Abstract
Recruitment into clinical research studies is a major challenge. This study was carried out to explore the perceptions and attitudes towards clinical research participation among the general public in Qatar. A population based questionnaire study was carried out at public events held in Qatar. Residents of Qatar, 18 years or above in age were surveyed, anonymously, following verbal consent. Descriptive and multivariate analyses were conducted. We administered 2517 questionnaires to examine clinical research participation, of which 2379 complete forms were analyzed. Those who had previously been approached to participate in research completed a more detailed assessment. Data showed that only 5.7% participants (n = 134) had previously been approached to participate in a clinical research study. Of these 63.4% (n = 85) had agreed to participate while 36.6% (n = 49) had declined. The main reasons for declining participation included: time constraint (47.8%, n = 11), ‘fear’ (13.0%, n = 3), lack of awareness about clinical research (8.7%, n = 2) and lack of interest (8.7%, n = 2). ‘To help others’ (31.8%, n = 27) and ‘thought it might improve my access to health care’ (24.7%, n = 21) were the prime motivators for participation. There was a general agreement among participants that their previous research experience was associated with positive outcomes for self and others, that the research conduct was ethical, and that opportunities for participation will be welcomed in future. More than ten years of stay within Qatar was a statistically significant determinant of willingness to participate, adjusted odds ratio 5.82 (95% CI 1.93–17.55), p = 0.002. Clinical research participation in Qatar needs improvement. Time constraints, lack of trust in and poor awareness about clinical research are main barriers to participation. Altruism, and improved health access are reported as prime motivators. Deeper insight in to the factors affecting clinical research participation is needed to devise evidence based policies for improvement in recruitment strategies.
Keywords: Attitudes, Perceptions, Barriers, Motivators, Clinical research, Qatar
1. Introduction
Recruitment into clinical research studies is a major continuing challenge [1]. The general public and patients are still not fully aware of the importance of participation in clinical research studies for the development and implementation of medical advances [2]. Not all clinical research studies meet their recruitment targets, and as few as 6% of eligible subjects may participate in a clinical research trial [3], [4].
Insufficient recruitment into research studies has significant implications [3]. Clinically important findings may be missed due to statistical non-significance, preventing or causing delays in demonstrating the value of interventions in clinical practice [2]. Many clinical research trials are abandoned or produce equivocal results due to recruitment difficulties. This leads to loss of return on the resources expended in designing, developing, setting up, and conducting a clinical research trial as well as implications for the reputation of study investigators and institutions associated with an unsuccessful study [2], [4].
The Middle East and North Africa (MENA) region is undergoing rapid development and population expansion. This has been accompanied by an increasing prevalence of both acute and chronic medical disorders. In particular, there is an alarming increase in the prevalence of obesity and type 2 diabetes mellitus, particularly in countries that are part of the Gulf Cooperation Council (GCC). To tackle the region's health challenges, there is an urgent need for clinical research studies, and in particular, randomized controlled trials. Clinical trial participant density in the MENA region is less than 1%, suggesting that a sizable population has not yet been engaged in clinical research [5]. Clinical research remains under-developed in the MENA region. Despite growing investment supported by the wealth of the region, clinical research in the MENA region accounts for 0.5% of the total global clinical trial sites compared to 66% in North America and Western Europe [6].
While there are structural and demographic challenges for the successful conduct of clinical research in the MENA region, little is known about perceptions of clinical research by potential participants. Surveys of attitudes to research participation in the MENA region have been limited [7]. To improve the success of clinical research studies, it is necessary to identify and explore the factors involved in low study enrollment and the public's perceptions towards research [2]. The aim of our study was to determine the perceptions towards clinical research in Qatar, a GCC country, where there is an increasing need to determine effective approaches to key medical disorders through the conduct of successful clinical research studies.
2. Methods
A questionnaire survey was conducted at two major public events held in Doha in Qatar between December 2014 and February 2015. National events are well attended in Qatar. The events for data collection marked Qatar's National Day and Qatar's National Sports Day, respectively. Both events attract large numbers of people from all backgrounds, therefore closely representing Qatar's multicultural population. Of the 2.3 million population in Qatar, the indigenous Qataris make up between 10 and 15% [8] [9], while the rest are expatriates. The proportion of Qataris in our study was 10.5% while remainder were expatriates.
The survey was conducted to explore the existing attitudes and behaviors prevalent among the population in Qatar. All visitors who came to a health awareness booth and who were able to speak, read and understand Arabic or English were approached about completing an anonymous survey and consented verbally by majority female clinical research coordinators. A semi structured questionnaire, available in English and Arabic, was then completed with residents of Qatar who were 18 years or above in age. Those who had previously participated in research studies were asked to rate a set of 23 pre-defined statements relating to their research experience on a Likert Scale ranging between Strongly Disagree to Strongly Agree. Tourists and visitors were excluded. The anonymous survey was approved and given exempt status by the Joint Institutional Review Board (JIRB) of Weill Cornell Medicine - Qatar and Hamad Medical Corporation (Doha, Qatar).
2.1. Statistical analysis
Completed surveys were analyzed on SPSS version 23. Descriptive analyses were conducted along with univariate and multivariate analyses to explore the relationships amongst different variables and willingness to participate in clinical research. Univariate analysis was performed by using Pearson's Chi Square tests and multivariate analysis using logistic regression. All potential confounding variables (gender, age, BMI, length of stay, education level, employment and comorbidity) were included in the final multivariate logistic regression model. Multicollinearity was assessed by performing bivariate linear regression between the variables and calculating the variance inflation factor (VIF). A VIF of <2.5 was deemed to indicate no evidence of multicollinearity [10]. A two sided P value of <0.05 was considered to be statistically significant. Participants were divided into three categories based upon their country or region of origin: Qataris (Q), Non-Qatari Arabs (NQA) and Non-Arabs (NA).
3. Results
A total of 2517 adults were surveyed with 2379 valid responses. Invalid surveys included those from ineligible participants (visitors or less than 18 years old individuals) or insufficient data (missing all or 90% or more of the responses to the survey). Approached subjects were approximately equally distributed by gender (females, 46%), and were employed (70%), living in Qatar ≤10 years, and had a good level of education (72% with college education and above) (Table 1). The majority of the surveyed population had never been approached or invited to participate in a clinical research study. These did not vary by length of stay (88.5% length of stay ≤10 years, 89.1% length of stay >10 years). Of the 5.6% participants (n = 134) who had previously been approached to participate in clinical research, 63.4% (n = 85) had agreed to participate while 36.6% (n = 49) had declined. Data for this question was missing for the remaining 5.6% (n = 132) surveyed.
Table 1.
Demographic characteristics of surveyed population with valid responses (n = 2379).
| Gender, n (%) | Males | 1267 (54.0) |
| Females | 1081 (46.0) | |
| Age, n (%) | 18-24 years | 208 (8.9) |
| 25-34 years | 934 (39.7) | |
| 35-44 years | 895 (38.1) | |
| 45-60 years | 281 (12.0) | |
| >60 years | 33 (1.4) | |
| Level of Education, n (%) | Below Elementary or None | 52 (2.2) |
| Elementary | 61 (2.6) | |
| Secondary | 539 (23.2) | |
| College and above | 1669 (71.9) | |
| Employment, n (%) | Currently Employed | 1643 (70.0) |
| Not employed | 709 (30.0) | |
| Workinga Hours/week, n (%) | ≤40 h | 899 (58.2) |
| >40 h | 645 (41.8) | |
| Length of Stay, n (%) | ≤10 years | 1438 (68.7) |
| >10 years | 655 (31.3) | |
| Comorbidity+, n (%) | Yes | 336 (14.4) |
| No | 2004 (85.6) | |
| Nationality, n (%) | Qatari | 201 (9.9) |
| Non Arab | 869 (42.6) | |
| Non Qatari Arab | 969 (47.5) |
Employed subjects only; +Comorbidity e.g. diabetes, hypertension.
3.1. Participants in clinical research
Among those who had previously participated in a clinical research study, 48.8% were Non-Arab nationalities (n = 41), 41.7% were Non-Qatari Arabs (n = 35) and 9.5% were Qatari participants (n = 8).
There were no significant differences observed in the demographic characteristics of the three groups. However, while the majority of Qatari participants were between 25 and 34 years of age, the Non-Qatari Arabs and Non-Arab participants were mostly in the age group between 35 and 44 years, with the majority having resided in Qatar for ten years or less. The majority of participants in all three groups were well-educated with four years of college or above education and employed at the time of the survey.
In those who had participated in research, male participants (60.2%, n = 50) outnumbered female participants (39.8% n = 33). The majority of participants were between 25 and 44 years of age (69.4%, n = 59) with a little over half having resided in Qatar for less than or equal to ten years. Participants in this group displayed high levels of education with approximately two thirds having attained a college degree or above. A similar proportion were employed at the time of survey. No participant in this cohort had received less than elementary education (Table 2).
Table 2.
Comparison of demographic characteristics of surveyed population who had agreed to participate in clinical research with those who did not participate in clinical research.
| Participated (n = 85) | Did not participate (n = 49) | P-value | ||
|---|---|---|---|---|
| Gender, n (%) | Male | 50 (60.2) | 31 (63.3) | 0.730 |
| Female | 33 (39.8) | 18 (36.7) | ||
| Missing | 2 | 0 | ||
| Age, n (%) | 18-24 years | 9 (10.6) | 6 (12.2) | 0.492 |
| 25-34 years | 28 (32.9) | 9 (18.4) | ||
| 35-44 years | 31 (36.5) | 22 (44.9) | ||
| 45-60 years | 15 (17.6) | 11 (22.5) | ||
| >60 years | 2 (2.4) | 1 (2.0) | ||
| Missing | 0 | 0 | ||
| Level of Education, n (%) | Below Elementary or None | 0 (0) | (0) | 0.781 |
| Elementary | 3 (3.5) | 1 (2.0) | ||
| Secondary | 11 (12.9) | 8 (16.3) | ||
| College and above | 71 (83.5) | 40 (81.6) | ||
| Missing | 0 | 0 | ||
| Employment, n (%) | Currently Employed | 67 (80.7) | 39 (78.6) | 0.875 |
| Not employed | 16 (19.3) | 10 (20.4) | ||
| Missing | 2 | 0 | ||
| Workinga Hours/week, n (%) | < or equal to 40 h | 35 (56.5) | 24 (61.5) | 0.614 |
| >40 h | 27 (43.5) | 15 (38.5) | ||
| Missing | 7 | 0 | ||
| Length of Stay, n (%) | < or equal to 10 years | 47 (61.0) | 37 (86.0) | 0.004 |
| >10 years | 30 (39.0) | 6 (14.0) | ||
| Missing | 8 | 6 | ||
| Comorbidity+, n (%) | Yes | 24 (28.6) | 10 (20.8) | 0.328 |
| No | 60 (71.4) | 38 (79.2) | ||
| Missing | 1 | 1 | ||
| Nationality, n (%) | Qatari | 8 (9.5) | 4 (8.3) | 0.509 |
| Non Arab | 41 (48.8) | 19 (39.6) | ||
| Non Qatari Arab | 35 (41.7) | 25 (52.1) | ||
| Missing | 1 | 1 | ||
Employed subjects only; +Comorbidity e.g. diabetes, hypertension.
Those who had lived in Qatar for over ten years were more likely to have participated in research than those who had lived for ten years or under. This was statistically significant in both the univariate (p = 0.004) and multivariate (p = 0.002) analyses (Table 3). Those who resided in Qatar for more than ten years were almost 6 times more likely to have participated in a clinical study, adjusted odds ratio 5.82 (95% CI 1.93–17.55).
Table 3.
Multivariate analysis of factors associated with participation in clinical research.
| Variable | Odds Ratio (95% CI) | P-value | |
|---|---|---|---|
| Gender | Male (n = 81) | 1.00 | 0.409 |
| Female (n = 51) | 1.57 (0.54–4.60) | ||
| Age | 18-24 years (n = 15) | 1.00 | 0.259 |
| 25-34 years (n = 37) | 1.43 (0.30–6.80) | ||
| 35-44 years (n = 53) | 0.43 (0.10–1.88) | ||
| 45-60 years (n = 26) | 0.40 (0.08–2.08) | ||
| >60 years (n = 3) | 0.94 (0.05–19.28) | ||
| Level of Education | College and above (n = 111) | 1.00 | 0.978 |
| Elementary (n = 4) | 0.75 (0.04–14.47) | ||
| Secondary or less (n = 19) | 0.93 (0.23–3.76) | ||
| Working Hours/week | ≤40 h (n = 59) | 1.00 | 0.683 |
| >40 h (n = 42) | 1.61 (0.54–4.79) | ||
| Length of Stay | ≤10 years (n = 84) | 1.00 | 0.002 |
| >10 years (n = 36) | 5.82 (1.93–17.55) | ||
| Co Morbid | No (n = 98) | 1.00 | 0.504 |
| Yes (n = 34) | 1.46 (0.48–4.46) | ||
| Nationality | Qatari (n = 12) | 1.00 | 0.474 |
| Non Arab (n = 60) | 2.13 (0.10–43.06) | ||
| Non Qatari Arab (n = 60) |
1.23 (0.06–23.59) | ||
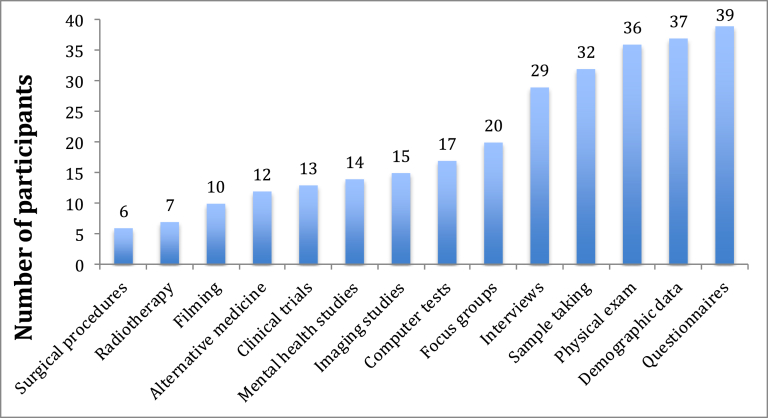
Participants had mostly participated in studies about diabetes (37%) and heart disease (7.4%), which are common health problems in Qatar. Simple data collection methods such as questionnaires (n = 39), demographic data (n = 37), physical examination (n = 36), biological sample collection (n = 32) and interviews (n = 29) were most abundantly used while clinical trials followed in fewer numbers (n = 13; Fig. 1).
Fig. 1.
Data collection methods used in studies that respondents participated in.
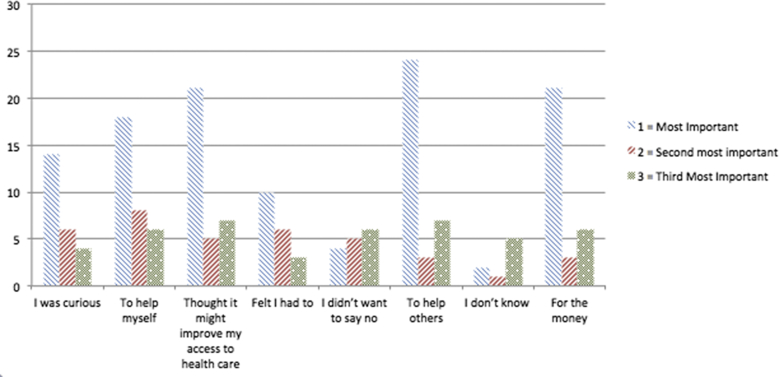
Participants were asked to rate their reasons for participation in clinical research on a scale of 1–3, where 1 was ‘the most important’, 2 was ‘second most important’ and 3 was ‘third most important’ factor (Fig. 2). Based on the responses; ‘to help others’ was the most important driving factor in research participation. The next most important motivators were ‘thought it might improve my access to healthcare’ (n = 21) and ‘to help myself’ (n = 21). Financial factors, ‘for the money’, were mostly considered as least important.
Fig. 2.
Motivators for participation rated on a scale 1 to 3.
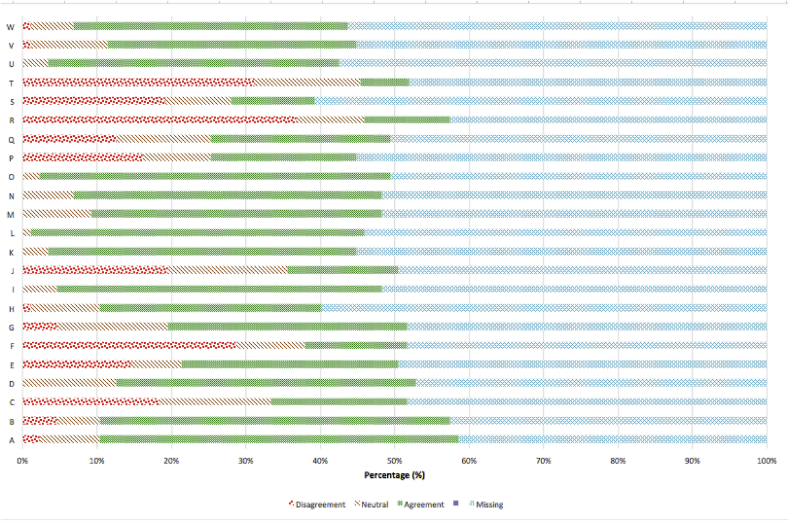
Fig. 3 shows responses regarding previous research participation. Participants showed high levels of agreement to statements that implied that their research experience was associated with positive outcomes for self and others (A, D, E, G, H, K, M, O), research conduct was ethical (I, L, Q, U, W), and opportunities for participation were and would be welcome (B, N, V). There was either no or little disagreement to these statements. Statements that gathered significant disagreement included those that indicated that research had strong emotional repercussions (C, E, J) and that the research procedures were ‘too long’, ‘boring’ or ‘inconvenient’ (R, S, T).
Fig. 3.
Respondents' agreement and disagreement on statements related to participation in clinical research.
A I gained something positive from participating.
B Knowing what I know now, I would participate in clinical research if given the opportunity.
C The research raised emotional issues for me that I had not expected.
D I gained insight about my experiences through research participation.
E The research made me think about things I didn't want to think about.
F I found the questions too personal.
G I found participating in the clinical research personally meaningful.
H I believe the clinical research results will be useful to others.
I I trusted that my replies would be kept private.
J I experienced intense emotions during the research session and/or parts of the study.
K I think clinical research is for a good cause.
L I was treated with respect and dignity when I participated in clinical research.
M I found participating in clinical research beneficial to me.
N I was glad to be asked to participate.
O I like the idea that I contributed to science.
P I was emotional during the research session.
Q I felt I could stop participating at any time.
R I found participating boring.
S The study procedures took too long.
T Participating in clinical research was inconvenient for me.
U Participation was a choice I freely made
V Had I known in advance what participating would be like I still would have agreed to participate.
W I understood the consent form.
3.2. Declined participation in research
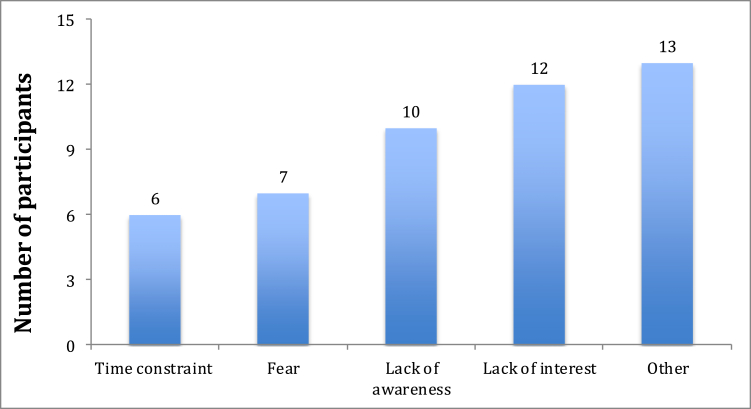
Of those who had declined consent to participate in research, 46.9% (n = 23) reported reasons for their decision. Time constraint (47.8%, n = 11) was the most prevalent reason for refusing participation followed by ‘fear’ (13.0%, n = 3), lack of awareness about clinical research (8.7%, n = 2), and lack of interest in research (8.7%, n = 2). Few stand-alone comments were classified as ‘others’ (21.7%, n = 5; Fig. 4). Of those who declined, 8.3% (n = 4) were Qataris, 52.1% (n = 25) were Non-Qatari Arabs and 39.6% (n = 19) were Non-Arab nationalities.
Fig. 4.
Reasons for declining consent to participate in clinical research.
Other reasons include:
1. ‘Because they (are not) raising awareness about these researches and the result is unknown’
2. ‘For personal reasons/I feel my rights aren't protected and I don't trust the research conductor’
3. ‘It depends on the type of participation’
4. ‘I didn't think about that’
5. ‘Recruitment team didn't call back when he expressed interest’.
4. Discussion
This was the first public survey of clinical research participation taking place in Qatar, and among the handful conducted in the Gulf Cooperation Council (GCC). The survey aimed to identify levels of research participation and to assess existing attitudes regarding clinical research participation in Qatar amongst those who had been approached to participate in research. There is an increasing need for clinical research studies in Qatar, making it essential to gain insight about the thoughts, beliefs and concerns of people regarding clinical research [11], [12]. Due to the common origin and similar socio-demographics of GCC countries, the findings may be generalizable for a region that is set on the path of developing novel interventions and clinical services.
Our survey demonstrated that, among participants who had been approached to participate in research, those who participated previously in research outnumbered those who had declined. Although comparable findings can be found in other studies from the GCC [13], [14] the present levels of participation need improvement. Killawi et al. observed that more often than not, their participants would return for the research interview after finishing with their doctor's appointment, especially if they had to see the doctor while in the middle of their interview [13].
There is a significant pool of potential subjects who are not approached for participation in clinical research. According to Gilliss and colleagues, since most recruitment is carried out in hospitals or clinical settings, the larger population becomes excluded from the recruitment process altogether [15]. This gap is rarely explored in the literature and therefore requires an in-depth insight to modify present recruitment strategies. Outreach strategies need to be devised for recruitment from this untapped, potentially eligible, population.
Our survey results show relatively consistent demographic findings for both ‘participated’ and ‘non-participated’ groups. Both groups comprised a majority of young to middle aged, well educated, employed individuals who work less than or equal to forty hours per week and have lived in Qatar for less than or equal to ten years. Males outnumbered females in both the groups. The difference, however, was less in the group that declined participation.
Existing research literature shows contrasting evidence about women's participation in clinical research. While there are studies that show increased likelihood of women to participate in research [16], [17] some conclude that they are underrepresented [18]. Some well recognized limiting factors identified in research literature include childcare, poverty and transport. There are certain patriarchal cultures in which women are either not fully empowered to take decisions independently [19] requiring consultation with family members before committing to a research study [13]. The participation of women in clinical research is generally encouraged [18]; however, none of the female participants in our survey reported any of the above reasons for non-participation. In fact, their reasons to decline or accept participation were similar to those reported by the male participants. This may be because of the affluence and greater family support in the GCC, where childcare, poverty, and lack of transportation are lesser issues.
4.1. Barriers to research participation
Time constraint was the most frequently cited reason for refusal to participate in clinical research followed by fear, lack of awareness and lack of interest. This was reported significantly by the expatriate population, especially in the Non-Arab group, as an overriding barrier to research participation. This is compatible with findings both in local and international literature [15], [16]. It is difficult for expatriates and ethnic minorities to attend somewhat lengthy and frequent study appointments as they often work long hours or over time, which may incur financial loss as well as job insecurity, leading to refusal to participate [14], [15].
While time has widely been reported as one of the prime barriers in participation in clinical research, ‘fear’, ‘mistrust’ and ‘misconceptions’ have been more thoroughly explored in the literature [7], [13], [14], [15], [16], [20], [21]. Our survey showed that among those who reported ‘fear’ and ‘mistrust’ as a reason for refusal to participate belonged mostly to Qatari and other Arab nationalities. Greater engagement between clinical researchers and the public can address some of the issues highlighted.
Fear was reported by at least three out of twenty-five participants from the Non-Qatari Arab group. While two plainly reported being just ‘afraid’ of participation, one described it as ‘fear of taking medications and their side effects’. Although the numbers of these participants is not large in our survey, this fear echoes notably in the literature where potential subjects have raised concerns about being treated as a ‘guinea pig’ or receiving ineffective treatment or placebo [20].
While current evidence shows that these barriers resonate primarily from marginalized or underserved minority ethnic groups [13], [15], [16], it is intriguing that these sentiments are raised by the local population and people who speak the native language, as well as share ethnic origins. Such misconceptions and more were also observed by a study conducted in Saudi Arabia while assessing awareness about clinical research trials among cancer patients and their families [14]. The study revealed misconceptions like ‘no clinical trials were conducted in the Arab world’ or that they were not performed under ‘regulatory authority supervision’ and ‘without the subject's consent.’
Fear and mistrust within the Arabic speaking participants may stem from a general ‘lack of awareness’ about clinical research, which is a fairly new, yet emerging phenomenon in this region. The ‘lack of awareness’ appeared to be unrelated to education as all the Arabic speaking participants of our survey (Qataris and Non-Qataris alike) had an above secondary level education with majority having completed four years of college.
The few primary studies from the Arab region have identified a lack of awareness or lack of familiarity with research as a leading cause of non-participation and declined consent [13], [14]. An absence of a research culture at large may be a key factor in discouraging participation in research [16]. While our survey results hinted at the ‘lack of knowledge’ about clinical research in responses e.g. fear and mistrust, there were at least two occasions where the participants (one Qatari and the other a Non-Arab) categorically admitted ‘lack of awareness’ verbatim.
Although none of the Non-Arab participants reported either fear or mistrust in our survey, there has been reference to insecurity in this group, especially among the Hindi speaking population, in work by Killawi et al. [13] where they observed vulnerability fears and concerns over negative repercussions to any (perceivably) undesirable responses. They were also hesitant upon sharing private information.
It was also observed that majority of the research participation refusals came from the Arabic-speaking participants in our survey. This finding is compatible with the empirical work done by Killawi et al. on recruitment and clinical research conduct in Qatar [13]. However, a study of similar nature done in Kuwait, a neighboring GCC country, showed a different trend where South Asians were the largest group to decline consent followed by Arabs and South-East Asians [16].
4.2. Motivators for research participation
According to our survey, altruism followed by personal and monetary benefits were the most frequently reported motivating factor in research participation across all nationalities. These findings compliment evidence found in previous literature [20], [21], [22]. Altruism has moral and ethical roots and may impart a sense of fulfillment or usefulness to the participants [22]. Improved access to health care or free lab investigations is a widely-reported facilitator to clinical research participation, especially for subjects who already have a certain disease or condition or have to pay for these services [15], [16], [20], [22], [23].
While monetary incentives are supported in international literature as effective facilitators in research participation [24], a local study [13] and our findings show contrasting trends where reimbursements or financial benefits were usually turned down. Monetary or financial benefits were generally considered to be least important by our participants. Both the Qatari and other Arab nationalities considered monetary incentives as the least important factor in participation. One third of the Non-Arab participants ranked it as the most important factor, while the remaining thought of it as the least important.
The length of stay in Qatar was a statistically significant predictor of research participation in our survey results. Those who had lived in Qatar for more than ten years (regardless of their region of origin) were almost six times more likely to participate in a research study. There were similar numbers of Arabic (n = 18) and non-Arabic (n = 14) speaking participants who had lived in Qatar for more than ten years and participated in a clinical study. There is sparse evidence in existing literature about the possible association between duration of stay and willingness to participate. It may be postulated that those who have stayed for ten years or longer are socially and economically more established, have less at stake, and therefore feel reasonably more confident while agreeing to participate in research. The greater length of stay may also allow greater exposure to ongoing research studies.
Maximum participation was seen among the South East Asian nationalities followed by other Arab and Qatari population. This trend is commensurate with findings by Tariq et al., whereby South-East Asians were the least likely to decline participation [16].
Prior experience with participation in a medical research study is a recognized determinant of willingness to participate [1]. Our study confirms this as majority of participants who agreed that their previous research experience had positive outcomes and was ethically conducted, showed strong inclination to the prospect of future research participation, if approached.
The participants in our survey did not find the research they had participated in as lengthy and tedious, or emotionally challenging. This may be correlated to the relatively simple research designs that the participants had participated in e.g. surveys, interviews etc. with very few having enrolled in more complex clinical research trials. There is evidence that the more complex the research procedures are (e.g. long consenting procedures and consent forms, more frequent visits, and changes in care), and despite potentially greater benefits, the more people choose to decline as it is ‘easier to say NO’ than to participate [25].
4.3. Strengths and limitations
This study is the first large-scale population based study examining research participation in Qatar and the Gulf Cooperation Council region. However, more in-depth exploration is needed through qualitative work to further determine the drivers of research participation/refusal further. Missing data were also a limitation of this study. Participants refused responses to either parts or whole of survey sheet after showing initial interest. A sampling plan was not utilized. The respondents were those who attended specific health awareness booths resulting in potential selection bias. The study did not explore attitudes towards clinical research amongst those who had not been approached to participate in clinical research. However, given that research participation requires explanation, discussion, and contemplation, it is unclear whether responses from those who have not been approached to participate will be reflective of their views when they are approached for research. Future work should explore this in more detail.
5. Conclusion
The levels of clinical research participation in Qatar need improvement. Access to the public for recruitment is limited and a large section of population is not approached for research participation. Time constraint has been the most frequently reported barrier, while altruism has been the prime facilitator in clinical research enrollment. Culturally compatible recruitment strategies must be devised for the diverse population of Qatar that demonstrates multispectral factors for accepting or declining participation. While this survey provides a window into an otherwise sparsely explored area, more in-depth research is required to assess the prevalent attitudes and behaviors regarding clinical research in Qatar.
Conflict of interest
The authors declare no conflict of interest.
Author's contributions
ST conceived the study and its design. HT, SC, and OC oversaw the collection and analysis of the data and contributed to the manuscript. SA, LA and AA were responsible for data handling and data checking. OO contributed to data analysis. HT and SC drafted the manuscript. ST and OC contributed further to the manuscript. All authors contributed to the final work.
Disclosure of interest
The authors declare that they have no conflicts of interest concerning this article.
Funding statement
The study was funded by the Qatar Foundation through the Biomedical Research Program at Weill Cornell Medicine in Qatar. ST has received funding from the Qatar National Research Fund (grants: NPRP 8-912-3-192 and NPRP 4-1392-3-345). The funding agencies had no influence on the design, development, conduct, conclusions, and reporting of the study.
Acknowledgements
The authors would like to thank all the colleagues at Clinical Research Core at Weill Cornell Medicine in Qatar for collecting and entering the survey data. We are grateful to Ms. Nouf Al Kuwari and Mr. Saif Hayek for their valuable input during data cleaning and checking.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.conctc.2017.10.010.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Trauth J.M., Musa D., Siminoff L., Jewell I.K., Ricci E. Public attitudes regarding willingness to participate in medical research studies. J. Health Soc. Policy. 2000;12(2):23–43. doi: 10.1300/J045v12n02_02. [DOI] [PubMed] [Google Scholar]
- 2.Burns K.E., Magyarody N., Jiang D., Wald R. Attitudes and views of the general public towards research participation. Intern Med. J. 2013;43(5):531–540. doi: 10.1111/j.1445-5994.2011.02433.x. [DOI] [PubMed] [Google Scholar]
- 3.Galli L., Knight R., Robertson S., Hoile E., Oladapo O., Francis D., Free C. Using marketing theory to inform strategies for recruitment: a recruitment optimisation model and the txt2stop experience. Trials. 2014;15:182. doi: 10.1186/1745-6215-15-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McCullagh M.C., Sanon M.A., Cohen M.A. Strategies to enhance participant recruitment and retention in research involving a community-based population. Appl. Nurs. Res. 2014;27(4):249–253. doi: 10.1016/j.apnr.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nair S.C., Ibrahim H., Celentano D.D. Clinical trials in the Middle East and North Africa (MENA) region: grandstanding or grandeur? Contemp. Clin. Trials. 2013;36(2):704–710. doi: 10.1016/j.cct.2013.05.009. [DOI] [PubMed] [Google Scholar]
- 6.Thiers F., Sinskey A., Berndt E. Trends in the globalization of clinical trials. Nat. Rev. Drug Discov. 2008;7:13–14. [Google Scholar]
- 7.Teschke K., Marino S., Chu R., Tsui J.K., Harris M.A., Marion S.A. Public opinions about participating in health research. Can. J. Public Health Revue Can. de sante publique. 2010;101(2):159–164. doi: 10.1007/BF03404364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DSouza P. Priya DSouza Communications; 2017. Population of Qatar by Nationality in 2017. [Google Scholar]
- 9.Qatar Population 2017 (Demographics, Maps, Graphs) [http://worldpopulationreview.com/countries/qatar-population/].
- 10.Allison P.D. SAS Institute; Cary (N.C.): 1999. Logistic Regression Using the SAS System Theory and Application. [Google Scholar]
- 11.Al-Kindi S., Al-Juhaishi T., Haddad F., Taheri S., Abi Khalil C. Cardiovascular disease research activity in the Middle East: a bibliometric analysis. Ther. Adv. Cardiovasc Dis. 2015;9(3):70–76. doi: 10.1177/1753944715578585. [DOI] [PubMed] [Google Scholar]
- 12.Choudhury S.M., Arora T., Alebbi S., Ahmed L., Aden A., Omar O., Taheri S. How do Qataris source health information? PLoS One. 2016;11(11):e0166250. doi: 10.1371/journal.pone.0166250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Killawi A., Khidir A., Elnashar M., Abdelrahim H., Hammoud M., Elliott H., Thurston M., Asad H., Al-Khal A.L., Fetters M.D. Procedures of recruiting, obtaining informed consent, and compensating research participants in Qatar: findings from a qualitative investigation. Bmc Med. Ethics. 2014;15:9. doi: 10.1186/1472-6939-15-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bazarbashi S., Hassan A., Eldin A.M., Soudy H., Hussain F. Awareness and perceptions of clinical trials in cancer patients and their families in Saudi Arabia. J. Cancer Educ. 2015;30(4):655–659. doi: 10.1007/s13187-015-0797-0. [DOI] [PubMed] [Google Scholar]
- 15.Gilliss C.L., Lee K.A., Gutierrez Y., Taylor D., Beyene Y., Neuhaus J., Murrell N. Recruitment and retention of healthy minority women into community-based longitudinal research. J. Womens Health Gend. Based Med. 2001;10(1):77–85. doi: 10.1089/152460901750067142. [DOI] [PubMed] [Google Scholar]
- 16.Tariq S., Goddard C.A., Elkum N. Barriers in participant recruitment of diverse ethnicities in the state of Kuwait. Int. J. Equity Health. 2013;12:93. doi: 10.1186/1475-9276-12-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dunn K.M., Jordan K., Lacey R.J., Shapley M., Jinks C. Patterns of consent in epidemiologic research: evidence from over 25,000 responders. Am. J. Epidemiol. 2004;159(11):1087–1094. doi: 10.1093/aje/kwh141. [DOI] [PubMed] [Google Scholar]
- 18.Cooley M.E., Sarna L., Brown J.K., Williams R.D., Chernecky C., Padilla G., Danao L.L. Challenges of recruitment and retention in multisite clinical research. Cancer Nurs. 2003;26(5):376–384. doi: 10.1097/00002820-200310000-00006. quiz 385–376. [DOI] [PubMed] [Google Scholar]
- 19.Daunt D.J. Ethnicity and recruitment rates in clinical research studies. Appl. Nurs. Res. 2003;16(3):189–195. doi: 10.1016/s0897-1897(03)00042-9. [DOI] [PubMed] [Google Scholar]
- 20.Udrea G., Dumitrescu B., Purcarea M., Balan I., Rezus E., Deculescu D. Patients' perspectives and motivators to participate in clinical trials with novel therapies for rheumatoid arthritis. J. Med. Life. 2009;2(2):227–231. [PMC free article] [PubMed] [Google Scholar]
- 21.Tanner A., Kim S.H., Friedman D.B., Foster C., Bergeron C.D. Promoting clinical research to medically underserved communities: current practices and perceptions about clinical trial recruiting strategies. Contemp. Clin. Trials. 2015;41:39–44. doi: 10.1016/j.cct.2014.12.010. [DOI] [PubMed] [Google Scholar]
- 22.Godskesen T., Hansson M.G., Nygren P., Nordin K., Kihlbom U. Hope for a cure and altruism are the main motives behind participation in phase 3 clinical cancer trials. Eur. J. Cancer Care (Engl) 2015;24(1):133–141. doi: 10.1111/ecc.12184. [DOI] [PubMed] [Google Scholar]
- 23.Jenkins V., Fallowfield L. Reasons for accepting or declining to participate in randomized clinical trials for cancer therapy. Br. J. Cancer. 2000;82(11):1783–1788. doi: 10.1054/bjoc.2000.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Watson J.M., Torgerson D.J. Increasing recruitment to randomised trials: a review of randomised controlled trials. BMC Med. Res. Methodol. 2006;6:34. doi: 10.1186/1471-2288-6-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.English R., Yeonwoo L., Griffin R. National Academy of Sciences. National Academic Press; Washington D.C. USA: 2010. Forum on Drug Discovery, Development, and Translation; Institute of Medicine. Transforming Clinical Research in the United States: Challenges and Opportunities: Workshop Summary. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.