Abstract
Xenotransplantation is frequently used to study normal and malignant hematopoiesis of human cells. However, conventional xenotransplantation mouse models lack essential human-specific bone marrow (BM) microenvironment-derived survival, proliferation, and self-renewal signals for engraftment of normal and malignant blood cells. As a consequence, many human leukemias and other hematologic disorders do not robustly engraft in these conventional models. Here, we describe a complete workflow for the generation of humanized ossicles with an accessible BM microenvironment that faithfully recapitulates normal BM niche morphology and function. The ossicles therefore allow for accelerated and superior engraftment of primary patient-derived acute myeloid leukemia (AML) and other hematologic malignancies such as myelofibrosis (MF) in mice. The humanized ossicles are formed by in situ differentiation of BM-derived mesenchymal stromal cells (MSCs). Human hematopoietic cells can subsequently be transplanted directly into the ossicle marrow space or via intravenous injection. Using this method, a humanized engraftable BM microenvironment can be formed within 6 – 10 weeks. Engraftment of human hematopoietic cells can be evaluated by flow cytometry 8 – 16 weeks after transplantation. This protocol describes a robust and reproducible in vivo methodology to study human normal and malignant hematopoiesis in a more physiologic setting.
Introduction
Xenotransplantation is currently the only reliable assay that facilitates the functional definition of human hematopoietic stem cells (HSCs) and their malignant counterparts, leukemia stem cells (LSCs). Xenotransplantation is therefore instrumental in developing a detailed understanding of human hematopoiesis and leukemogenesis. Humanized mouse models have become an important tool to investigate human normal and malignant hematopoiesis1–3, and progressively more immune-deficient mice strains have been developed to improve engraftment of hematopoietic cells.4–8 Furthermore, mice with human cytokine over-expression or knock-in into the endogenous mouse loci have been engineered to further enhance human engraftment. 9–15
Although previous xenotransplantation models are fairly advanced and can recapitulate many aspects of human normal hematopoiesis, several major limitations remained to be solved for the engraftment of malignant cells. A substantial proportion of primary AML patient samples, in particular less aggressive clinical subtypes such as those bearing mutations in core binding factor and those classified as acute promyelocytic leukemia (APL), failed to engraft in NOD/SCID/IL2R-gamma null (NSG) mice or did so at low levels that do not mimic clinical human disease 16–18. Furthermore, other more chronic hematopoietic neoplasms completely lacked engraftment in all of the available mouse strains and attempts to generate xenograft models of myelodysplastic syndrome (MDS), myeloproliferative neoplasms (MPN), and multiple myeloma met with limited success 19–21.
The reasons for the difficulty in xenotransplanting some human hematopoietic neoplasms remains largely unclear, but likely relates to the lack of cross-reactivity of specific factors and environmental clues that mediate hematopoietic cell homing, survival, and expansion. Human hematopoiesis is regulated by a specialized microenvironment, the BM niche.22 This specialized microenvironment, is necessary to fully recapitulate human disease in vivo by providing survival and maintenance signals to hematopoietic stem and progenitor cells (HSPCs) and leukemia-initiating cells which actively contribute to proper hematopoietic and disease development.23,24 These signals include: i) secreted species-specific cytokines, chemokines, and growth factors, and ii) the direct interaction of hematopoietic cells with microenvironmental stromal cells such as MSCs and extracellular matrix. To overcome these limitations we recently developed a novel xenotransplantation system by generating heterotopically localized bone organoid (hereafter defined as ossicles) - niches in mice to mimic the aforementioned human specific microenvironmental signals. Using this system we were able to successfully engraft the majority of AML samples including CBF-driven leukemias and APL. Furthermore this novel approach could be used for the first time to formally identify disease-initiating cells in human primary myelofibrosis and APL.25
This protocol is based on this recently published work and provides a step by step, user-friendly, reproducible instruction for the generation and subsequent use of such humanized microenvironments. Generation of BM-MSC-derived humanized ossicles will allow investigators to more successfully and faithfully perform xenotransplantation experiments.
We describe: 1) isolation and expansion of BM-derived mesenchymal stromal cells using a xenoprotein-free cell culture system; 2) transplantation and generation of subcutaneously localized humanized ossicles in NSG mice; 3) subsequent transplantation of normal or malignant hematopoietic cells into generated ossicles; and finally, 4) engraftment analysis from ossicle and other hematopoietic tissues in ossiclebearing mice. Collectively, this comprehensive protocol allows for the reproducible generation of immune-compromised mice bearing humanized BM-microenvironments that will lead to superior engraftment of normal and leukemic human hematopoietic cells, thereby providing an ideal tool to better recapitulate and model human hematopoietic development and hematopoietic malignancies in vivo. We anticipate this technology will contribute significantly to disease understanding and development of novel therapeutic approaches in the future.
This method has been successfully used in our investigations25,26 and other investigators have generated similar ectopic humanized marrow organs using different protocols (see Table 1) and also showed superior leukemia engraftment compared to naïve mice, further supporting the hypothesis that a humanized BM-microenvironment is beneficial for growth of human leukemia cells.27,28 Our humanized microenvironment was also sufficient to promote the engraftment of hematopoietic cells differentiated from AML-derived iPS cells.26
Table 1. Comparison of humanized BM niche models used for hematopoietic xenotransplantation.
| Mouse strain | Culture conditions/matrix and/or scaffold | Source and # of transplanted MSCs | Implantation of MSC | Time of hematopoietic transplantation | Route of hematopoietic transplantation | Type/number of transplanted hematopoietic cells | Conditioning | Reference # |
|---|---|---|---|---|---|---|---|---|
| NSG | Expansion in 10% pHPL containing media, extracellular matrix (Millipore) | BM 2 x 106 |
Subcutaneous injection | 6 – 10 weeks post MSC transplantation | Intraossicle iv |
UCB-CD34: 1 x 104 AML: 1 cell – 1.4 x 106 PMF: 2 x 103 – 5 x 104 |
Sublethal irradiation | 25 |
| NSG | Expansion in 10% pHPL-containing media, resuspended in extracellular matrix (Millipore), admixed with ECFC | BM 1.5 x 106 + 1.5 x 106 ECFC |
Subcutaneous injection | 8 weeks post MSC transplantation | Iv | UCB-MNC: 2 x 106 MOLM13: 2 x 106 |
Sublethal irradiation | 41 |
| NSG | Expansion in 10% pHPL-containing media, gene knock-down in MSC prior to transplantation, resuspended in extracellular matrix (Millipore), admixed with ECFC | BM 1.5 x 106 + 1.5 x 106 ECFC |
Subcutaneous injection | 8 weeks post MSC transplantation | iv | MOLM13: 2 x 106 Nalm6: 2 x 106 |
Sublethal irradiation | 40 |
| BRG | Expansion in 5% pHPL-containing media, seeded onto biphasic calcium phosphate (BCP) particles, incubated for an additional 7 days in vitro in osteogenic media | BM 2 x 105 MSCs |
Surgical implantation | 8 weeks post MSC transplantation | Intracardiac intraossicle |
UCB-CD34: 1 x 105 – 5 x 105 MM: 1 x 106 – 5 x 106 |
none | 31 |
| BRG & NSG | Expansion in 5% pHPL-containing media, seeded onto biphasic calcium phosphate (BCP) particles, incubated for an additional 7 days in vitro in osteogenic media | BM 2 x 105 MSCs |
Surgical implantation | 8 weeks post MSC transplantation | Intraossicle | AML: 5 x 104 – 4 x 106 |
Sublethal irradiation | 27 |
| NSG | Expansion in 5% pHPL-containing media, seeded onto biphasic calcium phosphate (BCP) particles, incubated for an additional 7 days in vitro in osteogenic media | BM 2 x 105 MSCs |
Surgical implantation | 8 weeks post MSC transplantation | Intraossicle | UCB-CD34 transduced with BCR-ABL or MLL-AF9 |
Sublethal irradiation | 42 |
| NSG | Expansion in 10% FBS containing media, seeded onto medical-grade polycaprolactone (mPCL) scaffolds coated with calcium phosphate (CaP), 4 weeks static, 4 week bioreactor | BM 3 x 105 MSCs |
Surgical implantation | 10 weeks post MSC transplantation | Iv (retro-orbital) |
UCB-CD34: 1.3 x 106 | Sublethal irradiation | 32 |
| NSG | Expansion in 10% FBS containing media, seeded onto medical-grade polycaprolactone (mPCL) scaffolds coated with calcium phosphate (CaP), 6–8 weeks differentiation in bioreactor, supplemented with rhBMP7 | BM 3 x 105 MSCs |
Surgical implantation | Up to 14 weeks post transplantation | Iv (retro-orbital) |
UCB/BM-CD34: 1 x 104 – 2 x 105 |
Sublethal irradiation | 33 |
| SCID-beige | Expansion in 10% FBS-containing media, 21 days of chondrogenic differentiation in Pellet culture | BM 3 x 105 MSCs |
Surgical implantation | 3 weeks post transplantation | iv | UCB-CD34: 3 x 105 | Sublethal irradiation | 37 |
| NSG | Expansion in 10% FBS-containing media, Seeding onto collagen sponges, culture for 2-7 days | BM 1 x 105 |
Surgical implantation | 48 hours before implantation | Intraossicle | UCB-CD34: 5 x 104 AML: 5 x 104 – 1 x 106 |
none | 28 |
BM: Bone Marrow, iv: intravenous; NSG: NOD-SCID IL-2Rγc-/-, BRG: BALB/c Rag2-/- IL-2Rγc-/-; MSC: Mesenchymal Stromal Cells, ECFC: Endothelial Colony Forming Cells, UCB: Umbilical Cord Blood, AML: Acute Myeloid Leukemia, PMF: Primary Myelofibrosis, pHPL: pooled Human Platelet Lysate; FBS: Fetal Bovine Serum
Application of the Method
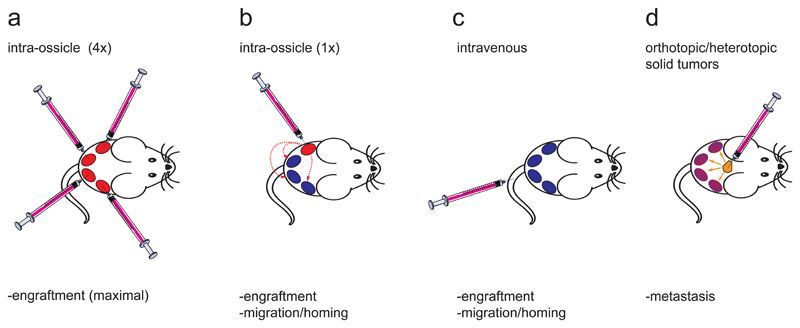
The humanized BM ossicle xentransplantation system can be used to study healthy and malignant human hematopoietic cells in a humanized BM microenvironment that more faithfully recapitulates physiological settings. In addition to providing a favorable microenvironment for human hematopoietic engraftment, direct intraossicle transplantation of hematopoietic cells into the ossicle marrow space minimizes the significant cell loss usually experienced after intravenous application and therefore allows for transplantation of lower cell numbers that will still enable robust engraftment. Importantly, disease-initiating cell frequencies within leukemia subpopulations can be studied and have been shown to be significantly higher using this xenotransplantation system compared to alternative NSG xenotransplantation models.25 Engraftment of cell populations with low engraftment potential can be augmented by injecting cells into multiple ossicles in the same mouse (Figure 1a).
Figure 1. Potential applications of humanized ossicle BM xenotransplantation.
After successful generation of 4 ossicles in an immunocompromised NSG mouse, hematopoietic cells can be (a) directly injected into all 4 humanized ossicle marrows (intraossicle) in order to maximize overall human engraftment rates. Homing and mobilization of human hematopoietic cells can be studied in non-injected ossicles if cells are (b) injected into only 1 out of 4 ossicles or (c) transplanted intravenously into ossicle-bearing mice. (d) Alternatively, humanized ossicles can be used to study bone-metastasis of solid tumor xenografts.
Our model also allows investigation of homing and migration of transplanted cells to other humanized niches when cells are injected by the intraossicle route into only 1 (out of 4 ossicles) of an individual mouse (Figure 1b), or can be used to study homing of injected cells to humanized sites in comparison to mouse BM if cells are injected intravenously (Figure 1c). Moreover, the humanized ossicle model is not restricted to investigation of human hematopoietic cell engraftment, but might also be a valuable tool to study bone metastasis behavior of solid tumors that exhibit poor metastasis behavior in conventional xenotransplantation models (Figure 1d).
Comparison to Other Methods
Table 1 summarises the major alternative methods. Many of these methods use osteo-conductive scaffold materials such as tri-calcium phosphate – hydroxyapatite (TCP-HA), bi-phasic calcium phosphate (BCP) or medical-grade polycaprolactone (mPCL) to form heterotopic bone constructs to mimic a BM microenvironment in vivo. Such methods require in vitro pre-coating of these materials with naïve or pre-differentiated BM-MSCs to force the cells into a pre-osteoblast state.27,29–33 Another approach is based on time-consuming in vitro manufacturing of late hypertrophic cartilage templates (with and without the use of collagen-based scaffold material) to generate ossicles that are then seeded with hematopoietic tissue to mimic physiological endochondral bone formation when implanted into immune-compromised mice.30,34–37 Both strategies require laborious cell culture to generate transplantable scaffolds or tissue, followed by surgical implantation of the in vitro generated tissue into subcutaneous pockets on the flanks of mice. In contrast, our protocol does not rely on osteo-conductive scaffold material, in vitro pre-differentiation, and surgical implantation. Following our protocol, in vitro expanded cells are simply injected into the subcutaneous space, spontaneously undergo differentiation in situ, and form a humanized BM microenvironment through endochondral ossification, a process recapitulating the normal development of the hematopoietic niche.38,39 The simple subcutaneous injection reduces surgery-induced morbidity and allows for the generation of many more experimental mice per time compared to time-consuming surgical implantation.
Experimental Design
Isolation and expansion of BM-MSCs (STEP 1 – 32)
Working with the optimal cellular material is key for the generation of proper human ossicles. BM was found to be the only source of MSCs with the inherent capacity to spontaneously undergo endochondral ossification and form an in situ BM microenvironment seeded with hematopoietic cells when injected in a heterotopic subcutaneous location.38,39 For the successful recapitulation of this protocol, we recommend the use of pooled human platelet lysate (pHPL) as media supplement instead of fetal bovine serum (FBS) for the isolation and expansion of human BM-MSCs 27,31,40–42 since we and others have recently shown that pooled HPL is highly efficient for expanding MSCs and is even superior to FBS for stimulation of cell proliferation.43–46 Therefor, using pHPL in BM-MSC cell culture will allow faster generation of cell quantities necessary for transplantation into mice. Several other independent reports also suggest that pHPL expanded MSCs can be successfully used to generate humanized BM in vivo 27,31,40–42 (for comprehensive comparison of different protocols see Table 1). We recommend producing pHPL by matching platelets of blood group O with plasma of blood group AB to avoid possible influences of ABH antigens and isoagglutinins (see [BOX 1] for pHPL production details).46–48
Box 1. Pooled human platelet lysate production: TIMING.
4 – 6 h hands on Platelet-derived growth factors efficiently stimulate cell proliferation in vitro. This effect has been reported for mesenchymal stromal cells (MSCs), fibroblasts, and endothelial colony-forming cells. 25,44,45,48,66 BM-MSCs isolated and expanded using pHPL, replacing fetal bovine serum (FBS), conserve their endochondral ossification capacity and reliably form humanized ossicles in vivo. One unit of platelet lysate (PL) is produced from one unit of platelet concentrate derived from 4 buffy coat units and one plasma unit (in total five blood donors), see also Figure Supplementary Figure 1. Ten PL units are then pooled to make one batch of pooled human platelet lysate (pHPL) (see also Supplementary Figure 1). So, for 1 PL unit the materials described in the following procedure are needed.
-
Take five units of regular whole blood donations (450 ± 50 mL each, one unit of blood group (BG) AB and four units of BG O) collected into a citrate-phosphate-dextrose containing quadruple bag system (e.g. by Fresenius HemoCare) derived from five healthy blood donors after written informed consent. Cool down the blood bag systems to 20 - 24°C.
PAUSEPOINT The blood can be kept at 22 ± 2°C for up to 24 hours until further processing.
Centrifuge the whole quadruple bag system for 13 minutes at 4,250 x g at 22°C for sedimentation of platelets to the buffy coat (BC) layer together with leukocytes.
Separate the fraction of red blood cells (RBCs) and the plasma supernatant from the middle BC portion by transfer into the respective satellite bags. Disconnect the RBC and plasma bags from the BC bag by sterile welding (Supplementary Figure 1a).
Mix 4 buffy coat units (derived from BG O donors, each unit represents an individual donor) with 1 unit of plasma (BG AB donor) in a 600 mL plasma bag and centrifuge for 6 minutes at 340 x g, 22°C
Transfer the supernatant to a platelet storage bag (blood group O, each unit represents an individual donor) and deplete leukocytes via inline filtration to obtain one unit of leukocyte-depleted platelet concentrate.
Freeze leukocyte-depleted platelet concentrate units at -30ºC for at least 48 hours to fragment platelets and release growth factors.
-
Repeat STEP 1 – 6 until 10 platelet concentrate units are collected (Supplementary Figure 1b).
CRITICAL STEP: Do not proceed until all 10 platelet concentrate units have been collected to prepare a well-standardized product guaranteeing appropriate growth factor concentrations. 45
Thaw 10 units of leukocyte-depleted platelet concentrate in a water bath at 37º until the ice clots disappear. The product is now called human platelet lysate (HPL). Do not warm the HPL.
Connect the HPL bags consecutively to a pooling double bag and transfer the HPL. Disconnect the individual HPL bags by welding.
After mixing all 10 units together, pool the final volume of 2 – 3 L, now called pooled HPL (pHPL), and aliquot into smaller size bags (e.g. 200 mL) or 50 mL conical tubes for storage.
Freeze-thaw pHPL for an additional time to increase rate of platelet fragmentation and the amount of released growth factors and transfer pHPL from 200 mL bags into 50 mL conical tubes for storage.
-
Remove platelet fragments form pHPL through centrifugation at 4,000 x g (15 minutes, 4°C).
CRITICAL STEP: This step is very important since platelet fragments aggregate during cell culture.
Pipette the supernatant plasma into the final storage containers (50 mL or 15 mL) and discard the platelet pellets.
Freeze aliquoted pHPL at -20°C. (Supplementary Figure 1c).
CRITICAL STEP: A sterility check of the final product has to be performed before using pHPL for cell culture. For sterility release criteria, we recommend including negative testing for bacteria, fungi, and mycoplasma.
PAUSEPOINT pHPL can be frozen at -20°C for up to two years prior to use.
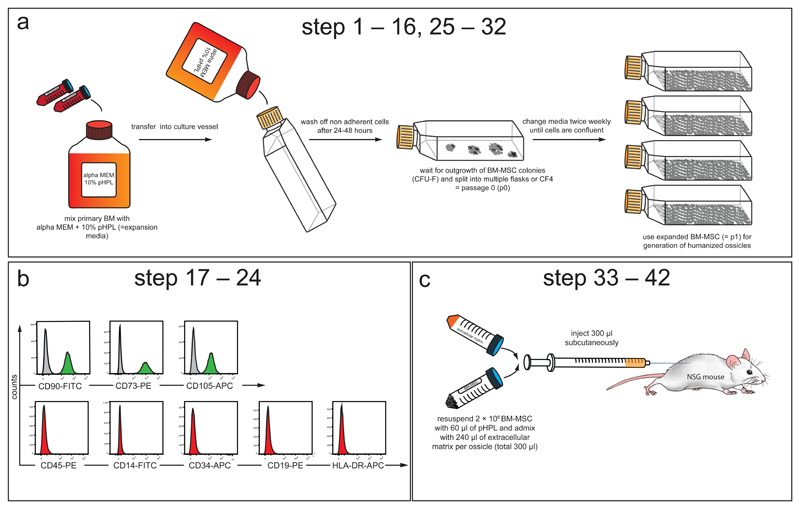
MSCs should be isolated by means of plastic adherence and initial plating of mononuclear cells (MNCs) should be done at frequencies between 5,000 –10,000 MNCs/cm2 cell culture area.46 This will optimize outgrowth of countable, individually separable fibroblastoid colony-forming units (CFU-Fs). Fresh BM aspirates (STEP 1 – 5) or filter washouts collected from BM-harvest equipment (STEP 6 – 8) can be used as starting material. We previously demonstrated that MSCs can be very efficiently isolated from unmanipulated BM (no lysis, no BM-MNC enrichment through density centrifugation)46 After initial outgrowth of CFU-Fs, adherent cells should be passaged and further expanded in appropriate sized cell culture flasks at cell seeding densities between 100 – 1000 cells per cm2. The cell culture surface area required for the expansion of MSCs depends on the final cell number needed to generate the intended number of ossicles. We recommend using 225cm2 flasks for cell isolation (passage [p] 0), followed by a change to multilayered cell-factories (CF-4) thereafter (p1 and later). To increase successful ossicle formation rates, we recommend only using early passage MSCs (no older than 20 – 25 population doublings, corresponding to p1 – p4) as these cells have a higher proliferative index. Backup cells from early passages should be cryopreserved in liquid nitrogen to guarantee sufficient material for repetitive experiments (Figure 2a). Allogeneic and autologous (with respect to transplanted hematopoietic cells) MSCs can be used for xenotransplantation assays.
Figure 2. BM-MSC isolation, propagation and phenotypic characterization.
This figure and figure legend has been slightly modified from its original publication in: A humanized bone marrow ossicle xenotransplantation model enables improved engraftment of healthy and leukemic human hematopoietic cells. Nat Med 22, 812-821, doi:10.1038/nm.4103 (2016).25 [AU: Step numbers in figure and legend need updating – we can request the copy editors modify the step numbers in the figure if you so wish to avoid you needing to upload this figure again?]
(a) Experimental schematic describing isolation and propagation of human BM-derived MSCs (corresponding to STEP 1 – 9 and 18 – 25). Human total BM cells (from fresh BM aspirates or bag washouts) are admixed with expansion medium consisting of alpha modified minimum essential medium (alpha MEM) supplemented with 10% pooled human platelet lysate (pHPL)47,67 and seeded into cell culture vessels. After 24 to 48 hours non-adherent cells are removed by rinsing with pre-warmed PBS and the adherent cells are further expanded until the outgrowth of colonies (colony unit forming fibroblasts, CFU-F) is observed. These cells are considered passage 0 (p0). All p0 cells are detached from the plastic and sub-passaged into multiple flasks or cell factories to provide enough culture surface area to allow for sufficient expansion. Expanded cells (p1) are frozen or immediately used for the generation of humanized ossicle niches. (b) Purity of isolated MSCs is analyzed by flow cytometry using a consensus panel of positive and negative markers (corresponding to STEP 10 – 17).49 Histograms show representative cell surface staining for CD90, CD73 and CD105 (green) compared to isotype control (grey). BM-MSCs do not express hematopoietic markers CD45, CD14, CD34, CD19, or HLA-DR (red). X-axis shows mean fluorescence intensity (MFI) and y-axis shows counts. (c) Experimental schematic describing subcutaneous BM-MSC transplantation (corresponding to STEP 26 – 36). 2 × 106 BM-MSCs are re-suspended in 60 µl of pHPL and admixed with 240 µl of extracellular matrix (Angiogenesis assay kit, Millipore) prior to subcutaneous injection (total volume: 300 µl per injection) into the flanks of 6 – 8 week old female NSG mice.
Cell characterization (STEP 17 – 24)
Criteria for the identification of MSCs from human BM include their fibroblast-like morphology combined with immunophenotypic characterization. Proper flow-cytometric quality control has to be performed to ensure MSC purity and integrity.49
A small number of extra cells should be expanded for immunophenotypic profiling in parallel to the large scale-expansion cells that will be used for generation of ossicles (Figure 2b). One day before the in vivo transplantation is planned, these extra cells should be harvested by trypsin digestion and stained with antibodies, as described in Table 1. Isotype-matched control antibodies should be used to exclude nonspecific staining and 7-aminoactinomycin D (7-AAD) should be used to evaluate cell viability. Flow cytometric analysis should show that isolated BM-MSCs are positive (>95%) for CD90, CD73, and CD105, but lack expression (<2%) of hematopoietic markers including CD45 (pan-leukocyte marker), CD34 (hematopoietic stem and progenitor cell and endothelial cell marker), CD14 or CD11b (macrophage and monocyte marker), CD79alpha or CD19 (B-cell marker) and HLA-DR (Figure 2b).49 Although absence of the described hematopoietic markers can exclude contamination with hematopoietic cells, expression of CD90, CD73, and CD105 is not specific to MSCs and unfortunately does not predict their functionality. Generally, cell surface immunophenotypes are highly overlapping between MSCs isolated from various tissue sources38 and can change during in vitro culture. 50
Transplantation of BM-MSCs to form humanized ossicles (STEP 25 – 45)
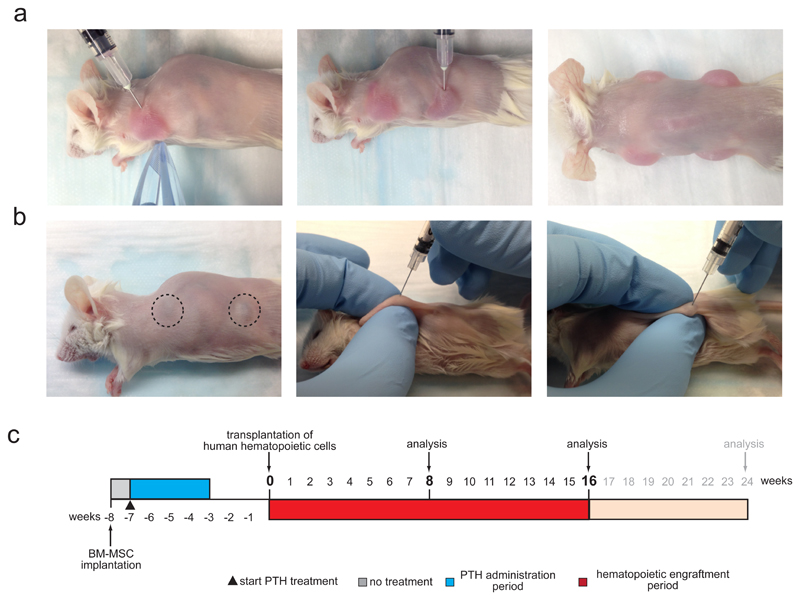
To form humanized ossicles, 2 x 106 BM-MSCs are transplanted subcutaneously into the flanks of immunocompromised NSG mice. Other strains of immunodeficient mice, such as nude, SCID-beige or BRG mice have been successfully used to form ectopic humanized bone marrow organs by transplanting BM-derived stromal cells together with scaffold materials 27,28,31–33,42 or after chondrogenic pre-differentiation30 (for a comprehensive comparison of humanized niche models used for hematopoietic xenotransplantation see Table 1). Different mouse strains might result in lower level of ossicle formation, as well as humanization and human hematopoietic cell engraftment. Up to four ossicles can be generated in a single mouse through injection of MSCs on the front, back, left, and right flanks (Figure 3b-c and Supplementary Video 1). Injections in the flanks are recommended because the flanks contain sufficient subcutaneous tissue space to fit the injection volume plus the mice cannot reach these areas with their limbs to disrupt ossicle development. Cells should be resuspended in liquid extracellular matrix, kept on ice, and transplanted as quickly as possible after trypsinization. After injection, extracellular matrix will solidify at mouse body temperature, a process that helps to keep the cells at the place of injection. Although we only used extracellular matrix from a single vendor, other commercially available extracellular matrix solutions have been successfully shown to support ossicle formation. For transplantation, we recommend using 6 – 10 week old female mice. Female mice are recommended because they show higher levels of human chimerism after transplantation of human HSPCs.51,52 Multiple injections in younger (smaller) mice are more difficult to perform and individual ossicles tend to fuse if cells are injected too close to each other. Starting from 3 days after injection, we recommend daily administration (subcutaneous) of anabolic doses of human 1-34 parathyroid hormone (PTH) to increase ossicle weight for a total of 28 consecutive days.38,53,54 As previously shown, PTH treatment does not increase the percentage nor the success of engraftment, rather it increases the number of engrafted cells that could be retrieved from successfully engrafted mice due to an increase in marrow cavity size.25 Transplanted MSCs will spontaneously differentiate into chondrocytes and subsequently undergo endochondral ossification to form a humanized BM microenvironment.38,39,41 The time course of this differentiation process should ideally be evaluated for each individual BM-MSC donor to estimate the ideal time point to start hematopoietic cell transplantations.38 Usually ossicle BM cavities start to develop 6 – 8 weeks post-MSC injection, and should be fully developed after a maximum of 10 weeks. This timeframe is in line with other reports using BM-MSC coated, surgically implanted scaffold materials to generate humanized hematopoietic niches.27,33 Appropriate marrow cavity formation can be indirectly evaluated by screening the ossicles (after shaving the areas of injection) for a color change from white/yellowish to purple, which indicates the presence of mouse hematopoietic tissue within the developing ossicle (Figure 3b and Supplementary Video 2).
Figure 3. Humanized ossicle niche formation. [AU: Update step numbers.].
(a) Representative images (corresponding to STEP 33 - 35) showing subcutaneous injection of cell-matrix mixtures into the left front (left image) and left back (middle image) flank of a shaved NSG mouse. Up to 4 injections were done per mouse (right image). (b) Representative images (corresponding to STEP 38) showing humanized ossicle-niches formed 8 weeks after MSC transplantation visible through the skin of shaved mice (left image). Dashed circles highlight a purple hue indicative of proper ossicle niche formation and murine hematopoietic engraftment. Humanized ossicle-niches are readily accessible for direct intra-ossicle transplantation of human hematopoietic cells (corresponding to STEP 40 – 46) or ossicle marrow aspiration (corresponding to STEP 49) (middle and right image). (c) Experimental timeline. Starting at 3 – 7 days after BM-MSC transplantation (week -7) daily treatment of anabolic doses (40 µg/kg BW) of human 1-34 parathyroid hormone (PTH) is carried out for 28 consecutive days. Ossicle formation is evaluated visually (color change to purple indicates hematopoietic engraftment and BM niche formation, see dashed lines f, left image) weekly starting at week -2. Human hematopoietic cells (HSPCs, AML) are transplanted and engraftment is analyzed subsequently at indicated time-points up to 24 weeks post transplantation. All mouse experiments were conducted in accordance with a protocol approved by the Institutional Animal Care and Use Committee (Stanford Administrative Panel on Laboratory Animal Care no. 22264) and in adherence with the US National Institutes of Health’s Guide for the Care and Use of Laboratory Animals.
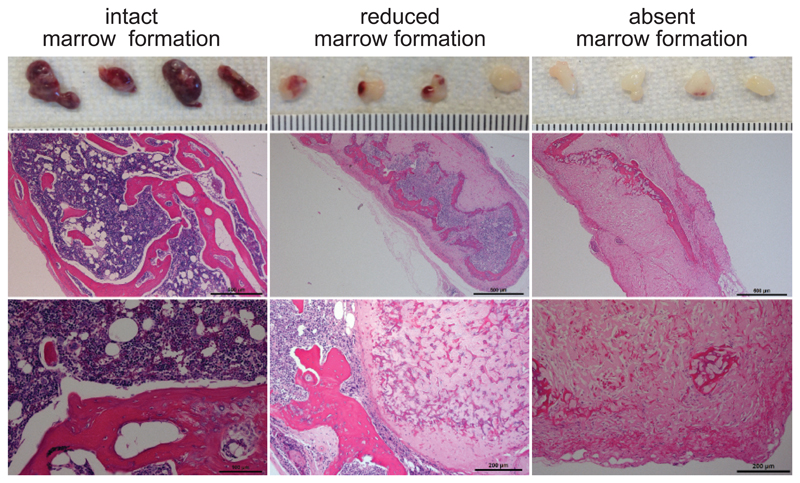
Despite their morphologic and immunophenotypic similarities in vitro, we observed an inherent variability in the ability to generate robust ossicles when transplanting BM-MSCs in vivo from different donors. Using AML patient-derived BM stromal cells, we could see sufficient ossicle formation in 44% (8/18) of the tested samples (Figure 4). Insufficient in vivo differentiation and marrow infiltration can lead to organoids with reduced marrow formation and only small areas of hematopoietic infiltration. (Figure 4, middle panel). Some BM-MSC donors are incapable of in vivo marrow formation (Figure 4, right panel). Therefore, we recommend evaluating in vivo endochondral ossification and marrow cavity formation from 3 – 5 individual donors on a smaller scale before initiating comprehensive experiments. Only donors that result in robust ossicle formation with intact marrow infiltration (Figure 4, left column) should be used for further studies.
Figure 4. In vivo ossicle formation.
Examples of humanized organoids formed 8 – 10 weeks after BM-MSC transplantation into NSG mice as described in the protocol. Gross macroscopic images of all four generated ossicles harvested from one mouse (top) and overview and high power microscopic images of hematoxylin and eosin stains performed on the same ossicles. Scale bar indicate a millimeter scale for gross macroscopic image, 500µm for overview and 100µm (left image) or 200µm (middle and right image) for high power images. Most BM-MSCs successfully undergo endochondral ossification and form an intact marrow cavity populated by hematopoietic cells (left panel). Reduced or absent ossification can cause limited or absent marrow formation (middle and right panel). All mouse experiments were conducted in accordance with a protocol approved by the Institutional Animal Care and Use Committee (Stanford Administrative Panel on Laboratory Animal Care no. 22264) and in adherence with the US National Institutes of Health’s Guide for the Care and Use of Laboratory Animals.
Transplantation of normal and malignant hematopoietic cells into humanized ossicles
Before transplanting human hematopoietic cells, ossicle-bearing mice need to be conditioned with a sublethal dose of irradiation (200rad). It is hypothesized that this opens the hematopoietic niches for incoming human hematopoietic cells. Alternative conditioning regimens such as busulfan chemotherapy 55–57 have not be tested in our model, but might facilitate elimination of radiation-induced damage to the niche, and could extend the use of the humanized ossicle model to investigators without access to animal irradiation. The humanized BM ossicle model allows the direct injection of human cells into a humanized microenvironment, thereby circumventing the substantial cell loss seen in intravenous transplantations (Figure 1a-b, Figure 2e and Supplementary Video 2). Using a 29G insulin needle, application of slight pressure and drilling movements will result in penetration of the cortical bone structure of the ossicle, subsequently allowing for the infusion of hematopoietic cells including sorted human HSPCs or subfractions, T-cell depleted bulk leukemia cells, or sorted leukemia subpopulations in a volume not exceeding 20 µl. Proper localization of the needle within the ossicle should be tested by aspiration before infusing the cells. Slight withdrawal of the plunger should result in aspiration of marrow visible within the syringe that will be reintroduced along with the transplanted cells (see Supplementary Video 2 and 3). If the experimental goal is to achieve maximal engraftment, all 4 ossicles can be transplanted (Figure 1a), but if migration to other ossicles is intended, only 1 ossicle should be used for transplantation (Figure 1b). Alternatively, cells can be injected intravenously in mice carrying humanized ossicles (Figure 1c).
Evaluation of human engraftment in humanized ossicles and mouse BM and peripheral blood (step 48 and onward and Figure 6)
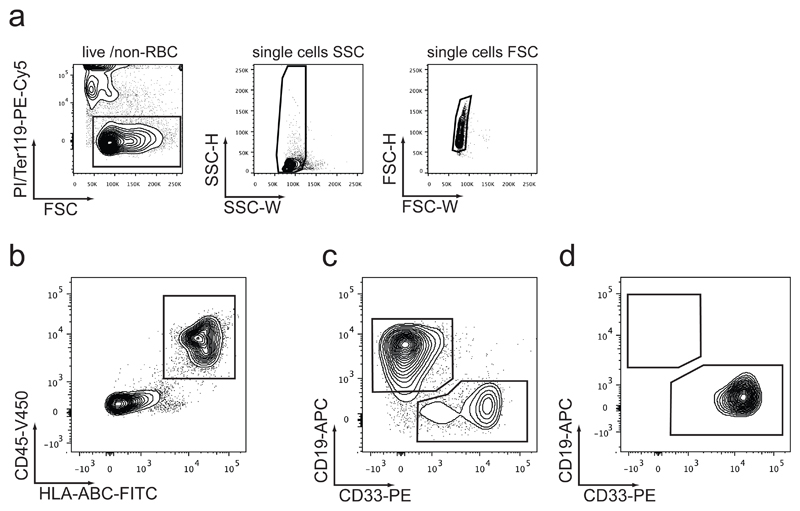
Figure 6. Flow cytometric analysis layout for human engraftment.
(a) Contaminating dead cells (PI+), mouse red blood cells (RBC, Ter119+) and cell doublets (low SSC-W and FSC-W) should be excluded prior to gating for human CD45/HLA-ABC double positive cells. (b) Transplanted and non-transplanted humanized ossicle bone marrow (BM), as well as murine BM (mBM) and murine peripheral blood (pB) should be analyzed by flow cytometry for the presence of human normal or leukemic CD45+HLA-ABC+ cells. Engrafted human cells should be defined by their expression of CD45 and HLA-ABC. These cells can be further gated for myeloid cells (CD45+HLA-ABC+CD33+) and B lymphoid cells (CD45+HLA-ABC+CD19+). Engrafted hematopoietic stem cells should result in bi-lineage engraftment (c), whereas myeloid leukemia engraftment should exclusively express CD33 and lack CD19 (d).
The easily accessible subcutaneous localization of the humanized ossicles allows for serial monitoring of hematopoietic engraftment by ossicle aspirations. We recommend intervals of at least 2 weeks between aspirates in order to minimize inaccurate engraftment assessments due to damage caused by prior aspirations. In order to guarantee reproducible engraftment measurements from aspirates, we recommend combining two individual aspirates from the two opposite poles of one ossicle. This should normalize for potential geographic differences of engraftment within individual ossicles. The extent of engraftment is determined by flow cytometry (Figure 6) with an antibody panel designed to detect human lymphoid (B-cells, T-cells) and myeloid cells (monocytes, granulocytes). To permit gating out of contaminating mouse leukocytes and erythrocytes, we typical add antibodies that detect these populations such as mouse CD45 and mouse Ter119 (Figure 6a).
Limitations of the Method
Although bone, cartilage, and mesenchymal stromal cells within the ossicle BM microenvironment are of human origin, the vasculature and the developing BM sinusoidal structures are mouse-derived. This is likely to limit the applicability of the model to addressing questions involving human specific niche factors associated with the endosteal and perivascular, but not the endothelial, niche. In our studies, all humanized ossicles are conditioned using sublethal irradiation to increase human normal and leukemic engraftment. Unfortunately, irradiation-based conditioning procedures significantly damage the BM-niche and will therefore influence studies of HSC regulation by the humanized microenvironment. Alternative conditioning regimens, which are less toxic to the niche, such as antibody based approaches 58–60 or feeding mice amino-acid deficient diets 61 could be explored to deplete hematopoietic elements. Furthermore, ossicles could be generated in genetically engineered mice that allow engraftment without irradiation such as NSGW41 to circumvent issues of conditioning-induced niche toxicity.62,63
Materials
Reagents
-
Human BM aspirates or BM collection bag filters, acquired per institutional protocol. Samples for this study were obtained according to the Administrative Panel on Human Subjects Research Institutional Review Board (IRB)-approved protocols (Stanford IRB no. 18329, no. 6453, and no. 5637) with informed consent.
CAUTION: Working with material collected from human subjects requires Institutional Review Board (IRB) approval. Human specimens must be collected with the informed consent of the donors.
-
Fresh or frozen human umbilical cord blood (UCB) for transplantation into ossicles acquired per institutional protocol. For this study, cord blood was collected with written informed consent from the mother, which was obtained before delivery of fullterm pregnancies at the Lucile Packard Children’s Hospital, according to IRB-approved protocols (Stanford IRB no. 5637).
CAUTION: Working with material collected from human subjects requires Institutional Review Board (IRB) approval. Human specimens must be collected with the informed consent of the donors.
CRITICAL Fresh or frozen umbilical cord blood or BM MNCs as well as enriched CD34+ HSPCs are commercially available from different vendors or can be sourced from full-term deliveries per institutionally approved protocols. In the latter case, collection and preparation of MNCs as well as CD34+ HSPC enrichment using MACS technology protocols should be performed as previously described by Park et al.64 Enrichment of CD34+ cells is recommended to minimize FACS sorting time for HSPC purification before transplantation. After MACS-based enrichment, typical yields are >90% purity with 30 – 50 % recovery. FACS sorted HSPC sub-fractions should yield >99% purity.
-
Frozen primary Leukemia samples acquired per institutional protocol. Samples for this study were obtained according to the Administrative Panel on Human Subjects Research Institutional Review Board (IRB)-approved protocols (Stanford IRB no. 18329, no. 6453, and no. 5637) with informed consent.
CAUTION: Primary leukemia samples should only be collected after patient’s informed consent following IRB approved protocols. Primary patient material should be handled according to institutional biosafety protocol requirements.
Alpha-modified minimum essential medium (a-MEM, Sigma, cat. no. M4526)
RPMI 1640 media, L-Glutamax supplemented + HEPES (Fisher, cat. no. 72400047)
Heparin, 1000 U/mL (Fresenius-Kabi, cat. no. 63323-540-57)
L-Glutamax 100x (200 mM, Fisher, cat. no 25-030-081)
Penicillin-Streptomycin (10,000 U/mL, Fisher, cat. no. 15-140-122)
40 buffy coat units derived from regular whole blood donations of blood group O
10 plasma units derived from regular whole blood donations of blood group AB
CRITICAL STEP: Human buffy coat units and human plasma can be sourced from various venders or should be obtained after informed consent from healthy volunteer blood donors fulfilling national requirements for blood donation. We obtain our human buffy coat units and plasma from collaborating partners at the Department of Blood Group Serology and Transfusion Medicine.
CRITICAL: Buffy coat and plasma units are required for platelet lysate production. For this, buffy coat units of blood group O should be mixed with plasma of blood group AB to reduce possible influences of ABH antigens and isoagglutinins.
BacT/ALERT BPA anaerob (bioMérieux Inc cat.no. 279044))
BacT/ALERT BNA aerob (bioMérieux Inc cat.no. 279045)
Mycoplasma test kit, MycoAlertTM (Lonza, cat. no. LT07-118)
TrypLE Express (Gibco, cat. no. 183-76-72)
1x PBS
-
Immune-deficient non-obese diabetic (NOD)-severe combined immunodeficiency (SCID), Il2rg−/− (NSG), 6-10 weeks old, female (NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ, The Jackson Laboratory). All mouse experiments were conducted in accordance with a protocol approved by the Institutional Animal Care and Use Committee (Stanford Administrative Panel on Laboratory Animal Care no. 22264)
CAUTION: Use of animals requires approval by local and national review committees.
Extracellular matrix (Angiogenesis assay kit, Millipore, cat. no. EC625)
1-34 human PTH (Tocris, cat. no. 3011)
BSA (Sigma, cat. no. A9647)
Deionized/distilled H2O (Fisher, cat. no. SH30538.02)
Ficoll-Paque Plus (GE-Healthcare, cat. no. 17-144-02)
Miltenyi human CD34 Microbead Kit (Miltenyi, cat. no 130-046-702)
Human CD3 positive selection Kit (StemCell Technologies, cat. no. 18051).
Trypan blue (Fisher, cat. no. 15250-061)
Red blood cell lysis buffer, 1X (eBioscience, cat. no. 00-4333-57)
Propidium Iodide 1 mg/mL (Sigma Aldrich, cat. no. P4864)
7-Amino-Actinomycin D (7-AAD, BD, cat. no. 559925)
Dextran (Sigma Aldrich, cat. no. 31392)
DNase (Deoxyribonuclease I, Worthington Biochemical Corporation, cat. no. LS002007)
Ultra pure 0.5 M EDTA (Fisher, cat.no. 155-75-020)
Fetal bovine serum (FBS, Omega Scientific, individual patch)
Bambanker cell freezing media (for freezing engrafted hematopoietic cells, Nippon Genetics, cat. no. BB01)
Dimethylsulfoxide (DMSO, Sigma, cat. no. D8414-250)
-
Antibodies for purification of human HSPCs
-
◦
CD34-APC (clone 8G12, BD, cat. no. 348053)
-
◦
CD38-PE-Cy7 (clone HB7, BD, cat. no. 555462)
-
◦
CD90-FITC (clone 5E10, BD, cat. no. 555595)
-
◦
CD45RA-BV605 (clone HI100, Biolegend, cat. no. 304134)
-
◦
CD10-APC-Cy7 (clone HI100, Biolegend, cat. no. 312212)
-
◦
-
Lineage antibodies for purification of human HSPCs
-
◦
CD2-PE-Cy5, clone, RPA-2.10 (BD, cat. no. 555328)
-
◦
CD3-PE-Cy5 (clone HIT3a, BD, cat. no. 555341)
-
◦
CD4-PE-Cy5 (clone RPA-T4, BD, cat. no. 555348)
-
◦
CD7-PE-Cy5 (clone M-T701, BD, cat. no. 555362)
-
◦
CD8-PE-Cy5 (clone RPA-T8, BD, cat. no. 555368)
-
◦
CD16-PE-Cy5 (clone 3G8, BD, cat. no. 555408)
-
◦
CD19-PE-Cy5 (clone H1B9, BD, cat. no. 555414)
-
◦
CD20-PE-Cy5 (clone 2H7, BD, cat. no. 555624)
-
◦
CD56-PE-Cy5 (B159, BD, cat. no. 555517)
-
◦
CD235a-PE-Cy5 (GA-R2, BD, cat. no. 559944)
-
◦
CD14-PerCP (clone MφP9, cat. no. 340585)
-
◦
-
Antibodies for human engraftment analysis
-
◦
HLA-A/B/C-FITC (clone W6/32, Biolegend, cat. no. 311426)
-
◦
CD45-V450 (clone HI30, BD, cat. no. 560368)
-
◦
CD33-PE (clone WM53, BD, cat. no. 555450)
-
◦
CD19-APC (clone HIB19, BD, cat. no. 555415)
-
◦
Ter119 PE-Cy5 (clone TER-119, eBioscience, cat. no. 15-5921-82)
-
◦
CD45.1-PE-Cy7 (clone A20, eBioScience, cat. no. 25-0453-82)
-
◦
CD3-APC-Cy7 (SK7, BD, cat. no. 561800)
-
◦
-
Antibodies for MSC characterization
-
◦
HLA-DR-APC (clone L243, BD, cat. no. 340549)
-
◦
CD14-PE (clone MφP9, BD, cat. no. 557742)
-
◦
CD19-PE (clone SJ25C1, BD, cat. no. 340364)
-
◦
CD34-APC (clone 8G12, BD, cat. no. 348053, same antibody is also used for human HSPC purification)
-
◦
CD45-PE (clone HI30, BD, cat. no. 555483)
-
◦
CD73-PE (clone AD2, BD, cat. no. 550257)
-
◦
CD90-FITC (clone 5E10, BD, cat. no. 555595, same antibody is also used for human HSPC purification)
-
◦
CD105-APC (clone SN6, Caltag, cat. no. PSI-76-874)
-
◦
Mouse IgG1 isotype control-FITC (clone X40, BD, cat. no. 349041)
-
◦
Mouse IgG2a isotype control-FITC (clone X39, BD, cat. no. 349051)
-
◦
Mouse IgG1 isotype control-PE (clone MOPC-21, BD, cat. no. 555749)
-
◦
Mouse IgG1 isotype control-APC (clone MOPC-21, BD, cat. no. 555751)
-
◦
Mouse IgG2a isotype control-APC (clone X39, BD, cat. no. 340473)
-
◦
Equipment
50 ml Falcon conical tubes (Thermo Fisher Scientific, cat. no. 14-432-22)
15 ml Falcon conical tubes (Thermo Fisher Scientific, cat. no. 12-565-268)
14 ml Polyproylene round bottom tubes (Fisher, cat.no. 14-959-11B)
5 ml tube with cell strainer snap cap (FACS-tubes, Fisher, cat. no. 08-771-23)
225 cm2 canted neck flask, tissue-culture treated (Fisher, cat. no. 07-200-62)
75 cm2 flask, tissue-culture treated (Fisher, cat. no. 10-126-11)
Easyfill cell factories (four layered, 2528 cm2, Fisher, cat. no. 12-567-302)
-
500 mL filter bottle (Stericup Express Plus, 0.22 µm, Millipore, cat. no. SCGPU05RE)
CRITICAL STEP: Stericup Express Plus has been tested to work for the filtration of pHPL-containing media. Other filter systems with same 0.22µm filter size are not recommended due to clogging problems.
Sterile cell strainer (70 µm, Fisher, cat. no. 07-201-43)
Cryotubes, 1.8 mL (Thermo, cat. no. 375418)
Serological pipettes (5 ml, 10 ml, 25 ml and 50 ml, Fisher, cat. no 357543, 357551, 357525, 357551)
3 mL syringes (VWR, cat. no. 53498-492)
needles, 25G x 5/8” (Covidien, cat. no. 8881250313)
35 mm Greiner dishes (Sigma, cat. no. P5112)
sterile gauze
plasma bags, 600 ml (Baxter, cat. no. R4R2021)
pooling double bag, 2 x 3.5 L (MacoPharma, cat. no. VDL 8000XQ, originally used for ascites puncture)
pooling bag set including leukocyte depletion filter for platelet concentrates (CompoStop 3F T&B, Fresenius-Kabi, cat. no. PT52600)
welding equipment (CompoSeal Mobilea II, Fresenius Kabi, model no. 9027011)
sterile tubing welder (TSCD®-II, Terumo Medical Corp, model no. 3ME-SC203A)
Luminometer (LucettaTM Lonza, model no. AAL–1001)
BacT/ALERT 3D Microbial Detection System (bioMérieux Inc., model no. 247001)
Heracell CO2 incubator (Thermo Fisher Scientific, cat. no. 51026282)
FACS Aria II cell sorter
FACSCalibur analyzer
Inverted microscope (microscope should fit a CF-4)
MACS MS and LS columns (Miltenyi)
Mini/Midi MACS seperator (Miltenyi)
29G Insulin syringes (Sure comfort, cat. no.)
27G Tuberculin syringes (BD, cat. no. 305136)
25G Monoinject 1ml syringes (Fisher, cat. no. 22-257-137)
Disposable sterile plastic forceps (Cole-Parmer, cat. no. EW-06443-20)
Extra fine bone scissors (FST, cat. no. 14084-08)
Disposable stile scalpel blades, blade No. 22, (VWR, cat. no. 21909-626)
High precision scalpel handle (Thermo Fisher Scientific, cat. no. 12-000-164)
Straight medium point forceps (Thermo Fisher Scientific, cat. no. 16-100-106)
Cabinet X-ray system (Faxitron, Model 43855F)
Isoflurane small animal and rodent anesthesia machine (Parkland Scientific, cat. no. V3000PK)
Mouse clipper (Wahl, model Chromado Lithium)
Reagent Setup
Pooled human platelet lysate (pHPL) See [BOX1] and Supplementary Figure 1 for details of how to produce pHPL.
Stromal cell culture media
Supplement 500 mL alpha-MEM with 5 mL L-Glutamax, 5mL Pen/Strep, 2 U/mL Heparin and 50 mL O/AB pHPL (10% v/v, see [BOX1 and Supplementary Figure 1] for production details). Heparin should be added to the medium before pHPL supplementation to avoid gel formation. Before use, the supplemented alpha-MEM should be sterile filtered through a 0.22 µm bottle filter. Media should be stored at 4ºC until required and can be used for up to 2 weeks. Repeated filtration might be necessary to get rid of newly formed particles.
BM harvesting media
Supplement 500mL RPMI1640 media with 5ml L-Glutamax, 5ml Pen/Strep, 10% (vol/vol) FBS. Store at 4ºC for maximal 2 weeks. Before use, add 2U/mL Heparin and 20U/ml DNase I.
BM collection media
Prepare 50 mL PBS containing 10mM EDTA (final) and aliqot 500µl into microcentrifuge tubes. Media can be stored at room temperature (RT; 22 – 25ºC) for up to 3 months.
Cell staining media (FACS Buffer)
Add 2% FBS (vol/vol) and 2mM EDTA to 500 mL of PBS. Mix well and sterile filter through a 0.22 µm filter bottle. Store at 4ºC and use within maximally 1 month.
BM-MSC cryopreservation media
Add 10% DMSO (vol/vol) to stromal cell culture media and store at 4ºC and use within 2 weeks.
2% dextran solution
Dissolve 1 g of dextran in 50ml of distilled, deionized water. Filter solution through a 0.2 µm filter. 2% dextran should be stored at 4ºC and can be used for up to 1 year.
Human HSPC antibody cocktail
We usually mix all lineage antibodies (see REAGENTS list above) resulting in a master-mix that reduces pipetting error and can be used to stain multiple samples. Typically we use all lineage antibodies (CD2, CD3, CD4, CD7, CD8, CD16, CD19, CD20, CD56, CD235a, CD14) at a 1:50 final dilution in FACS-Buffer (except CD235a, which is included at a dilution of 1:200). However optimal antibody-concentration should be determined empirically by the investigator before use. Antibodies for CD34, CD38, CD45RA, and CD90 are added individually with a final concentration ranging from 1:25 to 1:50, but optimal concentration should be determined empirically by each investigator. In our experience up to 1 x 107 UCB HSPCs can be stained in a total volume of 50µl staining buffer. Store at 4°C in the dark. Do not use staining master-mix for longer then a week.
Human engraftment antibody cocktail
Prepare a staining master-mix by combining all human engraftment antibodies (list provided in REAGENTS above) before adding them to the cells. Antibody dilutions range from 1:25 to 1:200 (final) but optimal antibody concentrations should be determined empirically and may vary between different antibody lots and antibody providers. In our experience up to 1 x 107 cells can be stained in a total volume of 50 µl staining buffer. Store at 4°C in the dark. Do not use staining master-mix for longer then a week.
Extracellular matrix
Thaw Angiogenesis Assay extracellular matrix solution and diluent overnight at 4ºC. Pre-cool 1000 µl pipette-tips and 14ml round bottom tubes on ice before use to limit matrix loss due to sticking to the inside of the tips while pipetting. We recommend pipetting in a cold room (if available). Add 110 µl of diluent to 1ml of extracellular matrix solution and mix well by pipetting up and down while trying to avoid generating air bubbles. Prepare at least 300 µl of extracellular matrix per single MSC injection to account for the inevitable matrix loss during pipetting. The final volume of extracellular matrix solution per single injection will only be 240 µl since the remaining volume (to make a total of 300 µl injection volume) comes from 2 x 106 MSCs resuspended in 60 µl pre-cooled pHPL. Leftover mixed diluted extracellular matrix solution should be used within one week.
CRITICAL: Angiogenesis assay kit (extracellular matrix) components should be kept at 4ºC to avoid solidifying.
Human 1-34 Parathyroid Hormone (PTH)
Reconstitute 1mg PTH powder with 10 ml of H2O + supplemented with 0.1% (w/vol) BSA. Further dilute stock solution 1:5 in PBS to reach a final concentration of 20 µg/ml. Freeze aliquots of 500µl at -20ºC and thaw fresh every day before use. Frozen aliquots can be used for up to 3 months.
Conditioning of ossicle-bearing NSG mice for hematopoietic transplantation
Mice should be ideally transplanted within 12 – 24 hours post-irradiation. To irradiate mice, transfer mice into a clean, institutionally approved irradiation chamber, lined with clean paper towels and apply 200 rad irradiation.
Procedure
Isolation of BM-MSC TIMING: 0.5 – 1 h; 2 days culturing
- Isolate BM-MSC from BM aspirates (option A) or BM harvesting filters (option B)
-
A)Isolation of BM-MSC from BM aspirates. TIMING: 0.5 – 1 h hands on; 2 days culturing
-
Take 200µl of the whole human BM into a microcentrifuge tube and lyse RBC by adding 500µl of RBC lysis buffer and incubating for 5min on ice.CRITICAL The remainder of the human BM is needed in step 5.
- Wash cells by adding 700µl PBS, centrifuging for 7min, 300g at 4ºC and resuspending in 200µl.
- Count 15µl of total nucleated cells (TNC) by diluting 1:1 in Trypan blue.
- Use remaining lysed TNC (from step 2) to determine the percentage of BM-MNCs (lymphocytes, monocytes) within TNC (also containing granulocytes) by flow-cytometric. Lymphocytes and monocytes are determined based on forward and side scatter properties of these cells (Supplementary Figure 2).
- Mix remaining total BM (without prior lysis and MNC enrichment) with pre-warmed stromal cell culture media and seed at a density corresponding to 5,000 – 10,000 BM-MNCs per cm2 cell culture surface area in T225 flasks and incubate at 37ºC, 5% CO2, 95% air humidity for 48 hours. Seed cells in 45 mL of media per T225 flask.
-
-
B)Isolation of BM-MSC from BM harvesting filters. TIMING: 0.5 – 1 h hands on; 2 days culturing
-
A)
CRITICAL As an alternative to option A, BM-MSCs can be isolated by washing out discarded filter units from BM-collection bags.
Add 45 ml pre-warmed stromal cell culture media to the BM collection bag filter unit and shake vigorously to loosen MSC-rich bone spicules from the filter membrane.
Collect cell-media mix in 50ml conical tubes and count TNCs and determine percentage of BM-MNC within TNC (see step 3 – 4).
Seed combined cell-media mix from of one BM-harvesting filter unit into T225 flasks at a density corresponding to 5,000 – 10,000 BM-MNCs per cm2 cell culture surface and incubate at 37ºC, 5% CO2, 95% air humidity for 48 hours.
CRITICAL STEP: Avoid unnecessary moving of the flask at the beginning of the culture period to reduce impairment of outgrowth of MSCs from bone spicules.
Removal of non-adherent cells and culture media changes. TIMING: 5 min each time during 10 – 14d culture.
-
2.
After the initial 48 hours incubating cells, remove media containing non-adherent cells and wash twice with 20mL pre-warmed PBS to get rid of remaining floating cells.
-
3.
Add 45 mL of pre-warmed fresh stromal cell culture media and continue incubation.
-
4.
Replace 1/3 of culture media with fresh pHPL-containing alpha-MEM twice weekly until outgrowing CFU-Fs reach confluence. These cells are considered passage 0.
Passaging and expansion of adherent BM-MSCs. TIMING: passaging: 0. 5h hands on, media changes: 5 min each time, 7 – 10 days culturing
-
5.
Remove media from flask and wash cells twice with pre-warmed PBS before adding 7 mL TrypLE Express to T225 flask. Incubate cells with enzyme for 5 min at 37ºC, tap the flask to lift cells. Quench TrypLE Express with 14 mL of stromal cell culture media, remove cells from flask and pellet in a 50 ml conical tube by centrifugation at 300g, 7min.
-
6.
Resuspend cell pellet in 5 ml of fresh stromal cell culture media and count cells using a hemocytometer and Trypan Blue exclusion. An average BM-aspirate or BM-harvest filter should yield 2 – 5 x 106 cells at this stage of cell culture.
PAUSE POINT: At this point MSCs can be cryopreserved and frozen cells can be used later for expansion. To freeze cells, pellet the cells, resuspend in freezing media before aliquoting 5 x 105 – 1 x 106 cells into cryovials (1 ml per vial) and placing into a slow-freeze cryovial chamber in a -80ºC freezer. Transfer vials into liquid nitrogen the following day.
CRITICAL STEP: If frozen cells are used for expansion, we recommend pre-culturing cells after thawing in 1x T225 flask for 3 – 5 days before transferring into CF-4 for large-scale expansion. This step maximizes viability of cells used to start BM-MSC expansion.
?TROUBLESHOOTING
-
7.
Seed 500 cells/cm2 in one or multiple 4-layered cell factories (CF-4, 2528 cm2) using 400 mL fresh stromal cell culture media per CF-4. Total cells needed per CF-4: 1,264,000 (= 500 x 2528). Liquids within CF-4s are distributed between individual layers by placing the closed cell factory onto its long side carrying the smaller port. The bigger size vent cap should be on top. Wait until the fluid levels within layers equilibrate before turning CF-4 to short side so that ports are oriented up and distribute media to cover whole surface area.
CRITICAL STEP: To avoid uneven seeding of cells due to un-level incubator shelves we highly recommend checking that the incubator is exactly level before starting large scale expansion in a CF-4.
CRITICAL STEP If using frozen cells, thaw a frozen aliquot of early passage cells and pre-expand in T225 flask before seeding into CF-4s as described in STEP 13.
-
8.
Seed an extra T75 flask at 1000 cells/cm2 for MSC characterization by flow cytometry (Figure 2b). CRITICAL STEP These cells will be harvested and analyzed for MSC marker expression prior to harvesting CF-4s.
-
9.
Change 1/3 of culture media twice weekly until cells reach confluence.
Immunophenotypic characterization of MSCs. Timing: 2 – 3h
-
10.
Harvest cells in 75 cm2 flask as described in Step 8.
-
11.
Resuspend cells in 250 µl FACS-buffer and block with 25 µl Fc-receptor blocking solution for 20 min on ice.
-
12.
Wash cells and resuspend pelleted cells with 250 µl FACS-buffer.
-
13.
Pipette MSC characterization antibodies and appropriate isotype control antibodies (see REAGENT LIST and Table 1 for antibody multi-color combinations and final dilutions) into FACS tubes and add 50 µl of cell suspension.
-
14.
Mix well and stain cells for 30 min on ice in the dark.
-
15.
To wash cells, centrifuge cells for 7 min, 300g, at 4ºC with cold FACS-buffer.
-
16.
Resuspend cells in 200 µl of FACS-buffer.
PAUSE POINT: Stained, resuspended cells can be stored at 4ºC in the dark for up to 6 hours before analysis.
-
17.
Add 5 µl of 7-AAD to FACS-tubes and analyze cells on FACSAria II, FACSCalibur, or other machines equipped with similar laser setup. Exclude dead cells based on 7-AAD positivity and collect at least 10,000 live events per tube (see Supplementary Figure 2c for full gating strategy). Export FCS-files to generate histograms as shown in Figure 2b using FlowJo or other 3rd party software confirming MSC purity and integrity. MSCs at this stage should be >95% positive for CD73, CD90, and CD105 and should lack (<2%) expression of CD45, CD14, HLA-DR, CD19, and CD34 (Figure 2b).49
CRITICAL STEP: Cells should only be injected into mice if expanded BM-MSCs meet the criteria described above.
Harvesting expanded cells from CF-4. TIMING: 2 – 3 h
-
18.
Confirm confluence of BM-MSCs grown in CF-4 by microscopy before starting the harvesting procedure.
-
19.
Pour out stromal cell culture media from smaller port into a 500 ml, 0.22 µm pore size bottle filter and keep filtered media for quenching TrypLE Express.
-
20.
Wash cells by carefully adding 150 ml of pre-warmed PBS.
CRITICAL STEP: When carrying CF-4s to and from incubators, always carry with ports upwards to prevent liquids moving from one layer to another.
-
21.
After removing PBS, add 70 mL of pre-warmed TrypLE. Distribute between layers as described in Step 7 and incubate for 7 min at 37ºC to lift the cells.
-
22.
Gently tap the CF-4 and quench TrypLE by adding 150 mL of filtered media that was collected at Step 19.
-
23.
Collect cells in a 500 mL centrifuge bottle and wash CF-4 once more with 150 ml of filtered media to collect remaining cells. Alternatively cells can be collected in multiple 50 ml conical tubes and pellets can be combined after individually being resuspended.
-
24.
Spin down at 300g, 4ºC for 7 min, aspirate media and resuspend pellet in a maximum of 12 mL of filtered media.
CRITICAL STEP: Carefully weigh collection bottles to be able to accurately balance the centrifuge.
-
25.
Count cells with a hemacytometer by Trypan blue exclusion. Typical yields from a single CF-4 are 60 – 80 x 106 MSCs, allowing the generation of up to 40 ossicles.
Preparing cells for in vivo application. TIMING: 0.5 – 1 h
-
26.
Transfer up to 44 x 106 MSCs (equivalent to the number of cells required for 22 injections) into a single 14 mL round bottom tube and spin down at 300g, 4ºC for 7 min.
CRITICAL STEP: We recommend calculating for an extra 10% to account for the volume loss caused by sticky extracellular matrix solution and pipetting.
-
27.
Per 2 x 106 MSCs (= one injection) use 60 µl (20% of final injection volume) of pre-cooled pure 0/AB pHPL for resuspension. e.g. 1.32 mL for 44 x 106 BM-MSCs.
-
28.
Use pre-cooled 1000 µl pipette-tips to add 240 µl of extracellular matrix solution (prepared as described in REAGENT SETUP) per 2 x 106 MSCs (e.g. 5.28 mL for 44 x 106 BM-MSCs) and mix well through pipetting up and down. Avoid using serological pipettes for this step.
-
29.
Keep cell-matrix mix on ice until injecting into mice. Cells can be kept on ice for up to two hours.
CRITICAL STEP: Minimize production of air bubbles during resuspension and keep cell-matrix mix on ice at all times.
MSC transplantation, PTH-injections, and evaluation of ossicle formation. Timing: depending on numbers of animals to transplant, 5 – 10 min per mouse (4 injections), 10 – 20 min for PTH injections, 6 – 10 weeks for in vivo ossicle formation
CRITICAL For further guidance regarding steps 30 – 36 see Supplementary Video 1.
-
30.
Anesthetize mice using isoflurane (2%, oxygen flow rate: 2 L/min) and carefully shave both flanks with a clipper.
-
31.
Clean sites of injection twice using 70% EtOH wipes (use fresh wipes each time)
-
32.
Draw 300 µl of cell-matrix mix form STEP 36 into 25G Monoinject syringes.
-
33.
Punch the mouse skin on the flank and then lift the needle to confirm subcutaneous localization. Lifting the needles additionally helps generate a pouch allowing for easy deposition the cell-matrix mix.
-
34.
Slowly apply the 300 µl cell-matrix mix and remove needle. Compress site of skin puncture for 10 sec with sterile forceps to minimize backflow.
-
35.
Repeat STEPS 31 – 34 to inject up to 4 sites per animal.
-
36.
Put animals into a clean cage placed under a heat source (red light) for recovery. Place mice on clean paper towels to avoid asphyxiation. After recovery (usually within a minute) put mice back into their original cage.
-
37.
Starting from day 3 post-MSC transplantation, subcutaneously inject (into dorsal neck fold) the transplanted mice daily with human 1-34 PTH. Use 50 µl of 20 µg/mL PTH (= 40 µg/kg BW of a 25 g mouse) for 28 consecutive days. PTH injections are not mandatory for proper ossicle formation but significantly increase the size.25
-
38.
After 6 – 8 weeks post transplantation shave the injection site to evaluate proper ossicle formation through visual inspection (development of a purple hue within the ossicle, Figure 2) and palpation (subcutaneously localized firm structure). When the ossicles are formed, proceed to the next section.
?TROUBLESHOOTING
Cell sorting TIMING: 1-3 hours
-
39.
If you plan to transplant human HSPCs into the ossicle, follow option A. If you plan to transplant human leukemia cells into the ossicle, follow option B.
-
A)Cell sorting of HSPCs. TIMING: 1 – 3 hours
- Resuspend fresh or frozen umbilical cord blood or BM MNCs or enriched CD34+ HSPC to a final concentration of up to 1 x 107 cells per 50 µl staining buffer.
- Add an equal volume of 2x HSPC antibody cocktail and incubate cells on ice for 30 min in the dark.
- Fill the tube with staining buffer and wash cells by spinning at 300g for 7 min at 4ºC.
- Aspirate supernatant and resuspend cells with staining buffer plus PI (1 µg/ml final). These cells should be left on ice, in the dark before they are then analysed and/or sorted.
-
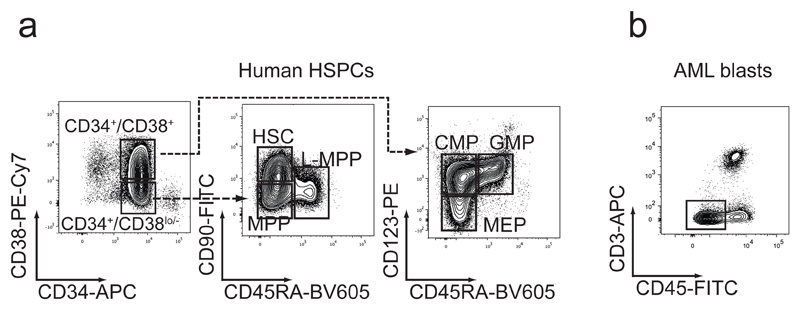
Define human HSPCs based on expression of CD34, CD38, CD90, CD45RA and CD123 (Figure 5a) on FACSAria II or other three laser machines with similar laser setup.CRITICAL STEP: It is very important to avoid contamination of the transplant with mature CD3+ T-cells, as T cells can proliferate in NSG mice and potentially cause fatal xenogenic graft versus host disease (Xeno-GvHD).
-
Sort cells into microcentrifuge tubes containing cold staining-buffer with PI. Typically >90% purity can be reached with a single sort. To increase purity to >99% we usually perform a double-sort.CRITICAL STEP: For all experiments, verify the purity of the sorted population by re-analyzing a small fraction of the collected cells.
- Spin down cells for 7 min, at 300g at 4ºC and aspirate staining buffer
-
Resuspend cells such that the final number of cells to be transplanted per individual ossicle is present in 20 µl of staining buffer. Minimize production of air bubbles.CRITICAL STEP: Sorted cells should be transplanted as quickly as possible following cell sorting since delay longer then 1 hour might affect cell viability.
-
B)Cell sorting of leukemia cells. Timing: 1 – 3 hours
- Thaw a frozen aliquot of primary human leukemia rapidly by placing vial into 37ºC water bath.
- Transfer cells to a sterile 50 mL conical tube filled with pre-warmed RPMI1640, supplemented with L-Glutamax, Pen/Strep, 10% vol/vol FBS, and 20 U/ml DNase I.
- Put cells for 10 min into 37ºC water bath to digest DNA released from dying cells before spinning at 300g, 7 min at 4ºC.
- Resuspend cells to a final concentration of up to 1 x 107 per 50 µl staining buffer and add CD45-PE and CD3 FITC antibodies.
- Mix well and incubate cells on ice for 30 min in the dark.
- Aspirate supernatant and resuspend cells with staining buffer plus PI (1 µg/ml final). These cells should be left on ice, in the dark before they are then analysed and/or sorted.
-
Define human leukemia blasts based on side-scatter properties and low CD45 expression (Figure 5b) on FACSAria II or other three laser machines with similar laser setup.CRITICAL STEP: It is very important to avoid contamination of the transplant with mature CD3+ T-cells, as T cells can proliferate in NSG mice and potentially cause fatal xenogenic graft versus host disease (Xeno-GvHD).CRITICAL STEP: If the blast count within primary samples exceeds 90%, or FACS sorting is not possible, contaminating donor T-cells can be eliminated using immunomagnetic micro-bead technologies. We usually perform CD3 enrichment and take the depleted cell fraction for transplantation using EASYSEP reagents (see REAGENT list).
-
Sort cells into microcentrifuge tubes containing cold staining-buffer with PI. Typically >90% purity can be reached with a single sort. To increase purity to >99% we usually perform a double-sort.CRITICAL STEP: For all experiments, verify the purity of the sorted population by re-analyzing a small fraction of the collected cells.
- Spin down cells for 7 min, at 300g at 4ºC and aspirate staining buffer
-
Resuspend cells such that the final number of cells to be transplanted per individual ossicle is present in 20 µl of staining buffer. Minimize production of air bubbles.CRITICAL STEP: Sorted cells should be transplanted as quickly as possible following cell sorting since delay longer then 1 hour might affect cell viability.
Figure 5. Sorting schemes for normal and leukemic hematopoietic cell populations.
(a) Fluorescence activated cell sorting (FACS) can be used to enrich hematopoietic stem and progenitor cell (HSPC) populations from human umbilical cord blood (UBC) or bone marrow (BM) samples. Hematopoietic stem cells (HSC), multipotent progenitors (MPP), lymphoid-primed multi-potent progenitors (L-MPP), common myeloid progenitors (CMP), granulocyte/macrophage progenitors (GMP) and megakaryocyte/erythroid progenitors (MEP) can be isolated from enriched total CD34+ cells based on differential expression of CD38, CD123, CD45RA, and CD90. HSC, MPP and LMPP reside within the CD34+/CD38lo/- fraction, whereas CMP, GMP, and MEP are found within the CD34+/CD38+ fraction. One representative cord blood sample with subpopulation gating is depicted. (b) Flow cytometry gating strategy of a representative primary patient leukemia sample shows that most human leukemia blasts can be discriminated from normal hematopoietic cells based on their lower surface expression of CD45. Gating on CD45low cells and additional staining of T-cells with CD3 allows for reliable enrichment of human leukemia blasts. Both sorting strategies concomitantly deplete donor T-cells, minimizing the risk of xenogeneic graft versus host disease (Xeno-GvHD).
Transplantation of sorted hematopoietic cells: TIMING: 0.5 – 1 h, 5 min per mouse CRITICAL This section should be carried out if you wish to transplant cells into the ossicle.
CRITICAL NSG mice bearing transplantable ossicles (see Figure 3b, evaluated by palpation and visual inspection as described in STEP 38) need to been conditioned by irradiating them with 200 rad 12 – 24 hours before transplantation, as described in REAGENT SETUP.
CRITICAL For further details of how to carry out steps 41 – 45, also watch Supplementary Video 2.
-
2
Anesthetize mice with isoflurane and prepare mice for injection as described in STEP 30 – 31
-
3
Draw 20 µl of cell suspension (1 cell – 3 x 106 cells per 20µl) from STEP 39 (or alternative, suitably prepared cell source) into 29 ½ G insulin syringes.
-
4
Localize ossicle and fix between thumb and index finger (see Figure 2)
-
5
Punch needle through skin and subcutaneous tissue until reaching the bony surface of the ossicle.
-
6
By applying pressure and a back and forth motion, use the tip of the needle to drill a whole into the cortical shell of the ossicle. When the needle goes through the bone layer a slight relief can be noticed. If performed appropriately the syringe should be fixed in the ossicle at this point.
-
7
Aspirate with a slight withdrawing pressure to confirm proper localization of the needle within the hematopoietic tissue in the humanized marrow cavity. If the needle is in the right position a tiny drop of blood should become visible in the syringe when aspirating.
-
8
Slowly apply cells into the ossicle marrow cavity over 10 – 15 seconds.
CRITICAL STEP: Injection volumes >20 µl, injecting too fast, or injecting air bubbles can lead to respiratory stress, embolism and possibly death.
? TROUBLESHOOTING
-
9
Clearly mark injected mice by punching their ears (or similar methods) and label cage card with localization of injected ossicles.
Evaluation of short term engraftment from ossicles (week 6 – 8): TIMING: 4 – 6 h CRITICAL For further details of how to carry out steps 49 – 51 also watch Supplementary Video 3.
CRITICAL As an alternative to this section, individual ossicles can be surgically removed and prepared for analysis as described in STEP 62 – 65 at various time points following institutional guidelines for surgical procedures.
-
10
At 6 – 8 weeks after hematopoietic cell transplantation anesthetize and prepare mice following STEP 30 – 31, before performing an ossicle BM aspiration with an empty 29G ½” insulin syringe from the ossicle as described in STEP 42 – 44.
CRITIAL STEP: Pre-coat syringe walls with EDTA-containing BM collection buffer to prevent coagulation.
-
11
Aspirate hematopoietic cells from the ossicle (use maximal withdrawing pressure) by quickly pulling the plunger of the syringe. If needle was placed correctly in the ossicle BM cavity, this will lead to the appearance of a tiny visible drop of ossicle marrow in the syringe. An average ossicle BM aspirate should yield 2 – 4 µl of marrow.
CRITICAL STEP: Occasionally needles can get clogged while drilling through the bone, making it impossible to aspirate BM from the ossicle marrow cavity. A clogged needle will lead to a scenario with the plunger moving back to its initial position after pulling out. In the rare case of a clogged needle, we recommend repeating the aspirate with a fresh pre-coated syringe.
?TROUBLESHOOTING
-
12
Transfer aspirated hematopoietic cells into a pre-labeled microcentrifuge tube filled with 500 µl BM-collection buffer (see REAGENT SETUP) and mix thoroughly.
-
13
Repeat STEP 49 – 50 on the opposite ossicle pole and combine both aspirates to account for geographical differences in engraftment levels.
PAUSE POINT: The ossicle aspirates can be left at room temperature for up to 3 hours while performing aspirates on additional ossicle sites and animals.
-
14
If you plan to compare human engraftment between humanized ossicle niches and the murine peripheral blood and/or murine BM, additionally collect peripheral blood (at least 5 drops) and/or perform a BM aspirate from the mouse femur using institutionally approved protocols for femur marrow collection and tail bleed sampling. A video protocol showing the femur collection has been previously published.64
-
15
Transfer ossicle marrow aspirated from microcentrifuge tubes into 5 ml round bottom tubes and fill with 3 ml pre-cooled staining buffer.
-
16
Spin for 7 min at 300g at 4ºC to pellet cells.
-
17
Resuspend with 500 µl red blood cell lysis buffer and incubate for 5 min on ice.
-
18
Fill the tubes with pre-cooled staining buffer and centrifuge 7 min at 300g at 4ºC to pellet cells.
-
19
Aspirate supernatant without disrupting cell pellet, such that 25 µl of buffer is left in the FACS tube.
-
20
To reduce nonspecific Fc receptor mediated antibody binding, block cells by adding 2.5 µl of Fc blocking reagent, mix well, and incubate for 5 – 10 min at RT. It is not necessary to wash cells between blocking and antibody staining.
-
21
Stain the cells by adding equal volume of 2x engraftment antibody master mix (see REAGENT SETUP) and incubate on ice for 30 min in the dark.
CRITICAL POINT: Always stain control human PB-MNCs (collected from discarded buffy coats and cryopreserved) as a positive control for the engraftment staining. PB-MNCs should contain CD45+CD33+ myeloid cells as well as CD45+CD19+ B-cell and CD45+CD3+ T-cell populations. All cells should be negative for expression of mouse CD45.1 and Ter119.
-
22
Wash cells by filling up FACS tube with pre-cooled staining buffer and spin for 7 min at 300g at 4ºC to pellet stained cells.
-
23
Aspirate the supernatant without disrupting the pellet and resuspend cells with 200 µl of staining buffer containing 1x PI (1 µg/mL final). Keep cells on ice in the dark until cells are analyzed on a flow cytometer using proper compensation settings and controls. See Figure 6 for a typical analysis layout optimized for evaluating human engraftment.
PAUSE POINT: Stained cells can be kept on ice for up to 6 hours before analysis on the flow cytometer.
Evaluation of long term engraftment from ossicles (week 12 – 16): TIMING: 6 – 8 h [AU: Can this be done after the earlier section or is it an alternative? I.e. can you remove BM from ossicles after 6-8 weeks and then remove the ossicles later as well?]
-
24
Euthanize engrafted mice by CO2 asphyxiation following an approved protocol.
-
25
Surgically remove ossicles individually. Cut the mouse skin approximately 5 – 10mm next to the ossicle and clear the subcutaneous space using sterile scissors. After localizing the ossicle, carefully excise it from its surrounding connective tissue using a sterile scalpel. Crush the ossicle using a mortar and pestle in 3ml of BM harvesting media.
CRITICAL POINT: For crushing, do not apply too much force since this can reduce cell viability. Gentle disruption of the ossicle is particularly important when working with engrafted fragile human primary leukemia samples.
-
26
Filter cells through a cell strainer snap cap and incubate tubes for 10 min in a 37ºC water bath to allow for sufficient digestion with DNase.
-
27
Spin down for 7 min, 300g at 4ºC and wash an additional time by filling up FACS tube with pre-cooled staining buffer.
-
28
Resupend cell pellet in 300µl of FACS-Buffer and keep cells on ice.
-
29
If human engraftment will be compared between humanized ossicle niches and the murine peripheral blood and/or murine BM, additionally harvest cells from mouse peripheral blood (option A) and/or mouse BM (option B).
-
(A)Harvesting cells from mouse peripheral blood
- Collect mouse peripheral blood by cardiac puncture using 27G Tuberculin syringe immediately after euthanasia.
- Transfer peripheral blood into microcentrifuge tubes containing PBS + 10 mM EDTA.
- Add 500 µl of 2% dextran, mix well, and incubate at 37ºC for 30 min, allowing red blood cells to settle in the bottom of the tube.
- Transfer white blood cell-containing upper fraction into a fresh FACS tube and wash with staining buffer by filling up tube and spinning for 7 min, 300g at 4ºC and then perform red blood cell lysis, blocking of non-specific antibody binding and staining as described in STEP 55 – 61.
-
(B)Harvesting cells from mouse BM
- Dissect bilateral femurs, tibias, and humerus and place into 3 cm petri-dishes containing pre-chilled BM harvesting media. Try to remove as much muscle and connective tissue from bones as possible using clean dissection technique. Remaining tissue should be pulled of with forceps or gauze.
- Remove epiphyses at the end of the marrow cavity using scissors.
- Insert 26G needle attached to a 3ml syringe and flush marrow with BM harvesting media. Flush bone cavities thoroughly until no more red BM is visible within the bones.
- Wash with staining buffer by filling up tube and spinning for 7 min, 300g at 4ºC and then perform red blood cell lysis and staining as described in STEP 55 – 61.
- Filter cells through a cell strainer snap cap, spin down for 7 min, 300g at 4ºC. Resuspend cells in 300 µl of staining buffer.
-
30
Transfer 25 µl of the ossicle cell solution (from step 66) into a separate FACS tube, block nonspecific antibody binding, and stain with engraftment antibody cocktails as described in STEP 58 – 61.
PAUSE POINT: Stained cells can be kept on ice for up to 6 hours before analysis on the flow cytometer.
-
31
If desired cryopreserve aliquots of the remaining cells from step 66 using cell freezing media or use remainder for secondary transplantation as described in Park et al.65 If secondary transplantation into ossicles is planned, careful experimental timing is important to have new ossicle-bearing mice available at the time of sacrificing primary recipients.
TIMING:
STEP 1 – 4: Isolation and culture of BM-MSCs (p0), hands on time 0.5 – 1 h, 10 – 14 days cell culture
STEP 5 – 9: Passaging and large-scale expansion of adherent BM-MSCs (p1), hands on time 0.5 – 1 h, 7 – 10 days cell culture
STEP 10 – 17: Immunophenotypic characterization of BM-MSCs, 2 – 3 h
STEP 18 – 25: Harvesting expanded cells from CF-4 cell factories, 2 – 3 h
STEP 26 – 29: Preparation of cells for in vivo application, 0.5 – 1 h
STEP 30 – 38: MSC transplantation, PTH-injections and in situ ossicle formation: 5 – 10 min per mouse (4 injections), 10 – 20 min for PTH injections, 6 – 10 weeks for in vivo ossicle formation.
STEP 39: Sorting of HSPCs (option A i – viii) or leukemia cells (option B i – x) for transplantation into ossicles, 1 – 3 h
STEP 40 – 47: Transplantation of sorted hematopoietic cell populations, 0.5 – 1 h (depending on number of ossicles to transplant, 5 min per mouse)
STEP 48 – 61: Evaluation of short-term engraftment, 4 – 6 h
STEP 62 – 69: Evaluation of long-term engraftment: 6 – 8 h
The completion of our complete outlined protocol (not including optional secondary transplantation) takes 28 – 32 weeks. We typically freeze multiple aliquots of early passage BM-MSCs (p0 or p1). Subsequently, frozen cell aliquots are expanded in CF-4s for large-scale production of cells before subcutaneous MSC transplantation. Using fresh BM-MSCs is not recommended for comprehensive experiments, since endochondral ossification potential should be pre-evaluated on a small scale for every new, fresh donor. Harvesting of BM-MSCs and transplanting MSCs into NSG mice has to be performed on the same day. Therefore, we recommend only generating a maximal number of cells sufficient to transplant 30 mice. Harvesting and transplantation of more mice is not recommended since delay in transplanting the BM-MSCs might negatively affect ossicle formation. Inexperienced users should start with a maximum of 5 mice (44 x 106 BM-MNC) per day. Transplanting hematopoietic cells into humanized BM ossicles is a rapid procedure, so that 20 – 30 mice can be transplanted on the same day. Depending on experience level, performing single BM ossicle niche aspirates takes about 2 – 5 min. However, a single mouse may contain up to four ossicles (plus optional tail bleed and mouse BM aspiration), therefore a maximum of 10 mice should be planned for evaluation of short-term engraftment on a single day. Long-term engraftment analysis is even more time consuming since ideally each ossicle (plus optional: mouse peripheral blood and mouse BM) needs to be excised, crushed, and analyzed separately. We recommend analyzing no more than 10 mice on the same day. If tissue processing takes longer than anticipated, prepared cells may be stored at 4ºC overnight and analyzed the next day without significant changes in results.
TROUBLESHOOTING
Troubleshooting advice can be found in Table 3
Table 3. Troubleshooting.
| Step | Problem | Possible reason | Solution |
|---|---|---|---|
| 6 | Poor BM-MSC cell yield | Cells were not distributed equally between individual cell layers in CF-4 when initially seeded. Incubator shelf is not level and cells accumulated on one side of the CF-4 rather than being equally distributed. |
Make sure that cells are well resuspended within the culture media and carefully check for equal liquid distribution between layers after filling CF-4s. Check the level of your incubator before placing CF-4s. |
| 38 | Ossicles are not properly visible or did not change color to purple after 6 – 10 weeks in vivo. | BM-MSCs have been injected intramuscular or intraperitoneal instead of subcutaneous. BM-MSCs did not differentiate appropriately and lack engraftment with hematopoietic tissue. |
Make sure that cells are injected into subcutaneous tissue by carefully lifting the skin with needle to visually confirm proper needle positioning (see Supplementary Video 1). Try to use earlier passage cells and/or test other BM-MSC donors to screen for cells with high in vivo endochondral ossification capacity. |
| 46 | Cells cannot be injected into the ossicle BM space. | The needle was clogged by tissue while drilling into ossicle bone marrow. | Remove needle, try to salvage cells from the syringe, and retry on opposite pole of the ossicle. Always make sure to have extra cells in case cells cannot be salvaged from syringe. |
| 49 | Cells cannot be aspirated from the BM space. | The needle was clogged by tissue while drilling into ossicle bone marrow. Needle tip is not positioned within ossicle BM space. |
Remove needle and retry with new needle on opposite pole of the ossicle. Always make sure to have extra syringes prepared. Slightly move needle further into BM space while continuously applying slight withdrawing pressure. |
Anticipated Results
Carefully following the steps provided in our protocol should result in the generation of high numbers of proliferative BM-derived stromal cells which will readily form individual CFU-Fs in the initial isolation step and thereafter can be extensively expanded to generate sufficient cells for preparing BM-MSC transplants. If non-adherent cells are removed as suggested and adherent stromal cells are properly passaged for further expansion, our resulting cell population usually meets required immunophenotypic criteria (Figure 2). Culture in large multi-layered tissue culture vessels guarantees sufficient expansion and the expected cell number per CF-4 (2528 cm2 cell surface area) should reach between 60 – 80 x 106 BM-MSCs, which will be enough material for up to 10 mice if 4 ossicles are injected per animal.
After subcutaneous implantation the injection site will initially have a soft bump due to the volume injected, which will reduce in size over the first few days post injection (Figure 2). Careful palpation of the transplants can be performed and should confirm progressive transformation of the tissue into a more rigid subcutaneous lump over a time-course of 1 – 3 weeks post-transplantation. We confirmed by histological analysis that these palpable changes in tissue stiffness can be attributed to early chondrogenic differentiation followed by progressive tissue remodeling reminiscent of endochondral ossification.38 Later in the development of the humanized BM ossicle niche, a continuous change of the color should be visible when carefully examining the developing subcutaneous ossicle in shaved mice. This transformation from a whitish/yellow to a purple hue is indicative of an increasing infiltration of the developing BM microenvironment with murine hematopoietic tissue (Figure 2). When a suitable BM-donor, capable of endochondral ossification and marrow formation has been identified (Figure 3, left panel), 75 – 95% of transplanted sites should form adequately sized ossicles that are suitable to be used for direct intra-ossicle transplantation of human hematopoietic cells after 6 – 10 weeks (Supplementary Video 2).
After conditioning irradiation, direct intra-ossicle injection of as little as 100 human HSPCs should lead to robust multi-lineage short-term (6 – 8 weeks) and long-term engraftment (16 weeks) in the injected ossicle (Figure 6c), but will eventually also lead to mobilization with subsequent homing and engraftment at distant human ossicles. As seen in naive NSG mice without ossicles, the amount of circulating cells in the mouse peripheral blood is relatively low and is not reflective of the actual engraftment in the ossicle or the mouse BM.
If leukemia cells are transplanted into humanized ossicles, we reproducibly found that humanized ossicles are superior in supporting leukemic engraftment of primary patient samples bearing diverse genetic lessions.25 Transplantation of acute myeloid leukemia blasts leads to fast and fulminant myeloid-restricted engraftment in the ossicle (Figure 6d) and preferred homing of leukemia cells to other humanized ossicle sites. Notably, when using leukemia samples previously confirmed as low (<1%) or non-engrafters for intra-ossicle and intravenous transplantation, human chimerism in the humanized BM ossicles usually reaches >90%, while human engraftment in the mouse BM remained very low or absent.25 This and additionally robust engraftment of acute promyelocytic leukemia (APL) and myelofibrosis (MF) samples confirms the superiority of the presented method and applying this protocol will allow investigators to substantially extend their repertoire of engraftable primary samples. This humanized BM ossicle xenotransplantation system will not only facilitate in vivo studies of diverse AML subtypes, but will broadly extend the use of xenotransplantation for in vivo investigation of hematological malignancies other than acute leukemias.
Supplementary Material
Editorial Summary.
Humanized bone marrow-ossicle niches are formed in mice via in situ differentiation of bone marrow-derived mesenchymal stromal cells and can be used for transplantation of normal and malignant human hematopoietic cells.
Tweet.
How to create humanized bone marrow-ossicle niches in mice
Table 2. MSC characterization panel with titrated antibody dilutions.
| FITC | PE | PerCP | APC | |
|---|---|---|---|---|
| 1 | IgG1 (1:100) | IgG1 (1:25) | 7-AAD | IgG1 (1:50) |
| 2 | IgG2b (1:100) | - | 7-AAD | IgG2a (1:50) |
| 3 | CD90 (1:50) | CD73 (1:20) | 7-AAD | CD105 (1:100) |
| 4 | CD3 (1:50) | CD19 (1:20) | 7-AAD | CD34 (1:50) |
| 5 | CD14 (1:50) | CD45 (1:50) | 7-AAD | HLA-DR (1:20) |
Acknowledgments
We acknowledge the Hematology Division Tissue Bank and the patients for donating their samples. We acknowledge F. Zhao for lab management, M. Stafford for technical help with the ossicle generation, and D. Strunk for providing critical reagents, advice, and intellectual support for the initial development of the protocol. A.R. was supported by an Erwin-Schroedinger Research Fellowship (Austrian Science Fund). D.C.H is a California Institute for Regenerative Medicine (CIRM) scholar. R.M. is a New York Stem Cell Foundation Robertson Investigator and Leukemia and Lymphoma Society Scholar. This research was supported by the Leukemia and Lymphoma Society, New York Stem Cell Foundation, and National Institutes of Health grants R01CA188055 and U01HL099999 to R.M. This work was also supported by funding from the European Union’s Horizon 2020 research and innovation program under grant agreement number 668724 (TECHNOBEAT) to Dirk Strunk (Paracelsus Medical University of Salzburg, Austria) and grant agreement number 731377 (MUSIC) to K.S.
Footnotes
Author Contributions. A.R. and R.M conceived and designed the project. A.R. and D.C.H performed the experimental work. A.R. analyzed all data, K.S. provided critical reagents, A.R. and R.M. wrote the protocol.
Competing Financial Interests. R. Majeti has an ownership interest (including patents) in Forty Seven Inc. and is a consultant/advisory board member for the same.
References
- 1.Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med. 1997;3:730–737. doi: 10.1038/nm0797-730. [DOI] [PubMed] [Google Scholar]
- 2.Pearce DJ, et al. AML engraftment in the NOD/SCID assay reflects the outcome of AML: implications for our understanding of the heterogeneity of AML. Blood. 2006;107:1166–1173. doi: 10.1182/blood-2005-06-2325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lapidot T, et al. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature. 1994;367:645–648. doi: 10.1038/367645a0. [DOI] [PubMed] [Google Scholar]
- 4.McCune JM, et al. The SCID-hu mouse: murine model for the analysis of human hematolymphoid differentiation and function. Science. 1988;241:1632–1639. doi: 10.1126/science.241.4873.1632. [DOI] [PubMed] [Google Scholar]
- 5.Agliano A, et al. Human acute leukemia cells injected in NOD/LtSz-scid/IL-2Rgamma null mice generate a faster and more efficient disease compared to other NOD/scid-related strains. Int J Cancer. 2008;123:2222–2227. doi: 10.1002/ijc.23772. [DOI] [PubMed] [Google Scholar]
- 6.Greiner DL, Hesselton RA, Shultz LD. SCID mouse models of human stem cell engraftment. Stem Cells. 1998;16:166–177. doi: 10.1002/stem.160166. [DOI] [PubMed] [Google Scholar]
- 7.Ishikawa F, et al. Development of functional human blood and immune systems in NOD/SCID/IL2 receptor {gamma} chain(null) mice. Blood. 2005;106:1565–1573. doi: 10.1182/blood-2005-02-0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Feuring-Buske M, et al. Improved engraftment of human acute myeloid leukemia progenitor cells in beta 2-microglobulin-deficient NOD/SCID mice and in NOD/SCID mice transgenic for human growth factors. Leukemia. 2003;17:760–763. doi: 10.1038/sj.leu.2402882. [DOI] [PubMed] [Google Scholar]
- 9.Goyama S, Wunderlich M, Mulloy JC. Xenograft models for normal and malignant stem cells. Blood. 2015;125:2630–2640. doi: 10.1182/blood-2014-11-570218. [DOI] [PubMed] [Google Scholar]
- 10.Wunderlich M, et al. AML xenograft efficiency is significantly improved in NOD/SCID-IL2RG mice constitutively expressing human SCF, GM-CSF and IL-3. Leukemia. 2010;24:1785–1788. doi: 10.1038/leu.2010.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nicolini FE, Cashman JD, Hogge DE, Humphries RK, Eaves CJ. NOD/SCID mice engineered to express human IL-3, GM-CSF and Steel factor constitutively mobilize engrafted human progenitors and compromise human stem cell regeneration. Leukemia. 2004;18:341–347. doi: 10.1038/sj.leu.2403222. [DOI] [PubMed] [Google Scholar]
- 12.Rongvaux A, et al. Development and function of human innate immune cells in a humanized mouse model. Nat Biotechnol. 2014;32:364–372. doi: 10.1038/nbt.2858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rongvaux A, et al. Human thrombopoietin knockin mice efficiently support human hematopoiesis in vivo. Proc Natl Acad Sci U S A. 2011;108:2378–2383. doi: 10.1073/pnas.1019524108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Strowig T, et al. Transgenic expression of human signal regulatory protein alpha in Rag2-/-gamma(c)-/- mice improves engraftment of human hematopoietic cells in humanized mice. Proc Natl Acad Sci U S A. 2011;108:13218–13223. doi: 10.1073/pnas.1109769108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Willinger T, et al. Human IL-3/GM-CSF knock-in mice support human alveolar macrophage development and human immune responses in the lung. Proc Natl Acad Sci U S A. 2011;108:2390–2395. doi: 10.1073/pnas.1019682108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sarry JE, et al. Human acute myelogenous leukemia stem cells are rare and heterogeneous when assayed in NOD/SCID/IL2Rgammac-deficient mice. J Clin Invest. 2011;121:384–395. doi: 10.1172/JCI41495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sanchez PV, et al. A robust xenotransplantation model for acute myeloid leukemia. Leukemia. 2009;23:2109–2117. doi: 10.1038/leu.2009.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Patel S, et al. Successful xenografts of AML3 samples in immunodeficient NOD/shi-SCID IL2Rgamma(-)/(-) mice. Leukemia. 2012;26:2432–2435. doi: 10.1038/leu.2012.154. [DOI] [PubMed] [Google Scholar]
- 19.Kim D, Park CY, Medeiros BC, Weissman IL. CD19-CD45 low/- CD38 high/CD138+ plasma cells enrich for human tumorigenic myeloma cells. Leukemia. 2012;26:2530–2537. doi: 10.1038/leu.2012.140. [DOI] [PubMed] [Google Scholar]
- 20.Kim D, et al. Anti-CD47 antibodies promote phagocytosis and inhibit the growth of human myeloma cells. Leukemia. 2012;26:2538–2545. doi: 10.1038/leu.2012.141. [DOI] [PubMed] [Google Scholar]
- 21.Pang WW, et al. Hematopoietic stem cell and progenitor cell mechanisms in myelodysplastic syndromes. Proc Natl Acad Sci U S A. 2013;110:3011–3016. doi: 10.1073/pnas.1222861110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schofield R. The relationship between the spleen colony-forming cell and the haemopoietic stem cell. Blood Cells. 1978;4:7–25. [PubMed] [Google Scholar]
- 23.Mendelson A, Frenette PS. Hematopoietic stem cell niche maintenance during homeostasis and regeneration. Nat Med. 2014;20:833–846. doi: 10.1038/nm.3647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schepers K, Campbell TB, Passegue E. Normal and leukemic stem cell niches: insights and therapeutic opportunities. Cell Stem Cell. 2015;16:254–267. doi: 10.1016/j.stem.2015.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reinisch A, et al. A humanized bone marrow ossicle xenotransplantation model enables improved engraftment of healthy and leukemic human hematopoietic cells. Nat Med. 2016;22:812–821. doi: 10.1038/nm.4103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chao MP, et al. Human AML-iPSCs Reacquire Leukemic Properties after Differentiation and Model Clonal Variation of Disease. Cell Stem Cell. 2016 doi: 10.1016/j.stem.2016.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Antonelli A, et al. Establishing human leukemia xenograft mouse models by implanting human bone marrow-like scaffold-based niches. Blood. 2016;128:2949–2959. doi: 10.1182/blood-2016-05-719021. [DOI] [PubMed] [Google Scholar]
- 28.Abarrategi A, et al. Versatile humanized niche model enables study of normal and malignant human hematopoiesis. J Clin Invest. 2017;127:543–548. doi: 10.1172/JCI89364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Janicki P, Kasten P, Kleinschmidt K, Luginbuehl R, Richter W. Chondrogenic pre-induction of human mesenchymal stem cells on beta-TCP: enhanced bone quality by endochondral heterotopic bone formation. Acta biomaterialia. 2010;6:3292–3301. doi: 10.1016/j.actbio.2010.01.037. [DOI] [PubMed] [Google Scholar]
- 30.Serafini M, et al. Establishment of bone marrow and hematopoietic niches in vivo by reversion of chondrocyte differentiation of human bone marrow stromal cells. Stem cell research. 2014;12:659–672. doi: 10.1016/j.scr.2014.01.006. [DOI] [PubMed] [Google Scholar]
- 31.Groen RW, et al. Reconstructing the human hematopoietic niche in immunodeficient mice: opportunities for studying primary multiple myeloma. Blood. 2012;120:e9–e16. doi: 10.1182/blood-2012-03-414920. [DOI] [PubMed] [Google Scholar]
- 32.Holzapfel BM, et al. Tissue engineered humanized bone supports human hematopoiesis in vivo. Biomaterials. 2015;61:103–114. doi: 10.1016/j.biomaterials.2015.04.057. [DOI] [PubMed] [Google Scholar]
- 33.Martine LC, et al. Engineering a humanized bone organ model in mice to study bone metastases. Nat Protoc. 2017;12:639–663. doi: 10.1038/nprot.2017.002. [DOI] [PubMed] [Google Scholar]
- 34.Scotti C, et al. Engineering of a functional bone organ through endochondral ossification. Proc Natl Acad Sci U S A. 2013;110:3997–4002. doi: 10.1073/pnas.1220108110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Scotti C, et al. Recapitulation of endochondral bone formation using human adult mesenchymal stem cells as a paradigm for developmental engineering. Proc Natl Acad Sci U S A. 2010;107:7251–7256. doi: 10.1073/pnas.1000302107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Scotti C, et al. Engineering Small-Scale and Scaffold-Based Bone Organs via Endochondral Ossification Using Adult Progenitor Cells. Methods Mol Biol. 2016;1416:413–424. doi: 10.1007/978-1-4939-3584-0_24. [DOI] [PubMed] [Google Scholar]
- 37.Pievani A, et al. Human umbilical cord blood-borne fibroblasts contain marrow niche precursors that form a bone/marrow organoid in vivo. Development. 2017;144:1035–1044. doi: 10.1242/dev.142836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reinisch A, et al. Epigenetic and in vivo comparison of diverse MSC sources reveals an endochondral signature for human hematopoietic niche formation. Blood. 2015;125:249–260. doi: 10.1182/blood-2014-04-572255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chan CK, et al. Endochondral ossification is required for haematopoietic stem-cell niche formation. Nature. 2009;457:490–494. doi: 10.1038/nature07547. nature07547 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Battula VL, et al. Connective tissue growth factor regulates adipocyte differentiation of mesenchymal stromal cells and facilitates leukemia bone marrow engraftment. Blood. 2013;122:357–366. doi: 10.1182/blood-2012-06-437988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen Y, et al. Human extramedullary bone marrow in mice: a novel in vivo model of genetically controlled hematopoietic microenvironment. Blood. 2012;119:4971–4980. doi: 10.1182/blood-2011-11-389957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sontakke P, et al. Modeling BCR-ABL and MLL-AF9 leukemia in a human bone marrow-like scaffold-based xenograft model. Leukemia. 2016;30:2064–2073. doi: 10.1038/leu.2016.108. [DOI] [PubMed] [Google Scholar]
- 43.Doucet C, et al. Platelet lysates promote mesenchymal stem cell expansion: A safety substitute for animal serum in cell-based therapy applications. J Cell Physiol. 2005;205:228–236. doi: 10.1002/jcp.20391. [DOI] [PubMed] [Google Scholar]
- 44.Reinisch A, et al. Humanized system to propagate cord blood-derived multipotent mesenchymal stromal cells for clinical application. Regen Med. 2007;2:371–382. doi: 10.2217/17460751.2.4.371. [DOI] [PubMed] [Google Scholar]
- 45.Burnouf T, Strunk D, Koh MB, Schallmoser K. Human platelet lysate: Replacing fetal bovine serum as a gold standard for human cell propagation? Biomaterials. 2016;76:371–387. doi: 10.1016/j.biomaterials.2015.10.065. [DOI] [PubMed] [Google Scholar]
- 46.Schallmoser K, et al. Rapid large-scale expansion of functional mesenchymal stem cells from unmanipulated bone marrow without animal serum. Tissue Eng Part C Methods. 2008;14:185–196. doi: 10.1089/ten.tec.2008.0060. [DOI] [PubMed] [Google Scholar]
- 47.Schallmoser K, et al. Human platelet lysate can replace fetal bovine serum for clinical-scale expansion of functional mesenchymal stromal cells. Transfusion. 2007;47:1436–1446. doi: 10.1111/j.1537-2995.2007.01220.x. TRF01220 [pii] [DOI] [PubMed] [Google Scholar]
- 48.Schallmoser K, Strunk D. Preparation of pooled human platelet lysate (pHPL) as an efficient supplement for animal serum-free human stem cell cultures. J Vis Exp. 2009 doi: 10.3791/1523. 1523 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dominici M, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 50.Buhring HJ, et al. Novel markers for the prospective isolation of human MSC. Ann N Y Acad Sci. 2007;1106:262–271. doi: 10.1196/annals.1392.000. [DOI] [PubMed] [Google Scholar]
- 51.Notta F, Doulatov S, Dick JE. Engraftment of human hematopoietic stem cells is more efficient in female NOD/SCID/IL-2Rgc-null recipients. Blood. 2010;115:3704–3707. doi: 10.1182/blood-2009-10-249326. [DOI] [PubMed] [Google Scholar]
- 52.Miller PH, et al. Analysis of parameters that affect human hematopoietic cell outputs in mutant c-kit-immunodeficient mice. Exp Hematol. 2017;48:41–49. doi: 10.1016/j.exphem.2016.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Song J, et al. An in vivo model to study and manipulate the hematopoietic stem cell niche. Blood. 2010;115:2592–2600. doi: 10.1182/blood-2009-01-200071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pettway GJ, et al. Anabolic actions of PTH (1-34): use of a novel tissue engineering model to investigate temporal effects on bone. Bone. 2005;36:959–970. doi: 10.1016/j.bone.2005.02.015. [DOI] [PubMed] [Google Scholar]
- 55.Chevaleyre J, et al. Busulfan administration flexibility increases the applicability of scid repopulating cell assay in NSG mouse model. PLoS One. 2013;8:e74361. doi: 10.1371/journal.pone.0074361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kang YK, et al. Humanizing NOD/SCID/IL-2Rgammanull (NSG) mice using busulfan and retro-orbital injection of umbilical cord blood-derived CD34(+) cells. Blood Res. 2016;51:31–36. doi: 10.5045/br.2016.51.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Saland E, et al. A robust and rapid xenograft model to assess efficacy of chemotherapeutic agents for human acute myeloid leukemia. Blood Cancer J. 2015;5:e297. doi: 10.1038/bcj.2015.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chhabra A, et al. Hematopoietic stem cell transplantation in immunocompetent hosts without radiation or chemotherapy. Science translational medicine. 2016;8:351ra105. doi: 10.1126/scitranslmed.aae0501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Czechowicz A, Kraft D, Weissman IL, Bhattacharya D. Efficient transplantation via antibody-based clearance of hematopoietic stem cell niches. Science. 2007;318:1296–1299. doi: 10.1126/science.1149726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Palchaudhuri R, et al. Non-genotoxic conditioning for hematopoietic stem cell transplantation using a hematopoietic-cell-specific internalizing immunotoxin. Nat Biotechnol. 2016;34:738–745. doi: 10.1038/nbt.3584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Taya Y, et al. Depleting dietary valine permits nonmyeloablative mouse hematopoietic stem cell transplantation. Science. 2016;354:1152–1155. doi: 10.1126/science.aag3145. [DOI] [PubMed] [Google Scholar]
- 62.McIntosh BE, et al. Nonirradiated NOD,B6.SCID Il2rgamma-/- Kit(W41/W41) (NBSGW) mice support multilineage engraftment of human hematopoietic cells. Stem cell reports. 2015;4:171–180. doi: 10.1016/j.stemcr.2014.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rahmig S, et al. Improved Human Erythropoiesis and Platelet Formation in Humanized NSGW41 Mice. Stem cell reports. 2016;7:591–601. doi: 10.1016/j.stemcr.2016.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chung YR, Kim E, Abdel-Wahab O. Femoral bone marrow aspiration in live mice. J Vis Exp. 2014 doi: 10.3791/51660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Park CY, Majeti R, Weissman IL. In vivo evaluation of human hematopoiesis through xenotransplantation of purified hematopoietic stem cells from umbilical cord blood. Nat Protoc. 2008;3:1932–1940. doi: 10.1038/nprot.2008.194. [DOI] [PubMed] [Google Scholar]
- 66.Reinisch A, et al. Humanized large-scale expanded endothelial colony-forming cells function in vitro and in vivo. Blood. 2009;113:6716–6725. doi: 10.1182/blood-2008-09-181362. blood-2008-09-181362 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bartmann C, et al. Two steps to functional mesenchymal stromal cells for clinical application. Transfusion. 2007;47:1426–1435. doi: 10.1111/j.1537-2995.2007.01219.x. TRF01219 [pii] [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.