Abstract
Background
A growing number of older adults use in-home Medicaid Waiver Home and Community Based services (HCBS) to facilitate aging-in-place. A primary service of this program is Home Care Aide assistance with activities of daily living and homemaker needs. Despite the known benefits of exercise, exercise programs are currently not offered to clients in the Medicaid Waiver system. Thus, the purpose of this paper is to describe a six-month Home Care Aide-led resistance exercise intervention protocol for frail older adults receiving Medicaid waiver services.
Methods/design
A randomized controlled trial will be used. We will enroll 126 Home Care Aide-client dyads for a 6-month exercise intervention. The intervention will consist of training phases to promote muscle strength, power, and endurance. We will use an intention to treat principle using mixed effects models for the quantitative outcomes. To analyze qualitative outcomes, we will use conventional content analysis to examine themes from participant program evaluations.
Discussion
As greater numbers of adults age in place with frailty and employ Home Care Aides to help manage functional limitations, interventions embedded within usual care services play a critical role in bringing exercise into the home setting. The research described in this protocol will provide important knowledge about the impact of a Home Care Aide-led exercise intervention in reducing frailty in older adults.
Clinical Trials Registration
ClinicalTrials.gov Identifier: NCT02942992;
Keywords: Frailty, Dyad, Formal caregivers, Resistance exercise
1. Introduction
Frailty, a collection of biomedical factors that reduce an individual's capacity to withstand stress [1], increases with age and is associated with adverse health outcomes, high health care expenditures, and nursing home placement [2], [3]. One of the most common classifications of frailty is the Fried phenotypic criteria in which an individual is classified as frail if he/she has at least three of the following five components: slow gait speed, weakness, low physical activity, unintentional weight loss, and fatigue [2]. Cumulatively, these deficits associated with frailty contribute to limitations in activities of daily living (ADL) that substantially increase the risk for nursing home placement [4].
Over 1.45 million persons in need of nursing home and long-term care services receive home and community-based care through Medicaid waiver programs in the United States [5]. The State of Illinois provides this service through its Community Care Program (CCP), which serves over 100,000 older adults annually [6]. The CCP targets low-income adults who are 60 years of age or older and have an assessed need for nursing home care. Services support aging-in-place by providing case management, adult day services, emergency response, and home care aides (HCAs), non-licensed paraprofessionals who provide non-medical care services such as assistance with ADLs and home management [6].
Current Medicaid Home and Community Based Services (HCBS) waiver programs are compensatory and do not attempt to improve the functioning of older adults through exercise interventions, despite known benefits of these interventions in improving functioning and health [7], [8], [9], [10], [11], [12]. Furthermore, functional limitations present a substantial barrier for clients in accessing community-based exercise programs [9]. Thus, there is a need to develop strategies to bring exercise interventions into the homes of this vulnerable population and promote services that improve function, rather than compensate for limitations.
While it is well established that progressive resistance exercise (PRE) improves muscle strength in both health and disease [7], [8], [9], [10], [11], [12], [13], [14], [15], it is not clear how best to engage frail older adults with limited community access in a PRE intervention [16]. It is also unclear the extent to which PRE can slow or reverse frailty [8]. Given the association between frailty, functional limitations, and the risk for nursing home placement [4], a home based intervention that can improve functioning and slow or reverse frailty will have a significant impact in helping older adults age-in-place. Importantly, delivering the intervention through HCBS providers will allow this intervention to be scaled nationally, reaching a significant segment of an underserved population. Thus, the purpose of this paper is to describe the protocol for recruiting, enrolling, and assessing a formal caregiver-older adult dyad in a research study of a resistance exercise intervention for frail older adults receiving the Medicaid HCBS waiver.
2. Methods
2.1. Design
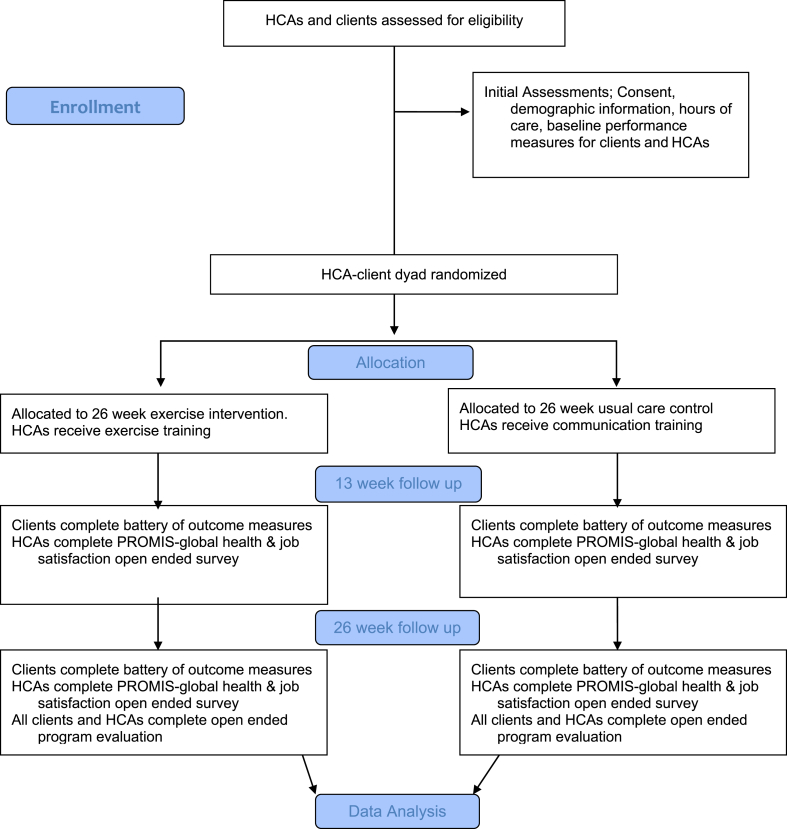
This study will be a pragmatic, assessor-masked randomized trial. We will randomize a home care aide and one of his or her clients (a HCA-client dyad) into the intervention or control group. Home care aides who are part of dyads randomly assigned to the intervention group will undergo intervention training and perform the intervention with his or her client two times per week in addition to usual care responsibilities. Home care aides who are part of dyads randomly assigned to the control group will receive training in improving communication with their clients and continue usual client care (e.g. housekeeping, meal preparation, and bathing). Dyad flow through the study is depicted in Fig. 1.
Fig. 1.
Clinical flow diagram.
A concurrent mixed-methods approach to analyzing both qualitative and quantitative outcomes will be used in this randomized controlled trial. This approach is used to corroborate quantitative and qualitative outcomes in order to cross-validate study findings. In addition to objective quantitative outcomes assessment and analysis, we will also administer open-ended program evaluation surveys for all HCAs and clients that will be analyzed using a conventional content analysis approach. This research has been approved by the Northwestern University Institutional Review Board.
2.2. Study setting
For this study, we have partnered with Help at Home, the largest provider of HCBS services in Illinois. Help at Home serves approximately 10,000 older adults in Illinois and employs nearly 4,500 HCAs.
2.3. Eligibility criteria and screening
Enrolling HCA-client dyads for this study involves a two-stage process. First, interested HCAs will be screened for eligibility. Screening inclusion criteria include being an employee of Help at Home with at least one client who receives HCA care from that HCA two or more days per week. For those all potentially eligible HCAs, information about the study will be mailed to all clients on that HCA's caseload who are seen two or more days per week with instructions to contact research staff if interested in participating in the study. We will then make telephone calls to all clients two weeks following mailing to facilitate an active recruitment process. If a client expresses interest in participation, clients will be initially screened over the telephone for eligibility to avoid an unnecessary home visit if the individual is not eligible. Inclusion criteria for clients include: > 60 years or age, receive HCA services from the same HCA two or more days per week, English-speaking, not currently participating in regular exercise as defined as 30 min three or more days per week, no health problems that contraindicate participation in exercise based on the EASY: Exercise Assessment and Screening for You [17], and Telephone Interview for Cognitive Status score > 26 [18]. If the client is determined provisionally eligible, an in-home appointment will be made with the client to further explain the study, sign informed consent, and participate in baseline assessments. Once the client has signed informed consent, the HCA-client dyad will be considered provisionally enrolled in the study. Home care aides will sign informed consent at the beginning of the training session. If the HCA does not attend training and subsequently does not sign informed consent, the client will no longer be eligible for the study. Likewise, if a client withdraws from the study, the HCA will no longer be eligible for the study.
2.4. Randomization procedure
After client baseline measurements are obtained and the dyad is considered provisionally enrolled, we will obtain the number of usual care hours the client receives weekly from the Help at Home office. HCA-client dyads will be randomized to one of two arms: 1) intervention plus usual care or 2) usual care only via a stratified block allocation scheme to promote equal allocation (1:1 across arms within each stratum). Participating dyads will be stratified by the number of weekly client usual care hours; the four categories of hours include: 1–8, 9–16, 17–24, and 25 h and above. Client hours are allocated from Help at Home based on the score on the Determination of Need, a measure that assesses client ADL and instrumental ADL performance with greater impairments correlated with a greater number of allocated care hours. Ideally, we would control functional impairment imbalance through treatment allocation procedures, but allocated hours serves as an easily attainable proxy for baseline client functional impairment. The study statistician will upload a pre-generated allocation sequence to the database platform that will not be accessible to study personnel. Randomization will occur within the REDCap (Research Electronic Data Capture) user interface, and all data will be collected and housed using REDCap tools hosted at Northwestern University. REDCap is a secure, web-based application designed to support data capture for research studies [26]. Study personnel will not have access to the randomization list, setup module, or dashboard, but the primary research coordinator will maintain ability to randomize. This will reduced selection bias (though it cannot be completely mitigated as blinding is not possible in this study). Randomization will occur prior to the HCA training session so that an unmasked member of the research team can direct HCAs to the appropriate training room based on the HCA's random assignment. This unblinded team member will not be involved in data collection. Research staff involved in data collection will be blinded to arm assignment. The statistician and primary research coordinator will be the only unblinded members of the research team and neither will be involved in data collection. It is impossible to blind participants and trainers to group assignment due to the nature of the exercise intervention.
2.5. Intervention
2.5.1. Exercise intervention
The functional resistance exercise intervention will be implemented face-to-face in the home during the HCA's usual care visits using a mobile application he or she will access through a tablet-computing device provided to all intervention group clients. The exercise intervention is based on a periodization training model [19]. The 26-week intervention consists of four phases with a two-week active recovery phase in between each phase. Table 1 details the exercise intervention. Exercises for each phase consist of bicep curls, tricep press, chest press, lateral row, squats, heel raises, sidestepping, and mini-lunges. Clients will have the option to select between a standing or seated version of the exercise program. Active recovery phases focus on functional tasks and will consist of 5 min of walking at a self-selected fast speed, 1 min of sit to stand exercises, and 1-min repetitions of balance activities including standing with feet together, semi-tandem, tandem, and one-legged. One minute dynamic balance tasks consist of lateral weight shifts and single steps in multiple directions. All exercises will use Theraband CLX resistance bands. As part of the baseline measurement, research staff will conduct an isometric maximum test for the biceps muscles to estimate an appropriate level of resistance band with which each client should exercise and will inform the HCA of this level at the training session.
Table 1.
Functional resistance exercise program training periods.
| Phase | Length | Goal | Exercise Prescription |
|---|---|---|---|
| Phase One: Tissue adaptation | 8 weeks | Increase structural strength of tissues to handle stresses of training and prepare for overload | 1 set; 10 reps; ∼40% of 1 rep max intensity |
| Phase Two: Strength | 4 weeks | Increase the size of the skeletal muscle | 2 sets; 12 reps; ∼70% of 1 rep max intensity |
| Phase Three: Power | 4 weeks | Develop muscle speed | 2 sets; 5 reps; instructed to perform safely with as fast of speed as possible |
| Phase Four: Endurance | 4 weeks | Improve ability to maintain power output over time with decreased fatigue | 30 reps; 1 set; ∼40% of 1 rep max intensity |
| Active recovery | 2 weeks in between each phase | Reduce muscle fatigue | 5 min of walking, 1 min of sit to stand, and 5 min of balance |
2.5.2. Control intervention
For dyads randomized to the control group, HCAs will receive communication training and continue providing usual care services to their client.
2.5.3. Strategies to improve exercise adherence
As part of the intervention training, HCAs will learn strategies to help clients overcome barriers to adherence. Using motivational interviewing strategies, trainers will educate HCAs on communication prompts to facilitate participation and overcome barriers such as pain, fatigue, and symptom exacerbation that may limit an older adult's exercise participation. Research staff will also contact each HCA during weeks 2, 4, 8, 12, 16, 20, and 24 following a scripted telephone fidelity checklist to ensure fidelity of intervention delivery. Should a fidelity issue be determined in these checks, research staff will make an in-home appointment as necessary for further HCA training. Research staff will also contact the HCAs in the control group to ensure matched attention between groups.
2.6. Outcome measures
2.6.1. Demographic characteristics
For clients, we will gather information on age, sex, race/ethnicity, education, living arrangement, and hours of weekly care allotted with their HCA. For HCAs, we will gather age, gender, race/ethnicity, education, and years of employment as a home care aide. Demographic characteristics will be collected at baseline only.
2.6.2. Primary outcome
The primary measure in this study is frailty as measured by the Survey of Health, Ageing, and Retirement in Europe-Frailty Index (SHARE-FI) [20]. This five-construct tool addresses fatigue, appetite, weakness, walking difficulties, and low physical activity; constructs from Fried's frailty phenotype criteria [3]. Fatigue is measured as a binary (yes/no) response to whether the client has too little energy to complete desired tasks. The appetite question inquires about the food intake over the last month with responses being diminished, same, or increased. Weakness is assessed by two repetitions of grip dynamometry on each hand. Walking difficulties are assessed by the ability to walk 100 m and climb one flight of stairs. Physical activity is measured via self-report of frequency of engagement in low to moderate physical activities. A composite frailty score is generated based on these items and that score will be used to classify individuals as non-frail, pre-frail, or frail. Since both the continuous and binary outcomes as determined by the SHARE-FI have clinical meaning, we plan to analyze both separately. The continuous outcome will serve as the primary outcome of interest for reporting purposes. The continuous measure allows for analysis of the quantitative degree in improvement in frailty score, and the categorical measure (secondary outcome) allows for determination of the percentage of clients that move between frailty categories at each measurement point.
The SHARE-FI tool was developed as an alternative to the Fried frailty phenotype, offering a simple and fast measure of frailty for primary care physicians and community practitioners [20]. Calculators for SHARE-FI scoring are freely available for females and males. If the predicted discrete factor score for females is < 0.315, the person is non-frail, pre-frail is 0.316–2.130, and scores of 2.131–6 are frail. For males, scores <1.212 are non-frail, 1.213 to 3.005 are pre-frail, and scores <7 are frail. The SHARE-FI has been shown to predict mortality [24] and disability [25] among pre-frail and frail persons.
2.6.3. Secondary outcomes
We will assess the self-reported overall health of both clients and HCAs using the PROMIS-global health. Overall health scores are based in responses to items from the five PROMIS domains of physical function, fatigue, pain, emotional distress, and social health [21]. We will administer the measure and calculate an overall score, as well as separate physical and mental health summary scores. We will use the 30-s chair rise test as a proxy test of quadriceps strength and will use the Timed Up and Go [22] as a measure of fall risk and mobility. Program evaluations will be conducted with both HCAs and clients using open-ended questions to ascertain the impact of the intervention or the communication training on quality of life, job satisfaction, functioning, and well-being.
2.7. Sample size
We plan to recruit 63 HCAs and one of their clients per study arm for a total sample size of 126 HCA-client dyads. In our pilot study of 42 clients, overall attrition was 17% [13]. Allowing for similar attrition, we project 50 subjects per arm with complete data at three- and six-month follow-up. Without much information on meaningful frailty effect sizes across arms and standard deviation, we chose to calculate power based on units of standard deviation differences across arms. With the anticipated 50 participants per arm with analyzable data at follow-up time points, we will have 80% power to detect a moderate to large effect size (mean/standard deviation, Cohen's d = 0.57) assuming a 5% type I error rate [27].
2.8. Recruitment
Because our participant enrollment is a two-stage process, we will also recruit in two-stages. Presentations about the study will occur at Help at Home weekly HCA in-services and flyers will be distributed. Once interested HCAs are determined eligible, we will mail recruitment flyers to all clients in the eligible HCA's caseload. We will follow-up with telephone calls after mailing to answer any questions clients have about the study and recruit interested participants. All participants will be reimbursed for their participation in the study; HCAs will receive $125 USD for their time in training and leading the exercise program and clients will receive $10 USD for participation in each measurement time point (baseline, 13 weeks, and 26 weeks).
2.9. Data collection
Research staff responsible for collecting data will be trained in data collection by a physical therapist board certified in geriatrics. Training will follow standardized protocols for data collection as outlined in the study manual of operations. Data collection will be obtained in the client's home at baseline, week 13, and week 27. The primary research coordinator will maintain a participant-tracking calendar to ensure that all HCA-client dyadic contacts are conducted in the appropriate time frame to minimize missing data. Other retention strategies include contacting both the HCA and client members of the dyad up to five times by phone and text message within a one-month time frame. If participants remain unreachable after these attempts, we will consider them lost to follow-up. If either a HCA or client do not wish to continue in the study, research staff will inquire as to the reason for withdrawal.
2.10. Data analysis
Descriptive statistics (mean ± standard deviation for continuous measures and frequency/percentages for categorical measures) will be used to summarize all baseline data in aggregate as well as across study arms. Analyses in general will assume parametric modeling assumptions are appropriate (e.g. normality for linear models); in cases of violation of these assumptions, nonparametric and/or transformations of variables may be explored. Analyses will be based on the intent-to-treat (ITT) dataset, and sensitivity analyses will examine the as treated dataset defined as clients who complete at least 75% of sessions. We will employ (generalized) linear mixed models with appropriate link function(s) (e.g. logit for categorical outcomes, identify for continuous outcomes) with random participant effects to account for within-participant association of outcomes. Fixed effects will include study arm, time since baseline (three or six months), and baseline frailty status. All quantitative statistical analyses will be performed using SAS software version 9.4 (The SAS Institute Inc, Cary, NC 2012). We will also complete program evaluation surveys for all intervention group home care aides and clients. These surveys will be analyzed using conventional content analysis with two coders independently reviewing surveys, identifying themes, and assigning initial codes. Coders will recode the surveys using the final coding structure and frequencies of responses will be calculated by theme. All analyses will assume a 5% level of significance, and we do not plan to correct for multiple hypothesis testing as this study will lay the groundwork for a larger phase III multicenter study, and we would hope to control type II error rate as opposed to type I error rate. Further, there are no planned pre-specified subgroup analyses or further adjusted analyses (for baseline variables other than the aforementioned baseline frailty). Any post hoc analyses will be reported as such.
3. Discussion
The primary aim of this randomized trial is to determine the impact of a functional resistance exercise intervention for frail older adults and their HCAs receiving Medicaid HCBS. This pragmatic trial has been designed to determine intervention effectiveness in both the short term (13 weeks) and long term (26 weeks) to lay the groundwork for a larger, phase III multicenter study across multiple HCBS agencies state-wide.
With an older adult population expected to double by 2060 [23], there is an urgent need to for interventions that promote successful aging. The development and implementation of interventions that improve the health of a rapidly expanding aging population with frailty is a critical public health issue. Despite the well-known benefits of exercise for older adults [8], [9], [10], [11], [12], [13], [14], [15], it remains unclear how best to prescribe and implement exercise in a frail population. The exercise intervention described in this paper attempts to address that gap by providing an intervention embedded within existing usual care services. Given the association between frailty, functional limitations, nursing home placement, and mortality, an intervention that can improve functioning and slow or reverse frailty will have a significant impact in helping older adults age-in-place. Importantly, delivering the intervention through HCBS providers will allow this intervention to be scaled nationally, reaching a significant segment of an underserved population.
Very few exercise programs have specifically examined the impact on the frailty construct [9], and as such, this research will provide new information regarding the impact of a 6-month resistance exercise intervention on the presentation of frailty. There is still much debate on the measurement of frailty in both clinical practice and research, and as such, this study will contribute new knowledge on assessing frailty in the community using the SHARE-FI and how SHARE-FI scores change in response to an intervention. In addition, the qualitative program evaluation will contribute information about the implementation and impact on well-being and quality of life that can best be explored through qualitative approaches. This mixed methods approach to data collection and analysis will provide an in-depth understanding of the impact of this intervention on the HCA-client dyad.
Source of support
This research is supported by a grant from the Retirement Research Foundation (Grant 2016-042) and Performance Health. Research reported in this publication was supported, in part, by the National Institutes of Health's National Center for Advancing Translational Sciences, Grant Number UL1TR001422. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- 1.Vincent, G., Velkoff, V. The Next Four Decades: the Older Population in the United States: 2010 to 2050. US Dept of Commerce. Issued May 2010. Accessed July 28, 2015 at https://www.census.gov/prod/2010pubs/p25-1138.pdf.
- 2.Lally F., Crome P. Understanding frailty. Postgrad. Med. J. 2007;83:16–20. doi: 10.1136/pgmj.2006.048587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fried L., Tangen C., Walston J., Newman A., Hirsch C., Gottdiener J., Seeman T., Kop W.J., Burke G., McBurnie M. Frailty in older adults: evidence for a phenotype. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2001;56(3):M146–M157. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 4.Gaugler J., Duval S., Anderson K., Kane R. Predicting nursing home admission in the U.S.: a meta-analysis. BMC Geriatr. 2007;7(13) doi: 10.1186/1471-2318-7-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Medicaid. 1915(c) Home- and Community-Based Waivers, 2014. Accessed February 1, 2015: http://www.medicaid.gov/medicaid-chip-program-information/by-topics/waivers/home-and-community-based-1915-c-waivers.html.
- 6.Community Care Program. Accessed July 28, 2015. https://www.illinois.gov/aging/CommunityServices/Pages/ccp.aspx.
- 7.Lutomski J., Baars M., Boter H., Buurman B., den Elzen W., Jansen A., Kempen G., Steunenberg B., Steyerberg E., Olde M., Melis R. Frailty, disability, and multi-morbidity: the relationship with quality of life and healthcare costs in elderly people. Ned. Tijdschr. Geneesd. 2014;158:A7297. [PubMed] [Google Scholar]
- 8.Liu C., Latham N. Progressive resistance strength training for improving physical function in older adults. Cochrane Database Syst. Rev. 2009;8(3) doi: 10.1002/14651858.CD002759.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu C., Fielding R. Exercise as an intervention for frailty. Clin. Geriatr. Med. 2012;27:101–110. doi: 10.1016/j.cger.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peterson M., Rhea M., Sen A., Gordon P. Resistance exercise for muscular strength in older adults: a meta-analysis. Ageing Res. Rev. 2010;9:226–237. doi: 10.1016/j.arr.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thiebaud R., Funk M., Abe T. Home-based resistance training for older adults: a systematic review. Geriatr. Gerontol. Int. 2014;14(4):750–757. doi: 10.1111/ggi.12326. [DOI] [PubMed] [Google Scholar]
- 12.Theou O., Stathokostas L., Roland K.P., Jakobi J., Patterson C., Vandervoort A., Jones G. The effectiveness of exercise interventions for the management of frailty: a systematic review. J. Aging Res. 2011;2011 doi: 10.4061/2011/569194. Article ID 569194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Danilovich M., Corcos D., Eisenstein A., Marquez D., Hughes S. Translating strong for life into the community care program: lessons learned. J. Appl. Gerontol. 2017;36(5):553–569. doi: 10.1177/0733464815625833. First published date: January-20-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Prodoehl J., Rafferty M., David F., Poon C., Vaillancourt D., Comella C., Leurgans S., Kohrt W., Corcos D., Robichaud J. Two-year exercise program improves physical function in Parkinson's disease: the PRET-PD randomized clinical trial. Neurorehabil Neural Repair. 2015;29:112–122. doi: 10.1177/1545968314539732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Corcos D., Robichaud J., David F., Leurgans S., Vaillancourt D., Poon C., Rafferty M., Kohrt W., Comella C. A two-year randomized controlled trial of progressive resistance exercise for Parkinson's disease. Mov. Disod. 2013;28:1230–1240. doi: 10.1002/mds.25380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hughes S., Seymour R., Campbell R., Whitelaw N., Bazzarre T. Best-practice physical activity programs for older adults: findings from the national impact study. Am. J. Public Health. 2009;99(2):362–368. doi: 10.2105/AJPH.2007.131466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Resnick B., Ory M., Hora K., Rogers M., Page P., Bolin J., Lyle R., Sipe C., Chodzlo-Zajko W., Bazzarre T. A proposal for a new screening paradigm and tool called exercise assessment and screening for you (EASY) J. Aging & Phys. Activity. 2008;16(2):215–233. doi: 10.1123/japa.16.2.215. [DOI] [PubMed] [Google Scholar]
- 18.Espeland M., Rapp S., Katula J., Andrews L., Felton D., Gaussoin S., Dagenbach D., Legaut C., Jennings J., Sink K. Telephone interview for cognitive status (TICS) screening for clinical trials of physical activity and cognitive training: the seniors health and activity research program pilot (SHARP-P) study. Int. J. Geriatric Psychiatry. 2011;26(2) doi: 10.1002/gps.2503. 10.1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Signorile J.F. Periodize training for the masters athlete. Sport Res. Intell. Sportive. 2007;5(5) Sept-Oct. [Google Scholar]
- 20.Romero-Ortuno R., Walsh C., Lawlor B., Kenny R. A frailty instrument for primary care: findings across the survey of health, ageing, and retirement in Europe (SHARE) BMC Geriatr. 2010;10:57. doi: 10.1186/1471-2318-10-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hays R., Bjorner J., Revicki R., Spritzer K., Cella D. Development of physical and mental health summary scores from the Patient Reported Outcomes Measurement Information System (PROMIS) global items. Qual. Life Res. 2009;18(7):873–880. doi: 10.1007/s11136-009-9496-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Podsiadlo D., Richardson S. The timed “Up & Go”: a test of basic functional mobility for frail elderly persons. J. Am. Geriatr. Soc. 1991;39(2) doi: 10.1111/j.1532-5415.1991.tb01616.x. [DOI] [PubMed] [Google Scholar]
- 23.The Next Four Decades: the Older Population in the United States. Current Population Reports. 2010. pp. P25–P1138. Vincent GaV, V. [Google Scholar]
- 24.Romero-Ortuno R. The Frailty Instrument of the Survey of Health, Ageing and Retirement in Europe (SHARE-FI) predicts mortality beyond age, comorbidities, disability, self-rated health, education and depression. Eur. Geriatr. Med. 2011;2(6):323–326. doi: 10.1016/j.eurger.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Romero-Ortuno R., O'Shea D., Kenny R. The SHARE frailty instrument for primary care predicts incident disability in a population-based sample. Qual. Prim. Care. 2011;19(5):301–309. [PubMed] [Google Scholar]
- 26.Harris P.A. Research electronic data capture (REDCap) - a metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inf. 2009;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cohen D. A power primer. Psychol. Bull. 1992;112(1):155–159. doi: 10.1037//0033-2909.112.1.155. [DOI] [PubMed] [Google Scholar]