Abstract
Purpose
The clinical benefit of targeted molecular therapy (TMT) in renal cell carcinoma (RCC) with an inferior vena cava (IVC) tumor thrombus remains controversial. The aim of this study was to investigate the effects of presurgical TMT on the heights and levels of IVC thrombi, and to assess its impact on surgical strategy.
Patients and methods
We retrospectively reviewed data from 18 patients with RCC involving IVC tumor thrombi who were treated at our hospital with presurgical TMT followed by an IVC thrombectomy. The changes in heights and levels of the IVC thrombi were compared using computed tomography or magnetic resonance imaging. Clinicopathological factors were also evaluated to assess their association with TMT efficacy.
Results
The tumor thrombus levels before TMT were stage I in 1 patient (5.6%), II in 12 patients (66.7%), III in 4 patients (22.2%), and IV in 1 patient (5.6%). After a median of two treatment cycles (range: 1–3), the thrombus height decreased measurably in 11 patients (61.1%) with an average shrinkage of 17.7%. The thrombus height remained stable in five patients (27.8%) and was enlarged in two (11.1%). Downstaging of the thrombus level occurred in four patients (22.2%); the surgical strategy was modified in three patients (16.7%) to avoid cardiopulmonary bypass and complicated liver mobilization under robot-assisted laparoscopy. Furthermore, a higher neutrophil count tended to be associated with a worse clinical TMT-associated outcome (P=0.056).
Conclusion
Our data suggest a limited influence of presurgical TMT with a positive benefit in RCC patients with level III and IV thrombus. Thrombus-level regression may potentially alter the surgical strategy, especially robotic surgery. However, our findings require validation with additional prospective investigations.
Keywords: presurgical TMT, surgical strategy, sorafenib, sunitinib
Introduction
Renal cell carcinoma (RCC) is a common malignant tumor of the genitourinary system and accounts for ~2%–3% of adult malignant tumors.1 According to a recent study, there were ~66,800 new diagnoses of RCC and 23,400 deaths due to this disease in China in 2015, and the trend in the morbidity of RCC has been increasing.2 Invasion of the renal vein and/or inferior vena cava (IVC) occurs in 4%–10% of patients, and it is more common in those with right kidney cancer.1,3 Radical nephrectomy (RN) with tumor thrombectomy is the standard approach for treating such challenging cases. These patients were able to obtain, as reported by the literature, better long-term survival, and the tumor-specific survival rate is up to 50%.4,5 However, this procedure has been shown to be associated with high morbidity and mortality rates.6,7 Therefore, preoperative systemic treatments are usually employed to shrink the tumor and IVC thrombus to reduce the complexity of the surgery.
In recent years, targeted molecular therapy (TMT) has been recognized as an effective strategy to significantly reduce the tumor burden and prolong progression-free survival of patients with advanced-stage cancer, especially metastatic renal cancer. TMTs have contributed to marked improvements in advanced RCC treatment and have been used as neoadjuvant therapies in recent years.8–10 A primary goal of neoadjuvant TMT is to shrink the primary lesions or metastases and enhance the safety and feasibility of surgical intervention. A number of case reports have described the efficacy of TMTs, including sunitinib, sorafenib, and axitinib, in reducing the tumor thrombus burden.11–13 Retrospective studies have produced more variable results, with decreased tumor thrombus levels observed in 7%–19% of patients.14–16 At present, there are no guidelines that recommend neoadjuvant TMT for locally advanced renal tumors. Although the clinical benefit of TMT against IVC tumor thrombi is controversial, improving surgical strategies and reducing surgical risks remain important goals. The aim of our study was to investigate the therapeutic effects of TMTs on the heights and levels of IVC tumor thrombi, as well as their safety profiles.
Patients and methods
Study design and patients
We retrospectively reviewed patients with RCC that involved an IVC tumor thrombus who were treated with presurgical TMT at our hospital between September 2012 and May 2017, followed by IVC thrombectomy. The indications for receiving presurgical TMT were to facilitate subsequent IVC thrombectomy by reducing the tumor size or to treat patients owing to the metastatic burden. Eighteen patients extracted from the hospital database met these inclusion criteria. The tumor histologies were confirmed by needle biopsies before TMT administration. All patients received TMT with the vascular endothelial growth factor receptor inhibitors sorafenib (Pfizer, Inc., New York, NY, USA), sunitinib (Pfizer, Inc.), and axitinib (Pfizer, Inc.). Sorafenib was administered orally at 400 mg twice a day during a 4-week cycle. Sunitinib was administered orally at 50 mg four times a day for a 6-week cycle (4 weeks followed by a break of 2 weeks). Axitinib was administered orally at a starting dose of 5 mg twice daily for a 4-week cycle. The main endpoints were the radiographic changes in the height and level of the tumor thrombus. The secondary endpoints included the safety and 1 of TMTs. After ~12 weeks of therapy, the feasibility of surgery and the surgical strategy were reevaluated. The study was approved by the Medical Ethics Committee of Chinese PLA General Hospital, and each patient provided written informed consent.
Assessment
We used the Response Evaluation Criteria in Solid Tumors, version 1.1, to assess the response to presurgical TMT through enhanced computed tomography or magnetic resonance imaging of the chest, abdomen, and pelvis. The IVC tumor thrombus level was evaluated according to the Mayo classification. The height of the tumor thrombus was defined as the distance beyond the renal vein level. Toxicity was recorded and graded throughout the treatment period according to the Common Terminology Criteria for Adverse Events (CTCAE), version 4.4.
The following clinical data were collected for analysis at baseline and after TMT: medical history and physical examination results, laboratory studies (including blood and hepatic parameters, biochemistry, and coagulation function), Eastern Cooperative Oncology Group (ECOG) performance status, clinical TNM staging (based on the TNM classification of the 2010 American Joint Committee on Cancer), and TMT agents. Perioperative data (operative time, estimated blood loss, transfusions, and perioperative complications graded according to the Clavien system) were also included in the analysis. Imaging and laboratory studies were performed every 3 months for follow-up surveillance.
The effects of sorafenib and sunitinib were compared. The patients who received axitinib were not included in our comparisons due to a small number of patients. Additionally, a comparison of clinical and pathological data between patients with a decreased tumor thrombus height vs those showing a stabilization or increase was performed.
Statistical analysis
Continuous data are presented as the mean ± SD when normally distributed. Data that conformed to nonnormal distributions are represented by the means and ranges. Continuous variables were compared using Student’s t-test or the Mann–Whitney U-test. The chi-square or Fisher’s exact test was performed to compare categorical variables between groups. Pearson’s correlation test was used to assess the relationship between two continuous variables. Statistical analyses were conducted using SPSS®, version 22 (IBM Corp., Armonk, NY, USA), and P<0.05 was deemed to be statistically significant.
Results
Baseline characteristics
A total of 18 patients with RCC involving an IVC tumor thrombus who received presurgical TMT were included in this study. All patients underwent a biopsy before receiving TMT to confirm the diagnosis of RCC. Baseline patient and tumor characteristics are shown in Table 1. Before TMT administration, the thrombus level was stage I in 1 patient (5.6%), II in 12 patients (66.7%), III in 4 patients (22.2%), and IV in 1 patient (5.6%). Of these, six patients (33.3%) received sorafenib, nine (50.0%) received sunitinib, and three (16.7%) received axitinib. The median duration of TMT was 2 cycles (range: 1–3 cycles).
Table 1.
Patient characteristics
| Characteristics | Total | Sunitinib | Sorafenib | P-value |
|---|---|---|---|---|
| Age, years; median (range) | 53.5 (33–75) | 47 (33–66) | 54 (34–68) | 0.72 |
| Sex, n (%) | 0.49 | |||
| Male | 16 (88.9) | 7 (77.8) | 6 (100.0) | |
| Female | 2 (11.1) | 2 (22.2) | 0 | |
| BMI, kg/m2; median (range) | 24.07 (18.09–30.42) | 23.94 (18.09–29.76) | 25.83 (22.41–30.42) | 0.20 |
| Tumor laterality, n (%) | 1.00 | |||
| Left | 4 (22.2) | 2 (22.2) | 1 (16.7) | |
| Right | 14 (77.8) | 7 (77.8) | 5 (83.3) | |
| ECOG performance status, n (%) | 0.32 | |||
| 0 | 2 (11.1) | 2 (22.2) | 0 | |
| 1 | 14 (77.8) | 6 (66.7) | 5 (83.3) | |
| 2 | 2 (11.1) | 1 (11.1) | 1 (16.7) | |
| TMT, n (%) | NA | |||
| Sorafenib | 6 (33.3) | 9 (100.0) | ||
| Sunitinib | 9 (50.0) | 6 (100.0) | ||
| Axitinib | 3 (16.7) | |||
| Cycles of TMT, weeks; median (range) | 2 (1–3) | 2 (1–3) | 2 (1–3) | 0.48 |
| Clinical stage, n (%) | NA | |||
| T3b | 17 (94.4) | 9 (100.0) | 6 (100.0) | |
| T3c | 1 (5.6) | 0 | 0 | |
| Nodal stage, n (%) | 0.58 | |||
| N0 | 12 (66.7) | 5 (55.6) | 5 (83.3) | |
| N1 | 6 (33.3) | 4 (44.4) | 1 (16.7) | |
| Metastatic disease, n (%) | 2 (11.1) | 0 | 1 (16.7) | 0.43 |
| Thrombus level, n (%) | 0.67 | |||
| I | 1 (5.6) | 0 | 1 (16.7) | |
| II | 12 (66.7) | 8 (88.9) | 4 (66.6) | |
| III | 4 (22.2) | 1 (11.1) | 1 (16.7) | |
| IV | 1 (5.6) | 0 | 0 | |
| Histology, n (%) | 0.66 | |||
| ccRCC | 15 (83.3) | 8 (88.9) | 5 (83.3) | |
| Papillary cell RCC | 2 (11.1) | 1 (11.1) | 0 | |
| Chromophobe RCC | 1 (5.6) | 0 | 1 (16.7) | |
| Fuhrman grade, n (%) | 0.11 | |||
| 2 | 3 (20.0) | 1 (12.5) | 2 (40.0) | |
| 3 | 9 (60.0) | 4 (50.0) | 3 (60.0) | |
| 4 | 3 (20.0) | 3 (37.5) | 0 | |
| ASA score, n (%) | 0.23 | |||
| 1+2 | 14 (77.8) | 6 (66.7) | 6 (100.0) | |
| 3+4 | 4 (22.2) | 3 (33.3) | 0 |
Abbreviations: ASA, American Society of Anesthesiologists; BMI, body mass index; ccRCC, clear cell renal cell carcinoma; ECOG, Eastern Cooperative Oncology Group; RCC, renal cell carcinoma; TMT, targeted molecular therapy.
Efficacy and toxicity of therapy
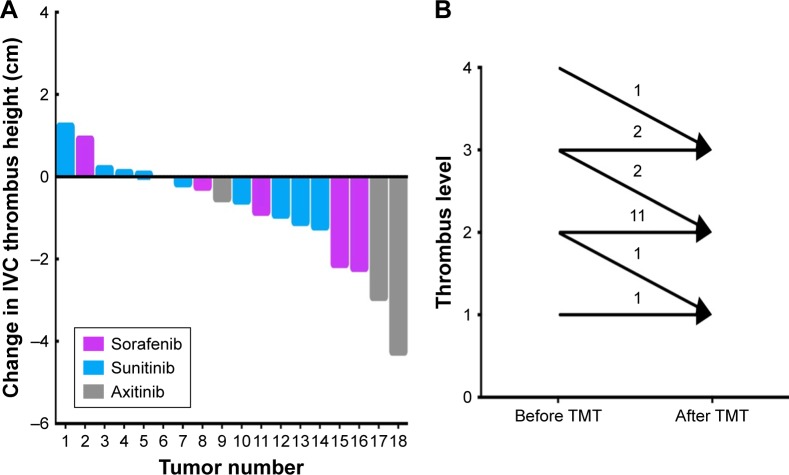
All patients were assessed for the clinical benefit of presurgical TMT (Table 2). The tumor thrombus height decreased measurably in 11 patients (61.1%), with an average shrinkage of 17.7%. The thrombi remained stable in five patients (27.8%) and grew larger in two patients (11.1%; Figure 1A). The median reduction in tumor thrombus height was −0.53 cm (range: −4.23 to +1.21 cm). The median change in the maximum diameter of the thrombus was −0.3 cm (range: −1.23 to +0.29 cm), with 12 patients showing a decreased thrombus diameter, 4 showing no change, and 2 experiencing an increase. The primary tumor size was decreased, stable, or increased in eight (44.4%), six (33.3%), and four (22.2%) of the patients, respectively. The median reduction in the primary tumor diameter was −0.16 cm (range: −1.2 to +2.1 cm). No statistically significant difference in tumor or thrombus shrinkage was found between the sorafenib-treated vs sunitinib-treated groups (Table 2).
Table 2.
Effect of TMTs and the comparison between sorafenib-treated vs sunitinib-treated groups
| Total | Sunitinib | Sorafenib | P-value | |
|---|---|---|---|---|
| Effect on IVC thrombus | ||||
| Change in thrombus height, n (%) | ||||
| Increased | 2 (11.1) | 1 (11.1) | 1 (16.7) | 0.56 |
| Stable | 5 (27.8) | 4 (44.4) | 1 (16.7) | |
| Decreased | 11 (61.1) | 4 (44.4) | 4 (66.7) | |
| Median (range), cm | −0.53 (−4.23 to +1.21) | −0.14 (−1.19 to +1.21) | −0.42 (−2.2 to +0.89) | 0.67 |
| Change in maximum diameter of thrombus, n (%) | ||||
| Increased | 2 (11.1) | 1 (11.1) | 1 (16.7) | 0.79 |
| Stable | 4 (22.2) | 3 (33.3) | 1 (16.7) | |
| Decreased | 12 (66.7) | 5 (55.6) | 4 (66.7) | |
| Median (range), cm | −0.3 (−1.23 to +0.29) | −0.18 (−1.25 to +0.29) | −0.50 (−1.9 to +0.12) | 0.29 |
| Change in thrombus level, n (%) | ||||
| Increased | 0 | 0 | 0 | 0.77 |
| Stable | 14 (77.8) | 8 (88.9) | 5 (83.3) | |
| Decreased | 4 (22.2) | 1 (11.1) | 1 (16.7) | |
| Effect on primitive tumor size, n (%) | ||||
| Increased | 4 (22.2) | 2 (22.2) | 1 (16.7) | 0.57 |
| Stable | 6 (33.3) | 4 (44.4) | 2 (33.3) | |
| Decreased | 8 (44.4) | 3 (33.3) | 3 (50.0) | |
| Median (range), cm | −0.16 (−1.2 to +2.1) | −0.11 (−1 to +2.1) | −0.11 (−1.2 to +0.8) | 0.91 |
| Primitive tumor size before TMT, cm; median (range) | 8.55 (4.9–16.4) | |||
| Thrombus height before TMT, cm; median (range) | 6.97 (2.0–13.8) |
Abbreviations: IVC, inferior vena cava; TMT, targeted molecular therapy.
Figure 1.
The changes in heights and levels of the IVC thrombi after TMTs.
Notes: (A) The measurable decrease in tumor thrombus height was shown according to types of target molecules. The median reduction of tumor thrombus height was −0.53 cm. (B) Downstaging of the thrombus level occurred in four patients after TMTs.
Abbreviations: IVC, inferior vena cava; TMT, targeted molecular therapy.
Following presurgical TMT, downstaging of the thrombus level occurred in four patients (level IV to III in one patient, level III to II in two patients, and level II to I in one patient). The thrombus levels in the other patients remained stable; none of the 18 patients experienced an increase (Figure 1B). The surgical strategy was reevaluated in three patients who underwent robot-assisted laparoscopic RN with tumor thrombectomy. For one patient (downstaged from level IV to III), a thoracoscope-assisted open atriotomy and cardiopulmonary bypass were avoided owing to presurgical TMT. Two patients (downstaged from level III to II) avoided height-proximal control of the suprahepatic IVC and mobilization of both lobes of the liver during robotic surgery, thus reducing the surgical risk.
Fourteen patients (77.8%) exhibited a concordant decrease or increase of both the primary tumor and tumor thrombus height, while the changes in the remaining four patients were not synchronous (Figure 2). We analyzed the association between the benefit on primary tumor size and tumor thrombus height (data not shown) and found no statistically significant correlation between the two parameters (r=0.085, P=0.738).
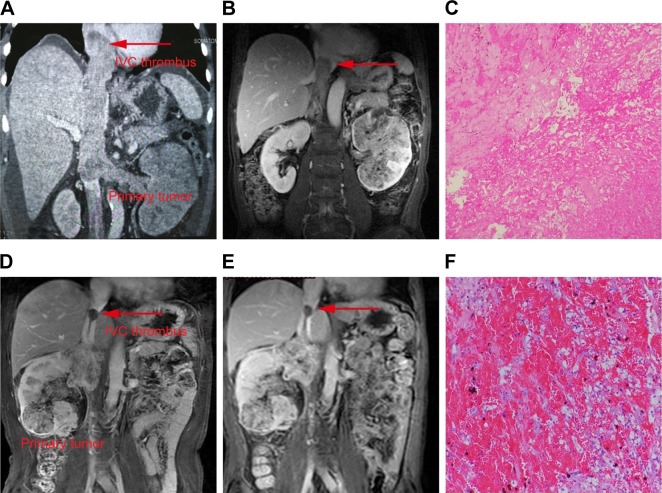
Figure 2.
Representative images of radiography and pathology after presurgical TMT.
Notes: After receiving 12 weeks of presurgical axitinib treatment, one patient experienced a remarkable decrease in both the thrombus height and primitive tumor. The arrow indicates the distal end of the tumor thrombus. (A, B) The level of thrombus changed from level IV to III. (C) Widespread necrosis and extensive degeneration were observed in the photomicrograph of tumor thrombus. One patient showed no response to primitive tumor and tumor thrombus with 8 weeks of presurgical sorafenib treatment. The arrow indicates the distal end of the tumor thrombus. (D, E) The height of tumor thrombus remained stable at the level of second hepatic portal. (F) The photograph of tumor thrombus after TMT showed massive thrombosis with viable tumor cells.
Abbreviations: IVC, inferior vena cava; TMT, targeted molecular therapy.
No CTCAE grade 4 or 5 AEs were observed owing to TMT. Three patients (16.7%) experienced grade 3 AEs, including two with hypertension and one with hand–foot syndrome. The common grade 1–2 AEs were hand–foot syndrome, alopecia, and diarrhea in the sorafenib-treated group and hypertension, fatigue, hand–foot syndrome, and diarrhea in the sunitinib-treated group (Table 3). One patient stopped treatment after 6 weeks owing to intolerable skin reactions and difficulty in walking.
Table 3.
Adverse events related to presurgical TMTs
| Total, n (%) | Sorafenib, n (%) | Sunitinib, n (%) | Axitinib, n (%) | |
|---|---|---|---|---|
| Hand–foot syndrome | 9 (50.0) | 4 (66.7) | 4 (44.4) | 1 (33.3) |
| Diarrhea | 8 (44.4) | 3 (50.0) | 3 (33.3) | 2 (66.7) |
| Fatigue | 7 (38.9) | 2 (33.3) | 4 (44.4) | 1 (33.3) |
| Hypertension | 9 (50.0) | 2 (33.3) | 5 (55.6) | 2 (66.7) |
| Mucositis | 5 (27.8) | 1 (16.7) | 3 (33.3) | 1 (33.3) |
| Alopecia | 5 (27.8) | 3 (50.0) | 2 (22.2) | 0 |
| Nausea | 2 (11.1) | 0 | 2 (22.2) | 0 |
| Anorexia | 3 (16.7) | 1 (16.7) | 2 (22.2) | 0 |
| Hypothyroidism | 1 (5.6) | 0 | 1 (11.1) | 0 |
| Thrombocytopenia | 1 (5.6) | 1 (16.7) | 0 | 0 |
| Neutropenia | 1 (5.6) | 0 | 1 (11.1) | 0 |
Abbreviation: TMT, targeted molecular therapy.
Perioperative data and follow-up
Following a median of two cycles of TMT, all patients successfully underwent RN with tumor thrombectomy (5 open and 13 laparoscopic/robotic). One patient had severe adhesion between the IVC and the surrounding tissue, and therefore only underwent left renal RN. The median operative time was 217 min (range 100–318 min), while the median estimated blood loss was 800 mL (range 20–5,000 mL). Twelve patients (66.7%) required transfusions during surgery, and all had drainage tubes placed for a median 3.5 days. Seven patients experienced postoperative complications; five had minor complications (grade I or II) that included wound-healing delay, anemia, and kidney dysfunction and were alleviated by conservative treatment. One patient experienced a grade IIIb complication (postoperative intra-abdominal hemorrhage) and underwent successful hemostasis following a laparoscopic second-look exploration. Considering wound errhysis without obvious bleeding points, the abdominal cavity blood was carefully cleaned and hemostatic gauzes were placed; the patient recovered after surgery. Finally, one patient experienced a grade V complication; this patient died of sudden cardiac death after robotic surgery because of various underlying primary diseases (a previous myocardial infarction, hypertension, and diabetes) as well as surgical trauma.
During follow-up (median duration, 15 months; range, 4–51 months), nine patients (50%) developed new metastases, including in the lung, liver, bone, and systematically. Five patients (28%) continued to receive TMT after surgery. Seven patients died of kidney cancer with systemic metastasis, of these, five were <50 years old.
Clinical and pathological factors associated with efficacy of therapy
To identify factors that predict a clinical benefit owing to presurgical TMT administration, patients were classified into those who had a decrease in tumor thrombus height and those who did not, and their clinical and pathological data were compared (Table 4). No factors were found to be significantly different between the two groups; however, elevated neutrophil counts tended to be associated with poorer therapeutic efficacy (P=0.056). Given the small sample size and the lack of statistically significant factors, multivariate analysis was not performed.
Table 4.
Comparison of the clinical and pathological factors associated with the effect of TMTs
| Decreased (n=11) | Increased or stable (n=7) | P-value | |
|---|---|---|---|
| Age, years; median (range) | 59 (34–75) | 46 (33–62) | 0.10 |
| Sex, n (%) | 0.50 | ||
| Male | 9 (81.8) | 7 (100) | |
| Female | 2 (18.2) | 0 | |
| Tumor laterality, n (%) | 1.00 | ||
| Left | 2 (18.2) | 2 (28.6) | |
| Right | 9 (81.8) | 5 (71.4) | |
| ECOG performance status, n (%) | 0.26 | ||
| 0 | 1 (9.1) | 1 (14.3) | |
| 1 | 10 (90.9) | 4 (57.1) | |
| 2 | 0 | 2 (28.6) | |
| TMT, n (%) | 0.23 | ||
| Sorafenib | 4 (36.4) | 5 (71.4) | |
| Sunitinib | 4 (36.4) | 2 (28.6) | |
| Axitinib | 3 (27.3) | 0 | |
| Cycles of TMT, weeks; median (range) | 2.5 (1–3) | 1.5 (1–3) | 0.24 |
| Clinical stage, n (%) | 1.00 | ||
| T3b | 10 (90.9) | 7 (100) | |
| T3c | 1 (9.1) | 0 | |
| Nodal stage, n (%) | 0.14 | ||
| N0 | 9 (81.8) | 3 (42.9) | |
| N1 | 2 (18.2) | 4 (57.1) | |
| Metastatic disease, n (%) | 1 (9.1) | 1 (14.3) | 1.00 |
| Thrombus level, n (%) | 0.17 | ||
| I | 0 | 1 (14.3) | |
| II | 7 (63.6) | 5 (71.4) | |
| III | 3 (27.3) | 1 (14.3) | |
| IV | 1 (9.1) | 0 | |
| Histology, n (%) | 1.00 | ||
| ccRCC | 9 (81.8) | 6 (85.7) | |
| Papillary cell RCC | 1 (9.1) | 1 (14.3) | |
| Chromophobe RCC | 1 (9.1) | 0 | |
| Fuhrman grade, n (%) | 0.42 | ||
| 2 | 1 (11.1) | 2 (33.3) | |
| 3 | 6 (66.7) | 3 (50.0) | |
| 4 | 2 (22.2) | 1 (16.7) | |
| ASA score, n (%) | 0.51 | ||
| 1+2 | 8 (72.7) | 4 (57.1) | |
| 3+4 | 3 (27.3) | 3 (42.9) | |
| Hemoglobin, g/L; median (range) | 128 (68–141) | 117 (83–139) | 0.23 |
| Neutrophil count/mL; median (range) | 3.83 (1.00–6.31) | 4.88 (3.67–7.23) | 0.056 |
| NLR, median (range) | 2.36 (0.65–4.56) | 4.09 (1.51–6.60) | 0.28 |
| Albumin, g/L; median (range) | 39.45 (32–43.4) | 37.25 (30.7–60) | 0.17 |
| Creatinine, µmol/L; median (range) | 96.75 (55.9–146.9) | 95.55 (79.4–127.2) | 0.77 |
| LDH, U/L; median (range) | 184.15 (110.9–288.3) | 181.63 (156.1–530.6) | 0.63 |
| ALP, U/L; median (range) | 71.15 (64–126.1) | 73.23 (34.8–241.3) | 0.85 |
Abbreviations: ALP, alkaline phosphatase; ASA, American Society of Anesthesiologists; ccRCC, clear cell renal cell carcinoma; ECOG, Eastern Cooperative Oncology Group; LDH, lactate dehydrogenase; RCC, renal cell carcinoma; TMT, targeted molecular therapy; NLR, neutrophil to lymphocyte ratio.
Discussion
RN with tumor thrombectomy is the standard approach for locally advanced RCC; however, this type of procedure has been shown to be associated with high surgical morbidity and mortality rates.17 The complication rates of IVC tumor thrombectomies range from 12.4% to 46.9%, with tumor thrombi levels of 0 to IV.6,17 Furthermore, estimated blood loss, transfusion rates, and postoperative complication rates have also been shown to increase with higher tumor thrombus levels.18 Given the considerable benefits observed with multiple targeted molecule treatments in patients with metastatic RCC,7 presurgical TMT ought to reduce the tumor thrombus burden, enhance the feasibility of surgery, and reduce complication rates.
However, the role and effect of presurgical TMT in patients with RCC exhibiting IVC tumor thrombi remain controversial. Several retrospective studies produced variable results, with the thrombus level decreases observed in 7%–19% of patients.15,19,20 Cost et al performed a retrospective study of 25 patients in which 3 (12%) showed a reduction in the level of thrombus and 1 (4%) experienced an increase; 44% of patients had a change in the tumor thrombus height following TMT. However, the impact on tumor thrombus level led to altering the surgical approach in only one patient who was downstaged from level IV to III.16
Based on one of the largest sample sizes at present, our study showed more beneficial results with measurably decreased heights in 11 patients (61.1%). For cases with tumor thrombus level III and IV, we observed a regression of level in three cases (60%). It is suggested that TMT may be of benefit in RCC patients with level III and IV thrombus. With the development of laparoscopy and robotic technology in recent years, the safety and feasibility of robot-assisted laparoscopic IVC thrombectomy (RAL-IVCTE) have been investigated at several centers.21,22 In our study, downstaging of the thrombus level occurred in four patients (22.2%), three of whom benefited from a surgical strategy modification to avoid cardiopulmonary bypass and complicated liver mobilization during RAL-IVCTE. TMT may potentially permit less invasive thrombectomy approaches and alter the course of the surgery, especially robotic surgery.
We did not observe a significant difference in efficacy between sorafenib and sunitinib treatments. However, all three patients who were treated with axitinib had a meaningful reduction in tumor thrombus size, two of whom experienced downstaging of the level of thrombus. Owing to the small sample size, further studies are required to determine whether axitinib is in fact superior to other targeted agents. Only one of our patients underwent dose reduction or intermittent administration during treatment. Surgical morbidity associated with TMT is another major clinical concern. Kondo et al observed two major and three minor complications out of nine patients after neoadjuvant treatment, mainly hemorrhage.23 In our study, the transfusion rate was 66.7%, and major complications occurred in two patients, including one who died of cardiac disease. Thus, preoperative evaluation of the vital organs and active preparation of blood are necessary in all cases.
As mentioned above, most patients (77.8%) experienced synchronous shrinking or growth in both the primary tumor and thrombus height. However, four patients had opposite primary tumor size/thrombus height outcomes. There was no statistically significant correlation between primary tumor size and thrombus height change. This may be due to the heterogeneity and compositional differences in renal tumors. Some studies indicated that genomic heterogeneity was associated with acquired resistance, which drives distinct responses to TMT.24,25
Moreover, clinical deterioration and potential progression should be taken into consideration during the presurgical treatment period.16 At present, there are no factors that can predict the response to TMT in locally advanced renal tumors or metastatic RCC.24,26 In our study, elevated neutrophil count tended to be associated with poor therapy outcomes; this was consistent with results from Fukuda et al’s study.14 Several reports have described higher blood neutrophils and blood neutrophil/lymphocyte ratios as being associated with poor clinical outcomes in RCC.27,28 After sunitinib treatment, altered neutrophil counts were associated with a specific change in plasma interleukin-8.29 Additionally, Huang et al reported that interleukin-8 expression was elevated in RCC tumors that were refractory to sunitinib treatment.30 Taken together, these studies suggest that the inflammatory microenvironment that comprises neutrophils and lymphocytes may impact the efficacy of treatment.
Limitations
Our study has several limitations. Its retrospective design and small number of patients may impact the reliability of the results. Furthermore, we were unable to control for selection bias and other unmeasurable confounding factors. We had no strict criteria for selecting patients before TMT; the patient population was mostly based on the physicians’ or patients’ treatment preferences. Long-term follow-up is required to investigate the oncological benefit of presurgical TMT. Thus, our observations require further prospective investigations with a larger number of patients to overcome these limitations.
Conclusion
Despite these limitations, our preoperative study investigates the therapeutic effects and safety on the height and level of IVC tumor thrombi during presurgical TMT. Our results suggest that TMT may be of benefit in RCC patients with level III and IV thrombus; in particular, the neutrophil count may tend to influence the clinical benefit of TMT. A change in thrombus level may potentially alter the surgical strategy to a less complicated method, especially in patients undergoing robotic surgery.
Acknowledgments
We are grateful to the patients for participating in this study. We also thank the investigators who contributed to this work.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Marshall FF, Dietrick DD, Baumgartner WA, Reitz BA. Surgical management of renal cell carcinoma with intracaval neoplastic extension above the hepatic veins. J Urol. 1988;139(6):1166–1172. doi: 10.1016/s0022-5347(17)42848-5. [DOI] [PubMed] [Google Scholar]
- 2.Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–132. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 3.Psutka SP, Leibovich BC. Management of inferior vena cava tumor thrombus in locally advanced renal cell carcinoma. Ther Adv Urol. 2015;7(4):216–229. doi: 10.1177/1756287215576443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haferkamp A, Bastian PJ, Jakobi H, et al. Renal cell carcinoma with tumor thrombus extension into the vena cava: prospective long-term followup. J Urol. 2007;177(5):1703–1708. doi: 10.1016/j.juro.2007.01.039. [DOI] [PubMed] [Google Scholar]
- 5.Kaushik D, Linder BJ, Thompson RH, et al. The impact of histology on clinicopathologic outcomes for patients with renal cell carcinoma and venous tumor thrombus: a matched cohort analysis. Urology. 2013;82(1):136–141. doi: 10.1016/j.urology.2013.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pouliot F, Shuch B, Larochelle JC, Pantuck A, Belldegrun AS. Contemporary management of renal tumors with venous tumor thrombus. J Urol. 2010;184(3):833–841. doi: 10.1016/j.juro.2010.04.071. [DOI] [PubMed] [Google Scholar]
- 7.Ficarra V, Novara G. Editorial comment on: update on the medical treatment of metastatic renal cell carcinoma. Eur Urol. 2008;54(2):324–325. doi: 10.1016/j.eururo.2008.04.058. [DOI] [PubMed] [Google Scholar]
- 8.Rini BI, Plimack ER, Takagi T, et al. A Phase II study of pazopanib in patients with localized renal cell carcinoma to optimize preservation of renal parenchyma. J Urol. 2015;194(2):297–303. doi: 10.1016/j.juro.2015.03.096. [DOI] [PubMed] [Google Scholar]
- 9.Powles T, Sarwar N, Stockdale A, et al. Safety and efficacy of pazopanib therapy prior to planned nephrectomy in metastatic clear cell renal cancer. JAM Oncol. 2016;2(10):1303–1309. doi: 10.1001/jamaoncol.2016.1197. [DOI] [PubMed] [Google Scholar]
- 10.Rini BI, Garcia J, Elson P, et al. The effect of sunitinib on primary renal cell carcinoma and facilitation of subsequent surgery. J Urol. 2012;187(5):1548–1554. doi: 10.1016/j.juro.2011.12.075. [DOI] [PubMed] [Google Scholar]
- 11.Horn T, Thalgott MK, Maurer T, et al. Presurgical treatment with sunitinib for renal cell carcinoma with a level III/IV vena cava tumour thrombus. Anticancer Res. 2012;32(5):1729–1735. [PubMed] [Google Scholar]
- 12.Peters I, Winkler M, Jüttner B, et al. Neoadjuvant targeted therapy in a primary metastasized renal cell cancer patient leads to down-staging of inferior vena cava thrombus (IVC) enabling a cardiopulmonary bypass-free tumor nephrectomy: a case report. World J Urol. 2014;32(1):245–248. doi: 10.1007/s00345-012-0955-5. [DOI] [PubMed] [Google Scholar]
- 13.Kroeger N, Gajda M, Zanow J, et al. Downsizing a tumor thrombus of advanced renal cell carcinoma with neoadjuvant systemic therapy and resulting histopathological effects. Urol Int. 2010;84(4):479–484. doi: 10.1159/000296301. [DOI] [PubMed] [Google Scholar]
- 14.Fukuda H, Kondo T, Takagi T, Iizuka J, Nagashima Y, Tanabe K. Limited benefit of targeted molecular therapy for inferior vena cava thrombus associated with renal cell carcinoma. Int J Clin Oncol. 2017;22(4):767–773. doi: 10.1007/s10147-017-1119-9. [DOI] [PubMed] [Google Scholar]
- 15.Bigot P, Fardoun T, Bernhard JC, et al. Neoadjuvant targeted molecular therapies in patients undergoing nephrectomy and inferior vena cava thrombectomy: is it useful? World J Urol. 2014;32(1):109–114. doi: 10.1007/s00345-013-1088-1. [DOI] [PubMed] [Google Scholar]
- 16.Cost NG, Delacroix SE, Jr, Sleeper JP, et al. The impact of targeted molecular therapies on the level of renal cell carcinoma vena caval tumor thrombus. Eur Urol. 2011;59(6):912–918. doi: 10.1016/j.eururo.2011.02.032. [DOI] [PubMed] [Google Scholar]
- 17.Karnes RJ, Blute ML. Surgery insight: management of renal cell carcinoma with associated inferior vena cava thrombus. Nat Clin Pract Urol. 2008;5(6):329–339. doi: 10.1038/ncpuro1122. [DOI] [PubMed] [Google Scholar]
- 18.Abel EJ, Thompson RH, Margulis V, et al. Perioperative outcomes following surgical resection of renal cell carcinoma with inferior vena cava thrombus extending above the hepatic veins: a contemporary multicenter experience. Eur Urol. 2014;66(3):584–592. doi: 10.1016/j.eururo.2013.10.029. [DOI] [PubMed] [Google Scholar]
- 19.Bex A, Kroon BK, de Bruijn R. Is there a role for neoadjuvant targeted therapy to downsize primary tumors for organ sparing strategies in renal cell carcinoma? Int J Surg Oncol. 2012;2012:250479. doi: 10.1155/2012/250479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sassa N, Kato M, Funahashi Y, Maeda M, Inoue S, Gotoh M. Efficacy of pre-surgical axitinib for shrinkage of inferior vena cava thrombus in a patient with advanced renal cell carcinoma. Jpn J Clin Oncol. 2014;44(4):370–373. doi: 10.1093/jjco/hyu014. [DOI] [PubMed] [Google Scholar]
- 21.Wang B, Li H, Ma X, et al. Robot-assisted laparoscopic inferior vena cava thrombectomy: different sides require different techniques. Eur Urol. 2016;69(6):1112–1119. doi: 10.1016/j.eururo.2015.12.001. [DOI] [PubMed] [Google Scholar]
- 22.Abaza R. Initial series of robotic radical nephrectomy with vena caval tumor thrombectomy. Eur Urol. 2011;59(4):652–656. doi: 10.1016/j.eururo.2010.08.038. [DOI] [PubMed] [Google Scholar]
- 23.Kondo T, Hashimoto Y, Kobayashi H, et al. Presurgical targeted therapy with tyrosine kinase inhibitors for advanced renal cell carcinoma: clinical results and histopathological therapeutic effects. Jpn J Clin Oncol. 2010;40(12):1173–1179. doi: 10.1093/jjco/hyq150. [DOI] [PubMed] [Google Scholar]
- 24.Russo M, Siravegna G, Blaszkowsky LS, et al. Tumor heterogeneity and lesion-specific response to targeted therapy in colorectal cancer. Cancer Discov. 2016;6(2):147–153. doi: 10.1158/2159-8290.CD-15-1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kwak EL, Ahronian LG, Siravegna G, et al. Molecular heterogeneity and receptor co-amplification drive resistance to targeted therapy in MET-amplified esophagogastric cancer. Cancer Discov. 2015;5(12):1271–1281. doi: 10.1158/2159-8290.CD-15-0748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abel EJ, Culp SH, Tannir NM, et al. Primary tumor response to targeted agents in patients with metastatic renal cell carcinoma. Eur Urol. 2011;59(1):10–15. doi: 10.1016/j.eururo.2010.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Donskov F. Immunomonitoring and prognostic relevance of neutrophils in clinical trials. Semin Cancer Biol. 2013;23(3):200–207. doi: 10.1016/j.semcancer.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 28.Azuma T, Matayoshi Y, Nagase Y, Oshi M. Neutrophil number after interferon-alfa treatment is an independent predictive marker of overall survival in metastatic renal cell carcinoma. Clin Genitourin Cancer. 2012;10(3):180–184. doi: 10.1016/j.clgc.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 29.Zhu AX, Duda DG, Ancukiewicz M, et al. Exploratory analysis of early toxicity of sunitinib in advanced hepatocellular carcinoma patients: kinetics and potential biomarker value. Clin Cancer Res. 2011;17(4):918–927. doi: 10.1158/1078-0432.CCR-10-0515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang D, Ding Y, Zhou M, et al. Interleukin-8 mediates resistance to antiangiogenic agent sunitinib in renal cell carcinoma. Cancer Res. 2010;70(3):1063–1071. doi: 10.1158/0008-5472.CAN-09-3965. [DOI] [PMC free article] [PubMed] [Google Scholar]