Abstract
Background
Recent meta-analytic reviews suggest exercise can reduce depression severity among adults with major depressive disorder (MDD); however, efficacy studies with depressed youth are limited. Few studies have investigated the efficacy of multi-modal exercise interventions in this population, addressed treatment engagement, or explored the differential effects of exercise on depressive symptom profiles.
Objectives
This paper describes the study protocol and recruitment pattern for an assessor blinded, two-arm randomised controlled trial investigating the efficacy of an integrated motivational interviewing (MI) and multi-modal exercise intervention in youth diagnosed with MDD. Associations between depressive symptom profiles (cognitive, somatic and affective) and psychological, physiological (fitness), and biological (blood biomarker) outcomes will also be examined.
Methods
Participants aged 15–25 years with current MDD were recruited. Eligible participants were randomised and stratified according to gender and depression severity to either an immediate or delayed (control) group. The immediate group received a brief MI intervention followed by a 12-week small group exercise intervention (3 times per week for 1 h), all delivered by personal trainers. The delayed control group received the same intervention 12-weeks later. Both groups were reassessed at mid-treatment or mid-control, post-treatment or post-control, and follow-up (12 weeks post-treatment).
Results
68 participants were recruited and randomly allocated to an intervention group.
Conclusion
This trial will increase our understanding of the efficacy of multi-modal exercise interventions for depression and the specific effects of exercise on depressive symptom profiles. It also offers a novel contribution by addressing treatment engagement in exercise efficacy trials in youth with MDD.
Keywords: Exercise, Depression, Motivational interviewing, Youth, Cognitive, Affective
1. Introduction
1.1. Background
Major depressive disorder (MDD) is a heterogeneous disorder characterised by a diverse symptom profile [1], [2]. MDD is highly prevalent in youth [3], [4] and associated with comorbid eating [5], [6], anxiety and substance use disorders, and increased risk of suicide completion [4], [7], [8], [9]. Established treatment approaches for youth are limited by accessibility to psychological therapies and side-effects of antidepressant medications [10], [11]. Exercise has physical and psychological benefits, with antidepressant effects documented among adult populations [12], [13], [14]. A small number of studies have reported similar benefits among youth populations [11], [15]; however, methodological issues have constrained the generalisability of findings [10], [16], [17]. Benefits have been established for aerobic exercise programs but not for those combining aerobic exercise and resistance training in a multi-modal approach [18], [19], [20]. Exercise studies across the lifespan have also predominately focused on change in global depression severity scores and little is known about the impact of exercise on depressive symptom profiles (cognitive, somatic and affective) or the relationship between depressive symptoms and psychological, physiological and biological factors.
Establishing the effectiveness of exercise for MDD is further constrained by high dropout rates particularly among people with more severe depressive symptoms [21]. Interventions that enhance readiness and engagement in exercise programs and address potential barriers may help address motivational constraints [13]. Substantial research supports the effectiveness of Motivational Interviewing (MI) in modifying health behaviour [22]. Recently, MI has also been applied as an effective pre-treatment engagement strategy [23], [24], but to the best of our knowledge its benefits as a pre-exercise intervention strategy among youth with MDD are yet to be determined.
We recently conducted a pilot study investigating the feasibility of integrating a pre-exercise MI intervention designed to engage young people with MDD in exercise training and assessed the effects of the intervention on depression symptom profiles [25]. In the current study, the pilot MI intervention was adapted for delivery by personal trainers and integrated with an exercise intervention in the context of a randomised controlled trial.
1.2. Objectives and hypothesis
The primary aim of this study is to evaluate the efficacy of a 12-week integrated MI and multi-modal exercise intervention on depression diagnosis and overall depression severity in youth diagnosed with MDD. Secondary aims are to determine whether there are: 1) differential changes in depressive symptoms (cognitive, somatic and affective); 2) persistence of treatment effects over 24 weeks (from recruitment) and any consequences of delaying treatment; 3) changes in anxiety symptoms; and 4) associations between changes in depression symptoms and program adherence, as well as changes in physical fitness, psychological and blood biomarker measures. It is hypothesised that the exercise program will improve overall depression severity scores in the intervention group relative to the control group, differentially effect depressive symptom subscales, and that these benefits will persist at follow-up.
2. Research design and methods
2.1. Study design
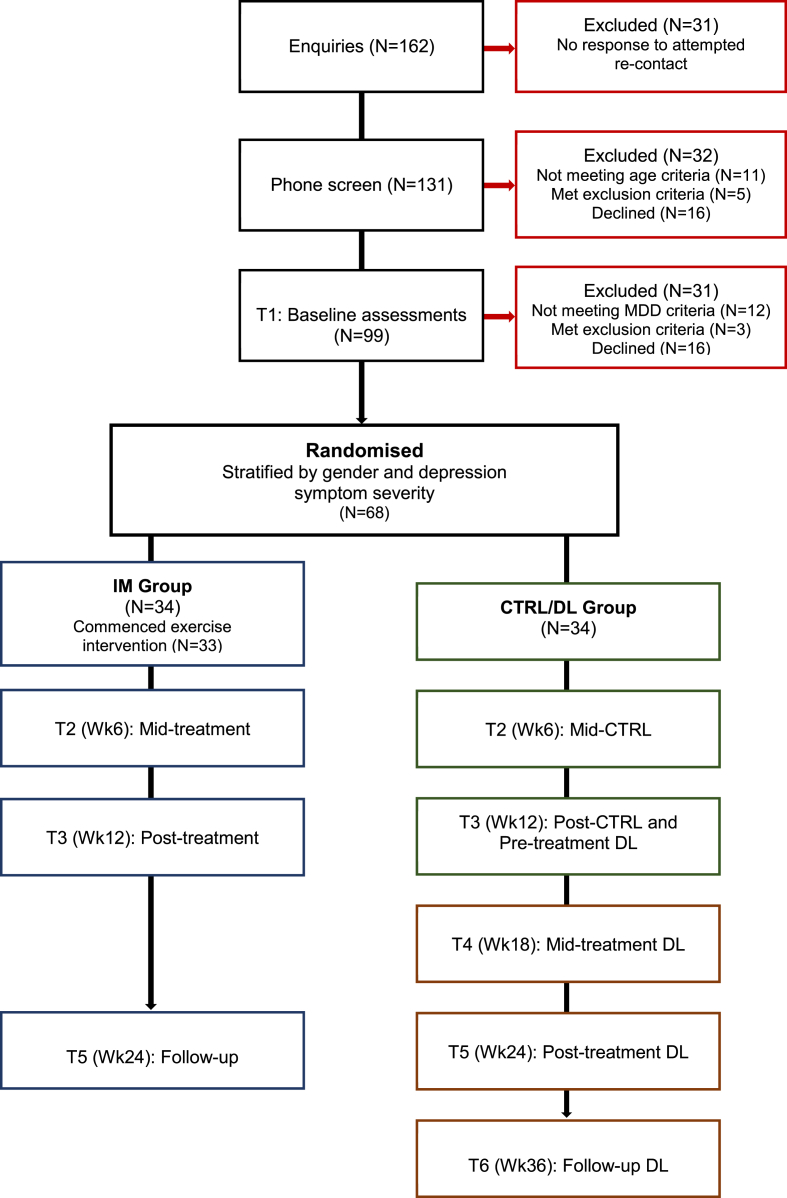
This is an assessor blinded, two-arm, randomised controlled trial (RCT) examining efficacy and differential depressive symptom effects of an integrated MI and multi-modal exercise intervention in youth with MDD. Participants were stratified by gender and overall depression severity and randomised either to the immediate (IM) intervention or control/delayed (CTRL/DL) group. A flow chart illustrating the study design and participant recruitment is presented in Fig. 1. Assessments were conducted at baseline (T1), mid-treatment or mid-control (T2, Wk6), post-treatment or post-control (T3, Wk12) (which doubled as the pre-treatment for DL), mid-treatment for DL (T4, Wk18), follow-up for IM or post-treatment for DL (T5, Wk24), and follow-up for DL (T6, Wk36). Data from the CTRL/DL group served both as a control condition (T1 to T3) and as a basis for evaluating treatment maintenance effects (T3 to T6) when combined with IM group data (T1 to T3, and T5). This research study was prospectively registered with the Australian New Zealand clinical trials registry (ANZCTR: ACTRN12613000638730) and approved by the University of Newcastle, Human Research Ethics Committee (H2012-0114) and the Hunter New England Local Health District Human Research Ethics Committee (15/04/15/4.05). The design, conduct and reporting of this study will comply with the Consolidated Standards of Reporting Trials (CONSORT) guidelines [26], [27].
Fig. 1.
Study design and participant recruitment for the intervention (IM) and control/delayed (CTRL/DL) groups.
2.2. Participants: eligibility, screening and recruitment
Participants were recruited from the local community and University population and fell within the World Health Organization youth age range of 15–25 years. They had to have current MDD, as assessed by a clinical psychologist utilising the structured clinical interview for DSM-IV Axis 1 disorders (SCID-1 Research Version) [28]. The SCID is widely regarded as the gold standard interview for diagnosis of depression. The eligibility and exclusion criteria for the study are detailed in Table 1. Individuals were excluded if they had medical contraindications to exercise or significant psychiatric co-morbidities such as bipolar or psychotic disorders or eating disorders where exercise was contraindicated. Participants were included whether or not they were receiving other treatment for MDD (counselling, pharmacotherapy), or if they reported engaging in modest levels of physical activity; this was intended to increase the generalisability of findings in this age group within the context of usual clinical practice.
Table 1.
Inclusion and exclusion criteria for the study.
| Inclusion criteria |
|
| Exclusion criteria |
|
2.2.1. Recruitment
Recruitment occurred across three waves between May 2013 and September 2015. A range of strategies were adopted to target recruitment of individuals with varied levels of depression severity (mild to severe). Strategies included: advertisements on community television, social media, YouTube, newspapers, community radio, University website, flyers on University and community notice boards, emails and letters to clinicians working across a range of local, youth health services, general and psychology practices. All participants were recruited from the Newcastle region of New South Wales, Australia.
2.2.2. Screening
Participants expressing interest in the study were screened using a specifically designed phone screen to determine their initial eligibility. This phone call was used to provide information about the study and collect relevant screening information using a structured interview. Verbal consent was obtained prior to screening. Participants were asked to provide relevant sociodemographic data (e.g., age, gender), pregnancy and current depression status. They were screened for current and past treatment of depression, suicidality, any associated comorbidities and family history of depression. Participants were also screened for any major medical problems that may interfere with their capacity to engage in exercise. Current physical activity and anthropometric information (estimated weight and height) were also collected.
2.2.3. Psychological screening assessment measures and suicide risk protocol
Participants were screened for current depression using the Beck Depression Inventory-Fast Screen (BDI-FS) [29] administered during the phone screen. The BDI-FS is a seven-item version of the Beck Depression Inventory (BDI-II) designed to assess depressive symptoms in accordance with DSM-IV criteria for a major depressive episode in adolescents (>13 years) and adults. The BDI-FS provides a valid and reliable measure of cognitive and affective depressive symptoms with item scores ranging from 0 to 3 and a maximum total score of 21 [29]. Participants who reported current suicidal ideation were further assessed to determine intent/plan for suicide using a specifically designed suicide risk assessment protocol. Participants considered to be at imminent risk of suicide were referred to relevant mental health services and/or authorities were contacted and they were excluded from the study. Participants were also screened for anxiety symptoms using the Generalised Anxiety Disorder Scale (GAD-7) [30]. The GAD-7 is a seven-item brief measure linked to DSM-IV criteria for assessing generalised anxiety symptoms, which has demonstrated reliability and validity [31].
2.3. Assessments
Psychological, physiological (physical fitness) and biological assessments were conducted (see Table 2). The incidence of depression was assessed by the SCID and overall depression severity and depressive symptom subscale scores were assessed by the BDI-II. Additional outcome variables included a comprehensive range of psychological measures, physiological (fitness) assessments and biological (blood marker) measures (see Table 2).
Table 2.
Main outcome indices and measures across the study phases.
| Main outcome indices and measures | Screen | Baseline | Mid- | Post- and Follow-up |
|---|---|---|---|---|
| Initial screening and study eligibility | ||||
| Phone screen protocol (incl. medical comorbidities) | x | |||
| Depression (BDI-FS) | x | |||
| Anxiety (GAD-7) | x | |||
| Inclusion/exclusion criteria | ||||
| SCID assessment (incl. psychiatric comorbidities) | x | |||
| Sociodemographic data | x | |||
| Online questionnaires | ||||
| Exercise related measures | ||||
| Physical activity levels | x | x | x | |
| Exercise readiness | x | x | ||
| Exercise self-efficacy and barriers (ESES; BARSE) | x | x | ||
| Confidence and support for exercise (SSES) | x | x | ||
| Program and outcome expectations | x | x | ||
| Resolve | x | x (Wk4 & 8) | x | |
| Sedentary behaviour (Sitting Time Questionnaire) | x | x | ||
| Psychological functioning measures | ||||
| Self-esteem (SISE) | x | x | ||
| Eating disorder behaviours (EDE-Q) | x | x | ||
| Quality of life and (Q-LES-Q-SF) | x | x | ||
| Life satisfaction (SWLS) | x | x | ||
| Physical self-concept and physique anxiety (PSDQ-S; SPAS) | x | x | ||
| Alcohol and other drug use (AUDIT) | x | x | ||
| Psychological assessment | ||||
| Depression and associated factors | ||||
| SCID assessment for current MDD | x | x | ||
| Depression severity (BDI-II) | x | x | x | |
| Anxiety (BAI) | x | x | ||
| Cognitive changes (ATQ; DASF1 & DASF2) | x | x | x | |
| Behavioural activation (BADS-SF) | x | x | x | |
| Somatic symptoms (DSSS; PHQ-15) | x | x | x | |
| Physical fitness and physiological assessments | ||||
| Exercise and Sports Science Australia (ESSA) screen | x | x | ||
| Sedentary behaviour (Sitting Time Questionnaire) | x | x | ||
| Exercise history | x | x | ||
| Developmental maturation | x | x | ||
| Maximal aerobic capacity | x | x | ||
| Muscular strength endurance | x | x | ||
| Anthropometrics (e.g., BMI, body composition) | x | x | ||
| Biological markers | ||||
| IL6, BDNF, blood samples stored | x | x | ||
2.3.1. Online questionnaires
The week prior to each psychological evaluation (T1, T3, T5, T6), participants were emailed a secure link instructing them to complete sociodemographic questions and pre-evaluation self-report questionnaires on the day prior to their evaluation appointment. An information statement describing the study and consent form were also emailed prior to the first appointment. All participants were asked to provide written, informed consent prior to proceeding with the study. Parental consent was required for participants under the age of 18 years. To reduce participant burden, shorter versions of established measures were adopted where possible. The questionnaires used are described below.
2.3.2. Exercise related questionnaires
Exercise Readiness. For this single-item readiness to exercise measure [32], participants are provided with a definition of regular exercise: “Regular exercise is any planned physical activity (e.g. brisk walking, aerobics, jogging, bicycling, swimming, rowing etc.) performed to increase physical fitness. Such activity should be performed 3–5 times per week for 20–60 min per session. Exercise does not have to be painful to be effective but should be done at a level that increases your breathing rate and causes you to break a sweat”. They are asked to indicate if they have been engaging in exercise in accordance with that criteria using a 5-point rating scale ranging from to 1 = ‘no and I do not intend to in the next 6 months’, and 5 = ‘yes I have been for more than 6 months’ which can be used to characterise participants along a stage of change continuum (pre-contemplation, contemplation, preparation, action and maintenance).
Exercise Self-Efficacy Scale (ESES) [33]. The ESES is an 18-item measure assessing confidence to exercise despite barriers, presented across 6 specific domains. Items are rated on a 5-point scale ranging from 1 = ‘not at all confident’ to 5 = ‘completely confident’. Higher scores indicate higher rates of exercise self-efficacy.
Barriers Self-Efficacy Scale (BARSE) [34]. The original BARSE is a 13-item exercise specific questionnaire assessing confidence to participate in exercise in the light of specific barriers. Items are rated on a 10-point scale ranging from 0 = ’not at all confident’ to 100 = ‘highly confident’. In the current study, we adapted the original scale by adding 9 additional barriers related to psychological symptoms such as depression and anxiety.
Confidence to Exercise. We created a single-item measure asking participants on a scale of 0–100% how likely it is that they would attend all their exercise sessions and get at least 30 min of moderate to vigorous exercise on all the days of the week over the next 3 months if they were in the immediate or delayed (control) group.
Social Support and Exercise Survey [35]. This 12-item measure assesses frequency of support for exercise and is completed twice; once for family and once for friends. Each item is rated on a 5-point scale ranging from 1 = ‘none’ to 5 = ‘very often’. The scale has 3 subscales: family participation, family rewards and participation, and friend participation. The scale has demonstrated reliability and validity [35].
2.3.3. Program and outcome expectations
We developed a four-item measure to assess participant's expectations about how they may benefit from the exercise program and its likelihood of success. Participants were asked to rate: 1 ‘How logical does the idea of exercise as treatment seem’, 2 ‘How successful they think the program will be in reducing their depression symptoms’, 3 ‘How confident they would be in recommending this program’, and 4 ‘How much improvement they expect to see/have seen?’. An additional eight-item measure was also created asking participants about outcome expectations. Each item is rated on a 5-point scale ranging from 1 = ‘strongly disagree’ to 5 = ‘strongly agree’. Participants are asked to rate how strongly they agreed or disagreed with statements about the outcomes of participating in the program. Sample questions included: ‘Regular exercise has helped me have a more positive outlook’ and ‘I have slept better when I got regular exercise’.
2.3.4. Psychological functioning measures
The Single Item Self-esteem Questionnaire (SISE) [36]. The single-item ‘I have high self-esteem’ is rated on a 5-point Likert scale ranging from 1 = ‘strongly disagree’ to 5 = ‘strongly agree’. The SISE has demonstrated convergent validity with the Rosenberg Self-esteem Scale [36].
Eating disorder questionnaire (EDE-Q) [37]. The EDE-Q is a self-report version of the Eating Disorder Examination Interview that is widely regarded as the instrument of choice for assessing eating disorders. The 22-item EDE-Q focuses on the past 28 days and is rated on a 7-point, forced choice rating scheme. There are 4 subscales (restraints, eating concerns, weight concern and shape concern) as well as a total score. The scale has demonstrated reliability and validity [38].
Quality of life enjoyment and satisfaction questionnaire–short form (Q-LES-Q-SF) [39]. The Q-LES-Q-SF is a 16-item questionnaire assessing satisfaction and enjoyment across a number of areas of functioning including mood, social relationships, and physical health. Items are rated on a 5-point, Likert point scale ranging from 1 = ‘very poor’ to 5 = ‘very good’. The scale has demonstrated reliability and validity [40].
Satisfaction With Life Scale (SWLS) [41]. The SWLS is a five-item questionnaire assessing satisfaction with life. The 5 items are rated on a 7-point, Likert scale ranging from 1 = ‘strongly disagree’ to 7 = ‘strongly agree’. The scale has demonstrated reliability and validity [42].
Physical self-description questionnaire short form (PSDQ-S) [43]. The PSDQ-S is a 40-item measure that retains all of the 11 subscales from the original PSDQ 70-item form. It assesses aspects relating to physical self-concept including: activity, appearance, body fat, endurance, coordination, flexibility, health, sport competence, strength, global physical self-concept, and global self-esteem. The PSDQ-S, has a 6-point rating scale with evaluative statements rated as ‘False, mostly false, more false than true, more true than false, mostly true and true’. The scale has demonstrated reliability and validity [43].
Social Physique Anxiety Scale (SPAS) [44]. The SPAS is a 12-item questionnaire assessing anxiety associated with physique. It is rated on a 5-point scale ranging from 1 = ’not at all characteristic of me’ to 5 = ‘extremely characteristic of me’, with total scores ranging from 12 to 60. Higher scores indicate higher levels of physique anxiety. The scale has demonstrated reliability and validity [44].
The Alcohol Use Disorders Identification Test (AUDIT) [45]. The AUDIT is a 10-item questionnaire which assesses current alcohol use, with two supplementary questions. The AUDIT has demonstrated reliability and validity [46].
To assess other drug use, participants were asked if they had ever used tobacco products, about usage in the past 3 months and about how many cigarettes they smoke each day. They were also asked similar questions about cannabis, and other substances such as cocaine, amphetamines and opioids.
2.3.5. Psychological evaluation
Following completion of the online questionnaires, eligible participants (as determined by initial screening) attended a baseline psychological assessment with a clinical psychologist (Y.N.) to confirm full study eligibility. Prior to the clinical interview, participants completed a number of additional self-report questionnaires that assessed overall depression severity, anxiety symptoms and cognitive, behavioural and somatic symptoms (see Table 2).
2.3.5.1. Overall depression severity and depressive symptom subscales
Beck Depression Inventory (BDI-II) [47]. The BDI-II is a 21-item self-report measure of depression symptoms corresponding to DSM-IV diagnostic criteria for MDD. The BDI-II measures overall depression severity via an assessment of the presence and severity of cognitive, affective and somatic depressive symptoms. Each item is rated on a 4-point scale (0–3), with higher scores indicating greater severity of depression. The BDI-II demonstrates good psychometric properties [47].
2.3.5.2. Anxiety symptoms
Beck Anxiety Inventory (BAI) [48]. This is a 21-item measure of anxiety symptoms rated on 4-point Likert scales ranging from 0 = ‘not at all’ to 3 = ‘severely’. Higher scores indicate greater severity of anxiety. The scale has good reliability and validity [49].
2.3.5.3. Cognition and activation
Automatic Thoughts Questionnaire (ATQ) [50]. The ATQ consists of 30 self-report items assessing frequency of negative automatic thoughts. Scores range from 30 to 150, with higher scores reflecting increased negative cognitions. The ATQ is a widely used measure with strong psychometric properties reported [51].
Dysfunctional Attitude Scale (DASF1 and DASF2) [52]. The original DAS-A is a 40-item measure that assesses dysfunctional attitudes in depression. We used the shorter versions of the scale: DASF1 and the DASF2. Each scale is comprised of 9 items rated on 4-point scales ranging from 1 = ‘totally agree’ to 4 = ‘totally disagree’. The authors conclude the shorter scales are strongly correlated with the original 40-item version, have adequate internal consistency, reliability and are strongly associated with each other [52].
Behavioural Activation for Depression Scale (BADS-SF) [53]. The BADS-SF is a measure of behavioural activation (activation and avoidance). This nine-item measure has a total score ranging from 0 to 54, with higher scores representing increased activation (and less avoidance). The authors report that the scale has an internal consistency of 0.82, with demonstrated construct validity and predictive validity [53].
2.3.5.4. Somatic symptoms
Depression Somatic Symptom Scale (DSSS) [54]. This 22-item questionnaire assesses depressive and somatic symptoms and consists of two subscales: depression and a somatic symptom subscale. Items are rated on 4-point scales ranging from 1 = ‘absent’ to 4 = ‘severe’. The DSSS has acceptable convergent, factorial and distinct group validities [54].
Physical Health Questionnaire (PHQ-15) [55]. The PHQ-15 is a 15-item subscale questionnaire assessing somatic symptoms over the past 4 weeks. Items are rated on 3-point Likert scales ranging from 1 = ‘not bothered at all’ to 3 = ‘bothered a lot’. It is derived from the Patient Health Questionnaire and is a reliable and valid measure routinely used in clinical practice [56].
2.3.5.5. SCID assessment
The participant was then interviewed by the psychologist using the SCID to confirm that they met current diagnostic criteria for MDD and whether they met any of the study's exclusion criteria (see Table 1). The procedure set out in the suicide risk assessment protocol developed as part of initial screening was repeated for participants who reported current suicidal ideation. Participants who met full study criteria were offered a place in the study. They completed a physical fitness assessment and blood test, which was scheduled as soon as possible following acceptance into the study.
2.3.6. Physical fitness, physical activity and maturation assessments
Physiological and fitness assessments were all undertaken in the Human Performance Laboratory at the University of Newcastle. All researchers performing the assessments were trained to follow standardised assessment protocols. Prior to the baseline fitness assessment, the Exercise and Sports Science Australia (ESSA) adult screening tool [57] was used to assess risks associated with exercise and any requirements for medical clearance. Exercise history and recent exercise participation were recorded. To assess sedentary behaviour participants completed the Sitting Time Questionnaire [58]. This is a five-item measure that assesses time spent sitting (hours and minutes) on a weekday and a weekend day in the following domains: 1 ‘Travelling to and from places’; 2 ‘At work’; 3 ‘Watching television’, 4 ‘Using a computer at home’; and 5 ‘For leisure’. The authors report acceptable reliability and validity, particularly for weekday sitting times [58]. Sedentary behaviour was reassessed at post-treatment and follow-up (see Table 2). Participants were asked if they had finished growing in height and the age at which final height was reached. Participants self-assessed their biological maturation status rating their secondary sex characteristics against Tanner reference diagrams [59]. Maturation was only reassessed if the participants indicated they had not reached maturation at their baseline assessment.
Maximal aerobic capacity. Maximal aerobic capacity (VO2max) is assessed using an incremental treadmill exercise test (GXT) (Powerjog JM100 treadmill, Expert Fitness UK) and indirect calorimetry (Vmax Spectra, CareFusion SensorMedics, USA or K4b2, COSMED, Italy). The same instruments were used at each time point. The electrocardiograph is monitored continuously in each participant using a portable 12-lead system (Quark T12, COSMED, Italy) and used to obtain heart rate (HR) data. Participants commence with a 4 min warm-up starting at 5 km/h, with speed increasing every 30 s until a heart rate between 60 and 70% of their age-predicted maximum is reached. That speed is maintained until 4 min has elapsed, after which the speed is increased every minute until the participant cannot run any faster, then the incline is increased by 2% every minute until exhaustion.
Upper and lower body muscular strength endurance. Upper body muscular strength endurance is assessed by the number of successful repetitions in the YMCA Bench Press Test. This test is a reliable and valid test of upper body muscular endurance. Lower body muscular strength endurance is assessed by measuring the number of repetitions on a horizontal seated leg press until fatigue with the load as 95–100% of their body mass in kg. This protocol was developed because no standardised test to assess non-isokinetic lower body muscular endurance has been established as safe for this group of novice lifters. The leg press is a machine guided, safe exercise requiring minimal skill. Participants are assessed on their ability (yes/no, repetitions) to leg press 95–100% of their body mass.
2.3.6.1. Anthropometric measures
Height in cm is measured without shoes on a Holtain stadiometer calibrated before each assessment session. Height is assessed twice with acceptable values within 0.3 cm, otherwise a third measure is taken. Weight in kg (to two decimal places) is measured without shoes and in light clothing on a calibrated digital scale. Weight is measured twice with acceptable values within 0.1 kg otherwise a third measure is taken. Body Mass Index (BMI) is calculated using the formula: weight (kg)/height (m2).
Waist circumference was measured in cm at the mid-point between the bottom rib and iliac crest at the end of a normal expiration. Waist circumference was assessed twice with acceptable values within 0.5 cm otherwise a third measure was taken. Hip circumference (maximum gluteal) was measured in cm and was measured twice with acceptable values within 0.5 cm otherwise a third measure was taken.
The final height, weight, waist and hip circumference estimates were the average of the respective two acceptable measures.
Body composition is measured in kg and percentages with a multi frequency bio-impedance device (InBody 720; Biospace Co., Ltd., Seoul, Korea). This device uses an eight-point tactile electrode system with demonstrated validity and reliability for skeletal muscle mass and percentage body fat [60], [61]. Measurements were taken in light clothing without shoes.
2.3.7. Blood biomarker measures
Venous blood samples are collected between 8:00 a.m. and 10:00 a.m. at each assessment in suitable vacutainers with EDTA as anticoagulant to obtain plasma and without additive to obtain serum for possible future investigation. Plasma and serum are obtained after centrifugation (15 min, 1000g, 4 °C). All samples obtained are stored at −70 °C until measurements are performed. Concentrations of brain-derived neurotrophic factor (BDNF) and Interleukin 6 (IL-6) are determined in serum using commercially available ultrasensitive solid-phase sandwich enzyme-linked immunosorbent assays (Quantikine Immunoassay, R&D Systems, Minneapolis, USA) with a spectrophotometric plate reader (Bio-Rad, Hercules, CA).
2.3.8. Intervention monitoring and compliance to the intervention
To monitor the effects of the intervention, the BDI-II and several online questionnaires assessing behavioural, cognitive and somatic factors were re-administered during week 6 of the exercise intervention (see Table 2). During weeks 4 and 8 of the exercise intervention a brief online survey was also completed assessing participants' goal evaluation and confidence in adhering to the exercise program. The seven ‘Resolve’ items were rated on a 7-point scale ranging from 0 = ‘strongly disagree’ to 7 = ‘strongly agree’. Sample questions include ‘Since starting the exercise program I have more confidence that I can exercise more regularly’ and ‘I feel that I am meeting my goals’.
Participants were asked to complete an online daily monitoring questionnaire and to rate their mood, sleep quality, energy levels and muscle soreness, as well as record their participation in the exercise program and/or any other physical activity. Participants were also asked to include how many hours spent in front of a screen (study/work and leisure), as well as the number of hours sleep they had the night before. For each participant, attendance rate in the intervention group was recorded as the average number and percentage of overall attendance at the supervised exercise sessions with the personal trainer.
2.3.9. Randomisation and blinding
Participants were randomly assigned to the IM (intervention) or control/delayed (CTRL/DL) group. A website-generated block randomisation procedure was used with variable block lengths from 4 to 8. Randomisation was concealed from the researchers by using numbered, opaque, sealed envelopes. Other than the 12-week wait period for the delayed group, participants across both groups were treated in the same way. Independent psychologists conducted the post- and follow-up psychological assessments (including the SCID), blinded to treatment allocation and baseline assessment scores. Personal trainers (PTs) providing the MI intervention were blinded to which group the participant was initially allocated (IM or CTRL/DL). Researchers conducting the physiological (fitness) assessments were also blinded to group allocation. All data were de-identified and preliminary data analyses were conducted using coded data with no identification of participants.
2.3.10. Participant reimbursement
Participants were provided with gift certificates as partial recompense for their travel and parking expenses at the rate of $5 per training or assessment session attended. All aspects of the study were free of charge.
2.4. Integrated MI and exercise intervention
The intervention comprised two components: an initial, brief MI intervention; and a 12-week multi-modal exercise training intervention, all designed to be delivered by the personal trainer (PT). The first phase of the intervention required the participants to attend an individual, 30-min, face-to-face MI intervention developed specifically to enhance participant motivation to engage with the exercise program. PTs used MI approaches to establish a positive working relationship with the participant, focus the conversation on exploring past experiences with exercise, exercise-mood link, and barriers to exercise. An ‘elicit-provide-elicit’ approach [62] was used in order to strengthen participants' intentions to exercise by helping increase their confidence that they could address barriers. PTs collaboratively developed an individualised exercise contract and elicited commitment from the participant. Within the MI framework, commitment to exercise was evoked from the participant rather than imposed. PTs used a specifically designed, one-page prompt sheet and exercise contract template to help facilitate the MI discussion. They were also encouraged to use MI communication approaches throughout the exercise intervention.
The MI intervention protocol ‘Train your mood: Exercise as Treatment for Depression’ was initially developed as a pre-exercise engagement strategy for young people with MDD and piloted as part of a previous study by the same researchers [25]. The revised MI protocol used in this trial “Exercise as treatment for depression: A guide for personal trainers” was adapted for delivery by PTs to increase engagement effects and the MI intervention was shortened to 30 min to promote dissemination and real-world application. The MI intervention was also adapted and streamlined to be consistent with industry approaches, including for example, use of contracts, and goal setting and monitoring.
2.4.1. MI treatment fidelity and integrity
The PTs received a 6-h MI training workshop led by a clinical psychologist (Y.N.) trained and experienced in the delivery of MI, utilising specifically developed MI training modules to ensure standardisation. The training provided a brief introduction to depression and working with clients with MDD. Five foundational MI elements were introduced based on the work of Miller and Rollnick [62] with regular interactive approaches including practice sessions, demonstrations and discussions provided throughout the training: 1) Background to MI; 2) Nature of change, ambivalence, and the righting reflex; 3) Spirit of MI; 4) Core MI skills (OARS: open questions, affirmations, reflections, and summary statements); and 5) Change talk and responding to sustain talk. The trainers were provided with a copy of the MI intervention protocol to allow additional practice of skills before their first MI session with a participant. Following the training, PTs received two, 1-h, individualised coaching sessions with the psychologist who provided the training. With verbal permission from the participants, PTs were asked to record all of their MI sessions using a specifically provided, digital audio recorder. The first two MI audio sessions with participants were reviewed as part of the coaching sessions. PTs were encouraged to draw on MI principles when interacting with participants throughout the exercise intervention.
In accordance with practice recommendations for the measurement of MI fidelity in clinical trials [63], the remainder of the recordings were reviewed as part of a fidelity assessment utilising the Motivational Interviewing Treatment Integrity (MITI) code version 3.1 [64]. Fidelity assessments were conducted to test how closely PTs adhered to the MI intervention. The fidelity assessments were performed by two independent psychologists (coders) both trained in MI and in the use of the MITI. All available recordings were double coded in their entirety (these were approximately 30 min sessions) to ensure we were representing PT skill level across time. Prior to coding the study sample, coders participated in six practice sessions to promote adherence to the MITI and to calculate interrater reliability. Coders only proceeded to rate the study sample after they had achieved adequate inter-rater reliability. Fortnightly face-to-face supervision was also provided to the coders to discuss questions and reach consensus when any difficulties emerged in coding.
2.4.2. Multimodal exercise intervention
Following the MI intervention, participants commenced a 12-week multi-modal exercise intervention at the University gym. The PTs were suitably qualified and experienced in the provision of individual and group based exercise programs. Participants exercised in supervised small groups (3–5 people) three times per week for 1 h. The structured exercise training protocol included resistance training for the development of local muscular strength, endurance and power and aerobic exercise for the development of cardiorespiratory fitness (aerobic fitness) across 4 blocks of progressive intensity (A, B, C and D), and adhered to National Strength and Conditioning Association guidelines [65]. In novices, progressive resistance training provides a form of exercise where progressive improvements are almost always observed, and this provides positive feedback about accomplishments to participants. Resistance training was monitored throughout the intervention period by recording work (sets x repetitions x load) performed at each session. For the resistance training component different blocks involved different types of exercises. This included leg press, leg curl, calf raise, leg extension, shoulder press, chest press, seated row, lat pull down, tricep push down, bicep curl, bench press, back extension, plank holds, bench squats, static lunges, dumbbell flys, dumbbell single arm pull, push ups, hammer curl, assisted chin ups, squat press, kettle bell swings, walking lunges, medicine ball slam/sprawl, bar bell overhead press, and supine pull up.
The aerobic exercise component incorporated interval training on cardio equipment: treadmills, stationary bikes, recumbent bikes, cross trainers, step machines, rowing machines. Aerobic conditioning was monitored using heart rate, rating of perceived exertion (RPE) for each session and the duration of each session. High intensity interval training (HIIT) was offered in block D to maximise improvement in aerobic fitness. Target heart rates were determined during initial exercise testing and used to maintain the relative intensity of activity over the training period. Heart rates were monitored using the heart rate monitors on the gym equipment and via a Polar team monitoring system interfaced to an iPad while performing resistance exercise. Ratings of perceived exertion for the session were recorded at the end of each exercise session by the PT.
Throughout the 12-week exercise intervention participants were asked to wear a pedometer each day and to record their steps in the daily online monitoring questionnaire. Step counts were measured using the Yamax SW200 pedometers (Yamax Corporation, Kumamoto City, Japan). The pedometers were provided to participants at their first personal training session and they were instructed on how to wear them (remove when sleeping, showering or swimming) and record their step counts using the daily online monitoring survey. Participants were encouraged to engage in some form of physical activity (walking, cycling, swimming etc.) on their non-group supervised exercise days. To help facilitate this, participants received a free, unlimited 12-week gym membership at the University Sports Centre with access to the pool and a range of exercise classes in addition to their supervised training during the intervention.
2.5. Statistical considerations
2.5.1. Sample size calculation
A sample size calculation was performed on the primary outcome measure of change in depression severity. Based on pilot work [25], a large differential effect was anticipated (effect size = 1), from which it was estimated that retention of 26 participants per group would provide 80% power to detect a post-treatment change in depression severity, using 0.01 level, two-tailed significance tests; conventional two-tailed significance tests were adopted as we would feel obliged to explore observed differences in the unexpected direction. Consequently, anticipating a retention rate of 75%, we planned to recruit 35 participants per group.
2.5.2. Data analysis
All statistical analyses will be performed using IBM SPSS software (Version 23: SPSS, Chicago, IL, USA). We will use multiple imputation to address any key missing data and, where appropriate, undertake sensitivity analyses comparing imputed and non-imputed data sets. Data will be analysed using an intention-to-treat equivalent analysis strategy to investigate change in outcome measures pre-to post-treatment and to assess the dosage effect of exercise participation on reductions in symptom severity across time points. Baseline clinical and demographic characteristics will be compared across the two groups. Participant baseline psychological, physiological and biological characteristics will be presented using descriptive statistics stratified by overall depression severity (mild/moderate versus severe). The primary statistical analyses will utilise generalised linear mixed models, and specifically generalised estimating equations (GEE), for examining both initial treatment effects (efficacy analyses) and the persistence of treatment effects across time points. This analysis strategy will use all available data for each participant, whilst accounting for individual variation (i.e., within-subject effects) and the cross-over aspects of the design. Planned comparisons will be used to examine change across assessment time points (baseline, 6 weeks, post-treatment, follow-up). We will also examine relationships between program engagement (e.g., session attendance and concurrent physical activity monitoring) and changes in depression symptoms in the combined post-treatment data. The significance level for all tests has been set at p ≤ 0.01 to partially control for potential Type 1 errors associated with multiple comparisons, with statistical trends also noted at p ≤ 0.05; this is the equivalent of a Bonferroni-adjusted family-wise error rate with 5 members per family (e.g., depression diagnosis, overall depression severity and cognitive, somatic and affective depressive symptom subscales).
3. Results
3.1. Recruitment patterns
Participants were recruited across three waves over a 2.5-year period from community, clinical and University settings (Wave 1: June to September 2013; Wave 2: February to October 2014; and Wave 3: February to October 2015). Recruitment was set to coincide with the start of school terms or University semesters and to finish before the end of year breaks when participants or families could be away on holidays. Across the three recruitment waves, there were 162 initial enquiries of 70, 56 and 36 respectively, with 131 participants confirming interest and participating in an initial phone screen. Of these, 99 participants proceeded to the next phase of the study and completed assessments and 32 were excluded (see Fig. 1). Reasons for exclusion included: did not meet age criteria (n = 11), met study exclusion criteria (n = 5; e.g., bipolar disorder, medical condition, or at imminent risk of suicide), and declined to participate (n = 16). Of the 99 participants who completed psychological, physiological and biological assessments, 31 participants were excluded. Reasons for exclusion included: not meeting MDD criteria (n = 12), meeting study exclusion criteria (n = 3; i.e., medical condition, suicide risk, bipolar), and declining to participate (n = 16).
The 68 recruited participants were stratified according to gender and overall depression severity and randomised to the IM (n = 34) or CTRL/DL groups (n = 34) (see Fig. 1). Thirty-four (48.5%) participants were randomised during Wave 1 (M = 6, F = 28) with an average age of 20.8 years. Nineteen (33.9%) participants were randomised during Wave 2 (M = 5, F = 19) with an average age of 20.6 years. Fifteen (41.6%) participants were randomised during Wave 3 (M = 4, F = 11) with an average age of 20.7 years. Across the waves, 43 (63.2%) participants had baseline BDI-II scores in the severe range (M = 8, F = 35) and 25 (36.8%) in the mild to moderate range (M = 7, F = 18).
4. Discussion
MDD is a highly prevalent, heterogeneous disorder with a diverse symptom profile affecting mind, body and behaviour [1], [66]. Prevalence rates are as high as one in five young people experiencing MDD before their 18th birthday [7] with many receiving limited or no treatment [10]. Given the comorbidities, costs and risks associated with depression [4], [7], [9], developing effective adjunctive treatments which support conventional treatment/s or offer an alternative where treatment is either unavailable or has limited efficacy [10], [67], [68] is a clinical and research priority. Several decades of research suggest exercise offers physical and psychological health benefits which extend to the treatment of depression, either as an adjunctive or stand-alone treatment [12], [69], [70], [71]. However, at this stage, the mechanisms of action are unclear, with researchers proposing both biological and psychological explanations for treatment effects [72], [73]. It is also not clear if treatment effects extend to younger age groups as the vast majority of efficacy trials have focused on adult and older populations. Promisingly, there have been a number of recent studies which suggest exercise may benefit young people, but, existing research is limited by methodological issues such as the use of non-clinical samples and an overreliance on self-report measures [16].
One unique aspect of this trial is the use of a combined exercise intervention, which incorporates both aerobic and resistance training exercise. A recent review [14] strongly supports the use of aerobic exercise interventions as treatment for depression in adults but concluded that there was insufficient evidence to evaluate the effects of resistance training or combined (multi-modal) exercise interventions. We chose a multi-modal intervention on the basis that it may have wider appeal to young people, and that the short-term gains in performance common when commencing resistance training may be encouraging and help maintain motivation.
High rates of non-compliance are commonly reported in exercise trials among individuals with severe depressive symptoms and this constrains research seeking to determine the efficacy of exercise for depression [21]. Individuals with depression are less likely to engage in exercise and report lower intention to exercise and reduced self-efficacy compared with non-depressed individuals [74], [75]. Symptom barriers such as motivational deficits [76] and somatic complaints may present additional constraints. To benefit from an exercise program, individuals need to be able to move beyond symptom barriers to engage in exercise on a frequent basis for a period of time [75]. Strategies which enhance intention to exercise, address exercise barriers and help generate coping skills may help increase exercise compliance [75]. Previous research has sought to increase compliance to exercise by using health education, with variable success. Other strategies have included goal setting, self-monitoring, enhancing social support, and modifying cognitions during exercise [77].
The current trial offers a unique contribution to existing research investigating efficacy of exercise in youth with MDD by addressing treatment engagement to help improve the outcomes of an exercise intervention among young people with a MDD. Our study seeks to determine if a single session of MI delivered as a pre-exercise intervention strategy increases engagement and improves the outcomes of an exercise intervention among young people with a MDD. The trial builds on previous pilot work conducted by the same research team, which integrated MI delivered by a psychologist with a multi-modal exercise training intervention delivered by a PT among youth diagnosed with MDD. In the current trial, a PT delivered both the MI and exercise intervention to the young person to help develop a collaborative and supportive environment to increase treatment engagement and retention.
This study will also add to our understanding of any differential effects of exercise on depressive symptom profiles and their relationship to cognitive, behavioural and biological factors. At this stage, little is known about how exercise targets depressive symptom domains in young people with depression. Preliminary results from our pilot study suggests exercise differentially targets depressive symptoms with largest overall improvement observed in the affective subscale followed by cognitive and somatic subscales [25]. Different depressive symptoms may show different response rates to exercise and understanding this may offer important insights into the potential mechanisms of action of exercise on mind, body and behaviour. Similarly, a closer examination of relationship between depression and depressive symptom subscales and exercise adherence will improve our understanding of the dose response relationship between exercise and depression. Exploring relationships between changes in cognitive variables such as automatic thoughts, dysfunctional attitudes, behavioural activation and somatic symptoms will also help build our understanding of the specific effects of exercise. Biomarkers such as markers of inflammation and BDNF are increasingly associated with depression and inclusion of these indices is another strength of the study.
Our trial includes an objective assessment of depression with a current clinical diagnosis of MDD addressing a key methodological constraint of previous research. We targeted recruitment of participants with varied depressive severity levels and our pragmatic design allowed young people who were currently exercising or receiving treatment to participate, increasing the generalisability of results to real-world clinical practice. We successfully recruited 68 participants across three waves and our recruitment strategy maximised youth participation in the study by actively recruiting during school or study terms to mitigate against the adverse impact of holiday disruptions.
Despite these strengths, there are a number of important limitations to this study. This includes potential bias, due to the 2.5 year time period over which the sample was recruited, to enable recruitment during school or study terms to mitigate against the impact of holiday disruptions. However, our results suggest that participant characteristics such as age and gender were similarly represented across waves minimising potential recruitment bias. It also highlighted the importance of considering family support and engagement in treatment when recruiting youth into exercise studies, where an ongoing commitment is also required from family members to support the young person in treatment (e.g. transportation to and from the training venue). To enhance treatment engagement, the MI intervention was integrated with exercise; however, a study design incorporating assessment of a third group who do not receive the MI component is required to further delineate the relative effects of MI on exercise engagement. Further, our study recruited young people with current MDD, some of whom were experiencing severe depressive symptoms and were at risk of imminent suicide. Notably almost two thirds of our recruited participants; disproportionately females, reported depression severity rates that fell into the severe range. Ensuring we were appropriately assessing suicide risk and linking young people to appropriate youth mental health services required careful consideration. The development of our suicide risk protocols from the first point of phone contact helped identify and support young people who were at risk of harm; however, it also increased the time required to conduct the initial phone screens, as depression is often associated with suicidal ideation. Similarly, the psychological evaluations also incorporated a detailed suicide risk assessment and were often time intensive. Despite these challenges, this work was an essential part of the study in order to ensure the safety of the young people who approached researchers as part of recruitment to the trial.
Although closely monitored, our pragmatic study design did not exclude participants who were currently participating in some form of physical activity or treatment for their depression, as we aimed to establish adjunctive exercise benefits for this age group and increase generalisability of findings. However, the synergistic effects of combined treatment effects cannot be ruled out. As a final point, given the limited number of youth efficacy trials in this field, our research sought to investigate the effects of the intervention from a psychological, physiological and biological perspective. This will help increase our understanding of specific treatment effects not only as they relate to depression diagnosis and severity but also by helping to identify specific information about how exercise targets depression symptom domains, the impact of exercise on other outcomes of interest, and how these characteristics interrelate to moderate or mediate treatment outcomes. However, to do this required extensive assessments and may have increased participant burden and reduced study acceptability, contributing to the longer time required for recruitment.
5. Conclusions
Given the limitations and accessibility of current psychological and pharmacotherapy treatment approaches, there is an urgent need to extend treatment options for youth with MDD. Data from this trial will provide additional information about the efficacy of an integrated MI and exercise intervention for the treatment of depression in youth, both in the short-term (post-treatment) and longer-term (three month follow-up) across both groups. It will contribute to our understanding of differential effects of exercise on depressive symptom profiles and their relationship to cognitive, behavioural and biological factors. This will help increase our understanding of the benefits of exercise across psychological, physiological, biological domains and determine the relative merits of exercise for treating depressive symptoms, which may be helpful to treating professionals and their young patients.
Conflicts of interest
The authors declare that they have no competing interests.
Funding
This work was supported by Hunter Medical Research Institute (HMRI) and Beyond Blue. Neither funding body had any role in the collection, analysis, or interpretation of data, or in writing this paper. Both Amanda Baker and Leanne Hides are currently supported by NHMRC (1041866, 119098) senior research fellowships. Leanne Hides was previously supported by an Australian Research Council Future Fellowship, FT120100780 (2012–2016).
Acknowledgements
The researchers would like to thank Adriana Giles for her assistance with the exercise intervention and assessments. We would also like to thank Katrina Bell and Rachelle Myers for their assistance with assessments.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.conctc.2017.11.007.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Fried E.I., Nesse R.M. Depression sum-scores don't add up: why analyzing specific depression symptoms is essential. BMC Med. 2015;13(1):1–11. doi: 10.1186/s12916-015-0325-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Olbert C.M., Rasmussen A., Gala G.J., Tupler L.A. Treatment outcome variation between depression symptom combinations in the STAR*D study. J. Affect. Disord. 2016;201:1–7. doi: 10.1016/j.jad.2016.04.050. [DOI] [PubMed] [Google Scholar]
- 3.Pössel P., Adelson J.L., Hautzinger M. A randomized trial to evaluate the course of effects of a program to prevent adolescent depressive symptoms over 12 months. Behav. Res. Ther. 2011;49(12):838–851. doi: 10.1016/j.brat.2011.09.010. [DOI] [PubMed] [Google Scholar]
- 4.Carrellas N.W., Biederman J., Uchida M. How prevalent and morbid are subthreshold manifestations of major depression in adolescents? A literature review. J. Affect. Disord. 2017;210:166–173. doi: 10.1016/j.jad.2016.12.037. [DOI] [PubMed] [Google Scholar]
- 5.Allen K.L., Byrne S.M., Oddy W.H., Crosby R.D. DSM–IV–TR and DSM-5 eating disorders in adolescents: prevalence, stability, and psychosocial correlates in a population-based sample of male and female adolescents. J. Abnorm. Psychol. 2013;122(3):720–732. doi: 10.1037/a0034004. [DOI] [PubMed] [Google Scholar]
- 6.Santos M., Steven Richards C., Kathryn Bleckley M. Comorbidity between depression and disordered eating in adolescents. Eat. Behav. 2007;8(4):440–449. doi: 10.1016/j.eatbeh.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 7.Delaney M. A critical evaluation of the role of cognitive behaviour therapy in children and adolescents with depression. Cogn. Behav. Ther. 2009;2(02):83. [Google Scholar]
- 8.Vitiello B. Prevention and treatment of child and adolescent depression: challenges and opportunities. Epidemiol. Psychiatr. Sci. 2011;20(01):37–43. doi: 10.1017/s2045796011000102. [DOI] [PubMed] [Google Scholar]
- 9.Rao U. Links between depression and substance abuse in adolescents. Am. J. Prev. Med. 2006;31(6):161–174. doi: 10.1016/j.amepre.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 10.Jorm A.F., Allen N.B., O'Donnell C.P., Parslow R.A., Purcell R., Morgan A.J. Effectiveness of complementary and self-help treatments for depression in children and adolescents. Med. J. Aust. 2006;185(7):368–372. doi: 10.5694/j.1326-5377.2006.tb00612.x. [DOI] [PubMed] [Google Scholar]
- 11.Hughes C.W., Barnes S., Barnes C., Defina L.F., Nakonezny P., Emslie G.J. Depressed Adolescents Treated with Exercise (DATE): a pilot randomized controlled trial to test feasibility and establish preliminary effect sizes. Ment. Health Phys. Act. 2013;6(2) doi: 10.1016/j.mhpa.2013.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cooney G.M., Dwan K., Greig C.A., Lawlor D.A., Rimer J., Waugh F.R., McMurdo M., Mead G.E. Exercise for depression. Cochrane Database Syst. Rev. 2013 doi: 10.1002/14651858.CD004366.pub6. (9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kvam S., Kleppe C.L., Nordhus I.H., Hovland A. Exercise as a treatment for depression: a meta-analysis. J. Affect. Disord. 2016;202:67–86. doi: 10.1016/j.jad.2016.03.063. [DOI] [PubMed] [Google Scholar]
- 14.Schuch F.B., Vancampfort D., Richards J., Rosenbaum S., Ward P.B., Stubbs B. Exercise as a treatment for depression: a meta-analysis adjusting for publication bias. J. Psychiatr. Res. 2016;77:42–51. doi: 10.1016/j.jpsychires.2016.02.023. [DOI] [PubMed] [Google Scholar]
- 15.Parker A.G., Hetrick S.E., Jorm A.F., Mackinnon A.J., McGorry P.D., Yung A.R., Scanlan F., Stephens J., Baird S., Moller B., Purcell R. The effectiveness of simple psychological and physical activity interventions for high prevalence mental health problems in young people: a factorial randomised controlled trial. J. Affect. Disord. 2016;196:200–209. doi: 10.1016/j.jad.2016.02.043. [DOI] [PubMed] [Google Scholar]
- 16.Larun L., Nordheim L.V., Ekeland E., Hagen K.B., Heian F. Exercise in prevention and treatment of anxiety and depression among children and young people. Cochrane Database Syst. Rev. 2006 doi: 10.1002/14651858.CD004691.pub2. (3) [DOI] [PubMed] [Google Scholar]
- 17.Brown H.E., Pearson N., Braithwaite R.E., Brown W.J., Biddle S.J. Physical activity interventions and depression in children and adolescents : a systematic review and meta-analysis. Sports Med. 2013;43(3):195–206. doi: 10.1007/s40279-012-0015-8. [DOI] [PubMed] [Google Scholar]
- 18.Dopp R.R., Mooney A.J., Armitage R., King C. Exercise for adolescents with depressive disorders: a feasibility study. Depress Res. Treat. 2012;2012:257472. doi: 10.1155/2012/257472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nabkasorn C., Miyai N., Sootmongkol A., Junprasert S., Yamamoto H., Arita M., Miyashita K. Effects of physical exercise on depression, neuroendocrine stress hormones and physiological fitness in adolescent females with depressive symptoms. Eur. J. Public Health. 2006;16(2):179–184. doi: 10.1093/eurpub/cki159. [DOI] [PubMed] [Google Scholar]
- 20.Hemat-Far A., Shahsavari A., Mousavi S.R. Effects of selected aerobic exercises on the depression and concentrations of plasma serotonin in the depressed female students aged 18 to 25. J. Appl. Res. 2012;12(1):47–52. [Google Scholar]
- 21.Stubbs B., Vancampfort D., Rosenbaum S., Ward P.B., Richards J., Soundy A., Veronese N., Solmi M., Schuch F.B. Dropout from exercise randomized controlled trials among people with depression: a meta-analysis and meta regression. J. Affect. Disord. 2016;190:457–466. doi: 10.1016/j.jad.2015.10.019. [DOI] [PubMed] [Google Scholar]
- 22.Hettema J., Steele J., Miller W.R. Motivational interviewing. Annu. Rev. Clin. Psychol. 2005;1(1):91–111. doi: 10.1146/annurev.clinpsy.1.102803.143833. [DOI] [PubMed] [Google Scholar]
- 23.Westra H.A., Aviram A., Doell F.K. Extending motivational interviewing to the treatment of major mental health problems: current directions and evidence. Can. J. Psychiatry. 2011;56(11):643–650. doi: 10.1177/070674371105601102. [DOI] [PubMed] [Google Scholar]
- 24.Romano M., Peters L. Evaluating the mechanisms of change in motivational interviewing in the treatment of mental health problems: a review and meta-analysis. Clin. Psychol. Rev. 2015;38(0):1–12. doi: 10.1016/j.cpr.2015.02.008. [DOI] [PubMed] [Google Scholar]
- 25.Nasstasia Y., Baker A.L., Halpin S.A., Lewin T.J., Hides L., Kelly B.J., Callister R. Pilot study of an exercise intervention for depressive symptoms and associated cognitive-behavioral factors in young adults with major depression. J. Nerv. Ment. Dis. 2017;205(8):647–655. doi: 10.1097/NMD.0000000000000611. [DOI] [PubMed] [Google Scholar]
- 26.Eldridge S.M., Chan C.L., Campbell M.J., Bond C.M., Hopewell S., Thabane L., Lancaster G.A. CONSORT 2010 statement: extension to randomised pilot and feasibility trials. Pilot Feasibility Stud. 2016;2:64. doi: 10.1186/s40814-016-0105-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moher D., Hopewell S., Schulz K.F., Montori V., Gotzsche P.C., Devereaux P.J., Elbourne D., Egger M., Altman D.G. CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. BMJ. 2010;340:c869. doi: 10.1136/bmj.c869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.First M.B., Spitzer R.L., Gibbon M., Williams J.B.W. non-patient edition. (SCID-I/NP), New York State Psychiatric Institute New York: Biometrics Research; 2002. Structured Clinical Interview for DSM-iv axis I Disorders, Research Version. [Google Scholar]
- 29.Beck A., Steer R., Brown G. Psychological Corporation; San Antonio, TX: 2000. BDI - Fast Screen for Medical Patients: Manual. [Google Scholar]
- 30.Spitzer R.L., Kroenke K., Williams J.B., Lowe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch. Intern. Med. 2006;166(10):1092–1097. doi: 10.1001/archinte.166.10.1092. [DOI] [PubMed] [Google Scholar]
- 31.Lowe B., Decker O., Muller S., Brahler E., Schellberg D., Herzog W., Herzberg P.Y. Validation and standardization of the generalized anxiety disorder screener (GAD-7) in the general population. Med. Care. 2008;46(3):266–274. doi: 10.1097/MLR.0b013e318160d093. [DOI] [PubMed] [Google Scholar]
- 32.Marcus B.H., Selby V.C., Niaura R.S., Rossi J.S. Self-efficacy and the stages of exercise behavior change. Res. Q. Exerc. Sport. 1992;63(1):60–66. doi: 10.1080/02701367.1992.10607557. [DOI] [PubMed] [Google Scholar]
- 33.Benisovich S.V., Rossi J.S., Norman G.J., Nigg C.R. New England Psychological Association; Boston, MA: 1998. A Multidimensional Approach to Exercise Self-efficacy: Relationship with Exercise Behavior and Attitudes towards Exercise. [Google Scholar]
- 34.McAuley E. The role of efficacy cognitions in the prediction of exercise behavior in middle-aged adults. J. Behav. Med. 1992;15(1):65–88. doi: 10.1007/BF00848378. [DOI] [PubMed] [Google Scholar]
- 35.Sallis J.F., Grossman R.M., Pinski R.B., Patterson T.L., Nader P.R. The development of scales to measure social support for diet and exercise behaviors. Prev. Med. 1987;16(6):825–836. doi: 10.1016/0091-7435(87)90022-3. [DOI] [PubMed] [Google Scholar]
- 36.Robins R.W., Hendin H.M., Trzesniewski K.H. Measuring global self-esteem: construct validation of a single-item measure and the Rosenberg Self-Esteem Scale. Pers. Soc. Psychol. Bull. 2001;27(2):151–161. [Google Scholar]
- 37.Fairburn C.G., Beglin S.J. Assessment of eating disorders: interview or self-report questionnaire? Int. J. Eat. Disord. 1994;16(4):363–370. [PubMed] [Google Scholar]
- 38.Luce K.H., Crowther J.H. The reliability of the eating disorder examination–self-report questionnaire version (EDE-Q) Int. J. Eat. Disord. 1999;25(3):349–351. doi: 10.1002/(sici)1098-108x(199904)25:3<349::aid-eat15>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 39.Endicott J., Nee J., Harrison W., Blumenthal R. Quality of life enjoyment and satisfaction questionnaire: a new measure. Psychopharmacol. Bull. 1993;29(2):321–326. [PubMed] [Google Scholar]
- 40.Rapaport M.H., Clary C., Fayyad R., Endicott J. Quality-of-life impairment in depressive and anxiety disorders. Am. J. Psychiatry. 2005;162(6):1171–1178. doi: 10.1176/appi.ajp.162.6.1171. [DOI] [PubMed] [Google Scholar]
- 41.Diener E., Emmons R.A., Larsen R.J. The satisfaction with life scale. J. Pers. Assess. 1985;49 doi: 10.1207/s15327752jpa4901_13. [DOI] [PubMed] [Google Scholar]
- 42.Pavot W., Diener E. Review of the satisfaction with life scale. Psychol. Assess. 1993;5 [Google Scholar]
- 43.Marsh H.W., Martin A.J., Jackson S. Introducing a short version of the Physical Self Description Questionnaire: new strategies, short-form evaluative criteria, and applications of factor analyses. J. Sport Exerc. Psychol. 2010;32(4):438–482. doi: 10.1123/jsep.32.4.438. [DOI] [PubMed] [Google Scholar]
- 44.Hart E.A., Rejeski W.J., Leary M.R. The measurement of social physique anxiety. J. Sport Exerc. Psychol. 1989;11(1):94–104. [Google Scholar]
- 45.Saunders J.B., Aasland O.G., Babor T.F., De La Fuente J.R., Grant M. Development of the alcohol use disorders identification test (AUDIT): WHO collaborative project on early detection of persons with harmful alcohol consumption-II. Addiction. 1993;88(6):791–804. doi: 10.1111/j.1360-0443.1993.tb02093.x. [DOI] [PubMed] [Google Scholar]
- 46.Reinert D.F., Allen J.P. The alcohol use disorders identification test: an update of research findings. Alcohol. Clin. Exp. Res. 2007;31(2):185–199. doi: 10.1111/j.1530-0277.2006.00295.x. [DOI] [PubMed] [Google Scholar]
- 47.Beck A., Steer R., Brown G. Psychological Corporation; San Antonio, TX: 1996. Manual for the Beck Depression Inventory-II. [Google Scholar]
- 48.Beck A., Steer R. Psychological Corporation; San Antonio, TX: 1993. Beck Anxiety Inventory Manual. [Google Scholar]
- 49.Fydrich T., Dowdall D., Chambless D.L. Reliability and validity of the beck anxiety inventory. J. Anxiety Disord. 1992;6(1):55–61. [Google Scholar]
- 50.Hollon S., Kendall P. Cognitive self-statements in depression: development of an automatic thoughts questionnaire. Cogn. Ther. Res. 1980;4(4):383–395. [Google Scholar]
- 51.Safren S.A., Heimberg R.G., Lerner J., Henin A., Warman M., Kendall P.C. Differentiating anxious and depressive self-statements: combined factor structure of the anxious self-statements questionnaire and the automatic thoughts questionnaire-revised. Cogn. Ther. Res. 2000;24(3):327–344. [Google Scholar]
- 52.Beevers C.G., Strong D.R., Meyer B., Pilkonis P.A., Miller I.W. Efficiently assessing negative cognition in depression: an item response theory analysis of the dysfunctional attitude scale. Psychol. Assess. 2007;19(2):199–209. doi: 10.1037/1040-3590.19.2.199. [DOI] [PubMed] [Google Scholar]
- 53.Manos R.C., Kanter J.W., Luo W. The behavioral activation for depression scale-short form: development and validation. Behav. Ther. 2011;42(4):726–739. doi: 10.1016/j.beth.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 54.Hung C.I., Weng L.J., Su Y.J., Liu C.Y. Depression and somatic symptoms scale: a new scale with both depression and somatic symptoms emphasized. Psychiatry Clin. Neurosci. 2006;60(6):700–708. doi: 10.1111/j.1440-1819.2006.01585.x. [DOI] [PubMed] [Google Scholar]
- 55.Kroenke K., Spitzer R.L., Williams J.B. The PHQ-15: validity of a new measure for evaluating the severity of somatic symptoms. Psychosom. Med. 2002;64 doi: 10.1097/00006842-200203000-00008. [DOI] [PubMed] [Google Scholar]
- 56.Zijlema W.L., Stolk R.P., Löwe B., Rief W., White P.D., Rosmalen J.G.M. How to assess common somatic symptoms in large-scale studies: a systematic review of questionnaires. J. Psychosom. Res. 2013;74(6):459–468. doi: 10.1016/j.jpsychores.2013.03.093. [DOI] [PubMed] [Google Scholar]
- 57.Norton K., Norton L. Exercise and Sports Science Australia; 2011. Pre-exercise Screening, Guide to the Australian Adult Pre-exercise Screening System. [Google Scholar]
- 58.Marshall A.L., Miller Y.D., Burton N.W., Brown W.J. Measuring total and domain-specific sitting: a study of reliability and validity. Med. Sci. Sports Exerc. 2010;42(6):1094–1102. doi: 10.1249/MSS.0b013e3181c5ec18. [DOI] [PubMed] [Google Scholar]
- 59.Tanner J.M. Growth and maturation during adolescence. Nutr. Rev. 1981;39(2):43–55. doi: 10.1111/j.1753-4887.1981.tb06734.x. [DOI] [PubMed] [Google Scholar]
- 60.Gibson A.L., Holmes J.C., Desautels R.L., Edmonds L.B., Nuudi L. Ability of new octapolar bioimpedance spectroscopy analyzers to predict 4-component-model percentage body fat in Hispanic, black, and white adults. Am. J. Clin. Nutr. 2008;87(2):332–338. doi: 10.1093/ajcn/87.2.332. [DOI] [PubMed] [Google Scholar]
- 61.Aandstad A., Holtberget K., Hageberg R., Holme I., Anderssen S.A. Validity and reliability of bioelectrical impedance analysis and skinfold thickness in predicting body fat in military personnel. Mil. Med. 2014;179(2):208–217. doi: 10.7205/MILMED-D-12-00545. [DOI] [PubMed] [Google Scholar]
- 62.Miller W.R., Rollnick S. third ed. Guilford Press; US, New York, NY: 2013. Motivational Interviewing: Helping People Change. [Google Scholar]
- 63.Jelsma J.G.M., Mertens V.-C., Forsberg L., Forsberg L. How to measure motivational interviewing fidelity in randomized controlled trials: practical recommendations. Contemp. Clin. Trials. 2015;43:93–99. doi: 10.1016/j.cct.2015.05.001. [DOI] [PubMed] [Google Scholar]
- 64.Moyers T.B., Martin T., Manuel J.K., Miller W.R., Ernst D. University of New Mexico; Albuquerque, NM: 2010. Motivational Interviewing Treatment Integrity 3.1.1: Revised Global Scales.http://casaa.unm.edu/download/mitii3_1.pdf Retrieved April 2014, from. [Google Scholar]
- 65.Faigenbaum A.D., Kraemer W.J., Blimkie C.J., Jeffreys I., Micheli L.J., Nitka M., Rowland T.W. Youth resistance training: updated position statement paper from the national strength and conditioning association. J. Strength Cond. Res. 2009;23(5 Suppl):S60–S79. doi: 10.1519/JSC.0b013e31819df407. [DOI] [PubMed] [Google Scholar]
- 66.Carragher N., Adamson G., Bunting B., McCann S. Subtypes of depression in a nationally representative sample. J. Affect. Disord. 2009;113(1–2):88–99. doi: 10.1016/j.jad.2008.05.015. [DOI] [PubMed] [Google Scholar]
- 67.Taliaz D., Nagaraj V., Haramati S., Chen A., Zangen A. Altered brain-derived neurotrophic factor expression in the ventral tegmental area, but not in the hippocampus, is essential for antidepressant-like effects of electroconvulsive therapy. Biol. Psychiatry. 2013;74(4):305–312. doi: 10.1016/j.biopsych.2012.07.025. [DOI] [PubMed] [Google Scholar]
- 68.Julious S.A. Efficacy and suicidal risk for antidepressants in paediatric and adolescent patients. Stat. Methods Med. Res. 2012;22(2):190–218. doi: 10.1177/0962280211432210. [DOI] [PubMed] [Google Scholar]
- 69.Blumenthal J.A., Babyak M.A., Doraiswamy P.M., Watkins L., Hoffman B.M., Barbour K.A., Herman S., Craighead W.E., Brosse A.L., Waugh R., Hinderliter A., Sherwood A. Exercise and pharmacotherapy in the treatment of major depressive disorder. Psychosom. Med. 2007;69(7):587–596. doi: 10.1097/PSY.0b013e318148c19a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lopresti A.L., Hood S.D., Drummond P.D. A review of lifestyle factors that contribute to important pathways associated with major depression: diet, sleep and exercise. J. Affect. Disord. 2013;148:12–27. doi: 10.1016/j.jad.2013.01.014. [DOI] [PubMed] [Google Scholar]
- 71.DiLorenzo T.M., Bargman E.P., Stucky-Ropp R., Brassington G.S., Frensch P.A., LaFontaine T. Long-term effects of aerobic exercise on psychological outcomes. Prev. Med. 1999;28(1):75–85. doi: 10.1006/pmed.1998.0385. [DOI] [PubMed] [Google Scholar]
- 72.Wipfli B., Landers D., Nagoshi C., Ringenbach S. An examination of serotonin and psychological variables in the relationship between exercise and mental health. Scand. J. Med. Sci. Sports. 2011;21(3):474–481. doi: 10.1111/j.1600-0838.2009.01049.x. [DOI] [PubMed] [Google Scholar]
- 73.Cerin E. Ways of unraveling how and why physical activity influences mental health through statistical mediation analyses. Ment. Health Phys. Act. 2010;3(2):51–60. [Google Scholar]
- 74.Crone D., Johnston L.H., Gidlow C., Henley C., James D.V.B. Uptake and participation in physical activity referral schemes in the UK: an investigation of patients referred with mental health problems. Issues Ment. Health Nurs. 2008;29(10):1088–1097. doi: 10.1080/01612840802319837. [DOI] [PubMed] [Google Scholar]
- 75.Krämer L.V., Helmes A.W., Seelig H., Fuchs R., Bengel J. Correlates of reduced exercise behaviour in depression: the role of motivational and volitional deficits. Psychol. Health. 2014;29(10):1206–1225. doi: 10.1080/08870446.2014.918978. [DOI] [PubMed] [Google Scholar]
- 76.Yang X.H., Huang J., Zhu C.Y., Wang Y.F., Cheung E.F.C., Chan R.C.K., Xie G.R. Motivational deficits in effort-based decision making in individuals with subsyndromal depression, first-episode and remitted depression patients. Psychiatry Res. 2014;220(3):874–882. doi: 10.1016/j.psychres.2014.08.056. [DOI] [PubMed] [Google Scholar]
- 77.Chao D., Foy C.G., Farmer D. Exercise adherence among older adults: challenges and strategies. Control. Clin. Trials. 2000;21(5, Supplement 1):S212–S217. doi: 10.1016/s0197-2456(00)00081-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.