Abstract
This article aims to review the clinical management strategies available for the rare iridocorneal endothelial syndrome. The different clinical variations as well as the imaging techniques available to aid diagnosis are discussed. We then present the evidence available to help the reader to understand how the condition can be managed medically and also the important surgical aspects of treatment. This involves raised intraocular pressure management in addition to the visual management options of partial or full thickness keratoplasty. We hope that this review provides an exhaustive but also succinct review of the literature available on what is a rare and difficult condition to treat.
Keywords: iridocorneal endothelial syndrome, endothelial syndrome, Cogan-Reese syndrome, Chandler syndrome, progressive iris atrophy
Introduction
Iridocorneal endothelial (ICE) syndrome is a rare and fascinating condition that can be challenging for ophthalmic surgeons to manage. It comprises a spectrum of three clinical entities: progressive essential iris atrophy, Cogan-Reese syndrome, and Chandler syndrome.1 It is characterized by proliferative and structural abnormalities of the corneal endothelium, progressive obliteration of the iridocorneal angle, and iris anomalies such as atrophy and polycoria.2 ICE syndrome is sporadic in presentation; it is usually unilateral and typically affects adult patients, females more often than males.
The purpose of this review is to highlight the difficulties associated with managing the condition, to discuss the evidence available for the clinical diagnosis and management of the condition.
Method of literature search
A PubMed search was performed using the search terms “ICE”, “Iridocorneal endothelial syndrome”, “Endothelial syndromes”, “Chandler syndrome”, “progressive iris atrophy” and “Cogan-Reese syndrome”. A full systematic review of the literature using the PubMed database was conducted up until 1/11/17. The articles used were written in English, with all articles accessed in full. Both review articles and original articles were used for this review.
Clinical diagnosis
ICE syndrome is characterized by proliferative and structural abnormalities of the corneal endothelium, progressive obstruction of the iridocorneal angle, and iris anomalies such as atrophy, correctopia, and polycoria. The consequences of these changes are corneal decompensation and secondary glaucoma, which represent the most frequent causes of visual loss in these patients.
Given the progressive nature of ICE syndrome and the wide spectrum of clinical presentations it is important to establish the diagnosis from similar conditions. Posterior polymorphous dystrophy (PPD) may show similar clinical features such as iridocorneal adhesions, membranes, and ectropion uveae which are typically associated with ICE syndrome. This can at times complicate diagnosis. PPD, in contrast to ICE syndrome, has a genetic component and is rarely progressive unlike ICE syndrome.3
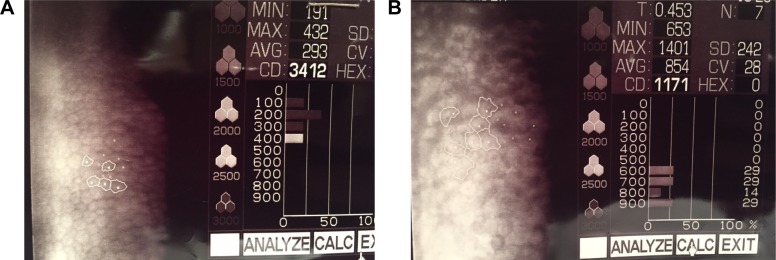
Specular microscopy can be used to differentiate between these two conditions. ICE cells are dark areas with a light central spot and a light peripheral zone. These are generally larger than normal endothelial cells, and occur in areas where the cornea appears to have a hammered silver appearance.3 These cells are regarded as pathognomonic of ICE syndrome and are termed “ICE cells” along with the tissue they form termed “ICE tissue”4 (Figure 1). Four basic patterns of ICE cells have been described by previous authors.4 More recent findings using in-vivo confocal microscopy have highlighted two main patterns of abnormal “epithelioid-like” endothelium, both characterized by marked hyperreflective nuclei and loss of regularity in cellular size and shape.5–10 Stromal nerve fibers in affected eyes were unusually thicker and distorted. It is suggested these signs can be examined to aid diagnosis, especially in edematous corneas.6,7
Figure 1.
(A) Specular microscopy from a normal cornea. (B) Specular microscopy from a patient with iridocorneal endothelial syndrome.
Anterior segment imaging in the form of ultrasound biomicroscopy or anterior segment optical coherence tomography (OCT) can be a useful tool for detecting peripheral anterior synechiae and iris atrophy more reliably than slit lamp microscopy and gonioscopy when corneal edema is present.11 Central anterior chamber depth is shown to be significantly less in patients with ICE syndrome (mean 2.25 (± SD 0.32) mm) than in normal subjects (2.76 (±0.32) mm).11 In contrast, PPD vesicles appear as dark rings with distinct, scalloped edges surrounding a lighter center.3 With repeated specular microscopy examination over several years, the PPD cells showed no changes in configuration or migration. No accelerated endothelial cell loss occurred. In the ICE patients, normal endothelial cell density was lost, although the amount of ICE cells remained constant.3 Both conditions show multilayered endothelial cells and thickened Descemet’s membrane.12
Corneas affected by ICE syndrome are said to exhibit extensive endothelial changes early in the course of the disease, before other manifestations are clinically apparent.2 Lymphocytes are often found in the endothelium, which may only be found early in the disease process.2 Epithelialization of the endothelial cell layer has been demonstrated using immunohistochemical studies,13 which results in cellular proliferation across the iridocorneal angle similar to that seen in epithelial downgrowth and posterior polymorphous endothelial dystrophy.13 Abnormal iris profiles have been reported using anterior segment OCT scanning.14
The ICE cells that border normal endothelial cells are said to be in a static, immobile state. These cells are often damaged or necrotic at boundary zones, suggesting that ICE cells may have a toxic effect on normal neighboring endothelial cells.15,16 This may explain why corneal failure in this syndrome is often slowly progressive.17 Some evidence suggests that a subclinical form may exist in the contralateral eye,18 predisposing to shallow or closed anterior chamber angles, which should be examined for gonioscopically.11 Contralateral endothelial cells are not reduced, although normal hexagonal-shaped cells are reduced.19
Three subtypes of the ICE syndrome exist, producing a spectrum of disease. These are progressive essential iris atrophy, Cogan-Reese syndrome, and Chandler syndrome.1 Differentiating between these conditions is difficult clinically, and most distinctions are made based on different levels of iris abnormalities, with Chandler syndrome said to be the most common subtype.20
Progressive iris atrophy involves significant iris atrophy often with hole formation. This is not diagnostic, however, as this can occur in the Cogan-Reese variant.21 Gonioscopy may show the presence of peripheral anterior synechiae as a result of angle endothelialization. This causes variable degrees of angle closure and consequent intraocular pressure (IOP) increase. Iris heterochromia and ectropion uveae can occur rarely.21
Cogan-Reese syndrome is characterized by proliferation of the corneal endothelium involving most of the iris and anterior chamber angle. This does not usually cause marked pupillary displacement. This subset involves the presence of multiple iris nodules, surrounded by iris stromal loss of crypts and a matted appearance. These nodules often develop late in the disease process and can appear as fine, yellowish nodules on the iris surface. Later in the disease course they become darker and larger.1,22
Chandler’s syndrome is said to have less iris involvement. Corneal edema, epithelial bullae, and a hammered silver appearance of the corneal endothelium. Mild iris atrophy can also occur but full thickness iris holes are rare.23
Medical management
Due to the unknown etiology of ICE syndrome, medical management of this condition can be difficult, and is often a temporizing measure. No therapy is available which targets the pathogenesis of ICE syndrome, meaning medical treatment is aimed at controlling IOP and corneal clarity. Due to the progressive nature of the disease and the younger presenting age of ICE patients, often with preexisting glaucoma, medical management is usually insufficient.24–26 Older reports suggest surgical intervention is required in up to 88% of cases,25 although these results were published prior to the advent of topical medications that are now ubiquitous. Newer data from an Indian cohort of 203 patients suggest 50% of patients require surgical intervention for IOP lowering and 14% require keratoplasy.24 These rates are certainly subject to geographical factors and availability of both medical and surgical treatment modalities, but adequately illustrate the difficulty associated with managing this condition medically.
Topical medication can be used in order to control IOP, with the reduction of IOP helping to reduce the amount of corneal edema. Hypertonic saline and hairdryer use can be applied in conjunction, as would be advised for other conditions affected by corneal edema.27 Aqueous suppressants are recommended as first-line agents, with prostaglandins suggested to be used cautiously due to the reported links between herpes viruses and ICE syndrome.1,28 Miotics are said to add little value, likely as a result of an abnormal iris.1,29
There is a suggestion within the literature that ICE syndrome has a viral etiology, after initial early reports of patients with concurrent anterior segment inflammation.21,30 One study reports polymerase chain reaction evidence of HSV DNA presence in 16 out of 25 (64%) ICE syndrome corneal specimens, potentially pointing toward a viral trigger or cause.31 Epstein–Barr virus (EBV) and varicella zoster virus were not detected,31 although other authors have suggested EBV may play a role after high titers of IgG antibodies to the EBV capsid antigen were found in ICE patients when compared with controls.32 Despite these findings, there is no available evidence to suggest that antiviral therapy alters the progression of ICE syndrome.
Glaucoma surgical management
Surgical intervention for IOP control is often required, given the high failure rate of medical treatment.
Data are scarce within the literature in regard to trabeculectomy. Only retrospective studies and case reports exist, although these report moderate success with augmented trabeculectomy.33–36 The largest involves 16 eyes all undergoing primary trabeculectomy with mitomycin-C, between the dates of 1991 and 2013,35 with no history of previous intraocular surgery. Median postoperative IOP was reduced significantly from 36 to 14 mmHg (P<0.001), with the median number of postoperative anti-glaucoma medications reduced from 3 to 0 (P<0.001). Complete success was defined as IOP ≤5 and ≥21 using no medications with the following outcomes reported: 75% at 6 months, 64% at 12 months, 57% at 36 months, and 33% at 60 months. These results are similar to those found in other cohorts,34,36 including one from Germany which showed a mean IOP of 12.1 mmHg, although the mean follow-up time was shorter (14.9 months).36
Common complications include hypotony, although this appears to resolve with conservative management in both studies.35,36 Encouragingly, the majority of eyes appear to maintain good visual acuity, with 10%–31% of patients losing more than two Snellen lines,35,36 with the commonest reason for reduced vision being corneal edema as opposed to inadequate IOP control.35 From the aforementioned study looking at 16 eyes, two required penetrating keratoplasty (PK) at 20 months and 59 months postoperatively.35
It is important to note that a significant proportion of eyes undergoing trabeculectomy for ICE syndrome (12.5%–53.8%) require a secondary glaucoma drainage implant (GDI) at some stage, suggesting augmented trabeculectomy does not always offer definitive treatment.
GDI surgery seems to offer an adequate alternative means to lower IOP. Again, only retrospective studies exist which show 70% of eyes maintaining an IOP of <21 mmHg using additional topical medications after Baerveldt tube, Molteno valve, or Ahmed valve implantation.34 Doe et al34 report a better success rate of 66% without topical medication required for additional control. It is important to note that the vast majority of the eyes reported from these studies have previously undergone glaucoma surgery, usually in the form of enhanced trabeculectomy. In some cases, the surgery was performed 30 years ago using older techniques. The findings of these studies are therefore difficult to compare directly with the trabeculectomy outcomes above, as these are not primary procedures and may have higher levels of preoperative conjunctival fibrosis increasing the risk of failure. This may inadvertently select for more difficult or refractory cases, and more research is required for this to be determined. In both studies, significant proportions (50%37 and 26.3%34 respectively) of patients required tube revisions or further GDI implantation in an attempt to achieve adequate pressure control.34,37 About 20% of eyes were reported to have a recurrence of an ICE membrane after GDI, causing blockage of the tube.37 50% of the eyes suffered corneal decompensation at mean follow-up of 55 months, despite some having undergone PK.37
Filtering surgery is said to fail due to the continued growth of the endothelial membrane over the osteum site,34,38 which is often only apparent when examined histologically. Contraction and synechial closure of the ostium are also suspected.34 Marked subconjunctival fibrosis has also been noted in trabeculectomy cases postoperatively, leading to speculation that these eyes may be predisposed to a more aggressive inflammatory response partly due to the fact that this disease tends to involve a younger patient cohort.26,34 One suggestion from the literature for GDI insertion in this cohort of patients is to route the tube along the sclera and to lengthen the tube in order to allow for repositioning.34 These authors also suggest keeping the tube away from potential endothelial proliferation sites such as the iris and endothelium. Peripheral iridectomy could allow the tube to sit in the sulcal plane. Entry through the pars plana can be used in pseudophakic, aphakic, or vitrectomized eyes. Despite these suggestions, however, only one patient in this study was stated to have a tube placed into the vitreous cavity and unfortunately still suffered corneal decompensation.
New minimally invasive glaucoma surgical devices offer an exciting option for future management of complex glaucoma such as ICE syndrome. Studies have shown the efficacy of Xen45 gel stents (allergan affiliate; AqueSys Inc., Aliso Viejo, CA, USA) in primary open angle glaucoma patients both in clinical trials and clinical practice.39–41 One case report exists reporting the successful implantation of a Xen45 stent in a patient with ICE syndrome who had also undergone prior Descemet’s membrane endothelial keratoplasty surgery (Figure 2). Additional intraoperative and postoperative anti-VEGF injections were given to optimize postoperative scarring.42 It would be reasonable to hypothesize that other angle-based surgery such as using the iStent (Glaukos Corp., San Clemente, CA, USA) and Hydrus stent (Ivantis, Irvine, CA, USA) may not be the intervention of choice, given the likely proliferation of endothelial cells over the stent opening, although no evidence currently exists to support this.
Figure 2.
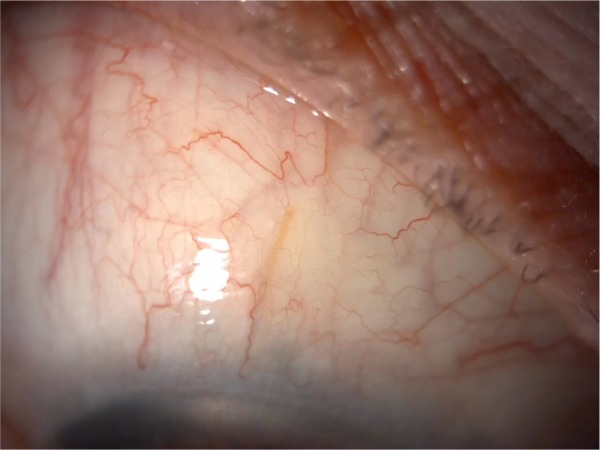
Xen45 stent (AqueSys Inc., Aliso Viejo, CA, USA) visible in the conjunctival space in a patient with iridocorneal endothelial syndrome.
Corneal surgical management
Although controlling IOP is crucial for maintaining long-term visual potential, ICE syndrome is associated with significant corneal symptoms as a result of the abnormal proliferation of an ectopic membrane over the anterior chamber angle. Lowering IOP was often the main intervention to reduce the rate of corneal decompensation.
There is a paucity of literature in regard to keratoplasty techniques in this syndrome, although replacement of the full corneal thickness with PK has been shown to be an effective treatment for corneal symptoms in early case series.43–45 The largest case series provides 5-year follow-up data on 14 ICE eyes undergoing PK. Repeat grafts in six eyes were required, although 12 of the 14 had clear corneas at the end of follow-up. This fits with findings from other data sets, as ICE syndrome has been reported alongside stromal dystrophies as being the commonest indication for undergoing three or more corneal transplants.46 From the data provided in the above cohort, it is reassuring to see that all of the eyes either improved or maintained their preoperative visual acuity. About 50% of eyes had a visual acuity of better than 20/100, with three having better than 20/40.43 Allograft rejection rates were high, however, with 11 eyes having primary graft rejection episodes.43 This agrees with the findings of other authors, who report similar visual outcomes with PK.45,47 An interesting suggestion was that simultaneous extracapsular cataract extraction did not result in a poorer visual prognosis of the graft.47 Older series also show PK to be effective at reducing morbidity from pain caused by bullous epithelial disease.
With the advances in lamellar corneal surgery, endothelial keratoplasty has been shown to be a viable option for ICE patients. Descemet stripping automated endothelial keratoplasty (DSAEK) may be theoretically advantageous for many reasons, but has the potential to be more technically challenging in this group of patients due to peripheral anterior synechiae formation, shallow anterior chambers, and iris abnormalities meaning graft positioning can be difficult (Figure 3).48,49 Different techniques have been suggested as a means to overcoming this, including suture dragging techniques,50 donor fixation,49 and pupilloplasties.51 However, most of the procedures reported in the literature use standard techniques48,49,52 and, despite this, graft dislocation rates requiring rebubbling in 22%49 of eyes are comparable with non-ICE procedures.53 Again, only small retrospective patient cohort reviews exist, but it is reassuring to see that good initial visual outcomes are reported from multiple centers around the world.49,52,54–57 Many studies fail to report follow-up periods beyond 24 months and lack data on endothelial cell counts.52,54,56,57 Authors from India report good visual outcomes and corneal clarity at a mean follow-up period of 1 year using automated endothelial graft techniques.54 It is important to note that more than half of these patients had a postoperative IOP rise that required oral medication to control, which can be problematic in a group of patients with preexisting glaucomatous damage. Only one patient was noted to have optic nerve changes due to glaucoma, although the follow-up period was short.54 No comment is made on the rejection profile of these transplants.
Figure 3.

Broad peripheral anterial synechiae seen in iridocorneal endothelial syndrome.
A smaller cohort with longer follow-up shows the rejection profile of DSAEK grafts to be high, with 50% of the eyes having a rejection episode,49 which is higher than the reported rejection rate of non-ICE patients from larger series.58 Corneal clarity and visual acuity were not well maintained beyond the 12-month mark, where only 12% of the grafts had a best corrected visual acuity of 6/12. 77.7% of the grafts failed at a mean age of 18 months in conjunction with a reduction in endothelial cell count and increased central corneal thickness.49 The endothelial cell loss appears to be rapid in ICE patients when compared with a DSEK cohort performed for mainly for pseudophakic or aphakic bullous keratopathy (79% vs 38% cell loss at 6 months respectively).49,59 Both groups of patients had undergone previous glaucoma surgery, which is known to be an independent factor for cell loss after corneal transplantation,59,60 suggesting there is a component of the ICE pathophysiology that causes grafts to fail more rapidly than in other conditions.
Due to the rarity of ICE syndrome and the availability of data within the literature, it is difficult to say with certainty whether partial thickness or full lamellar transplantation is favorable in this condition. Direct comparison of individual studies is difficult due to differences in available data and small patient numbers, meaning statistical significance is hard to ascertain. Only one study using data from Singapore exists comparing PK with DSAEK. Kaplan–Meier survival analysis showed no significant difference between either group with a mean follow-up period of nearly 6 years. This study echoes the findings of other authors49 which suggest the PK survival rate is longer than that of DSAEK (9 vs 4.6 years respectively).48 Two PKs (11.7%) had rejection episodes leading to graft failure, while none were reported in the DSAEK group. Overall, around 40% of eyes required treatment for raised IOP, with no statistical difference found between the two groups. The double-edged sword of glaucoma surgery is nicely represented by the results from this study.48 On the one hand, those eyes that had undergone previous glaucoma surgery were significantly less likely to require escalation of IOP-lowering therapy post keratoplasty (9.1% vs 50%, P=0.034). However, these grafts were more likely to fail (50% vs 32.6%, P=0.432), although the difference did not reach statistical significance. No significant difference between a glaucoma drainage device and trabeculectomy groups was found in terms of survival.
Nonetheless, DSAEK appears to offer rapid and better visual rehabilitation with reduced astigmatic effect,49,52 albeit with survival times of generally less than 24 months. Although there is potential for regrafting post DSAEK, the concept of repeating surgery every 2 years is far from ideal. Rejection rates appear to be lower in ICE patients undergoing DSAEK vs PK (33% vs 79% respectively),43,49 which despite being more frequent in this subgroup of patients is consistent with findings from other studies49,58 (Figure 4).
Figure 4.
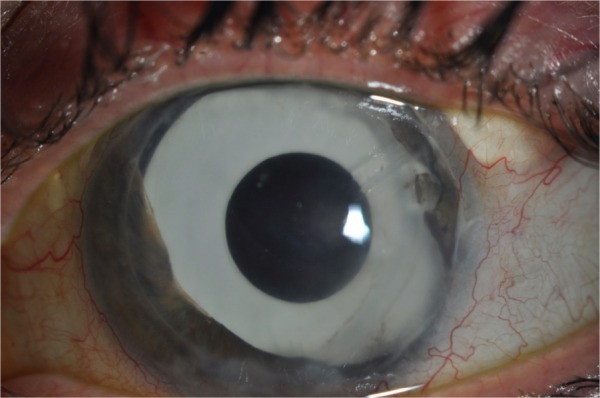
A patient who has undergone multiple procedures for iridocorneal endothelial syndrome. A clear Descemet stripping automated endothelial keratoplasty graft is visible, with a supertemporal Baerveldt tube in situ. A prosthetic iris implant can also be seen.
Older, pre-DSAEK techniques such as deep lamellar endothelial keratoplasty have reported similar visual outcomes to those mentioned above.61 No cases of graft failure were reported, although the maximum follow-up period was only 20 months.61 Deep lamellar endothelial keratoplasty requires the excision of host stroma as opposed to just a descemetorhexis. This can be time consuming and prolongs visual recovery but can be advantageous in phakic eyes with shallower anterior chambers, allowing the donor button to be inserted more easily.
Although lamellar surgery is evolving rapidly, few cases are reported using Descemet’s membrane endothelial keratoplasty for ICE syndrome.42,62 This technique has many potential advantages and may provide benefits in the future. One paper describes the technique being used successfully in three cases, all of which had an improved best corrected visual acuity and reduction in corneal thickness.62 Two of the three cases required rebubbling of the graft, although advances in intraoperative OCT may help to improve postoperative graft adherence in the future.63
Boston type 1 keratoprosthesis is an option in patients who have had failed allogenic transplants.64 Four cases have been reported, three of which had an improved visual acuity of 6/30 Snellen equivalent 4 years postoperatively, with all grafts being retained in situ. As expected, glaucomatous progression is a known complication along with retroprosthetic membrane formation,64 both of which may require surgical intervention.61
Cosmetic and diplopic interventions
Patients with ICE syndrome may develop a myriad of iris changes, which can vary as the syndrome persists over time. These may manifest as stromal atrophy, corectopia, pseudopolycoria, and the induced nodular irregularity of iris nevus syndrome. If the pupil is displaced, or the stroma is insufficient to absorb light, glare and other unwanted optical phenomena such as monocular diplopia may occur. One report shows how combined cataract extraction and iris reconstruction using a multipiece endocapsular iris prosthesis can help to alleviate symptoms and improve cosmesis.65 Suturing techniques may not be possible due to the friable nature of the iris.66 Visual acuity postoperatively was 6/9 Snellen equivalent with a reduction in glare. It is worth noting that this patient had undergone previous trabeculectomy and glaucoma drainage device insertion, and IOP remained stable postoperatively.
Femtosecond-assisted corneal keratopigmentation (tattooing) is a new technique that has been proposed for cosmetic improvement of opaque corneas67 for cosmetic purposes. This technique has been used to aid functional eye disability related to a severe iris and pupil defect from ICE syndrome. Femtosecond laser technology allows tattoos to be created using intralamellar dissections. It is necessary to enter into the anterior chamber in order to remove atrophic parts of iris that are obscuring the visual axis. Excellent visual acuity is reported postoperatively.68
Conclusion
This review highlights the complexities associated with managing patients with ICE syndrome. There is clearly no one single intervention that can treat the condition for an entire lifetime. Due to the rarity of the syndrome and the associated lack of high level evidence it is difficult to ascertain the best strategy for managing these patients. What is clear is that many patients will require multiple interventions in order to control IOP and corneal clarity. It may be wise to control IOP before tackling visual enhancement in order to promote long-term visual outcomes. However, as discussed above, previous glaucoma procedures result in poorer corneal transplantation outcomes. The minimally invasive glaucoma surgical devices may offer less invasive approaches to control IOP, with the potential to be less problematic for the cornea. Ultimately, frank discussion with the patient is required early on into the disease process so that appropriate expectations are set. This helps to guide the patient and clinician as to which future interventions are chosen.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Sacchetti M, Mantelli F, Marenco M, Macchi I, Ambrosio O, Rama P. Diagnosis and management of iridocorneal endothelial syndrome. Biomed Res Int. 2015;2015:763093. doi: 10.1155/2015/763093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Levy SG, Kirkness CM, Moss J, Ficker L, McCartney AC. On the pathology of the iridocorneal-endothelial syndrome: the ultrastructural appearances of “subtotal-ice”. Eye (Lond) 1995;9(Pt 3):318–323. doi: 10.1038/eye.1995.62. [DOI] [PubMed] [Google Scholar]
- 3.Laganowski HC, Sherrard ES, Muir MG, Buckley RJ. Distinguishing features of the iridocorneal endothelial syndrome and posterior polymorphous dystrophy: value of endothelial specular microscopy. Br J Ophthalmol. 1991;75(4):212–216. doi: 10.1136/bjo.75.4.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sherrard ES, Frangoulis MA, Muir MG, Buckley RJ. The posterior surface of the cornea in the irido-corneal endothelial syndrome: a specular microscopical study. Trans Ophthalmol Soc U K. 1985;104(Pt 7):766–774. [PubMed] [Google Scholar]
- 5.Garibaldi DC, Schein OD, Jun A. Features of the iridocorneal endothelial syndrome on confocal microscopy. Cornea. 2005;24(3):349–351. doi: 10.1097/01.1co.0000138842.83044.34. [DOI] [PubMed] [Google Scholar]
- 6.Le QH, Sun XH, Xu JJ. In-vivo confocal microscopy of iridocorneal endothelial syndrome. Int Ophthalmol. 2009;29(1):11–18. doi: 10.1007/s10792-007-9187-x. [DOI] [PubMed] [Google Scholar]
- 7.Chiou AG, Kaufman SC, Beuerman RW, Ohta T, Yaylali V, Kaufman HE. Confocal microscopy in the iridocorneal endothelial syndrome. Br J Ophthalmol. 1999;83(6):697–702. doi: 10.1136/bjo.83.6.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sheppard JD, Jr, Lattanzio FA, Jr, Williams PB, Mitrev PV, Allen RC. Confocal microscopy used as the definitive, early diagnostic method in Chandler syndrome. Cornea. 2005;24(2):227–229. doi: 10.1097/01.ico.0000141233.41343.d4. [DOI] [PubMed] [Google Scholar]
- 9.Levy SG, McCartney AC, Sawada H, Dopping-Hepenstal PJ, Alexander RA, Moss J. Descemet’s membrane in the iridocorneal-endothelial syndrome: morphology and composition. Exp Eye Res. 1995;61(3):323–333. doi: 10.1016/s0014-4835(05)80127-7. [DOI] [PubMed] [Google Scholar]
- 10.Howell DN, Damms T, Burchette JL, Jr, Green WR. Endothelial meta-plasia in the iridocorneal endothelial syndrome. Invest Ophthalmol Vis Sci. 1997;38(9):1896–1901. [PubMed] [Google Scholar]
- 11.Zhang M, Chen J, Liang L, Laties AM, Liu Z. Ultrasound biomicros-copy of Chinese eyes with iridocorneal endothelial syndrome. Br J Ophthalmol. 2006;90(1):64–69. doi: 10.1136/bjo.2005.074864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bromley JG, Randleman JB, Stone D, Stulting RD, Grossniklaus HE. Clinicopathologic findings in iridocorneal endothelial syndrome and posterior polymorphous membranous dystrophy after Descemet stripping automated endothelial keratoplasty. Cornea. 2012;31(9):1060–1064. doi: 10.1097/ICO.0b013e31823fb978. [DOI] [PubMed] [Google Scholar]
- 13.Hirst LW, Bancroft J, Yamauchi K, Green WR. Immunohistochemical pathology of the corneal endothelium in iridocorneal endothelial syndrome. Invest Ophthalmol Vis Sci. 1995;36(5):820–827. [PubMed] [Google Scholar]
- 14.Holló G, Naghizadeh F. Optical coherence tomography characteristics of the iris in Cogan-Reese syndrome. Eur J Ophthalmol. 2014;24(5):797–799. doi: 10.5301/ejo.5000465. [DOI] [PubMed] [Google Scholar]
- 15.Haemmerli G, Felix H. Shape and motility, two interdependent features. Scan Electron Microsc. 1982;(Pt 2):731–739. [PubMed] [Google Scholar]
- 16.Polliack A, Gordon S. Scanning electron microscopy of murine macrophages. Surface characteristics during maturation, activation, and phagocytosis. Lab Invest. 1975;33(5):469–477. [PubMed] [Google Scholar]
- 17.Bourne WM, Brubaker RF. Progression and regression of partial corneal involvement in the iridocorneal endothelial syndrome. Am J Ophthalmol. 1992;114(2):171–181. doi: 10.1016/s0002-9394(14)73981-9. [DOI] [PubMed] [Google Scholar]
- 18.Malhotra C, Pandav SS, Gupta A, Jain AK. Phenotypic heterogeneity of corneal endothelium in iridocorneal endothelial syndrome by in vivo confocal microscopy. Cornea. 2014;33(6):634–637. doi: 10.1097/ICO.0000000000000122. [DOI] [PubMed] [Google Scholar]
- 19.Lucas-Glass TC, Baratz KH, Nelson LR, Hodge DO, Bourne WM. The contralateral corneal endothelium in the iridocorneal endothelial syndrome. Arch Ophthalmol. 1997;115(1):40–44. doi: 10.1001/archopht.1997.01100150042006. [DOI] [PubMed] [Google Scholar]
- 20.Wilson MC, Shields MB. A comparison of the clinical variations of the iridocorneal endothelial syndrome. Arch Ophthalmol. 1989;107(10):1465–1468. doi: 10.1001/archopht.1989.01070020539035. [DOI] [PubMed] [Google Scholar]
- 21.Scheie HG, Yanoff M. Iris nevus (Cogan-Reese) syndrome. A cause of unilateral glaucoma. Arch Ophthalmol. 1975;93(10):963–970. doi: 10.1001/archopht.1975.01010020761004. [DOI] [PubMed] [Google Scholar]
- 22.Eagle RC, Jr, Font RL, Yanoff M, Fine BS. Proliferative endotheliopathy with iris abnormalities. The iridocorneal endothelial syndrome. Arch Ophthalmol. 1979;97(11):2104–2111. doi: 10.1001/archopht.1979.01020020422002. [DOI] [PubMed] [Google Scholar]
- 23.Chandler PA. Atrophy of the stroma of the iris, endothelial dystrophy, corneal edema, and glaucoma. Trans Am Ophthalmol Soc. 1955;53:75–89. discussion, 89–93. [PMC free article] [PubMed] [Google Scholar]
- 24.Chandran P, Rao HL, Mandal AK, Choudhari NS, Garudadri CS, Senthil S. Glaucoma associated with iridocorneal endothelial syndrome in 203 Indian subjects. PLoS One. 2017;12(3):e0171884. doi: 10.1371/journal.pone.0171884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kidd M, Hetherington J, Magee S. Surgical results in iridocorneal endothelial syndrome. Arch Ophthalmol. 1988;106(2):199–201. doi: 10.1001/archopht.1988.01060130209027. [DOI] [PubMed] [Google Scholar]
- 26.Laganowski HC, Kerr Muir MG, Hitchings RA. Glaucoma and the iridocorneal endothelial syndrome. Arch Ophthalmol. 1992;110(3):346–350. doi: 10.1001/archopht.1992.01080150044025. [DOI] [PubMed] [Google Scholar]
- 27.Siu GD, Young AL, Jhanji V. Alternatives to corneal transplantation for the management of bullous keratopathy. Curr Opin Ophthalmol. 2014;25(4):347–352. doi: 10.1097/ICU.0000000000000062. [DOI] [PubMed] [Google Scholar]
- 28.Saleem AA, Ali M, Akhtar F. Iridocorneal endothelial syndrome. J Coll Physicians Surg Pak. 2014;24(Suppl 2):S112–S114. [PubMed] [Google Scholar]
- 29.Wand M, Gilbert CM, Liesegang TJ. Latanoprost and herpes simplex keratitis. Am J Ophthalmol. 1999;127(5):602–604. doi: 10.1016/s0002-9394(99)00050-1. [DOI] [PubMed] [Google Scholar]
- 30.Shields MB. Progressive essential iris atrophy, Chandler’s syndrome, and the iris nevus (Cogan-Reese) syndrome: a spectrum of disease. Surv Ophthalmol. 1979;24(1):3–20. doi: 10.1016/0039-6257(79)90143-7. [DOI] [PubMed] [Google Scholar]
- 31.Alvarado JA, Underwood JL, Green WR, et al. Detection of herpes simplex viral DNA in the iridocorneal endothelial syndrome. Arch Ophthalmol. 1994;112(12):1601–1609. doi: 10.1001/archopht.1994.01090240107034. [DOI] [PubMed] [Google Scholar]
- 32.Tsai CS, Ritch R, Straus SE, Perry HD, Hsieh FY. Antibodies to Epstein-Barr virus in iridocorneal endothelial syndrome. Arch Ophthalmol. 1990;108(11):1572–1576. doi: 10.1001/archopht.1990.01070130074034. [DOI] [PubMed] [Google Scholar]
- 33.Jain VK, Sharma R, Ojha S, et al. Trabeculectomy with mitomycin-C in patients with iridocorneal endothelial syndrome: a case series. J Clin Diagn Res. 2016;10(5):NR05–NR06. doi: 10.7860/JCDR/2016/16506.7782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Doe EA, Budenz DL, Gedde SJ, Imami NR. Long-term surgical outcomes of patients with glaucoma secondary to the iridocorneal endothelial syndrome. Ophthalmology. 2001;108(10):1789–1795. doi: 10.1016/s0161-6420(01)00725-4. [DOI] [PubMed] [Google Scholar]
- 35.Chandran P, Rao HL, Mandal AK, Choudhari NS, Garudadri CS, Senthil S. Outcomes of primary trabeculectomy with mitomycin-C in glaucoma secondary to iridocorneal endothelial syndrome. J Glaucoma. 2016;25(7):e652–e656. doi: 10.1097/IJG.0000000000000268. [DOI] [PubMed] [Google Scholar]
- 36.Lanzl IM, Wilson RP, Dudley D, Augsburger JJ, Aslanides IM, Spaeth GL. Outcome of trabeculectomy with mitomycin-C in the iridocorneal endothelial syndrome. Ophthalmology. 2000;107(2):295–297. doi: 10.1016/s0161-6420(99)00077-9. [DOI] [PubMed] [Google Scholar]
- 37.Kim DK, Aslanides IM, Schmidt CM, Jr, Spaeth GL, Wilson RP, Augsburger JJ. Long-term outcome of aqueous shunt surgery in ten patients with iridocorneal endothelial syndrome. Ophthalmology. 1999;106(5):1030–1034. doi: 10.1016/S0161-6420(99)00529-1. [DOI] [PubMed] [Google Scholar]
- 38.Shields MB, McCracken JS, Klintworth GK, Campbell DG. Corneal edema in essential iris atrophy. Ophthalmology. 1979;86(8):1533–1550. doi: 10.1016/s0161-6420(79)35384-2. [DOI] [PubMed] [Google Scholar]
- 39.Sheybani A, Lenzhofer M, Hohensinn M, Reitsamer H, Ahmed II. Phacoemulsification combined with a new ab interno gel stent to treat open-angle glaucoma: pilot study. J Cataract Refract Surg. 2015;41(9):1905–1909. doi: 10.1016/j.jcrs.2015.01.019. [DOI] [PubMed] [Google Scholar]
- 40.Sheybani A, Dick HB, Ahmed II. Early clinical results of a novel ab interno gel stent for the surgical treatment of open-angle glaucoma. J Glaucoma. 2016;25(7):e691–e696. doi: 10.1097/IJG.0000000000000352. [DOI] [PubMed] [Google Scholar]
- 41.Tan SZ, Walkden A, Au L. One-year result of XEN45 implant for glaucoma: efficacy, safety, and postoperative management. Eye (Lond) 2018;32(2):324–332. doi: 10.1038/eye.2017.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hohberger B, Welge-Lüen UC, Lämmer R. ICE-syndrome: a case report of implantation of a microbypass Xen gel stent after DMEK transplantation. J Glaucoma. 2017;26(2):e103–e104. doi: 10.1097/IJG.0000000000000584. [DOI] [PubMed] [Google Scholar]
- 43.Alvim PT, Cohen EJ, Rapuano CJ, et al. Penetrating keratoplasty in iridocorneal endothelial syndrome. Cornea. 2001;20(2):134–140. doi: 10.1097/00003226-200103000-00003. [DOI] [PubMed] [Google Scholar]
- 44.Buxton JN, Lash RS. Results of penetrating keratoplasty in the iridocorneal endothelial syndrome. Am J Ophthalmol. 1984;98(3):297–301. doi: 10.1016/0002-9394(84)90319-2. [DOI] [PubMed] [Google Scholar]
- 45.Crawford GJ, Stulting RD, Cavanagh HD, Waring GO., 3rd Penetrating keratoplasty in the management of iridocorneal endothelial syndrome. Cornea. 1989;8(1):34–40. [PubMed] [Google Scholar]
- 46.Yildiz EH, Hoskins E, Fram N, et al. Third or greater penetrating keratoplasties: indications, survival, and visual outcomes. Cornea. 2010;29(3):254–259. doi: 10.1097/ICO.0b013e3181b31b6f. [DOI] [PubMed] [Google Scholar]
- 47.Chang PC, Soong HK, Couto MF, Meyer RF, Sugar A. Prognosis for penetrating keratoplasty in iridocorneal endothelial syndrome. Refract Corneal Surg. 1993;9(2):129–132. [PubMed] [Google Scholar]
- 48.Quek DT, Wong CW, Wong TT, et al. Graft failure and intraocular pressure control after keratoplasty in iridocorneal endothelial syndrome. Am J Ophthalmol. 2015;160(3):422.e1–429.e1. doi: 10.1016/j.ajo.2015.05.024. [DOI] [PubMed] [Google Scholar]
- 49.Fajgenbaum MA, Hollick EJ. Descemet stripping endothelial keratoplasty in iridocorneal endothelial syndrome: postoperative complications and long-term outcomes. Cornea. 2015;34(10):1252–1258. doi: 10.1097/ICO.0000000000000530. [DOI] [PubMed] [Google Scholar]
- 50.Bradley JC, McCartney DL. Descemet’s stripping automated endothelial keratoplasty in intraoperative floppy-iris syndrome: suture-drag technique. J Cataract Refract Surg. 2007;33(7):1149–1150. doi: 10.1016/j.jcrs.2007.03.043. [DOI] [PubMed] [Google Scholar]
- 51.Groat B, Ying MS, Vroman DT, Fernández de Castro LE. Descemet-stripping automated endothelial keratoplasty technique in patients with anterior chamber intraocular lenses. Br J Ophthalmol. 2007;91(6):714. [Google Scholar]
- 52.Price MO, Price FW., Jr Descemet stripping with endothelial keratoplasty for treatment of iridocorneal endothelial syndrome. Cornea. 2007;26(4):493–497. doi: 10.1097/ICO.0b013e318030d274. [DOI] [PubMed] [Google Scholar]
- 53.Lee WB, Jacobs DS, Musch DC, Kaufman SC, Reinhart WJ, Shtein RM. Descemet’s stripping endothelial keratoplasty: safety and outcomes: a report by the American Academy of Ophthalmology. Ophthalmology. 2009;116(9):1818–1830. doi: 10.1016/j.ophtha.2009.06.021. [DOI] [PubMed] [Google Scholar]
- 54.Chaurasia S, Ramappa M, Garg P, Murthy SI, Senthil S, Sangwan VS. Endothelial keratoplasty in the management of iridocorneal endothelial syndrome. Eye (Lond) 2013;27(4):564–566. doi: 10.1038/eye.2012.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mohamed A, Chaurasia S, Murthy SI, et al. Endothelial keratoplasty: a review of indications at a tertiary eye care centre in South India. Asia Pac J Ophthalmol (Phila) 2014;3(4):207–210. doi: 10.1097/APO.0b013e3182a75304. [DOI] [PubMed] [Google Scholar]
- 56.Bahar I, Kaiserman I, Buys Y, Rootman D. Descemet’s stripping with endothelial keratoplasty in iridocorneal endothelial syndrome. Ophthalmic Surg Lasers Imaging. 2008;39(1):54–56. doi: 10.3928/15428877-20080101-01. [DOI] [PubMed] [Google Scholar]
- 57.Kymionis GD, Kontadakis GA, Agorogiannis GI, Bennett M, Angelidou F. Descemet stripping automated endothelial keratoplasty combined with phacoemulsification in Chandler syndrome. Eur J Ophthalmol. 2011;21(4):495–497. doi: 10.5301/EJO.2010.6210. [DOI] [PubMed] [Google Scholar]
- 58.Ezon I, Shih CY, Rosen LM, Suthar T, Udell IJ. Immunologic graft rejection in descemet’s stripping endothelial keratoplasty and penetrating keratoplasty for endothelial disease. Ophthalmology. 2013;120(7):1360–1365. doi: 10.1016/j.ophtha.2012.12.036. [DOI] [PubMed] [Google Scholar]
- 59.Anshu A, Price MO, Price FW. Descemet’s stripping endothelial keratoplasty: long-term graft survival and risk factors for failure in eyes with preexisting glaucoma. Ophthalmology. 2012;119(10):1982–1987. doi: 10.1016/j.ophtha.2012.04.031. [DOI] [PubMed] [Google Scholar]
- 60.Aldave AJ, Chen JL, Zaman AS, Deng SX, Yu F. Outcomes after DSEK in 101 eyes with previous trabeculectomy and tube shunt implantation. Cornea. 2014;33(3):223–229. doi: 10.1097/ICO.0000000000000028. [DOI] [PubMed] [Google Scholar]
- 61.Huang T, Wang Y, Ji J, Gao N, Chen J. Deep lamellar endothelial keratoplasty for iridocorneal endothelial syndrome in phakic eyes. Arch Ophthalmol. 2009;127(1):33–36. doi: 10.1001/archophthalmol.2008.537. [DOI] [PubMed] [Google Scholar]
- 62.Weller JM, Tourtas T, Kruse FE. Feasibility and outcome of Descemet membrane endothelial keratoplasty in complex anterior segment and vitreous disease. Cornea. 2015;34(11):1351–1357. doi: 10.1097/ICO.0000000000000625. [DOI] [PubMed] [Google Scholar]
- 63.Siebelmann S, Bachmann B, Lappas A, et al. Intraoperative optical coherence tomography in corneal and glaucoma surgical procedures. Ophthalmologe. 2016;113(8):646–650. doi: 10.1007/s00347-016-0320-y. [DOI] [PubMed] [Google Scholar]
- 64.Phillips DL, Goins KM, Greiner MA, Alward WL, Kwon YH, Wagoner MD. Boston type 1 keratoprosthesis for iridocorneal endothelial syndromes. Cornea. 2015;34(11):1383–1386. doi: 10.1097/ICO.0000000000000616. [DOI] [PubMed] [Google Scholar]
- 65.Khng C, Snyder ME. Iris reconstruction with a multipiece endocapsular prosthesis in iridocorneal endothelial syndrome. J Cataract Refract Surg. 2005;31(11):2051–2054. doi: 10.1016/j.jcrs.2005.04.030. [DOI] [PubMed] [Google Scholar]
- 66.Burk SE, Da Mata AP, Snyder ME, Cionni RJ, Cohen JS, Osher RH. Prosthetic iris implantation for congenital, traumatic, or functional iris deficiencies. J Cataract Refract Surg. 2001;27(11):1732–1740. doi: 10.1016/s0886-3350(01)01124-5. [DOI] [PubMed] [Google Scholar]
- 67.Kim JH, Lee D, Hahn TW, Choi SK. New surgical strategy for corneal tattooing using a femtosecond laser. Cornea. 2009;28(1):80–84. doi: 10.1097/ICO.0b013e318181a83c. [DOI] [PubMed] [Google Scholar]
- 68.Alió JL, Rodriguez AE, Toffaha BT, Piñero DP, Moreno LJ. Femtosecond-assisted keratopigmentation for functional and cosmetic restoration in essential iris atrophy. J Cataract Refract Surg. 2011;37(10):1744–1747. doi: 10.1016/j.jcrs.2011.08.003. [DOI] [PubMed] [Google Scholar]