Abstract
Although there are a few studies of portions of the vestibular system such as the vestibulocerebellar tract and the neural connectivity of the vestibular nuclei (VN), no study of the ipsilateral vestibulothalamic tract (VTT) (originating from the VN and mainly connecting to the lateral thalami nuclei) has been reported. In the current study, using diffusion tensor tractography (DTT), we investigate the reconstruction method and characteristics of the ipsilateral VTT in normal subjects. Thirty-three subjects were recruited for this study. For the ipsilateral VTT, the seed region of interest (ROI) was placed on the VN, which was isolated on the FA map using adjacent structures as follows: the reticular formation (anterior boundary), posterior margin of medulla and pons (posterior boundary), medial lemniscus (medial boundary) and restiform body (lateral boundary). The target ROI was placed at the lateral thalamic nuclei using known anatomical locations. The DTT parameters of the ipsilateral VTT were measured. The ipsilateral VTTs that originated from the vestibular nuclei ascended postero-laterally to the upper pons and antero-medially to the upper midbrain via the medial longitudinal fasciculus, and terminated the lateral thalamic nuclei. No significant differences were observed in DTT parameters of the ipsilateral VTT between the right and left hemispheres (p > 0.05). Using DTT, we reconstructed the ipsilateral VTT and observed the anatomical characteristics of the ipsilateral VTT in normal subjects. We believe that the methodology and results in this study could be helpful to researchers and clinicians in this field.
Keywords: Vestibulothalamic tract, Vestibular nuclei, Lateral thalamic nuclei, Diffusion tensor tractography
Introduction
The vestibular system consists of various structures including the cochlea, vestibular nuclei (VN), cerebellum, thalamus, and cerebral cortex, and it has a unique function in sensorimotor control and perception [1,2]. Among these structures, the VN, located at the pons and medulla oblongata, is a pivotal structure for vestibular function with connection to the thalamic nuclei [2,3]. The divergent vestibular projections to the thalamic nuclei have been reported in the rats, cats, and monkeys using tracing and electrophysiological techniques [4,5,6,7,8,9,10,11]. In detail, the ipsilateral vestibulothalamic tract (VTT) that originates from the VN mainly connects to the lateral thalami nuclei via the medial longitudinal fasciculus, and is involved in conscious perception of movement and spatial orientation [2,3,12]. Many animal studies reported on the pathways of the ipsilateral VTT using tracing and electrophysiological techniques. A few studies reported that the VN is functionally associated with activation of the thalamic nuclei in the human brain. However, research is limited in the live human brain because of the deep location and difficult identification of both the VN and thalamus in conventional brain magnetic resonance imaging (MRI) [2,13,14,15,16].
Recent diffusion tensor tractography (DTT), derived from diffusion tensor imaging (DTI), has the unique capability to estimate and visualize the neural tract three-dimensionally in the live human brain by detection of characteristics of water diffusion [17,18]. Since the introduction of DTT, several neural tracts not previously identified in the human brain began to be reported [19,20,21]. A few studies have described some portions of the vestibular system such as the vestibulocerebellar tract and the neural connectivity of the VN [22,23,24]. However, no study of the VTT in the human brain has been reported.
In the current study, using DTT, we investigate the reconstruction method and characteristics of the ipsilateral VTT in normal subjects.
Methods
Subjects
Thirty-three healthy subjects (males: 18, females: 15, mean age: 37.2 years, range: 20~ 56 years) with no previous history of neurological, physical, or psychiatric illness were recruited for this study. All subjects understood the purpose of this study and provided written, informed consent prior to participation. The study protocol was approved by our local Institutional Review Board.
Data acquisition
A six-channel head coil on a 1.5T Philips Gyroscan Intera (Philips, Ltd, Best, The Netherlands) with single-shot echo-planar imaging was used for acquisition of DTI data. For each of the 32 gradients, 70 contiguous slices were acquired parallel to the anterior commissure-posterior commissure line. Imaging parameters of DTI were as follows: acquisition matrix = 96 × 96; reconstructed to matrix = 192 × 192; field of view = 240 × 240mm2; repetition time = 10,398ms; echo time = 72ms; parallel imaging reduction factor = 2; echo-planar imaging factor = 59; b = 1000s/ mm2; number of excitations = 1; and a slice thickness of 2.5mm.
Probabilistic fiber tracking
Analysis of DTI data was performed using the Oxford Centre for Functional Magnetic Resonance Imaging of the Brain (FMRIB) Software Library (FSL; www.fmrib.ox.ac.uk/fsl). Eddy current correction was applied to correct the head motion effect and image distortion. Fiber tracking was performed using probabilistic tractography, and applied in the default tractography option in FMRIB Diffusion Software (5000 streamline samples, 0.5 mm step lengths, curvature thresholds = 0.2) [18,25]. The probabilistic tracking method enables estimation of more than one fiber population in each imaging voxel and uses an algorithm that models intravoxel crossing fibers where the probability corresponds to multiple fiber populations [18,25]. For reconstrucion of the ipsilateral VTT, the seed region of interest (ROI) was placed on the VN that was isolated on the FA map at the three levels from the medulla to the midpons using adjacent structures: the reticular formation (anterior boundary), posterior margin of medulla and pons (posterior boundary), medial lemniscus (medial boundary) and restiform body (lateral boundary) [24,26]. The target ROI was given at the lateral thalamic nuclei with the option of termination using the known anatomical locations based on a study by Morel et al. that provided exact locations and size of the lateral thalamic nuclei on the axial slice [27]. The average axial slice for ROI was 7.73 ± 0.73 mm above the anterior commissure. A threshold of five streamlines was applied for the results of fiber tracking. Values of fractional anisotropy (FA), mean diffusivity (MD), and tract volume for the ipsilateral VTT were measured.
Statistical analysis
SPSS software (SPSS Inc. Released 2006. SPSS for Windows, Version 15.0. Chicago, SPSS Inc.) was used for the analysis. An independent t-test was used for determination of variances in the value of FA, MD, and tract volume between the right and left hemispheres. Null hypotheses of no difference were rejected if p-values were less than .05.
Results
The ipsilateral VTTs that originated from the vestibular nuclei at the medulla and pons levels ascended postero-laterally to the upper pons and antero-medially to the upper midbrain via the medial longitudinal fasciculus, and terminated in the lateral thalamic nuclei (Fig. 1).
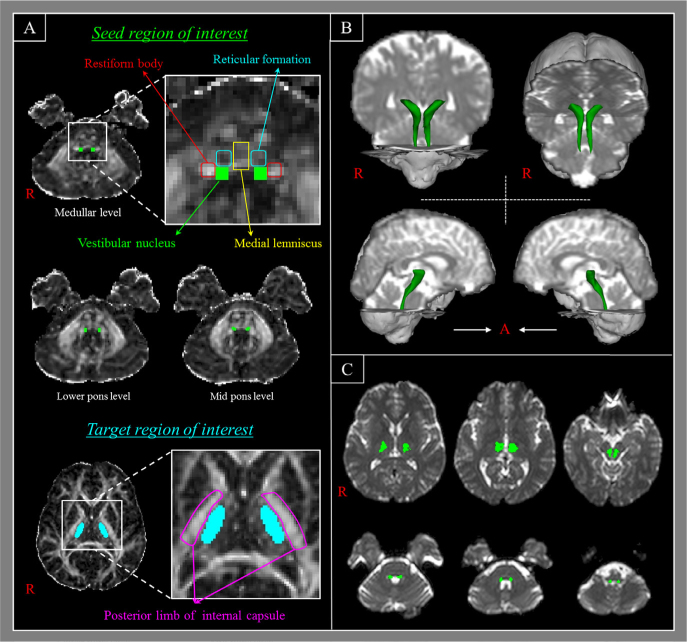
Fig. 1.
The region of interest (ROI) and results of diffusion tensor tractography for the ipsilateral vestibulothalamic tract (VTT). (A) Seed ROI is given at the vestibular nuclei (VN, green color) on fractional anisotropy map at the three levels from the medulla to the midpons, identified by adjacent structures as follows: the reticular formation (anterior boundary), posterior margin of medulla and pons (posterior boundary), medial lemniscus (medial boundary) and restiform body (lateral boundary). A target ROI is placed on the lateral thalamic nuclei with option of the termination. (B) The ipsilateral VTTs are visualized three-dimensionally in both hemispheres. (C) The pathway of the ipsilateral VTTs is shown at each level of the axial images from the medulla to the thalamus.
The mean value for FA was 0.41, for MD 0.82, and for tract volume, 610.5. No significant differences were observed in FA, MD, and tract volume between the right and left hemispheres (p>0.05) (Table 1).
Table 1.
Diffusion tensor imaging parameters for the ipsilateral vestibulothalamic tract
| Hemisphere | FA | MD | Tract volume | p-value | |
|---|---|---|---|---|---|
| Right | 0.41 (0.03) | 0.83 (0.04) | 590.4 (195.9) | 0.307 | |
| Left | 0.42 (0.03) | 0.82 (0.05) | 630.7 (162.3) | 0.738 | |
| Both | 0.41 (0.03) | 0.82 (0.05) | 610.6 (179.7) | 0.366 |
Values represent mean (±standard deviation), FA: fractional anisotropy, MD: mean diffusivity, MD×10–3(mm2/s).
Discussion
In the current study, we reconstructed the ipsilateral VTT in the human brain using DTT. In the field of DTT study, selection of ROIs is fundamental to the methods. Our seed ROI was placed on the VN with boundary of the reticular formation anteriorly, posterior margin of medulla and pons posteriorly, medial lemniscus medially, and the restiform body laterally on FA map, which were clearly localized. We used three axial slices to place on the whole VN. In the target ROI, we referred to the study by Morel et al. that provided exact location and size of the lateral thalamic nuclei on the axial slice in the human brain to give the target ROI for lateral thalamic nuclei, because localization of the thalamic nuclei is difficult, particularly neuroimaging technique [2]. We found that the ipsilateral VTTs originated from the VN ascended postero-laterally to the upper pons and antero-medially to the upper midbrain and terminated in the lateral thalamic nuclei. Therefore, we believe that the reconstructed ipsialteral VTTs would be precise because we used identified seed and target ROIs, and the pathway of our reconstructed ipsialteral VTTs coincided with the known pathway of the ipsialteral VTT in earlier research [4,5,6,7,8,9,10,11,12,13,14,15,16,22,24].
A few studies report an association between the VN and the thalamic nuclei in the human brain [13,14,15,16]. Bense et al. (2001) reported that galvanic vestibular stimulation increased the blood-oxygenation-level-dependent signals on the paramedian and dorsolateral thalamic nuclei in six healthy subjects using functional MRI [13]. Two studies by Dieterich et al. in 2003 (12 healthy subjects) and 2005 (eight patients with lesions on the posterolateral thalamus) suggested that the posterolateral thalamic nucleus was associated with the VN using positron emission tomography [14,15]. In 2008, Zwergal et al. observed that the ipsilateral VTT ran from the VN to the posterolateral thalamic nucleus in 14 patients with pontomesencephalic infarctions using MRI with electrophysiological technique [16]. To the best of our knowledge, two studies using DTT have described the connectivity between the VN and the thalamus [22,24]. In 2016, Kirsch et al. observed that the VN showed ipsilaterally connectivity to the parietoinsular vestibular cortex via the thalamus in either the posterolateral or paramedian nuclei in 24 normal subjects [22]. In 2017, Kwon et al. investigated structural neural connectivity of the VN to almost the entire brain in 37 normal subjects and found the connectivity of VN to the thalamus in all subjects [24]. Compared to our study, although these studies described the connectivity of the VN to the thalamus, they did not exactly identify the ipsilateral VTT three dimensionally using selection of two ROIs (seed ROI: the VN and target ROI: the lateral thalamic nuclei). Thus, we believe that this is the first DTT study to identify the ipsilateral VTT in the live human brain.
In conclusion, using DTT, we reconstructed the ipsilateral VTT and observed the anatomical characteristics of the ipsilateral VTT in the normal subjects. We believe that the methodology and results reported in this study could be helpful to researchers and clinicians in this field. However, several limitations of this study should be considered. First, we could not identify the discrete pathways of the ipsilateral VTTs between specific VN such as superior VN and medial VN and the specific lateral thalamic nuclei such as ventrolateral and posterolateral thalamic nuclei due to limitation of present neuroimaging technique. Second, ROI drawing technique has operatordependent Third, due to the partial volume effect, DTI can produce both false positive and negative results throughout the white matter of the brain [28,29]. Therefore, we suggest further studies including large numbers of patients and overcoming the limitations of this study.
Acknowledgement
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2015R1D1A4A01020385)
References
- [1].Siegel A., Sapru H.N., Siegel H.. Essential neuroscience. (Third edition) [Google Scholar]
- [2].Lopez C., Blanke O.. The thalamocortical vestibular system in animals and humans. Brain Res Rev. 2011;67:119–146. doi: 10.1016/j.brainresrev.2010.12.002. [DOI] [PubMed] [Google Scholar]
- [3].Wijesinghe R., Protti D.A., Camp A.J.. Vestibular Interactions in the Thalamus. Front Neural Circuits. 2015;9:79. doi: 10.3389/fncir.2015.00079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Lang W., Buttner-Ennever J.A., Buttner U.. Vestibular projections to the monkey thalamus: an autoradiographic study. Brain Res. 1979;177:3–17. doi: 10.1016/0006-8993(79)90914-4. [DOI] [PubMed] [Google Scholar]
- [5].Kotchabhakdi N., Rinvik E., Walberg F., Yingchareon K.. The vestibulothalamic projections in the cat studied by retrograde axonal transport of horseradish peroxidase. Exp Brain Res. 1980;40:405–418. doi: 10.1007/BF00236149. [DOI] [PubMed] [Google Scholar]
- [6].Maciewicz R., Phipps B.S., Bry J., Highstein S.M.. The vestibulothalamic pathway: contribution of the ascending tract of Deiters. Brain Res. 1982;252:1–11. doi: 10.1016/0006-8993(82)90973-8. [DOI] [PubMed] [Google Scholar]
- [7].Nakano K., Kohno M., Hasegawa Y., Tokushige A.. Cortical and brain stem afferents to the ventral thalamic nuclei of the cat demonstrated by retrograde axonal transport of horseradish peroxidase. J Comp Neurol. 1985;231:102–120. doi: 10.1002/cne.902310109. [DOI] [PubMed] [Google Scholar]
- [8].Nagata S.. The vestibulothalamic connections in the rat: a morphological analysis using wheat germ agglutinin-horseradish peroxidase. Brain Res. 1986;376:57–70. doi: 10.1016/0006-8993(86)90899-1. [DOI] [PubMed] [Google Scholar]
- [9].Shiroyama T., Kayahara T., Yasui Y., Nomura J., Nakano K.. Projections of the vestibular nuclei to the thalamus in the rat: a Phaseolus vulgaris leucoagglutinin study. J Comp Neurol. 407. 1999:318–332. [PubMed] [Google Scholar]
- [10].Matesz C., Bacskai T., Nagy E., Halasi G., Kulik A.. Efferent connections of the vestibular nuclei in the rat: a neuromorphological study using PHA-L. Brain Res Bull. 2002;57:313–315. doi: 10.1016/s0361-9230(01)00726-2. [DOI] [PubMed] [Google Scholar]
- [11].Meng H., May PJ., Dickman J.D., Angelaki D.E.. Vestibular signals in primate thalamus: properties and origins. J Neurosci. 2007;27:13590–13602. doi: 10.1523/JNEUROSCI.3931-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Barmack N.H.. Central vestibular system: vestibular nuclei and posterior cerebellum. Brain Res Bull. 2003;60:511–541. doi: 10.1016/s0361-9230(03)00055-8. [DOI] [PubMed] [Google Scholar]
- [13].Bense S., Stephan T., Yousry T.A., Brandt T., Dieterich M.. Multisensory cortical signal increases and decreases during vestibular galvanic stimulation (fMRI) J Neurophysiol. 2001;85:886–899. doi: 10.1152/jn.2001.85.2.886. [DOI] [PubMed] [Google Scholar]
- [14].Dieterich M., Bense S., Lutz S., Drzezga A., Stephan T., Bartenstein P.. et al. Dominance for vestibular cortical function in the non-dominant hemisphere. Cereb Cortex. 2003;13:994–1007. doi: 10.1093/cercor/13.9.994. [DOI] [PubMed] [Google Scholar]
- [15].Dieterich M., Bartenstein P., Spiegel S., Bense S., Schwaiger M., Brandt T.. Thalamic infarctions cause side-specific suppression of vestibular cortex activations. Brain. 2005;128:2052–2067. doi: 10.1093/brain/awh551. [DOI] [PubMed] [Google Scholar]
- [16].Zwergal A., Buttner-Ennever J., Brandt T., Strupp M.. An ipsilateral vestibulothalamic tract adjacent to the medial lemniscus in humans. Brain. 2008;131:2928–2935. doi: 10.1093/brain/awn201. [DOI] [PubMed] [Google Scholar]
- [17].Parker G.J., Alexander D.C.. Probabilistic anatomical connectivity derived from the microscopic persistent angular structure of cerebral tissue. Philos Trans R Soc Lond B Biol Sci. 2005;360:893–902. doi: 10.1098/rstb.2005.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Behrens T.E., Berg H.J., Jbabdi S., Rushworth M.F., Woolrich M.W.. Probabilistic diffusion tractography with multiple fibre orientations: What can we gain? Neuroimage. 2007;34:144–155. doi: 10.1016/j.neuroimage.2006.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Kwon H.G., Hong J.H., Hong C.P., Lee D.H., Ahn S.H., Jang S.H.. Dentatorubrothalamic tract in human brain: diffusion tensor tractography study. Neuroradiology. 2011;53:787–791. doi: 10.1007/s00234-011-0878-7. [DOI] [PubMed] [Google Scholar]
- [20].Yim S.H., Kim J.H., Han Z.A., Jeon S., Cho J.H., Kim G.S.. et al. Distribution of the corticobulbar tract in the internal capsule. J Neurol Sci. 2013;334:63–68. doi: 10.1016/j.jns.2013.07.015. [DOI] [PubMed] [Google Scholar]
- [21].Jang S.H., Kwon H.G.. The ascending reticular activating system from pontine reticular formation to the hypothalamus in the human brain: a diffusion tensor imaging study. Neurosci Lett. 2015;590:58–61. doi: 10.1016/j.neulet.2015.01.071. [DOI] [PubMed] [Google Scholar]
- [22].Kirsch V., Keeser D., Hergenroeder T., Erat O., Ertl-Wagner B., Brandt T.. et al. Structural and functional connectivity mapping of the vestibular circuitry from human brainstem to cortex. Brain Struct Funct. 2016;221:1291–1308. doi: 10.1007/s00429-014-0971-x. [DOI] [PubMed] [Google Scholar]
- [23].Jang S.H., Kim J.H., Kim D.H., Kwon H.G.. The vestibulocerebellar tract in the human brain: a diffusion tensor tractography Study. Current Medical Imaging Reviews. 2017 In press. [Google Scholar]
- [24].Jang S.H., Lee M.Y., Yeo S.S., Kwon H.G.. Structural neural connectivity of vestibular nuclei in the human brain: a diffusion tensor imaging study. Neural Regen Res. 2017 doi: 10.4103/1673-5374.230304. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Smith S.M., Jenkinson M., Woolrich M.W., Beckmann C.F., Behrens T.E., Johansen-Berg H.. et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(1):S208–219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- [26].Naidich T.P., Duvernoy H.M. Duvernoy’s atlas of the human brain stem and cerebellum : high-field MRI : surface anatomy, internal structure, vascularization and 3D sectional anatomy. Wien; New York: Springer: 2009. [Google Scholar]
- [27].Morel A., Magnin M., Jeanmonod D.. Multiarchitectonic and stereotactic atlas of the human thalamus. J Comp Neurol. 1997;387:588–630. doi: 10.1002/(sici)1096-9861(19971103)387:4<588::aid-cne8>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- [28].Yamada K., Sakai K., Akazawa K., Yuen S., Nishimura T.. MR tractography: a review of its clinical applications. Magn Reson Med Sci. 2009;8:165–174. doi: 10.2463/mrms.8.165. [DOI] [PubMed] [Google Scholar]
- [29].Fillard P., Descoteaux M., Goh A., Gouttard S., Jeurissen B., Malcolm J.. et al. Quantitative evaluation of 10 tractography algorithms on a realistic diffusion MR phantom. Neuroimage. 2011;56:220–234. doi: 10.1016/j.neuroimage.2011.01.032. [DOI] [PubMed] [Google Scholar]