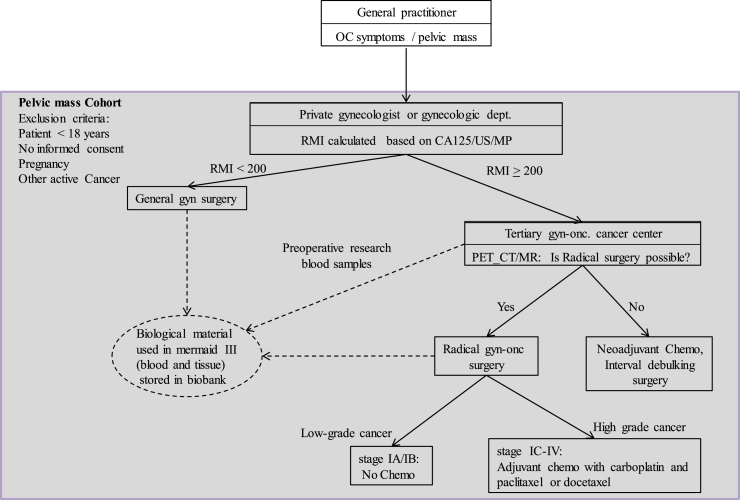
Fig. 1.
Treatment of OC patients in Denmark, and inclusion in the Pelvic mass cohort. If a pelvic mass is suspected, the patient is examined by a gynecologist, whom will calculate RMI based on ultrasound, CA125 and menopause status. Based on RMI the patient is either sent to general gynecological surgery, or a tertiary gyn-onc cancer center. PET_CT/MR scanning determines the patient for either radical surgery with adjuvant chemotherapy or neoadjuvant chemotherapy with interval surgery. Women with a pelvic mass are enrolled in the pelvic mass cohort when they are forwarded to surgery. OC: ovarian cancer, CA125: cancer antigen 125, US: ultrasound scanning, MP: menopausal state, RMI: Risk of malignancy Index, gyn: gynecological, gyn-onc: gynecologic-oncology, PET_CT: Positron emission tomography–computed tomography, MR: Magnetic Resonance scanning.
The best prognosis is obtained after radical surgery but unfortunately this is not always possible [4], [12]. Standard treatment is surgery followed by platinum-based chemotherapy combined with taxane drugs, except for patients with stage IA-IB low grade and IA-IB clear cell OC [4]. If complete resection is not possible, neoadjuvant chemotherapy before interval surgery is an alternative [13], but targeting patients for the relevant treatment is difficult [14]. Moreover, the majority of the patients respond to platinum-based treatment initially, but 80% develop resistance and experience disease relapse [15]. A new promising field in cancer-research is personalized precision medicine, where the molecular profile of a patients tumor is taken into account during treatment [16]. However, predicting how the patients will respond to medication is challenging. Biomarkers that support specific treatment strategies could improve treatment. The search for new biomarkers will also improve knowledge of the molecular mechanisms and help to identify new drug targets.
Mermaid is an organization with focus on gynecological cancers. Earlier Mermaid projects have been very successful resulting in more than 70 publications [17]. Resent technical improvements in molecular biology have made it possible to investigate several molecular markers simultaneously in large patient cohorts. The aim of the study described here is to identify and validate new diagnostic, predictive and prognostic biomarkers for OC, by using the underlying molecular characteristics of the disease. The goal is to increase survival of OC patients by improving diagnosis, ease treatment planning and by discovery of new drug targets and possible screening markers.
2. Methods and design
2.1. Patients
All patient material used in the study will be from women enrolled in “The pelvic mass study”, a prospective, ongoing cohort study initiated in September 2004. Patients are included if they are 18 years or older and admitted to surgery of a potentially malignant pelvic mass at the Gynecologic clinic at Copenhagen University Hospital, Rigshospitalet (Fig. 1). Patients are examined according to the Danish Cancer Fast Track Guidelines and admitted to radical surgery or biopsy for diagnosis followed by neoadjuvant chemotherapy [18]. Patients are excluded from the pelvic mass study if they are unable to give informed consent, are pregnant, have known relapse of previous cancer or have another active cancer. All patient tissue is examined by specialized pathologists and the patients are registered in the Danish Gynecologic Cancer Database (DGCD, http://www.dgcg.dk/), where information about clinical data, treatment and survival of the patient is updated regularly.
2.2. Blood and tissue
Blood is sampled within 2 weeks prior to surgery and fractionated into serum, buffy coat, EDTA plasma and whole blood within 6 h after collection. Representative tissues are sampled and verified by the pathologist. All blood and tissue samples (fresh frozen, RNA-later treated, formalin fixed and paraffin embedded tissues (FFPE) and O.C.T. compounds) are registered and stored in Danish Cancer Biobank (www.cancerbiobank.dk), and handled according to their guidelines. FFPE tissue is kept at room temperature whereas all other tissue and blood samples are stored at - 80 °C.
2.3. Ethics
The Danish Ethical Committee approved the protocol according to the rules used in International Conference on Harmonization/Good Clinical Practice (ICH/GCP) recommendations and the Helsinki and Tokyo conventions (KF01-227/03 and KF01-143/04, H-3-2010-022).
2.4. Sub-studies
2.4.1. MicroRNA
The small, single stranded, non-coding microRNAs (miRNAs) have gone from being largely unknown to becoming widely recognized as major players in gene regulation in less than 25 years. miRNAs bind to partially complimentary mRNA sequences an inhibit translation, thereby silencing gene expression in plants and animals [19]. miRNAs are involved in the regulation of several important cellular processes, including proliferation, differentiation, apoptosis and DNA repair, and deregulated miRNAs are often found in cancer tissue [20], [21]. Like genes, miRNAs can be oncogenic, contributing to cancer progression by being wrongly upregulated, or tumor suppressors, if cancer progression is caused by lowered expression of the miRNA [22]. Typically, an oncogenic miRNA will inhibit expression of tumor suppressor genes, for instance miRNA-21, which promotes proliferation, invasion and migration in OC by inhibiting expression of the tumor suppressor PTEN [23]. On the other hand, the let-7 family of miRNAs is considered to be tumor suppressors, and one of their actions is to suppress the oncogene KRAS [24]. Several miRNAs has been shown to be deregulated in OC, and miRNA expression profiles correlates with pathological conditions including disease development, survival and drug-response [25], [26], [27]. miRNAs therefore hold a promising potential as both diagnostic and predictive markers.
In order to identify new predictive miRNA markers, a correlation between miRNA expression and chemotherapy resistance was investigated in a retrospective study. The results of this study part have been published elsewhere [28]. A total of 198 patients were included (Fig. 2). All patients included had tumors of epithelial histology and had completed primary surgery followed by treatment with a minimum of two cycles of adjuvant chemotherapy with Carboplatin and Paclitaxel or Docetaxel.
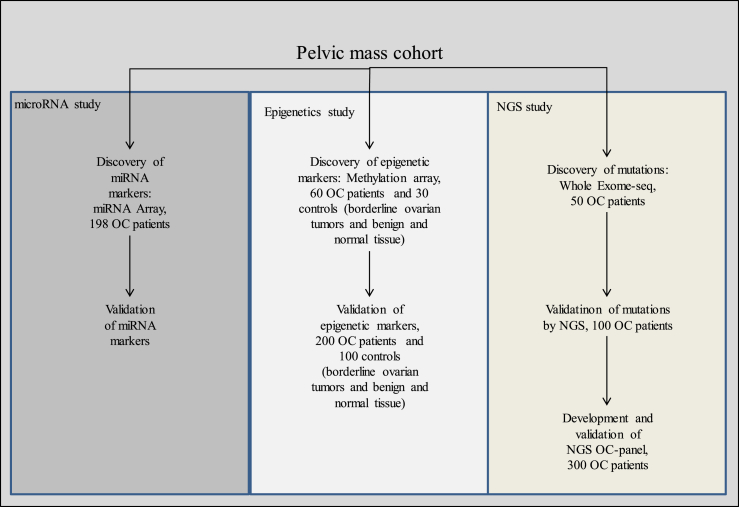
Fig. 2.
Flow chart/study overview. Flow chart of the study “Biomarkers and/or prognostic markers”. All patient material used is collected from the Pelvic mass cohort. The study contains 3 branches; a miRNA sub-study, an epigenetic sub-study and a NGS sub-study. Each sub-study aims at discovering and validating new biomarkers. OC: ovarian cancer, NGS: Next Generation Sequencing, Exome-seq: Exome sequencing.
The correlation between miRNA expression and resistance to drug treatment was predicted by the Medical Prognosis Institute (MPI) as described previously [29], [30] Growth inhibition (GI50) for a panel of human cancer cell lines, the NCI60 cell line panel, when treated with Carboplatin, Paclitaxel or Docetaxel, were compared to miRNA expression levels for each combination of miRNA and drug.
Total RNA was extracted from patient FFPE tissues using the RecoverAll™ total nucleic acid isolation kit from Ambion at the Molecular Unit at Herlev hospital. miRNAs was labeled using FlashTag™ HSRTM Biotin RNA Labeling Kit from Genisphere and the expression of 847 miRNAs was measured using an Affymetrix® GeneChip miRNA 1.0 array.
20 identified miRNA prediction markers, who showed the best correlation with drug sensitivity, were then used to develop prediction scores for each OC patient. The prediction scores were blindly validated, by comparing the clinical data on drug treatment and resistance, overall survival (OS) and progression free survival (PFS) to the measured expression of the predictive miRNAs. Drug resistance was defined as relapse or progressive disease less than 6 months after treatment.
The miRNA expression data will also be compared to patient data regarding tumor type, disease stage and grade, in order to investigate if miRNA markers holds potential as diagnostic or prognostic markers. Since patient blood samples are stored at −80 °C in the biobank, further studies of miRNA expression in blood are possible to support data, and regarding the use of circulating miRNAs to differentiate between benign tumors and malignancy.
The labeled patient RNA will be used in similar investigations using mRNA arrays, to look at the correlation between gene expression and clinical data like pathology, drug treatment and survival.
Identifying miRNAs, whose regulation can predict resistance to chemotherapy, would greatly improve the treatment of OC patients. Not only would the patients avoid a toxic treatment with more side-effects than effects, the predictions made could also ease and speed up the unset of alternative more personalized treatment, increasing the chance of survival.
miRNAs may hold a great potential as diagnostic markers, and circulating miRNAs are stable in body fluids and can therefore be detected in blood-tests [31]. It has been shown that cancer-specific miRNAs circulating in tumor exosomes can be detected already in early stages of OC, and the level of exosome miRNAs might hold the potential to differentiate between benign disease and stage I cancer [32], [33].
miRNAs has also gained focus as possible therapeutic agents. Because miRNAs recognize their targets by partial complementarity, one miRNA often regulate several different genes. Even though the complexity of this system can be challenging, it also has a huge advantage, since drugs that mimic one miRNA might be capable of silencing several oncogenes at the same time, thereby attacking the cancer through several different pathways. This tactic has led to the first phase 1 trial of a drug that mimics a miRNA, namely miR-34a, which targets more than 30 oncogenes and is often downregulated in cancer [34].
2.4.2. Epigenetics
The new knowledge, that gene-regulation can be altered in an inheritable way, carried in the chromosome but without changing the DNA sequence, opens for new possibilities when it comes to cancer-treatment. It is easier to change epigenetic regulation of genes than it is to repair mutated or damaged DNA in the patient [35]. One of the most well studied epigenetic mechanisms has also been related to cancer. DNA methylation, where methyl groups are added to the nucleotide cytosine, is a process that can alter gene expression in a stable manner [36]. High levels of methylation in a promoter region inhibit gene transcription. The process of DNA methylation is essential in embryonal development, where it is involved in silencing of genes important for cell differentiation in embryonic stem-cells and in X-chromosome inactivation. However, erroneous changes in DNA methylation-patterns are often seen in carcinogenesis [37]. Increasing levels of DNA methyltransferase and localized hypermethylation has been observed, especially in the promotors of tumor suppressors [38], [39]. However, reduced methylation of oncogene promoters or genome wide hypomethylation, resulting in chromosome instability, has also been observed in cancer [40], [41]. In some OC patients, the PolyComb Group Target genes (PCGTs), essential for differentiation, are hypermethylated [42], [43]. Another group of genes, MESC, which are named this as they are normally highly Methylated in Embryonic Stem Cells, become hypomethylated in OC. While the PCGT hypermethylation can be detected very early, before unset of carcinogenesis, the hypomethylation of MESC progresses from primary cancer to metastasis [43]. In this way, the status of specific methylation sites of cancer cell DNA can both be used to diagnose early stages of cancer and work as prognostic indicators.
Patterns of DNA methylation which can be used either diagnostically, in screening, or as prognostic or predictive markers, will be investigated in a retrospective study. 60 OC patients and 30 age-matched controls will be included (Fig. 2). Patients from whom we have both blood samples, cytological cervix samples from smear testing and both fresh frozen- and FFPE tissue will be prioritized. The cancer samples will include both early and late stage epithelial cancers and both blood and tissue will be analyzed. Corresponding cervical smear samples will also be included for 40 patients, diagnosed with early and late OC stages. The reason for including cervical smear samples will be explained later. Both platinum-resistant and platinum-responsive patients will be assessed. The non-cancerous controls included as references will both include benign or borderline tumors as well as normal tissue from healthy women.
For discovery of global methylation profiles and relevant methylation sides, patient DNA will be purified from blood, fresh frozen tissues or FFPE, using the Maxwell® rsc DNA purification systems from Promega, at the Molecular Unit, Department of Pathology at Herlev University Hospital. As DNA from FFPE samples may be partly degraded, these samples will be restored with the Infinium® HD FFPE restore kit. To begin with, fresh frozen and FFPE samples will be run in parallel in order to ensure concordance between results using DNA from FFPE samples and DNA from frozen material. The DNA will be bisulfite treated with the EZ DNA Methylation™ kit (Zymo Research). Bisulfite converts non-methylated cytosine to uracil, thereby changing the genetic code in non-methylated areas. Global methylation profiles will be identified using the Infinium® MethylationEPIC BeadChip Kit (Illumina) in the lab of Associate Professor Jesper B. Andersen at the Biotech Research and Innovation Center, University of Copenhagen. The group already has the Infinium® Methylation EPIC array technology implemented in the lab, studying other cancer types, and the OC samples will be run under his supervision. The methylation array distinguishes between methylated DNA or the bisulfite converted non-methylated DNA by hybridization to DNA-probes specific for either the methylated or non-methylated allele. The methylation pattern of 850.000 cancer related targets, including promotors and enhancers of tumor suppressor genes, will be investigated.
Changes in gene expression of affected genes will be validated by qPCR for a maximum of 5–10 methylation sites discovered by the array. Patient RNA will be purified from both fresh frozen tissue and from FFPE tissue from 200 OC tumors and 100 benign tumors.
The obtained methylation data will be blindly correlated with clinical data for the involved patients. The focus will be both on data regarding drug resistance, to identify predictive and prognostic markers, on data regarding malignancy and cancer stage, to identify diagnostic markers and finally on the correlations between methylation patterns in different sample types of early stage cancers, to identify screening markers.
Changes in methylation patterns can occur very early in cancer development, and therefore holds potential for screening and differentiating between benign conditions and cancers [43], [44]. Another important parameter that underscores the diagnostic potential of methylation patterns is that tumor-specific, methylated DNA is accessible pre-surgical. It has been shown that it can be obtained from serum or plasma as circulating DNA, and that the cell free, tumor-specific, methylated DNA is highly biologically stable, and therefore holds a biomarker potential [45], [46]. High grade ovarian carcinomas, which constitute the largest and most aggressive group of OC tumors, have been shown to originate primarily from the fallopian tube rather than from ovarian tissue [47], [48]. Fluid and oocytes pass from the tubes and through cervix and the tubes and cervix are parts of the same organ. It can therefore be speculated that stable biomarkers from the ovaries, like methylated DNA, which indicate changes in pre-malignant or malignant tissue of tubal origin, can be detected in the cervical canal [49]. If a highly specific and sensitive methylation-based marker for OC was identified, it is hypothetically possible that it could be detected in a cervical smear test, and that screening for ovarian cancer could be included in the already implemented smear-testing and screening for cervix cancer.
Reversing false DNA methylation also holds potential for treatment. Drugs inhibiting the enzyme DNA methyltransferases has been extensively studied for this purpose, and several preclinical studies have shown that their hypomethylating effect can reactivate transcription and probably re-sensitize patients to treatment [50].
Promising results from this sub-study will possibly be followed up by a larger, national, prospective multicenter study, and if possible an international study as well.
2.4.3. Next Generation Sequencing (NGS)
Cancer can arise because of deregulation of genes important for growth, development or other crucial processes, but often the cause of cancer is mutation, deletion or even duplication of the gene itself [51]. The mutation may interfere with the function of a gene involved in tumor suppression or turn a proto-oncogene into an oncogene by increasing its activity. Several cancer-related mutations are already known and used as biomarkers for other cancers. Mutations in the gene encoding the tumor suppressor p53 or in one of the RAS proto-oncogenes, HRAS, KRAS and NRAS, are amongst the most abundant mutations found in cancer and also amongst the most studied genes [52], [53].
The gene coding for p53 is mutated in approximately 45% of OCs [54], and previous data from the Danish MALOVA study (MALignant OVArian cancer study) has indicated that p53 may function as a prognostic marker in OC patients, as a significantly shorter survival was observed for patients with missense mutations in the gene encoding p53 [55]. On the other hand, mutations in KRAS are found in a smaller percentage of OCs and primarily in low-grade serous carcinomas and mucinous carcinomas [56].
Another set of mutations which are relevant for OC is the BRCA mutations found in approximately 10% of OCs. BRCA genes have an important function in DNA-repair and can be inherited from the parents and they are a major risk factor for developing OC with a lifetime risk of 40–60% [57], [58].
Sequencing technology and related DNA tools are constantly evolving and the development of Next Generation Sequencing (NGS) has opened for a wide array of new possibilities when it comes to investigating mutations in cancer patient cohorts [59]. Sequencing of several hundreds of genes simultaneously in large patient cohorts will facilitate the discovery of new cancer-related mutations and the investigation of the correlation between mutations and clinical data, like patient survival. Moreover, the data that can be generated with NGS can help us to a better understanding of the etiologies of OC, and to a more thorough investigation of the involvement of the known cancer-related genes, like TP53 and KRAS, in OC.
This study part will be initiated with a genome-wide discovery phase. In order to identify mutations that can be used as prognostic markers for ovarian-cancer, all protein-coding DNA sequences in the genome will be investigated by exome-sequencing for selected patients. DNA from 50 OC patients will be sufficient to obtain a satisfying statistical power and reliable results for this part of the study (Fig. 2). Patients with different clinical outcomes will be included. The patient samples will cover epithelial tumors of different stages, histological subtypes and grades, and each group should be represented by at least 5 patients.
Maxwell® rsc DNA purification systems from Promega will be used to purify patient DNA from fresh frozen or FFPE tissue. Exome enrichment of patient DNA is done by multiplex PCR Sequencing of the Exome library will be done on a chip (Ion 540 chip kit) with the Ion 540™ Kit-OT2 (Thermo Fisher Scientific).
Mutations discovered by the exome sequencing will be validated with NGS using the Ion torrent Oncomine comprehensive panel, supplemented with primers for additional targets identified in the exome sequencing if necessary. Approximately 400 genes will be included (hot-spots), comprising tp53, KRAS, HRAS, NRAS and the BRCA genes. DNA from 100 patients will be included and sequenced. Datamining will be performed using existing software.
Data on patient mutational state will be blindly compared with the clinical data for the involved patients, including disease stage, grade, chemotherapy resistance and survival (OS and PFS) and tumor type.
Mutations that holds potential as prognostic or predictive markers, discovered and validated by exome sequencing and targeted/panel sequencing, will be used to design a primer panel specialized for OC patients. The panel will be tested on DNA from 300 patients. The patient samples should represent a broad spectrum of epithelial OCs, grouped by stage, histological subtype and grade, also including benign ovarian tumors.
The assessment of predictive mutations in cancer related genes is already an integral part of the clinical work. An example of this is that KRAS mutational status is routinely examined and used for patients with colorectal cancer [60], [61]. KRAS mutations in a tumor predict a poor response to certain kinds of targeted treatment [62]. This strategy spares the relevant patients from numerous side effects, and the hospital for wasting expensive drugs on those who do not benefit from it.
In OC treatment BRCA mutations are used to assess which OC patients will benefit from treatment with poly(adenosine diphosphate [ADP]-ribose) polymerase (PARP)-inhibitors [4], [63]. Both PARP and the BRCA proteins are involved in DNA repair, and PARP-inhibition can therefore kill cancer cells with BRCA mutations. However, a great breakthrough came recently in this area when Mirza et al. showed that the PARP-inhibitor Niraparib can prolong PFS significantly in all platinum-sensitive OC-patients, even though the effect was most significant in patients with BRCA mutations [64].
Identification of new similar predictive mutations in OC patients could likewise improve the treatment of OC patients, but also improve the understanding of the molecular mechanism in OC etiologies. New knowledge in this area can lead to new drug targets. Moreover, new predictive biomarkers are of the highest importance for the improvement of cancer treatment, as more personalized and targeted treatment calls for new possibilities to identify the patients who will benefit from a specific treatment.
3. Results
The study is expected to generate a vast amount of results and we plan to publish 10–15 papers in relation to it. The first promising results, regarding miRNAs and prediction of resistance to platinum-based drugs, has already been published in the paper “Clinical validation of chemotherapy predictors developed on global microRNA expression in the NCI60 cell line panel tested in ovarian cancer” [28].
4. Discussion
Traditionally, cancer has been treated medically with chemotherapy which interferes with the cell cycle [4]. It has been a “one size fits all”-approach were cancer is treated as a primarily homogenous disease. However, not all patients respond equally well to chemotherapeutic treatment, and the risk of developing resistance is high. A primary reason for the diversity in reaction-pattern is, that cancer is a very heterogeneous condition [51], [65]. With the continuing improvement in molecular areas like ”omics,” and the increasing knowledge of molecular disease etiologies, cancer is now seen as a large array of individual diseases rather than the limited number of cancers grouped by organ and histology that we have operated with until now. This enables a more personalized and effective treatment, and even treatment targeted directly at specific molecules. However, it is a prerequisite for this development, that clinical data from patient cohorts will be collected systematically, along with tissue and blood samples [16]. In this way, small patient groups with similar disease profiles, or even individuals, can be studied and followed retrospectively in order to identify markers that characterize the cancer at an individual level and predict which patients will benefit from a particular specialized therapy. The thorough collection of information is a great challenge as it takes a lot of resources. However the Danish Cancer Biobank and DGCD are working ahead of this problem, as we already are collecting and storing both information and biological samples from all cancer patients who agree to it. The thorough collection of patient data and material is unique to Denmark and holds a great research potential.
One of the great benefits of storing patient material and data is that it makes translational research possible and eases the work of identifying new biomarkers. New biomarkers are needed, as CA125 levels are only increased in 50% of patients with stage I OC, and CA125 can be elevated in other cancers or benign conditions [8], [9], [10]. A lot of work is already being done in this field. In 2009 FDA approved the use of a multivariate index assay called OVA1, which evaluate serum concentrations of 5 different markers (CA125-II, transferrin, transthyretin, apolipoprotein AI, and beta 2 microglobulin) [66], [67]. The test has a high sensitivity compared to CA125 alone, but a low specificity.
Another biomarker for OC which has gained a lot of interest recently is the glycoprotein Human Epididymis Protein 4 (HE4). It is highly expressed in the epithelial OC with no expression in healthy ovaries [68]. It has been shown that HE4 might be a better diagnostic OC marker than CA125, as it differentiates not only between benign and malignant disease with a higher sensitivity for cancer, but also between early FIGO stages of epithelial OC and benign conditions [69], [70], [71], [72]. However, an even bigger effect is seen if HE4 and CA125 are combined, and this led Moore et al. to develop the Risk of Ovarian Malignancy Algorithm (ROMA), which besides from Serum HE4 and CA125 levels also includes menopausal status [70], [73]. The majority of OC patients are postmenopausal when diagnosed [74]. The authors own results suggest that ROMA can improve differentiation between OC and benign disease compared to RMI, but the results of other studies have been diverging when it comes to the performance of the ROMA index [69], [72], [75]. By combining serum levels of HE4 and CA125 with age instead of menopausal stage, Karlsen et al. has developed the Copenhagen Index (CPH-I), and according to their results CPH-I, ROMA and RMI perform equally well when it comes to differentiating between benign conditions and OC [76], [77]. This has been confirmed by another study [78]. CPH-I might have an advantage compared to the other two indices, in that it only takes a blood test and knowledge of patient age, which makes it highly reproducible and easy to apply in a clinical setting.
Screening markers, which have high sensitivity and specificity and which could be measured pre-surgery in a blood test or cervix smear, has long been wished for. Recently, the UK Collaborative Trial of OC Screening (UKCTOCS) was published [11]. More than 200.000 women were included in this study, which is the largest randomized controlled study to evaluate a strategy for screening of OC. Participants were randomly screened using transvaginal ultrasound or the use of the Risk of Ovarian Cancer Algorithm (ROCA). ROCA calculates a statistical risk of OC from a series of CA125 serum level measurements, rather than the use of a single cutoff value [79]. This method omits some of the problems with the natural variation in CA125 levels, as focus is on the changes in CA125 level compared with a woman's own baseline level. However, the results of the large UKCTOCS screening were not as promising as hoped for, as there was no significant reduction in mortality in the primary analysis [11]. A significantly reduced mortality rate was observed when prevalent cases were removed, indicating that it may be possible to identify early stage OCs by the use of screening. Yet, OC is still the leading cause of death from gynecological malignancies and new markers and a better understanding of the disease etiology are needed if we wish to see a significant decline in deaths. Large screenings for cancer-related molecular changes, as the ones initiated under this study, holds the potential of bringing us closer to this goal, especially because we are doing a combined effort, not only investigating genetics, but also two different mechanism of gene regulation. Dysregulation of both DNA methylation and miRNAs have been shown to be highly involved in carcinogenesis. We need to study several types of biomarkers in order to identify sensitive and specific markers for both differentiation between disease and benign conditions and prediction of sensitivity to treatment, especially since there is a slight variation in the challenges and advantages between different kinds of molecular markers. The more classical way of working with nucleotides as biomarkers, is by assessing genetic changes like mutations. Cancer genetics is a well-studied field, and mutations have already proven useful as predictive and prognostic markers. However, mutations are not well suited in early diagnostics and screening, as ovarian cancer tissue is only accessible under surgery. Even though tumor DNA from dead cancer-cells can be found in circulation in the blood, it is not easily detectable due to the large background of cell free DNA present in circulation [80]. However, genetic changes are still important when it comes to precision medicine. Exome sequencing has made it possible to investigate the whole coding region of genomes, increasing the chance of discovering new biomarkers and drug-targets. When developing new drugs, it may also be easier to avoid side effects if the target is a single gene, and not regulatory units like miRNAs which may have many targets that will be affected simultaneously.
Both miRNAs and methylated DNA has great potential as prognostic and diagnostic markers, as changes in miRNA expression and DNA methylation has been correlated to different pathogenic processes including early carcinogenesis, metastasis and to sensitivity to treatment. DNA methylation is particularly interesting for screening and early diagnosis, as changes in methylation often precedes carcinogenesis and since circulating, methylated tumor DNA is more stable than RNAs or protein [81]. Moreover, methylation patterns are easier to detect than mutations as they are binary signals and can be amplified by methylation specific PCR-based techniques. Since DNA methylation is a reversible process, hypomethylating agents also has a promising potential in treating cancer and chemo resistance caused by erroneous methylation of promotors of tumor suppressors.
Despite the fact that body-fluids contain nucleases that degrade RNA, miRNAs in circulation are stable and protected, which makes them very suitable as biomarkers [31]. miRNAs, or miRNA antagonists, also has a huge therapeutic potential, as one miRNA often regulates several oncogenes or tumor suppressor genes, affecting more than one pathway.
The latest research in the area of OC has indicated that screening and early prognosis may be possible in the future and that resistance to treatment can be overcome by precision medicine. We might already be moving towards making OC a chronic disease which the patient can live with if well-treated. However, the goal is to make it a cancer which, in most cases, is discovered and treated before it spreads and becomes untreatable, even with the use of therapy, thereby improving survival. Mermaid III will reveal new and extensive information which can possibly be used to extend or save the lives of OC patients.
Acknowledgements
We thank the Danish Cancer Biobank and the Danish Gynecological Cancer Database (DGCD) for making this study possible by giving us access to biological samples and clinical data respectively. Funding: This work is supported by Mermaid III, who had no involvement in the decision to write this paper.
References
- 1.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2016. CA Cancer J. Clin. 2016;66(1):7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.DGCG . 2014-2015. DGCD Årsreport.http://www.dgcg.dk/images/rsrapport_DGCD_2014-15.pdf [Google Scholar]
- 3.Høgdall L.T.C.K., Nielsen M.L.S. 2011. DGCD Årsreport.http://www.dgcg.dk/images/DGCD%20rsrapport%202011.pdf [Google Scholar]
- 4.DGCG . 2016. Ovariecancer Guidelines.http://www.dgcg.dk/index.php/guidelines/ovariecancer-guidelines [Google Scholar]
- 5.Prat J. Figo Committee on Gynecologic Oncology, Staging classification for cancer of the ovary, fallopian tube. Perit. Int. J. Gynaecol. Obstet. 2014;124(1):1–5. doi: 10.1016/j.ijgo.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 6.Hamilton W., Peters T.J., Bankhead C., Sharp D. Risk of ovarian cancer in women with symptoms in primary care: population based case-control study. BMJ. 2009;339 doi: 10.1136/bmj.b2998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jacobs I., Oram D., Fairbanks J., Turner J., Frost C., Grudzinskas J.G. A risk of malignancy index incorporating CA 125, ultrasound and menopausal status for the accurate preoperative diagnosis of ovarian cancer. Br. J. Obstet. Gynaecol. 1990;97(10):922–929. doi: 10.1111/j.1471-0528.1990.tb02448.x. [DOI] [PubMed] [Google Scholar]
- 8.Nossov V., Amneus M., Su F., Lang J., Janco J.M., Reddy S.T., Farias-Eisner R. The early detection of ovarian cancer: from traditional methods to proteomics. Can we really do better than serum CA-125? Am. J. Obstet. Gynecol. 2008;199(3):215–223. doi: 10.1016/j.ajog.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 9.Sundar S., Neal R.D., Kehoe S. Diagnosis of ovarian cancer. BMJ. 2015;351 doi: 10.1136/bmj.h4443. [DOI] [PubMed] [Google Scholar]
- 10.Buamah P. Benign conditions associated with raised serum CA-125 concentration. J. Surg. Oncol. 2000;75(4):264–265. doi: 10.1002/1096-9098(200012)75:4<264::aid-jso7>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 11.Jacobs I.J., Menon U., Ryan A., Gentry-Maharaj A., Burnell M., Kalsi J.K., Amso N.N., Apostolidou S., Benjamin E., Cruickshank D., Crump D.N., Davies S.K., Dawnay A., Dobbs S., Fletcher G., Ford J., Godfrey K., Gunu R., Habib M., Hallett R., Herod J., Jenkins H., Karpinskyj C., Leeson S., Lewis S.J., Liston W.R., Lopes A., Mould T., Murdoch J., Oram D., Rabideau D.J., Reynolds K., Scott I., Seif M.W., Sharma A., Singh N., Taylor J., Warburton F., Widschwendter M., Williamson K., Woolas R., Fallowfield L., McGuire A.J., Campbell S., Parmar M., Skates S.J. Ovarian cancer screening and mortality in the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS): a randomised controlled trial. Lancet. 2016;387(10022):945–956. doi: 10.1016/S0140-6736(15)01224-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vergote I., Amant F., Kristensen G., Ehlen T., Reed N.S., Casado A. Primary surgery or neoadjuvant chemotherapy followed by interval debulking surgery in advanced ovarian cancer. Eur. J. Cancer. 2011;47(Suppl 3):S88–S92. doi: 10.1016/S0959-8049(11)70152-6. [DOI] [PubMed] [Google Scholar]
- 13.Vergote I., Trope C.G., Amant F., Kristensen G.B., Ehlen T., Johnson N., Verheijen R.H., van der Burg M.E., Lacave A.J., Panici P.B., Kenter G.G., Casado A., Mendiola C., Coens C., Verleye L., Stuart G.C., Pecorelli S., Reed N.S., R European Organization for, G. Treatment of Cancer-Gynaecological Cancer, N.C.T. Group, Neoadjuvant chemotherapy or primary surgery in stage IIIC or IV ovarian cancer. N. Engl. J. Med. 2010;363(10):943–953. doi: 10.1056/NEJMoa0908806. [DOI] [PubMed] [Google Scholar]
- 14.Karlsen M.A., Fago-Olsen C., Hogdall E., Schnack T.H., Christensen I.J., Nedergaard L., Lundvall L., Lydolph M.C., Engelholm S.A., Hogdall C. A novel index for preoperative, non-invasive prediction of macro-radical primary surgery in patients with stage IIIC-IV ovarian cancer-a part of the Danish prospective pelvic mass study. Tumour Biol. 2016;37(9):12619–12626. doi: 10.1007/s13277-016-5166-z. [DOI] [PubMed] [Google Scholar]
- 15.Martin L.P., Schilder R.J. Management of recurrent ovarian carcinoma: current status and future directions. Semin. Oncol. 2009;36(2):112–125. doi: 10.1053/j.seminoncol.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 16.Barker R. Precision medicine: what's all the fuss about? Scand. J. Clin. Lab. Invest. Suppl. 2016;245:S2–S5. doi: 10.1080/00365513.2016.1206434. [DOI] [PubMed] [Google Scholar]
- 17.Mermaid, http://www.mermaidprojektet.dk/wp-content/uploads/2015/09/Evaluering-af-forskningen-i-MERMAID-I.pdf).
- 18.Sundhedsstyrelsen . 2016. Pakkeforløb for kræft i æggestokkene.https://www.sst.dk/da/udgivelser/2016/∼/media/765a142bc73446399c769a5d8b54c3dc.ashx [Google Scholar]
- 19.Bartel D.P. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116(2):281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 20.Flynt A.S., Lai E.C. Biological principles of microRNA-mediated regulation: shared themes amid diversity. Nat. Rev. Genet. 2008;9(11):831–842. doi: 10.1038/nrg2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang W., Dahlberg J.E., Tam W. MicroRNAs in tumorigenesis: a primer. Am. J. Pathol. 2007;171(3):728–738. doi: 10.2353/ajpath.2007.070070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zaman M.S., Maher D.M., Khan S., Jaggi M., Chauhan S.C. Current status and implications of microRNAs in ovarian cancer diagnosis and therapy. J. Ovarian Res. 2012;5(1):44. doi: 10.1186/1757-2215-5-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lou Y., Yang X., Wang F., Cui Z., Huang Y. MicroRNA-21 promotes the cell proliferation, invasion and migration abilities in ovarian epithelial carcinomas through inhibiting the expression of PTEN protein. Int. J. Mol. Med. 2010;26(6):819–827. doi: 10.3892/ijmm_00000530. [DOI] [PubMed] [Google Scholar]
- 24.Johnson S.M., Grosshans H., Shingara J., Byrom M., Jarvis R., Cheng A., Labourier E., Reinert K.L., Brown D., Slack F.J. RAS is regulated by the let-7 microRNA family. Cell. 2005;120(5):635–647. doi: 10.1016/j.cell.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 25.Yang H., Kong W., He L., Zhao J.J., O'Donnell J.D., Wang J., Wenham R.M., Coppola D., Kruk P.A., Nicosia S.V., Cheng J.Q. MicroRNA expression profiling in human ovarian cancer: miR-214 induces cell survival and cisplatin resistance by targeting PTEN. Cancer Res. 2008;68(2):425–433. doi: 10.1158/0008-5472.CAN-07-2488. [DOI] [PubMed] [Google Scholar]
- 26.Yang N., Kaur S., Volinia S., Greshock J., Lassus H., Hasegawa K., Liang S., Leminen A., Deng S., Smith L., Johnstone C.N., Chen X.M., Liu C.G., Huang Q., Katsaros D., Calin G.A., Weber B.L., Butzow R., Croce C.M., Coukos G., Zhang L. MicroRNA microarray identifies Let-7i as a novel biomarker and therapeutic target in human epithelial ovarian cancer. Cancer Res. 2008;68(24):10307–10314. doi: 10.1158/0008-5472.CAN-08-1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kapetanakis N.I., Uzan C., Jimenez-Pailhes A.S., Gouy S., Bentivegna E., Morice P., Caron O., Gourzones-Dmitriev C., Le Teuff G., Busson P. Plasma miR-200b in ovarian carcinoma patients: distinct pattern of pre/post-treatment variation compared to CA-125 and potential for prediction of progression-free survival. Oncotarget. 2015;6(34):36815–36824. doi: 10.18632/oncotarget.5766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Prahm K.P., Hogdall C., Karlsen M.A., Christensen I.J., Novotny G.W., Knudsen S., Hansen A., Jensen P.B., Jensen T., Mirza M.R., Ekmann-Gade A.W., Nedergaard L., Hogdall E. Clinical validation of chemotherapy predictors developed on global microRNA expression in the NCI60 cell line panel tested in ovarian cancer. PLoS One. 2017;12(3) doi: 10.1371/journal.pone.0174300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Knudsen S., Hother C., Gronbaek K., Jensen T., Hansen A., Mazin W., Dahlgaard J., Moller M.B., Ralfkiaer E., Brown Pde N. Development and blind clinical validation of a microRNA based predictor of response to treatment with R-CHO(E)P in DLBCL. PLoS One. 2015;10(2) doi: 10.1371/journal.pone.0115538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Winther M., Knudsen S., Dahlgaard J., Jensen T., Hansen A., Jensen P.B., Tramm T., Alsner J., Nordsmark M. Clinical impact of a novel MicroRNA chemo-sensitivity predictor in gastrooesophageal cancer. PLoS One. 2016;11(2) doi: 10.1371/journal.pone.0148070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mitchell P.S., Parkin R.K., Kroh E.M., Fritz B.R., Wyman S.K., Pogosova-Agadjanyan E.L., Peterson A., Noteboom J., O'Briant K.C., Allen A., Lin D.W., Urban N., Drescher C.W., Knudsen B.S., Stirewalt D.L., Gentleman R., Vessella R.L., Nelson P.S., Martin D.B., Tewari M. Circulating microRNAs as stable blood-based markers for cancer detection. Proc. Natl. Acad. Sci. U. S. A. 2008;105(30):10513–10518. doi: 10.1073/pnas.0804549105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Taylor D.D., Gercel-Taylor C. MicroRNA signatures of tumor-derived exosomes as diagnostic biomarkers of ovarian cancer. Gynecol. Oncol. 2008;110(1):13–21. doi: 10.1016/j.ygyno.2008.04.033. [DOI] [PubMed] [Google Scholar]
- 33.Zheng H., Zhang L., Zhao Y., Yang D., Song F., Wen Y., Hao Q., Hu Z., Zhang W., Chen K. Plasma miRNAs as diagnostic and prognostic biomarkers for ovarian cancer. PLoS One. 2013;8(11) doi: 10.1371/journal.pone.0077853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Beg M.S., Brenner A.J., Sachdev J., Borad M., Kang Y.K., Stoudemire J., Smith S., Bader A.G., Kim S., Hong D.S. Phase I study of MRX34, a liposomal miR-34a mimic, administered twice weekly in patients with advanced solid tumors. Invest. New Drugs. 2017;35(2):180–188. doi: 10.1007/s10637-016-0407-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Karpf A.R., Jones D.A. Reactivating the expression of methylation silenced genes in human cancer. Oncogene. 2002;21(35):5496–5503. doi: 10.1038/sj.onc.1205602. [DOI] [PubMed] [Google Scholar]
- 36.Wolffe A.P., Matzke M.A. Epigenetics: regulation through repression. Science. 1999;286(5439):481–486. doi: 10.1126/science.286.5439.481. [DOI] [PubMed] [Google Scholar]
- 37.Baylin S.B., Jones P.A. A decade of exploring the cancer epigenome - biological and translational implications. Nat. Rev. Cancer. 2011;11(10):726–734. doi: 10.1038/nrc3130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xiao X., Cai F., Niu X., Shi H., Zhong Y. Association between P16INK4a promoter methylation and ovarian cancer: a meta-analysis of 12 published studies. PLoS One. 2016;11(9) doi: 10.1371/journal.pone.0163257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Saito Y., Kanai Y., Nakagawa T., Sakamoto M., Saito H., Ishii H., Hirohashi S. Increased protein expression of DNA methyltransferase (DNMT) 1 is significantly correlated with the malignant potential and poor prognosis of human hepatocellular carcinomas. Int. J. Cancer. 2003;105(4):527–532. doi: 10.1002/ijc.11127. [DOI] [PubMed] [Google Scholar]
- 40.Feinberg A.P., Vogelstein B. Hypomethylation distinguishes genes of some human cancers from their normal counterparts. Nature. 1983;301(5895):89–92. doi: 10.1038/301089a0. [DOI] [PubMed] [Google Scholar]
- 41.Eden A., Gaudet F., Waghmare A., Jaenisch R. Chromosomal instability and tumors promoted by DNA hypomethylation. Science. 2003;300(5618):455. doi: 10.1126/science.1083557. [DOI] [PubMed] [Google Scholar]
- 42.Widschwendter M., Fiegl H., Egle D., Mueller-Holzner E., Spizzo G., Marth C., Weisenberger D.J., Campan M., Young J., Jacobs I., Laird P.W. Epigenetic stem cell signature in cancer. Nat. Genet. 2007;39(2):157–158. doi: 10.1038/ng1941. [DOI] [PubMed] [Google Scholar]
- 43.Zhuang J., Jones A., Lee S.H., Ng E., Fiegl H., Zikan M., Cibula D., Sargent A., Salvesen H.B., Jacobs I.J., Kitchener H.C., Teschendorff A.E., Widschwendter M. The dynamics and prognostic potential of DNA methylation changes at stem cell gene loci in women's cancer. PLoS Genet. 2012;8(2) doi: 10.1371/journal.pgen.1002517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Teschendorff A.E., Jones A., Fiegl H., Sargent A., Zhuang J.J., Kitchener H.C., Widschwendter M. Epigenetic variability in cells of normal cytology is associated with the risk of future morphological transformation. Genome Med. 2012;4(3):24. doi: 10.1186/gm323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ibanez de Caceres I., Battagli C., Esteller M., Herman J.G., Dulaimi E., Edelson M.I., Bergman C., Ehya H., Eisenberg B.L., Cairns P. Tumor cell-specific BRCA1 and RASSF1A hypermethylation in serum, plasma, and peritoneal fluid from ovarian cancer patients. Cancer Res. 2004;64(18):6476–6481. doi: 10.1158/0008-5472.CAN-04-1529. [DOI] [PubMed] [Google Scholar]
- 46.Talens R.P., Boomsma D.I., Tobi E.W., Kremer D., Jukema J.W., Willemsen G., Putter H., Slagboom P.E., Heijmans B.T. Variation, patterns, and temporal stability of DNA methylation: considerations for epigenetic epidemiology. FASEB J. 2010;24(9):3135–3144. doi: 10.1096/fj.09-150490. [DOI] [PubMed] [Google Scholar]
- 47.Kurman R.J. Origin and molecular pathogenesis of ovarian high-grade serous carcinoma. Ann. Oncol. 2013;24(Suppl 10):x16–21. doi: 10.1093/annonc/mdt463. [DOI] [PubMed] [Google Scholar]
- 48.Kurman R.J., Shih Ie M. The dualistic model of ovarian carcinogenesis: revisited, revised, and expanded. Am. J. Pathol. 2016;186(4):733–747. doi: 10.1016/j.ajpath.2015.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bartlett T.E., Chindera K., McDermott J., Breeze C.E., Cooke W.R., Jones A., Reisel D., Karegodar S.T., Arora R., Beck S., Menon U., Dubeau L., Widschwendter M. Epigenetic reprogramming of fallopian tube fimbriae in BRCA mutation carriers defines early ovarian cancer evolution. Nat. Commun. 2016;7:11620. doi: 10.1038/ncomms11620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Matei D.E., Nephew K.P. Epigenetic therapies for chemoresensitization of epithelial ovarian cancer. Gynecol. Oncol. 2010;116(2):195–201. doi: 10.1016/j.ygyno.2009.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bolton K.L., Ganda C., Berchuck A., Pharaoh P.D., Gayther S.A. Role of common genetic variants in ovarian cancer susceptibility and outcome: progress to date from the Ovarian Cancer Association Consortium (OCAC) J. Intern Med. 2012;271(4):366–378. doi: 10.1111/j.1365-2796.2011.02509.x. [DOI] [PubMed] [Google Scholar]
- 52.Surget S., Khoury M.P., Bourdon J.C. Uncovering the role of p53 splice variants in human malignancy: a clinical perspective. Onco Targets Ther. 2013;7:57–68. doi: 10.2147/OTT.S53876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Downward J. Targeting RAS signalling pathways in cancer therapy. Nat. Rev. Cancer. 2003;3(1):11–22. doi: 10.1038/nrc969. [DOI] [PubMed] [Google Scholar]
- 54.Kmet L.M., Cook L.S., Magliocco A.M. A review of p53 expression and mutation in human benign, low malignant potential, and invasive epithelial ovarian tumors. Cancer. 2003;97(2):389–404. doi: 10.1002/cncr.11064. [DOI] [PubMed] [Google Scholar]
- 55.Hogdall E.V., Kjaer S.K., Blaakaer J., Christensen L., Glud E., Vuust J., Hogdall C.K. P53 mutations in tissue from Danish ovarian cancer patients: from the Danish "MALOVA" ovarian cancer study. Gynecol. Oncol. 2006;100(1):76–82. doi: 10.1016/j.ygyno.2005.07.131. [DOI] [PubMed] [Google Scholar]
- 56.Christie M., Oehler M.K. Molecular pathology of epithelial ovarian cancer. J. Br. Menopause Soc. 2006;12(2):57–63. doi: 10.1258/136218006777525794. [DOI] [PubMed] [Google Scholar]
- 57.Mavaddat N., Peock S., Frost D., Ellis S., Platte R., Fineberg E., Evans D.G., Izatt L., Eeles R.A., Adlard J., Davidson R., Eccles D., Cole T., Cook J., Brewer C., Tischkowitz M., Douglas F., Hodgson S., Walker L., Porteous M.E., Morrison P.J., Side L.E., Kennedy M.J., Houghton C., Donaldson A., Rogers M.T., Dorkins H., Miedzybrodzka Z., Gregory H., Eason J., Barwell J., McCann E., Murray A., Antoniou A.C., Easton D.F. Embrace, Cancer risks for BRCA1 and BRCA2 mutation carriers: results from prospective analysis of EMBRACE. J. Natl. Cancer Inst. 2013;105(11):812–822. doi: 10.1093/jnci/djt095. [DOI] [PubMed] [Google Scholar]
- 58.King M.C., Marks J.H., Mandell J.B., G New York Breast Cancer Study, Breast and ovarian cancer risks due to inherited mutations in BRCA1 and BRCA2. Science. 2003;302(5645):643–646. doi: 10.1126/science.1088759. [DOI] [PubMed] [Google Scholar]
- 59.Paolillo C., Londin E., Fortina P. Next generation sequencing in cancer: opportunities and challenges for precision cancer medicine. Scand. J. Clin. Lab. Invest. Suppl. 2016;245:S84–S91. doi: 10.1080/00365513.2016.1210331. [DOI] [PubMed] [Google Scholar]
- 60.Mack G.S. FDA holds court on post hoc data linking KRAS status to drug response. Nat. Biotechnol. 2009;27(2):110–112. doi: 10.1038/nbt0209-110c. [DOI] [PubMed] [Google Scholar]
- 61.Kamel-Reid S., Zhang T., Persons D.L., Nikiforova M.N., Halling K.C., P Molecular Oncology Resource Committee of the College of American, Validation of KRAS testing for anti-EGFR therapeutic decisions for patients with metastatic colorectal carcinoma. Arch. Pathol. Lab. Med. 2012;136(1):26–32. doi: 10.5858/arpa.2011-0220-OA. [DOI] [PubMed] [Google Scholar]
- 62.Dahabreh I.J., Terasawa T., Castaldi P.J., Trikalinos T.A. Systematic review: anti-epidermal growth factor receptor treatment effect modification by KRAS mutations in advanced colorectal cancer. Ann. Intern Med. 2011;154(1):37–49. doi: 10.7326/0003-4819-154-1-201101040-00006. [DOI] [PubMed] [Google Scholar]
- 63.Ledermann J., Harter P., Gourley C., Friedlander M., Vergote I., Rustin G., Scott C.L., Meier W., Shapira-Frommer R., Safra T., Matei D., Fielding A., Spencer S., Dougherty B., Orr M., Hodgson D., Barrett J.C., Matulonis U. Olaparib maintenance therapy in patients with platinum-sensitive relapsed serous ovarian cancer: a preplanned retrospective analysis of outcomes by BRCA status in a randomised phase 2 trial. Lancet Oncol. 2014;15(8):852–861. doi: 10.1016/S1470-2045(14)70228-1. [DOI] [PubMed] [Google Scholar]
- 64.Mirza M.R., Monk B.J., Herrstedt J., Oza A.M., Mahner S., Redondo A., Fabbro M., Ledermann J.A., Lorusso D., Vergote I., Ben-Baruch N.E., Marth C., Madry R., Christensen R.D., Berek J.S., Dorum A., Tinker A.V., du Bois A., Gonzalez-Martin A., Follana P., Benigno B., Rosenberg P., Gilbert L., Rimel B.J., Buscema J., Balser J.P., Agarwal S., Matulonis U.A., Investigators E.-O.N. Niraparib maintenance therapy in platinum-sensitive, recurrent ovarian cancer. N. Engl. J. Med. 2016;375(22):2154–2164. doi: 10.1056/NEJMoa1611310. [DOI] [PubMed] [Google Scholar]
- 65.N. Cancer Genome Atlas Research Integrated genomic analyses of ovarian carcinoma. Nature. 2011;474(7353):609–615. doi: 10.1038/nature10166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ueland F.R., Desimone C.P., Seamon L.G., Miller R.A., Goodrich S., Podzielinski I., Sokoll L., Smith A., van Nagell J.R., Jr., Zhang Z. Effectiveness of a multivariate index assay in the preoperative assessment of ovarian tumors. Obstet. Gynecol. 2011;117(6):1289–1297. doi: 10.1097/AOG.0b013e31821b5118. [DOI] [PubMed] [Google Scholar]
- 67.Ware Miller R., Smith A., DeSimone C.P., Seamon L., Goodrich S., Podzielinski I., Sokoll L., van Nagell J.R., Jr., Zhang Z., Ueland F.R. Performance of the American College of Obstetricians and Gynecologists' ovarian tumor referral guidelines with a multivariate index assay. Obstet. Gynecol. 2011;117(6):1298–1306. doi: 10.1097/AOG.0b013e31821b1d80. [DOI] [PubMed] [Google Scholar]
- 68.Hellstrom I., Raycraft J., Hayden-Ledbetter M., Ledbetter J.A., Schummer M., McIntosh M., Drescher C., Urban N., Hellstrom K.E. The HE4 (WFDC2) protein is a biomarker for ovarian carcinoma. Cancer Res. 2003;63(13):3695–3700. [PubMed] [Google Scholar]
- 69.Sandri M.T., Bottari F., Franchi D., Boveri S., Candiani M., Ronzoni S., Peiretti M., Radice D., Passerini R., Sideri M. Comparison of HE4, CA125 and ROMA algorithm in women with a pelvic mass: correlation with pathological outcome. Gynecol. Oncol. 2013;128(2):233–238. doi: 10.1016/j.ygyno.2012.11.026. [DOI] [PubMed] [Google Scholar]
- 70.Moore R.G., Brown A.K., Miller M.C., Skates S., Allard W.J., Verch T., Steinhoff M., Messerlian G., DiSilvestro P., Granai C.O., Bast R.C., Jr. The use of multiple novel tumor biomarkers for the detection of ovarian carcinoma in patients with a pelvic mass. Gynecol. Oncol. 2008;108(2):402–408. doi: 10.1016/j.ygyno.2007.10.017. [DOI] [PubMed] [Google Scholar]
- 71.Holcomb K., Vucetic Z., Miller M.C., Knapp R.C. Human epididymis protein 4 offers superior specificity in the differentiation of benign and malignant adnexal masses in premenopausal women. Am. J. Obstet. Gynecol. 2011;205(4):e1–6. doi: 10.1016/j.ajog.2011.05.017. 358. [DOI] [PubMed] [Google Scholar]
- 72.Montagnana M., Danese E., Ruzzenente O., Bresciani V., Nuzzo T., Gelati M., Salvagno G.L., Franchi M., Lippi G., Guidi G.C. The ROMA (Risk of Ovarian Malignancy Algorithm) for estimating the risk of epithelial ovarian cancer in women presenting with pelvic mass: is it really useful? Clin. Chem. Lab. Med. 2011;49(3):521–525. doi: 10.1515/CCLM.2011.075. [DOI] [PubMed] [Google Scholar]
- 73.Moore R.G., McMeekin D.S., Brown A.K., DiSilvestro P., Miller M.C., Allard W.J., Gajewski W., Kurman R., Bast R.C., Jr., Skates S.J. A novel multiple marker bioassay utilizing HE4 and CA125 for the prediction of ovarian cancer in patients with a pelvic mass. Gynecol. Oncol. 2009;112(1):40–46. doi: 10.1016/j.ygyno.2008.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cancer_Research_UK, 2014).
- 75.Karlsen M.A., Sandhu N., Hogdall C., Christensen I.J., Nedergaard L., Lundvall L., Engelholm S.A., Pedersen A.T., Hartwell D., Lydolph M., Laursen I.A., Hogdall E.V. Evaluation of HE4, CA125, risk of ovarian malignancy algorithm (ROMA) and risk of malignancy index (RMI) as diagnostic tools of epithelial ovarian cancer in patients with a pelvic mass. Gynecol. Oncol. 2012;127(2):379–383. doi: 10.1016/j.ygyno.2012.07.106. [DOI] [PubMed] [Google Scholar]
- 76.Karlsen M.A., Hogdall E.V., Christensen I.J., Borgfeldt C., Kalapotharakos G., Zdrazilova-Dubska L., Chovanec J., Lok C.A., Stiekema A., Mutz-Dehbalaie I., Rosenthal A.N., Moore E.K., Schodin B.A., Sumpaico W.W., Sundfeldt K., Kristjansdottir B., Zapardiel I., Hogdall C.K. A novel diagnostic index combining HE4, CA125 and age may improve triage of women with suspected ovarian cancer - an international multicenter study in women with an ovarian mass. Gynecol. Oncol. 2015;138(3):640–646. doi: 10.1016/j.ygyno.2015.06.021. [DOI] [PubMed] [Google Scholar]
- 77.Hogdall E. Approaches to the detection of ovarian cancer. Scand. J. Clin. Lab. Invest. Suppl. 2016;245:S49–S53. doi: 10.1080/00365513.2016.1208452. [DOI] [PubMed] [Google Scholar]
- 78.Yoshida A., Derchain S.F., Pitta D.R., Andrade L.A., Sarian L.O. Comparing the Copenhagen Index (CPH-I) and Risk of Ovarian Malignancy Algorithm (ROMA): two equivalent ways to differentiate malignant from benign ovarian tumors before surgery? Gynecol. Oncol. 2016;140(3):481–485. doi: 10.1016/j.ygyno.2016.01.023. [DOI] [PubMed] [Google Scholar]
- 79.Skates S.J. Ovarian cancer screening: development of the risk of ovarian cancer algorithm (ROCA) and ROCA screening trials. Int. J. Gynecol. Cancer. 2012;22(Suppl 1):S24–S26. doi: 10.1097/IGC.0b013e318256488a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Henry N.L. The pathway to clinical use of a cancer biomarker. Scand. J. Clin. Lab. Invest. Suppl. 2016;245:S17–S21. doi: 10.1080/00365513.2016.1206441. [DOI] [PubMed] [Google Scholar]
- 81.Barton C.A., Hacker N.F., Clark S.J., O'Brien P.M. DNA methylation changes in ovarian cancer: implications for early diagnosis, prognosis and treatment. Gynecol. Oncol. 2008;109(1):129–139. doi: 10.1016/j.ygyno.2007.12.017. [DOI] [PubMed] [Google Scholar]