Abstract
Essential tremor (ET) is one of the most common neurologic disorders, and genetic factors are thought to contribute significantly to disease etiology. There has been a relative lack of progress in understanding the genetic etiology of ET. This could reflect a number of factors, including the presence of substantial phenotypic and genotypic heterogeneity. Thus, a meticulous approach to phenotyping is important for genetic research. A lack of standardized phenotyping across studies and patient centers likely has contributed to the relative lack of success of genomewide association studies in ET. To dissect the genetic architecture of ET, whole-genome sequencing will likely be of value. This will allow specific hypotheses about the mode of inheritance and genetic architecture to be tested. A number of approaches still remain unexplored in ET genetics, including the contribution of copy number variants, uncommon moderate-effect alleles, rare variant large-effect alleles (including Mendelian and complex/polygenic modes of inheritance), de novo and gonadal mosaicism, epigenetic changes, and noncoding variation.
INTRODUCTION
Essential tremor (ET) is a chronic, progressive neurologic disease (Louis, 2001). The hallmark motor feature of ET is a 4–12-Hz kinetic tremor (i.e., a tremor that occurs during voluntary movements such as writing or eating) that involves the hands and arms, but which may also eventually spread to involve the head (i.e., neck), voice, jaw, and other body regions (Louis et al., 2013b). Given the presence of etiologic, clinical, pharmacologic response profile and pathologic heterogeneity, there is increasing support for the notion that ET may be a family of diseases whose central defining feature is kinetic tremor of the arms, and which might more appropriately be referred to as “the essential tremors” (Louis, 2013a).
CLINICAL MANIFESTATIONS
The cardinal feature of ET is kinetic tremor (Louis, 2001). The tremor is typically mildly asymmetric (Louis et al., 1998). In approximately 5% of patients, the tremor is markedly asymmetric or even unilateral (Phibbs et al., 2009). In about 50% of patients (Louis et al., 2009), the tremor has an intentional component. Postural tremor also occurs in ET, and is generally worse in the wing-beat position than when the arms are held straight in front of the patient. The postural tremor of ET is generally greatest in amplitude at the wrist joint rather than more proximal (i.e., shoulder, elbow) or distal (metacarpophalangeal and phalangeal) joints (Sternberg et al., 2013). As a rule, the amplitude of kinetic tremor exceeds that of postural tremor (Louis, 2013b). Tremor at rest, without other cardinal features of parkinsonism, occurs in 20% of patients with ET who are attending a specialty clinic, but in contrast to that of Parkinson disease, it is a late feature and it has only been observed in the arm rather than the arm and leg (Cohen et al., 2003). Aside from tremor, another motor feature of ET is gait ataxia, which is in excess of that seen in similarly aged controls (Singer et al., 1994; Earhart et al., 2009; Kronenbuerger et al., 2009). In most patients, this is mild, although in some it may be of moderate severity (Louis et al., 2013a) and associated with functional problems (Louis et al., 2013a). In several studies, mild saccadic eye movement abnormalities have also been detected in ET patients (Helmchen et al., 2003; Gitchel et al., 2013).
In recent years, an increasing appreciation of the presence of nonmotor features in ET has emerged (Findley, 2004). Sensory changes include diminished hearing in excess of that seen in age-matched controls (Ondo et al., 2003) as well as a mild olfactory deficit in some, though not all, studies (Louis et al., 2002). Cognitive changes are in excess of those seen in similarly aged controls, and range from mild changes across several domains (but especially executive function) to mild cognitive impairment and dementia, both of which occur to a greater extent than seen in age-matched controls (Lombardi et al., 2001; Bermejo-Pareja, 2011; Benito-Leon et al., 2011). Psychiatric manifestations include both secondary anxiety and depression (Louis et al., 2007a). A harm-avoidant personality has been reported in studies that have assessed personality traits (Thenganatt and Louis, 2012).
Initially the tremor may be mild and asymptomatic, but in most individuals the tremor worsens over time (Critchley, 1949). Several patterns of progression have been described (Louis, 2013a). There are few natural history studies, but from these, the best estimates of rate of change suggest that arm tremor worsens by 2–5%/year (Louis et al., 2011). With the progression of time, there is a tendency for the tremor to spread beyond the arms to cranial structures (neck, voice, jaw), particularly in women, among whom the risk of neck tremor is several-fold higher than that of men (Hubble et al., 1997; Hardesty et al., 2004).
The term “benign essential tremor” is no longer considered appropriate (Louis and Okun, 2011). Indeed, the majority of patients have some tremor-related disability, and 15–25% of ET patients are disabled by the high-amplitude shaking and cannot continue to work (Rautakorpi et al., 1982; Busenbark et al., 1991; Bain et al., 1993).
ET and Parkinson disease seem to co-occur (Tan et al., 2008; Fekete and Jankovic, 2011), and epidemiologic studies indicate that ET patients have a fourfold increased risk of developing incident Parkinson disease (Benito-Leon et al., 2009).
EPIDEMIOLOGY
ET is among the most prevalent adult-onset movement disorders. It may occur at any age, and pediatric cases have been reported (Louis et al., 2001a), yet most cases arise later in life. In a recent metaanalysis of data from 28 population-based prevalence studies in 19 countries, the pooled prevalence of ET across all ages was 0.9% (Louis and Ferreira, 2010). This prevalence increases markedly with age (Dogu et al., 2003). In the metaanalysis, prevalence among persons aged 65 years and older was 4.6%, and in some studies, the prevalence among persons aged 95 and older reached values in excess of 20% (Louis and Ferreira, 2010). The rate at which new ET cases arise (i.e., the incidence rate of ET) has been estimated to be 619 per 100,000 person-years among individuals aged 65 and older, and incidence rises with age (Rajput et al., 1984; Benito-Leon et al., 2005). Established risk factors for ET include older age and family history of ET (Louis et al., 2013c).
PATHOPHYSIOLOGY
The traditional model of ET, the olivary model, was first proposed in the early 1970s; the model posited that a tremor pacemaker in the inferior olivary nucleus was responsible for ET (Llinas and Volkind, 1973). However, there are major problems with this model, and its relevance to ET has been increasingly called into question (Louis, 2014). Recent mechanistic research on ET has focused more on the cerebellum and the role it plays in the biology of this disorder. Interest in the cerebellum was initially motivated by neuroimaging studies, which strongly implicate the importance of this brain region in ET (Wills et al., 1994; Pagan et al., 2003; Quattrone et al., 2008; Passamonti et al., 2012), and clinical studies, which have consistently noted the presence of cerebellar signs in patients with ET (Singer et al., 1994; Bares et al., 2012). More recently, controlled postmortem studies have revealed an array of changes in the cerebellar cortex, primarily involving the Purkinje cell and surrounding neuronal populations, in the majority of ET cases (Louis et al., 2007b; Babij et al., 2013). In some studies, there is actual Purkinje cell loss. A smaller group of ET cases demonstrate a pattern of Lewy bodies that are relatively restricted to the locus coeruleus (Louis et al., 2007b). Of mechanistic interest is that the noradrenergic neurons of the locus coeruleus project to the cerebellum and synapse with Purkinje cells, suggesting that the cerebellum and its outflow tracts may be the final common pathway for this disease. The notion that ET is a degenerative disease or diseases, linked to the cerebellum, has gained increasing momentum in recent years (Louis, 2009; Bonuccelli, 2012; Grimaldi and Manto, 2013).
ETIOLOGY – GENETIC VS. ENVIRONMENTAL FACTORS
Both genetic and environmental (toxic) factors are likely contributors to disease etiology. Many large kindreds show an autosomal-dominant pattern of inheritance (Bain et al., 1994), and in a familial aggregation study, first-degree relatives of ET patients are approximately five times more likely to develop the disease than are members of the general population, and 10 times more likely if the proband’s tremor began at an early age (Louis et al., 2001b). Twin studies reveal a concordance of approximately 60% in monozygotic twins (Tanner et al., 2001; Lorenz et al., 2004). However, the existence of sporadic cases, variability in age at onset in familial cases, and lack of complete disease concordance in monozygotic twins all argue for nongenetic (i.e., environmental) causes as well (Louis, 2008). A number of environmental toxins are under investigation, including β-carboline alkaloids (e.g., harmane, a dietary toxin) and lead (Louis, 2008), and the search for ET genes is ongoing and intensive (Tan and Schapira, 2008). The remainder of this chapter will focus on the genetics of ET, as the disease clearly has a strong familial component.
MODES OF INHERITANCE AND TRANSMISSION IN ESSENTIAL TREMOR
Introduction
The genetic architecture of familial and sporadic ET is likely to be explained by several modes of inheritance and transmission, including both Mendelian and complex disease patterns. These modes of inheritance and transmission are unlikely to be mutually exclusive, as we have learned from other common complex diseases, including Parkinson disease (Gasser et al., 2011), Alzheimer disease (Karch et al., 2014) and schizophrenia (Gratten et al., 2014), and it is likely that in ET both Mendelian/monogenic and complex disease patterns contribute to the genetic architecture. Historically, most studies have assumed a Mendelian inheritance pattern because of the high heritability and aggregation of ET in families (Bain et al., 1994; Findley, 2000; Tanner et al., 2001; Lorenz et al., 2004). Aggregation studies indicate that on the order of 30–70% of ET patients have a family history, with the vast majority (>80%) of young-onset (≤40 years old) cases reporting ≥1 affected first-degree relative (Louis and Dogu, 2007). While other modes of inheritance, including autosomal-recessive and complex inheritance patterns, are possible, published family and linkage studies suggest an autosomal-dominant mode of inheritance with reduced penetrance (Gulcher et al., 1997; Higgins et al., 1997; Kovach et al., 2001; Shatunov et al., 2006). Echoing this, our own experience with ET pedigrees strongly suggests an autosomal-dominant mode of inheritance with reduced penetrance.
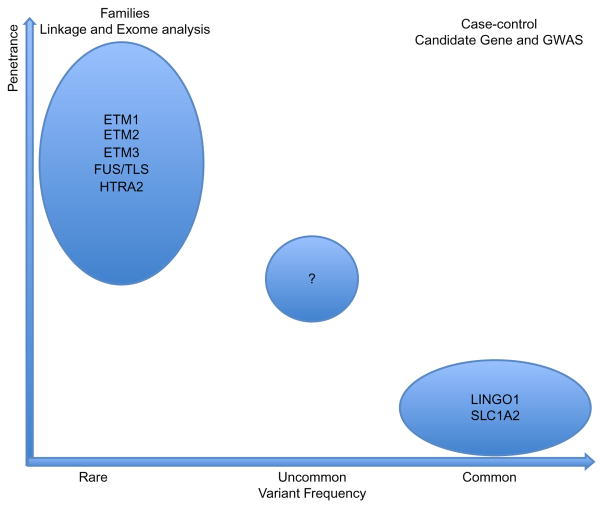
Progress in ET genetics has been limited and the slow rate of ET gene identification may be due to a number of confounding factors that we discuss later in this chapter (Clark and Louis, 2015). As of 2015, only a handful of genes and risk factors have been identified in ET families and ET case-control cohorts (Fig. 15.1).
Fig. 15.1.
Schematic summarizing essential tremor (ET) genes identified to date. The penetrance (y-axis) and variant frequency (x-axis; rare, uncommon, and common variants) are indicated. FUS/TLS, fused in sarcoma/translated in liposarcoma; GWAS, genomewide association studies. (Adapted from Verstraeten A, Theuns J, Van Broeckhoven C (2015) Progress in unraveling the genetic etiology of Parkinson disease in a genomic era. Trends Genet 31: 140–149.)
Mendelian inheritance and monogenic genes: family studies and linkage
To date, only three published genomewide linkage scans have been performed, all in north American or Icelandic ET families (Gulcher et al., 1997; Higgins et al., 1997; Shatunov et al., 2006). These studies led to the identification of genetic loci harboring ET genes on chromosomes 3q13 (ETM1; OMIM: 190300) (Gulcher et al., 1997), 2p22-p25 (ETM2; OMIM: 602134) (Higgins et al., 1997), and 6p23 (ETM3; OMIM: 611456) (Shatunov et al., 2006). Several studies have attempted to replicate linkage to ETM1 (Kovach et al., 2001; Illarioshkin et al., 2002; Lucotte et al., 2006), ETM2 (Higgins et al., 1998, 2005; Kovach et al., 2001), and ETM3, without success (no logarithm of the odds score >2.0), and the genes and causal mutations for these loci (ETM1, ETM2, and ETM3) have yet to be identified despite the reporting of linkage many years ago. Using a linkage and whole-exome sequencing approach, a p.Q290X mutation in the fused in sarcoma/translated in liposarcoma (FUS/TLS) gene was identified as the cause of ET in a large Quebec family (Merner et al., 2012). Subsequent studies (Labbe et al., 2013; Ortega-Cubero et al., 2013; Parmalee et al., 2013) suggest that mutations in FUS are an extremely rare or family-specific cause of ET, and without functional studies, the pathogenicity of mutations identified so far (p.Q290X (Merner et al., 2012)andR377Wreportedin1patientwithafamilyhistory of ET (Rajput et al., 2014)) is unknown. More recently, in a six-generation consanguineous Turkish kindred with both ET and Parkinson disease, the mitochondrial serine protease HTRA2 p.G399S variant was shown to segregate with both phenotypes (Parkinson disease and ET). All affected individuals in the family were either heterozygous or homozygous for the HTRA2 variant and homozygosity was associated with earlier age at onset of tremor (p < 0.0001), more severe postural tremor (p < 0.0001), and more severe kinetic tremor (p = 0.0019) (Unal Gulsuner et al., 2014). Follow-up studies in ET family studies and case-control studies will be needed to determine whether HTRA2 represents a major ET susceptibility gene (Clark and Louis, 2015).
Genetic association studies of candidate genes
Although numerous genes have been evaluated as positional or functional candidates for ET, the majority of genes tested do not provide evidence of association (reviewed in Testa, 2013 and Jiménez-Jiménez et al., 2013). However, more recently a rare variant (p.R47H) in TREM2 was identified as a risk factor for ET in a Spanish population and this gene may turn out to be a promising candidate in other populations as well. A rare amino acid substitution (p.R47H; rs75932628) in the TREM2 protein (triggering receptor expressed on myeloid cells 2; OMIM: 605086) has been identified as a risk factor for several neurodegenerative diseases, including Alzheimer disease (Jonsson et al., 2013; Ruiz et al., 2014), Parkinson disease (Benitez et al., 2013), fronto-temporal dementia (Ruiz et al., 2014; Thelen et al., 2014), and amyotrophic lateral sclerosis (Cady et al., 2014). A large cross-sectional multicenter international study that included a discovery ET case-control cohort from Spain (n = 456 ET and n = 2715 controls) and a replication case-control series from different populations (Italy, Germany, North America, and Taiwan; n = 897 ET and n = 1449 controls) reported a significant association between TREM2 p.R47H and ET in the Spanish cohort (odds ratio (OR), 5.97; 95% confidence interval, 1.2–29.6; p = 0.042). However, the association was not replicated in the other populations, which may suggest population-specific differences, and allelic heterogeneity at TREM2 (Ortega-Cubero et al., 2015). Resequencing of TREM2 in different ET case-control ethnic populations may lead to the identification of other rare variants that are risk factors in specific populations.
Candidate genes that have been tested with null findings in ET are summarized in Table 15.1. We and others have also evaluated additional genes that are associated with other neurodegenerative diseases, such as Parkinson disease, dystonia, spinocerebellar ataxias, and fragile X tremor ataxia syndrome. We did not observe an association with the Parkinson disease genes synuclein, alpha (non A4 component of amyloid precursor) (SNCA) (Ross et al., 2011), leucine-rich repeat kinase 2 (LRRK2) (Clark et al., 2010a), glucocerebrosidase (GBA) (Clark et al., 2010a), or microtubule-associated protein tau (MAPT) (Clark et al., 2014), nor did we identify pathogenic repeat expansions in the 10 common degenerative loci (SCA-1 (ATXN1), SCA-2 (ATXN2), SCA-3 (ATXN3), SCA-6 (CACNA1A), SCA-7 (ATXN7), SCA-8 (ATXN8OS), SCA-10 (ATXN10), SCA-12 (PPP2R2B), SCA-17 (TBP), and DRPLA (ATN1) or FRAXA (unpublished results) (Clark and Louis, 2015).
Table 15.1.
Candidate genes for essential tremor
| Gene | Function/pathway | Published citation | Variant/SNP frequency | Significance |
|---|---|---|---|---|
| Dopamine receptor D3 (DRD3) | Dopamine receptor, activity mediated by G proteins which inhibit adenyl cyclase/dopamine neurotransmitter receptor activity |
Jeanneteau et al. (2006); Lucotte et al. (2006); Tan et al. (2007); Blair et al. (2008); Vitale et al. (2008); Garcia-Martin et al. (2009); Lorenz et al. (2009) |
Ser9Gly variant of DRD3 |
Jeanneteau et al. (2006): p = 0.039 Garcia-Martin et al. (2009): p<0.017 (genotype) and p<0.005 (allele) All other studies not significant |
| HS1-binding protein 3 (HS1BP3) | May be a modulator of IL-2 signaling (by similarity) | Deng et al. (2005); Shatunov et al. (2005) | Ala265Gly variant of HS1BP3 | Not significant |
| Solute carrier family 1 (glial high-affinity glutamate transporter) member 2 (SLC1A2) | Solute transporter. Clears excitatory neurotransmitter glutamate at synapses in CNS | Thier et al. (2012); Garcia-Martin et al. (2013); Tan et al. (2013); Ross et al. (2014) | rs3794087 |
Thier et al. (2012): p = 6.95 3 10–5 Tan et al. (2013): p = 0.009 Other studies not significant |
| Microtubule-associated protein 2 (MAPT) | Promotes microtubule assembly and stability. MAPT gene mutations and risk SNPs associated with several neurodegenerative diseases | Vilarino-Guell et al. (2011); Garcia-Martin et al. (2012); Clark et al. (2014) | rs1052553 | Clark et al. (2014): metaanalysis of published studies not significant |
| Methylene tetrahydrofolate reductase (NAD(P)H)(MTFHR) | Catalyzes the conversion of 5,10-methylenetetrahydrofolate to 5-methyltetrahydrofolate | Sazci et al. (2004) | MTHFR 677T allele | p = 0.005 |
| Cytochrome P450, family 2, subfamily C, polypeptide 19 (CYP2C19) | Member of the cytochrome P450 family of enzymes. Monooxygenase that catalyzes reactions involved in drug metabolism, synthesis of cholesterol, steroids, and other lipids | Alonso-Navarro et al. (2006) | CYP2C19 allelic variants | p = 0.0044 |
| Cytochrome P450, family 2, subfamily C, polypeptide 9 (CYP2C9) | Member of the cytochrome P450 family of enzymes | Martinez et al. (2007b) | CYP2C9 allelic variants | CYP2C9*2: p = 0.05 CYP2C9*3: p = 0.07 |
| Cytochrome P450, family 2, subfamily C, polypeptide 8 (CYP2C8) | Member of the cytochrome P450 family of enzymes | Martinez et al. (2007b) | CYP2C8 allelic variants | CYP2C8*3: p = 0.006 |
| Alcohol dehydrogenase 1B, beta polypeptide (ADH2) | Alcohol dehydrogenase family member. Metabolizes several substrates, including ethanol, retinol, aliphatic alcohols, hydroxysteroids, lipid peroxidation products | Martinez et al. (2007a) | ADH2*1/ADH2*2 | Not significant |
| Glutathione S-transferase pi 1 (GSTP1), | GSTP1: conjugation of reduced glutathione to a number of exogenous and endogenous hydrophobic electrophiles | Martinez et al. (2008) | GSTP1Val allele (rs1695) | Not significant |
| Gamma-aminobutyric acid (GABA) receptor genes | Ligand-gated chloride channels that bind GABA, the major inhibitory neurotransmitter in the brain | Deng et al. (2006); Garcia-Martin et al. (2011); Thier et al. (2011) | Tagging SNPs | Not significant |
| Fused in sarcoma/translated in liposarcoma (FUS/TLS) | Component of the heterogeneous nuclear riboprotein (hnRNP) complex. Mutations in FUS/TLS associated with amyotrophic lateral sclerosis | Merner et al. (2012); Labbe et al. (2013); Ortega-Cubero et al. (2013); Parmalee et al. (2013) | Complete or partial gene sequencing | Merner et al. (2012): Fus p.Gln290* in a single essential tremor family. Other published studies no evidence for pathogenic mutations or association of SNPs/variants in FUS/TLS in essential tremor cases |
| Synuclein, alpha (non-A4 component of amyloid precursor) (SNCA) | Localizes to and enriched at synapses and lipid-rich membrane structures. Mutations in SNCA associated with PD | Ross et al. (2011) | 20 different variants at the SCNA locus | Not significant |
| Leucine-rick repeat kinase 2 (LRRK2) | Large multidomain protein kinase. May play a role in phosphorylation of proteins central to PD. Mutations in LRRK2 associated with PD | Clark et al. (2010a) | 6 LRRK2 mutations: G2019S, I2020T, R1441C, Y1699C, L1114L and I1122V and 19LRRK2 SNPs | Not significant |
| Glucosidase, beta, acid (GBA) | Hydrolase that catalyzes the cleavage of glucosylceramide. Homozygous mutations in GBA cause Gaucher disease. Heterozygous mutations in GBA associated with PD and dementia with Lewy bodies | Clark et al. (2010a); Sun et al. (2013) |
Clark et al. (2010a): all GBA exons sequenced Sun et al. (2013): L444P mutation only |
Not significant |
| Triggering receptor expressed on myeloid cells 2 (TREM2) | Encodes a membrane protein that forms a receptor signaling complex with the TYRO protein tyrosine kinase-binding protein | Ortega-Cubero et al. (2015) | rs75932628 (p.R47H) | p = 0.042 |
CNS, central nervous system; PD, Parkinson disease; SNP, single-nucleotide polymorphism.
Genomewide association studies (GWAS) and risk gene identification
To date, two published GWAS variably identified single-nucleotide polymorphisms (SNPs) in the leucine-rich repeat and Ig domain containing 1 (LINGO1) gene or an intronic variant in the SLC1A2 gene, which reached genomewide significance and are associated with increased risk for ET.
LINGO1
A genomewide SNP association study of ET in an Icelandic population identified an association with a marker in the LINGO1 gene (Stefansson et al., 2009). Since the initial report, numerous studies have replicated the association in independent ET case-control samples worldwide (Tan et al., 2009; Clark et al., 2010b; Thier et al., 2010; Vilarino-Guell et al., 2010a, b, c; Zuo et al., 2010; Wu et al., 2011; Bourassa et al., 2011; Lorenzo-Betancor et al., 2011; Jiménez-Jiménez et al., 2012; Radovica et al., 2012; Clark et al., 2010b). Collectively, these data suggest that the LINGO1 SNP rs9652490 confers modest risk, with ORs in the range of 1.2–1.7, across different studies and populations. Although the majority of studies positively replicate the LINGO1 SNP rs9652490 association, some studies did not observe an association (Zuo et al., 2010; Lorenzo-Betancor et al., 2011; Wu et al., 2011; Radovica et al., 2012). One explanation for this lack of association may be allelic heterogeneity, and that rs9652490 does not confer risk in these populations, and that other variants in LINGO1 are risk factors. Alternatively, clinical and genetic heterogeneity in ET may explain the lack of association. We have also shown that a highly related LINGO1 family member (61% amino acid identity), the leucine-rich repeat (LRR) and Ig domain containing two genes (LINGO2), is also a risk factor for ET and Parkinson disease, providing further evidence that the LRR gene pathway may be perturbed in ET pathogenesis (Vilarino-Guell et al., 2010b). To date, SNPs in LINGO1 provide the strongest evidence for association with ET; however, data are not completely consistent.
SLC1A2
The SNP, rs3794087, located in SLC1A2, was identified in a two-stage European GWAS in a total of 990 ETcases and 1537 controls, with an OR of ~1.4 (Thier et al., 2012). To date, four studies have attempted to replicate the association of rs3794087 with ET in different populations, with conflicting results, some of which are positive (Chinese, Taiwanese) and others of which are negative (Spanish, North American) (Garcia-Martin et al., 2013; Tan et al., 2013; Ross et al., 2014). While further studies in different populations are needed to confirm the role of SLC1A2 in ET, rs3794087 is unlikely to represent a major risk factor for ET.
To summarize, there is a lack of consistent and robust associations from candidate gene studies and GWAS. Furthermore, the paucity of genomewide significant SNP associations from GWAS argues against the common disease common variant hypothesis in ET, and that common SNPs with small effect are unlikely to contribute to the heritability of ET (Clark and Louis, 2015).
CONFOUNDING FACTORS IN GE’NE IDENTIFICATION IN ESSENTIAL TREMOR
Why has the field of ET genetics made so little progress? Despite significant efforts to identify genes for ET there has been a slow rate of gene identification. There are a number of possible explanations, some of which we highlight below (Clark and Louis, 2015).
Phenotyping
We would rank this as perhaps the top problem. A meticulous approach to phenotyping is critical for genetic research in ET. The ET diagnosis relies on clinical evaluation. The only tool for phenotyping is clinical. Thus, there is currently no serum/imaging biomarker or defining neuropathologic feature (e.g., a protein aggregate specific to ET or a distinctive imaging finding) that can be used for diagnosis, and there is clinical overlap with other disorders such as Parkinson disease and dystonia. These issues greatly complicate the diagnosis of ET; thus, in some studies, as many as 30–50% of cases labeled as “ET” have later been found to carry other diagnoses (e.g., dystonia, Parkinson disease, and other disorders) rather than ET (Jain et al., 2006). Poor attention to phenotyping (e.g., merely defining ET as an “action tremor”) is likely a major issue in some family studies of ET, and this as well as lack of standardized phenotyping across studies and patient centers is likely to be the major contributor to the lack of success of GWAS. A related issue is the possibility, as discussed above, that ET may represent a family of diseases rather than a single clinical-pathologic entity.
Genetic heterogeneity and sample size
Linkage and GWAS provide evidence for genetic heterogeneity in ET. Genetic heterogeneity has been observed in other neurodegenerative disorders such as Parkinson disease, but does not preclude the identification of ET gene(s). One method to deal with the effects of genetic heterogeneity is to increase the sample size.
Mode of inheritance
Mendelian and complex disease inheritance patterns may play a role in ET. While most studies have assumed an autosomal-dominant mode of inheritance with reduced penetrance, other modes of inheritance, including complex disease inheritance patterns, may be important.
Molecular methods and statistical analysis of rare variants
A major roadblock to gene discovery, until recently, has been the lack of technologic and analytic advances in genomewide sequencing and statistical models for multiple rare variants. Recent advances in next-generation sequencing techniques, particularly for whole-genome sequencing (WGS), sequence data processing, decreased cost of WGS, and faster computational power, together with new analytic methods to study rare variants, mean that WGS and rare variant analysis are now feasible. While whole-exome sequencing identifies on the order of ~20,000 coding variants per exome sequenced, WGS provides more uniform coverage and captures a range of genetic variation to allow analysis of both coding and noncoding variants in addition to indels and structural variation, with on average of ~4,000,000 variants per genome (Clark and Louis, 2015).
NOVEL APPROACHES TO ESSENTIAL TREMOR GENE IDENTIFICATION
In understanding the genetic architecture of ET and novel approaches that can be used in gene identification, we turn to approaches being used to identify genes in other common complex diseases such as neuropsychiatric and neurodegenerative disease, and child neurodevelopmental disorders (e.g., autism).
To dissect the genetic architecture of ET, WGS in carefully characterized and well-phenotyped discovery and replication datasets of large case-control and familial cohorts is needed. This will allow specific hypotheses about the mode of inheritance and genetic architecture to be tested. A number of approaches remain unexplored in ET genetics, including copy number variants, the contribution of uncommon moderate-effect alleles, rare variant large-effect alleles (including Mendelian and complex/polygenic modes of inheritance), de novo and gonadal mosaicism, epigenetic changes, and the contribution of noncoding variation. These approaches are likely to yield new ET genes (Clark and Louis, 2015).
References
- Alonso-Navarro H, Martinez C, Garcia-Martin E, et al. CYP2C19 polymorphism and risk for essential tremor. Eur Neurol. 2006;56:119–123. doi: 10.1159/000095702. [DOI] [PubMed] [Google Scholar]
- Babij R, Lee M, Cortes E, et al. Purkinje cell axonal anatomy: quantifying morphometric changes in essential tremor versus control brains. Brain. 2013;136:3051–3061. doi: 10.1093/brain/awt238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bain PG, Mally J, Gresty M, et al. Assessing the impact of essential tremor on upper limb function. J Neurol. 1993;241:54–61. doi: 10.1007/BF00870673. [DOI] [PubMed] [Google Scholar]
- Bain PG, Findley LJ, Thompson PD, et al. A study of hereditary essential tremor. Brain. 1994;117:805–824. doi: 10.1093/brain/117.4.805. [DOI] [PubMed] [Google Scholar]
- Bares M, Husarova I, Lungu OV. Essential tremor, the cerebellum, and motor timing: towards integrating them into one complex entity. Tremor Other Hyperkinet Mov (N Y) 2012:2. [PMC free article] [PubMed] [Google Scholar]
- Benitez BA, Cruchaga C United States-Spain Parkinson’s Disease Research Group. TREM2 and neurodegenerative disease. N Engl J Med. 2013;369:1567–1568. doi: 10.1056/NEJMc1306509#SA4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benito-Leon J, Bermejo-Pareja F, Louis ED. Incidence of essential tremor in three elderly populations of central Spain. Neurology. 2005;64:1721–1725. doi: 10.1212/01.WNL.0000161852.70374.01. [DOI] [PubMed] [Google Scholar]
- Benito-Leon J, Louis ED, Bermejo-Pareja F. Risk of incident Parkinson’s disease and parkinsonism in essential tremor: a population based study. J Neurol Neurosurg Psychiatry. 2009;80:423–425. doi: 10.1136/jnnp.2008.147223. [DOI] [PubMed] [Google Scholar]
- Benito-Leon J, Louis ED, Mitchell AJ, et al. Elderly-onset essential tremor and mild cognitive impairment: a population-based study (NEDICES) J Alzheimers Dis. 2011;23:727–735. doi: 10.3233/JAD-2011-101572. [DOI] [PubMed] [Google Scholar]
- Bermejo-Pareja F. Essential tremor – a neurodegenerative disorder associated with cognitive defects? Nat Rev Neurol. 2011;7:273–282. doi: 10.1038/nrneurol.2011.44. [DOI] [PubMed] [Google Scholar]
- Blair MA, Ma S, Phibbs F, et al. Reappraisal of the role of the DRD3 gene in essential tremor. Parkinsonism Relat Disord. 2008;14:471–475. doi: 10.1016/j.parkreldis.2007.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonuccelli U. Essential tremor is a neurodegenerative disease. J Neural Transm. 2012;119:1383–1387. doi: 10.1007/s00702-012-0878-8. [DOI] [PubMed] [Google Scholar]
- Bourassa CV, Riviere JB, Dion PA, et al. LINGO1 variants in the French-Canadian population. PLoS One. 2011;6:e16254. doi: 10.1371/journal.pone.0016254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busenbark KL, Nash J, Nash S, et al. Is essential tremor benign? Neurology. 1991;41:1982–1983. doi: 10.1212/wnl.41.12.1982. [DOI] [PubMed] [Google Scholar]
- Cady J, Koval ED, Benitez BA, et al. TREM2 variant p. R47H as a risk factor for sporadic amyotrophic lateral sclerosis. JAMA Neurol. 2014;71:449–453. doi: 10.1001/jamaneurol.2013.6237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark LN, Louis ED. Challenges in essential tremor genetics. Revue Neurologique. 2015;171:466. doi: 10.1016/j.neurol.2015.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark LN, Kisselev S, Park N, et al. Mutations in the Parkinson’s disease genes, leucine rich repeat kinase 2 (LRRK2) and glucocerebrosidase (GBA), are not associated with essential tremor. Parkinsonism Relat Disord. 2010a;16:132–135. doi: 10.1016/j.parkreldis.2009.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark LN, Park N, Kisselev S, et al. Replication of the LINGO1 gene association with essential tremor in a North American population. Eur J Hum Genet. 2010b;18:838–843. doi: 10.1038/ejhg.2010.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark LN, Liu X, Parmalee NL, et al. The microtubule associated protein tau H1 haplotype and risk of essential tremor. Eur J Neurol. 2014;21:1044–1048. doi: 10.1111/ene.12335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen O, Pullman S, Jurewicz E, et al. Rest tremor in patients with essential tremor: prevalence, clinical correlates, and electrophysiologic characteristics. Arch Neurol. 2003;60:405–410. doi: 10.1001/archneur.60.3.405. [DOI] [PubMed] [Google Scholar]
- Critchley M. Observations of essential (heredofamilial) tremor. Brain. 1949;72:113–139. doi: 10.1093/brain/72.2.113. [DOI] [PubMed] [Google Scholar]
- Deng H, Le WD, Guo Y, et al. Extended study of A265G variant of HS1BP3 in essential tremor and Parkinson disease. Neurology. 2005;65:651–652. doi: 10.1212/01.wnl.0000173033.32535.23. [DOI] [PubMed] [Google Scholar]
- Deng H, Xie WJ, Le WD, et al. Genetic analysis of the GABRA1 gene in patients with essential tremor. Neurosci Lett. 2006;401:16–19. doi: 10.1016/j.neulet.2006.02.066. [DOI] [PubMed] [Google Scholar]
- Dogu O, Sevim S, Camdeviren H, et al. Prevalence of essential tremor: door-to-door neurologic exams in Mersin Province, Turkey. Neurology. 2003;61:1804–1806. doi: 10.1212/01.wnl.0000099075.19951.8c. [DOI] [PubMed] [Google Scholar]
- Earhart GM, Clark BR, Tabbal SD, et al. Gait and balance in essential tremor: variable effects of bilateral thalamic stimulation. Mov Disord. 2009;24:386–391. doi: 10.1002/mds.22356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fekete R, Jankovic J. Revisiting the relationship between essential tremor and Parkinson’s disease. Mov Disord. 2011;26:391–398. doi: 10.1002/mds.23512. [DOI] [PubMed] [Google Scholar]
- Findley LJ. Epidemiology and genetics of essential tremor. Neurology. 2000;54:10. [PubMed] [Google Scholar]
- Findley LJ. Expanding clinical dimensions of essential tremor. J Neurol Neurosurg Psychiatry. 2004;75:948–949. doi: 10.1136/jnnp.2004.041293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Martin E, Martinez C, Alonso-Navarro H, et al. Dopamine receptor D3 (DRD3) genotype and allelic variants and risk for essential tremor. Mov Disord. 2009;24:1910–1915. doi: 10.1002/mds.22518. [DOI] [PubMed] [Google Scholar]
- Garcia-Martin E, Martinez C, Alonso-Navarro H, et al. Gamma-aminobutyric acid (GABA) receptor rho (GABRR) polymorphisms and risk for essential tremor. J Neurol. 2011;258:203–211. doi: 10.1007/s00415-010-5708-z. [DOI] [PubMed] [Google Scholar]
- Garcia-Martin E, Martinez C, Alonso-Navarro H, et al. H1-MAPT and the risk for familial essential tremor. PLoS One. 2012;7:e41581. doi: 10.1371/journal.pone.0041581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Martin E, Martinez C, Alonso-Navarro H, et al. No association of the SLC1A2 rs3794087 allele with risk for essential tremor in the Spanish population. Pharmacogenet Genomics. 2013;23:587–590. doi: 10.1097/FPC.0b013e328364db9d. [DOI] [PubMed] [Google Scholar]
- Gasser T, Hardy J, Mizuno Y. Milestones in PD genetics. Mov Disord. 2011;26:1042–1048. doi: 10.1002/mds.23637. [DOI] [PubMed] [Google Scholar]
- Gitchel GT, Wetzel PA, Baron MS. Slowed saccades and increased square wave jerks in essential tremor. Tremor Other Hyperkinet Mov (N Y) 2013:3. doi: 10.7916/D8251GXN. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratten J, Wray NR, Keller MC, et al. Large-scale genomics unveils the genetic architecture of psychiatric disorders. Nat Neurosci. 2014;17:782–790. doi: 10.1038/nn.3708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimaldi G, Manto M. Is essential tremor a Purkinjopathy? The role of the cerebellar cortex in its pathogenesis. Mov Disord. 2013;28:1759–1761. doi: 10.1002/mds.25645. [DOI] [PubMed] [Google Scholar]
- Gulcher JR, Jonsson P, Kong A, et al. Mapping of a familial essential tremor gene, FET1, to chromosome 3q13. Nat Genet. 1997;17:84–87. doi: 10.1038/ng0997-84. [DOI] [PubMed] [Google Scholar]
- Hardesty DE, Maraganore DM, Matsumoto JY, et al. Increased risk of head tremor in women with essential tremor: longitudinal data from the Rochester Epidemiology Project. Mov Disord. 2004;19:529–533. doi: 10.1002/mds.20096. [DOI] [PubMed] [Google Scholar]
- Helmchen C, Hagenow A, Miesner J, et al. Eye movement abnormalities in essential tremor may indicate cerebellar dysfunction. Brain. 2003;126:1319–1332. doi: 10.1093/brain/awg132. [DOI] [PubMed] [Google Scholar]
- Higgins JJ, Pho LT, Nee LE. A gene (ETM) for essential tremor maps to chromosome 2p22-p25. Mov Disord. 1997;12:859–864. doi: 10.1002/mds.870120605. [DOI] [PubMed] [Google Scholar]
- Higgins JJ, Loveless JM, Jankovic J, et al. Evidence that a gene for essential tremor maps to chromosome 2p in four families. Mov Disord. 1998;13:972–977. doi: 10.1002/mds.870130621. [DOI] [PubMed] [Google Scholar]
- Higgins JJ, Lombardi RQ, Pucilowska J, et al. A variant in the HS1-BP3 gene is associated with familial essential tremor. Neurology. 2005;64:417–421. doi: 10.1212/01.WNL.0000153481.30222.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubble JP, Busenbark KL, Pahwa R, et al. Clinical expression of essential tremor: effects of gender and age. Mov Disord. 1997;12:969–972. doi: 10.1002/mds.870120620. [DOI] [PubMed] [Google Scholar]
- Illarioshkin SN, Rakhmonov RA, Ivanova-Smolenskaia IA, et al. Molecular genetic analysis of essential tremor. Genetika. 2002;38:1704–1709. [PubMed] [Google Scholar]
- Jain S, Lo SE, Louis ED. Common misdiagnosis of a common neurological disorder: how are we misdiagnosing essential tremor? Arch Neurol. 2006;63:1100–1104. doi: 10.1001/archneur.63.8.1100. [DOI] [PubMed] [Google Scholar]
- Jeanneteau F, Funalot B, Jankovic J, et al. A functional variant of the dopamine D3 receptor is associated with risk and age-at-onset of essential tremor. Proc Natl Acad Sci USA. 2006;103:10753–10758. doi: 10.1073/pnas.0508189103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiménez-Jiménez FJ, Garcia-Martin E, Lorenzo-Betancor O, et al. LINGO1 and risk for essential tremor: results of a meta-analysis of rs9652490 and rs11856808. J Neurol Sci. 2012;317:52–57. doi: 10.1016/j.jns.2012.02.030. [DOI] [PubMed] [Google Scholar]
- Jiménez-Jiménez FJ, Alonso-Navarro H, Garcia-Martin E, et al. Update on genetics of essential tremor. Acta Neurol Scand. 2013;128:359–371. doi: 10.1111/ane.12148. [DOI] [PubMed] [Google Scholar]
- Jonsson T, Stefansson H, Steinberg S, et al. Variant of TREM2 associated with the risk of Alzheimer’s disease. N Engl J Med. 2013;368:107–116. doi: 10.1056/NEJMoa1211103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karch CM, Cruchaga C, Goate AM. Alzheimer’s disease genetics: from the bench to the clinic. Neuron. 2014;83:11–26. doi: 10.1016/j.neuron.2014.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovach MJ, Ruiz J, Kimonis K, et al. Genetic heterogeneity in autosomal dominant essential tremor. Genet Med. 2001;3:197–199. doi: 10.1097/00125817-200105000-00009. [DOI] [PubMed] [Google Scholar]
- Kronenbuerger M, Konczak J, Ziegler W, et al. Balance and motor speech impairment in essential tremor. Cerebellum. 2009;8:389–398. doi: 10.1007/s12311-009-0111-y. [DOI] [PubMed] [Google Scholar]
- Labbe C, Soto-Ortolaza AI, Rayaprolu S, et al. Investigating the role of FUS exonic variants in essential tremor. Parkinsonism Relat Disord. 2013;19:755–757. doi: 10.1016/j.parkreldis.2013.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinas R, Volkind RA. The olivo-cerebellar system: functional properties as revealed by harmaline-induced tremor. Exp Brain Res. 1973;18:69–87. doi: 10.1007/BF00236557. [DOI] [PubMed] [Google Scholar]
- Lombardi WJ, Woolston DJ, Roberts JW, et al. Cognitive deficits in patients with essential tremor. Neurology. 2001;57:785–790. doi: 10.1212/wnl.57.5.785. [DOI] [PubMed] [Google Scholar]
- Lorenz D, Frederiksen H, Moises H, et al. High concordance for essential tremor in monozygotic twins of old age. Neurology. 2004;62:208–211. doi: 10.1212/01.wnl.0000103236.26934.41. [DOI] [PubMed] [Google Scholar]
- Lorenz D, Klebe S, Stevanin G, et al. Dopamine receptor D3 gene and essential tremor in large series of German, Danish and French patients. Eur J Hum Genet. 2009;17:766–773. doi: 10.1038/ejhg.2008.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzo-Betancor O, Garcia-Martin E, Cervantes S, et al. Lack of association of LINGO1 rs9652490 and rs11856808 SNPs with familial essential tremor. Eur J Neurol. 2011;18:1085–1089. doi: 10.1111/j.1468-1331.2010.03251.x. [DOI] [PubMed] [Google Scholar]
- Louis ED. Clinical practice. Essential tremor. N Engl J Med. 2001;345:887–891. doi: 10.1056/NEJMcp010928. [DOI] [PubMed] [Google Scholar]
- Louis ED. Environmental epidemiology of essential tremor. Neuroepidemiology. 2008;31:139–149. doi: 10.1159/000151523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis ED. Essential tremors: a family of neurodegenerative disorders? Arch Neurol. 2009;66:1202–1208. doi: 10.1001/archneurol.2009.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis ED. ‘Essential tremor’ or ‘the essential tremors’: is this one disease or a family of diseases? Neuroepidemiology. 2013a;42:81–89. doi: 10.1159/000356351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis ED. The primary type of tremor in essential tremor is kinetic rather than postural: cross-sectional observation of tremor phenomenology in 369 cases. Eur J Neurol. 2013b;20:725–727. doi: 10.1111/j.1468-1331.2012.03855.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis E. Re-thinking the biology of essential tremor: from models to morphology. Parkinsonism Related Disord. 2014;20(suppl 1):S88–S93. doi: 10.1016/S1353-8020(13)70023-3. [DOI] [PubMed] [Google Scholar]
- Louis ED, Dogu O. Does age of onset in essential tremor have a bimodal distribution? Data from a tertiary referral setting and a population-based study. Neuroepidemiology. 2007;29:208–212. doi: 10.1159/000111584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis ED, Ferreira JJ. How common is the most common adult movement disorder? Update on the worldwide prevalence of essential tremor. Mov Disord. 2010;25:534–541. doi: 10.1002/mds.22838. [DOI] [PubMed] [Google Scholar]
- Louis ED, Okun MS. It is time to remove the ‘benign’ from the essential tremor label. Parkinsonism Relat Disord. 2011;17:516–520. doi: 10.1016/j.parkreldis.2011.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis ED, Wendt KJ, Pullman SL, et al. Is essential tremor symmetric? Observational data from a community-based study of essential tremor. Arch Neurol. 1998;55:1553–1559. doi: 10.1001/archneur.55.12.1553. [DOI] [PubMed] [Google Scholar]
- Louis ED, Dure LSt, Pullman S. Essential tremor in childhood: a series of nineteen cases. Mov Disord. 2001a;16:921–923. doi: 10.1002/mds.1182. [DOI] [PubMed] [Google Scholar]
- Louis ED, Ford B, Frucht S, et al. Risk of tremor and impairment from tremor in relatives of patients with essential tremor: a community-based family study. Ann Neurol. 2001b;49:761–769. doi: 10.1002/ana.1022. [DOI] [PubMed] [Google Scholar]
- Louis ED, Bromley SM, Jurewicz EC, et al. Olfactory dysfunction in essential tremor: a deficit unrelated to disease duration or severity. Neurology. 2002;59:1631–1633. doi: 10.1212/01.wnl.0000033798.85208.f2. [DOI] [PubMed] [Google Scholar]
- Louis ED, Benito-Leon J, Bermejo-Pareja F. Self-reported depression and anti-depressant medication use in essential tremor: cross-sectional and prospective analyses in a population-based study. Eur J Neurol. 2007a;14:1138–1146. doi: 10.1111/j.1468-1331.2007.01923.x. [DOI] [PubMed] [Google Scholar]
- Louis ED, Faust PL, Vonsattel JP, et al. Neuropathological changes in essential tremor: 33 cases compared with 21 controls. Brain. 2007b;130:3297–3307. doi: 10.1093/brain/awm266. [DOI] [PubMed] [Google Scholar]
- Louis ED, Frucht SJ, Rios E. Intention tremor in essential tremor: prevalence and association with disease duration. Mov Disord. 2009;24:626–627. doi: 10.1002/mds.22370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis ED, Agnew A, Gillman A, et al. Estimating annual rate of decline: prospective, longitudinal data on arm tremor severity in two groups of essential tremor cases. J Neurol Neurosurg Psychiatry. 2011;82:761–765. doi: 10.1136/jnnp.2010.229740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis ED, Galecki M, Rao AK. Four essential tremor cases with moderately impaired gait: how impaired can gait be in this disease? Tremor Other Hyperkinet Mov (N Y) 2013a:3. doi: 10.7916/D8QV3K7G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis ED, Gerbin M, Galecki M. Essential tremor 10, 20, 30, 40: clinical snapshots of the disease by decade of duration. Eur J Neurol. 2013b;20:949–954. doi: 10.1111/ene.12123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis ED, Hernandez N, Rabinowitz D, et al. Predicting age of onset in familial essential tremor: how much does age of onset run in families? Neuroepidemiology. 2013c;40:269–273. doi: 10.1159/000345253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucotte G, Lagarde JP, Funalot B, et al. Linkage with the Ser9Gly DRD3 polymorphism in essential tremor families. Clin Genet. 2006;69:437–440. doi: 10.1111/j.1399-0004.2006.00600.x. [DOI] [PubMed] [Google Scholar]
- Martinez C, Garcia-Martin E, Alonso-Navarro H, et al. Alcohol dehydrogenase 2 genotype and allelic variants are not associated with the risk for essential tremor. Clin Neuropharmacol. 2007a;30:196–200. doi: 10.1097/wnf.0b013e3180413d94. [DOI] [PubMed] [Google Scholar]
- Martinez C, Garcia-Martin E, Alonso-Navarro H, et al. Changes at the CYP2C locus and disruption of CYP2C8/9 linkage disequilibrium in patients with essential tremor. Neuromolecular Med. 2007b;9:195–204. doi: 10.1007/BF02685892. [DOI] [PubMed] [Google Scholar]
- Martinez C, Garcia-Martin E, Alonso-Navarro H, et al. Glutathione-S-transferase P1 polymorphism and risk for essential tremor. Eur J Neurol. 2008;15:234–238. doi: 10.1111/j.1468-1331.2007.02040.x. [DOI] [PubMed] [Google Scholar]
- Merner ND, Girard SL, Catoire H, et al. Exome sequencing identifies FUS mutations as a cause of essential tremor. Am J Hum Genet. 2012;91:313–319. doi: 10.1016/j.ajhg.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ondo WG, Sutton L, Dat Vuong K, et al. Hearing impairment in essential tremor. Neurology. 2003;61:1093–1097. doi: 10.1212/01.wnl.0000086376.40750.af. [DOI] [PubMed] [Google Scholar]
- Ortega-Cubero S, Lorenzo-Betancor O, Lorenzo E, et al. Fused in sarcoma (FUS) gene mutations are not a frequent cause of essential tremor in Europeans. Neurobiol Aging. 2013;34:2441e9–2441.e11. doi: 10.1016/j.neurobiolaging.2013.04.024. [DOI] [PubMed] [Google Scholar]
- Ortega-Cubero S, Lorenzo-Betancor O, Lorenzo E, et al. TREM2 R47H variant and risk of essential tremor: a cross-sectional international multicenter study. Parkinsonism Relat Disord. 2015;21:306–309. doi: 10.1016/j.parkreldis.2014.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagan FL, Butman JA, Dambrosia JM, et al. Evaluation of essential tremor with multi-voxel magnetic resonance spectroscopy. Neurology. 2003;60:1344–1347. doi: 10.1212/01.wnl.0000065885.15875.0d. [DOI] [PubMed] [Google Scholar]
- Parmalee N, Mirzozoda K, Kisselev S, et al. Genetic analysis of the FUS/TLS gene in essential tremor. Eur J Neurol. 2013;20:534–539. doi: 10.1111/ene.12023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passamonti L, Cerasa A, Quattrone A. Neuroimaging of essential tremor: what is the evidence for cerebellar involvement? Tremor Other Hyperkinet Mov (N Y) 2012:2. doi: 10.7916/D8F76B8G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phibbs F, Fang JY, Cooper MK, et al. Prevalence of unilateral tremor in autosomal dominant essential tremor. Mov Disord. 2009;24:108–111. doi: 10.1002/mds.22113. [DOI] [PubMed] [Google Scholar]
- Quattrone A, Cerasa A, Messina D, et al. Essential head tremor is associated with cerebellar vermis atrophy: a volumetric and voxel-based morphometry MR imaging study. AJNR Am J Neuroradiol. 2008;29:1692–1697. doi: 10.3174/ajnr.A1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radovica I, Inashkina I, Smeltere L, et al. Screening of 10 SNPs of LINGO1 gene in patients with essential tremor in the Latvian population. Parkinsonism Relat Disord. 2012;18:93–95. doi: 10.1016/j.parkreldis.2011.06.006. [DOI] [PubMed] [Google Scholar]
- Rajput AH, Offord KP, Beard CM, et al. Essential tremor in Rochester, Minnesota: a 45-year study. J Neurol Neurosurg Psychiatry. 1984;47:466–470. doi: 10.1136/jnnp.47.5.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajput A, Rajput AH, Rajput ML, et al. Identification of FUS p.R377W in essential tremor. Eur J Neurol. 2014;21:361–363. doi: 10.1111/ene.12231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rautakorpi I, Takala J, Marttila RJ, et al. Essential tremor in a Finnish population. Acta Neurol Scand. 1982;66:58–67. doi: 10.1111/j.1600-0404.1982.tb03129.x. [DOI] [PubMed] [Google Scholar]
- Ross OA, Conneely KN, Wang T, et al. Genetic variants of alpha-synuclein are not associated with essential tremor. Mov Disord. 2011;26:2552–2556. doi: 10.1002/mds.23909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross JP, Rayaprolu S, Bernales CQ, et al. SLC1A2 rs3794087 does not associate with essential tremor. Neurobiol Aging. 2014;35(935):e939–910. doi: 10.1016/j.neurobiolaging.2013.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz A, Dols-Icardo O, Bullido MJ, et al. Assessing the role of the TREM2 p.R47H variant as a risk factor for Alzheimer’s disease and frontotemporal dementia. Neurobiol Aging. 2014;35:444.e1–444.e4. doi: 10.1016/j.neurobiolaging.2013.08.011. [DOI] [PubMed] [Google Scholar]
- Sazci A, Ergul E, Bayulkem K. Association of the C677T and A1298C polymorphisms of methylenetetrahy-drofolate reductase gene in patients with essential tremor in Turkey. Mov Disord. 2004;19:1472–1476. doi: 10.1002/mds.20254. [DOI] [PubMed] [Google Scholar]
- Shatunov A, Jankovic J, Elble R, et al. A variant in the HS1-BP3 gene is associated with familial essential tremor. Neurology. 2005;65:1995. doi: 10.1212/01.wnl.0000200984.10076.e5. author reply 1995. [DOI] [PubMed] [Google Scholar]
- Shatunov A, Sambuughin N, Jankovic J, et al. Genomewide scans in North American families reveal genetic linkage of essential tremor to a region on chromosome 6p23. Brain. 2006;129:2318–2331. doi: 10.1093/brain/awl120. [DOI] [PubMed] [Google Scholar]
- Singer C, Sanchez-Ramos J, Weiner WJ. Gait abnormality in essential tremor. Mov Disord. 1994;9:193–196. doi: 10.1002/mds.870090212. [DOI] [PubMed] [Google Scholar]
- Stefansson H, Steinberg S, Petursson H, et al. Variant in the sequence of the LINGO1 gene confers risk of essential tremor. Nat Genet. 2009;41:277–279. doi: 10.1038/ng.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternberg EJ, Alcalay RN, Levy OA, et al. Postural and intention tremors: a detailed clinical study of essential tremor vs. Parkinson’s disease. Front Neurol. 2013;4:51. doi: 10.3389/fneur.2013.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun QY, Guo JF, Han WW, et al. Genetic association study of glucocerebrosidase gene L444P mutation in essential tremor and multiple system atrophy in mainland china. J Clin Neurosci. 2013;20:217–219. doi: 10.1016/j.jocn.2012.01.055. [DOI] [PubMed] [Google Scholar]
- Tan EK, Schapira AH. Hunting for genes in essential tremor. Eur J Neurol. 2008;15:889–890. doi: 10.1111/j.1468-1331.2008.02226.x. [DOI] [PubMed] [Google Scholar]
- Tan EK, Prakash KM, Fook-Chong S, et al. DRD3 variant and risk of essential tremor. Neurology. 2007;68:790–791. doi: 10.1212/01.wnl.0000256773.87498.2f. [DOI] [PubMed] [Google Scholar]
- Tan EK, Lee SS, Fook-Chong S, et al. Evidence of increased odds of essential tremor in Parkinson’s disease. Mov Disord. 2008;23:993–997. doi: 10.1002/mds.22005. [DOI] [PubMed] [Google Scholar]
- Tan EK, Teo YY, Prakash KM, et al. LINGO1 variant increases risk of familial essential tremor. Neurology. 2009;73:1161–1162. doi: 10.1212/WNL.0b013e3181bacfc9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan EK, Foo JN, Tan L, et al. SLC1A2 variant associated with essential tremor but not Parkinson disease in Chinese subjects. Neurology. 2013;80:1618–1619. doi: 10.1212/WNL.0b013e31828f1903. [DOI] [PubMed] [Google Scholar]
- Tanner CM, Goldman SM, Lyons KE, et al. Essential tremor in twins: an assessment of genetic vs environmental determinants of etiology. Neurology. 2001;57:1389–1391. doi: 10.1212/wnl.57.8.1389. [DOI] [PubMed] [Google Scholar]
- Testa CM. Key issues in essential tremor genetics research: where are we now and how can we move forward? Tremor Other Hyperkinet Mov (N Y) 2013:3. doi: 10.7916/D8Q23Z0Z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thelen M, Razquin C, Hernandez I, et al. Investigation of the role of rare TREM2 variants in frontotemporal dementia subtypes. Neurobiol Aging. 2014;35(2657):e2613–2659. doi: 10.1016/j.neurobiolaging.2014.06.018. [DOI] [PubMed] [Google Scholar]
- Thenganatt MA, Louis ED. Personality profile in essential tremor: a case-control study. Parkinsonism Relat Disord. 2012;18:1042–1044. doi: 10.1016/j.parkreldis.2012.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thier S, Lorenz D, Nothnagel M, et al. LINGO1 polymorphisms are associated with essential tremor in Europeans. Mov Disord. 2010;25:717–723. doi: 10.1002/mds.22887. [DOI] [PubMed] [Google Scholar]
- Thier S, Kuhlenbaumer G, Lorenz D, et al. GABA (A) receptor- and GABA transporter polymorphisms and risk for essential tremor. Eur J Neurol. 2011;18:1098–1100. doi: 10.1111/j.1468-1331.2010.03308.x. [DOI] [PubMed] [Google Scholar]
- Thier S, Lorenz D, Nothnagel M, et al. Polymorphisms in the glial glutamate transporter SLC1A2 are associated with essential tremor. Neurology. 2012;79:243–248. doi: 10.1212/WNL.0b013e31825fdeed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unal Gulsuner H, Gulsuner S, Mercan FN, et al. Mitochondrial serine protease HTRA2 p.G399S in a kindred with essential tremor and Parkinson disease. Proc Natl Acad Sci U S A. 2014;111:18285–18290. doi: 10.1073/pnas.1419581111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilarino-Guell C, Ross OA, Wider C, et al. LINGO1 rs9652490 is associated with essential tremor and Parkinson disease. Parkinsonism Relat Disord. 2010a;16:109–111. doi: 10.1016/j.parkreldis.2009.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilarino-Guell C, Wider C, Ross OA, et al. LINGO1 and LINGO2 variants are associated with essential tremor and Parkinson disease. Neurogenetics. 2010b;11:401–408. doi: 10.1007/s10048-010-0241-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilarino-Guell C, Wider C, Ross OA, et al. LINGO1 and LINGO2 variants are associated with essential tremor and Parkinson disease. Neurogenetics. 2010c;11:401–408. doi: 10.1007/s10048-010-0241-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilarino-Guell C, Soto-Ortolaza AI, Rajput A, et al. MAPT H1 haplotype is a risk factor for essential tremor and multiple system atrophy. Neurology. 2011;76:670–672. doi: 10.1212/WNL.0b013e31820c30c1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitale C, Gulli R, Ciotti P, et al. DRD3 Ser9Gly variant is not associated with essential tremor in a series of Italian patients. Eur J Neurol. 2008;15:985–987. doi: 10.1111/j.1468-1331.2008.02164.x. [DOI] [PubMed] [Google Scholar]
- Wills AJ, Jenkins IH, Thompson PD, et al. Red nuclear and cerebellar but no olivary activation associated with essential tremor: a positron emission tomographic study. Ann Neurol. 1994;36:636–642. doi: 10.1002/ana.410360413. [DOI] [PubMed] [Google Scholar]
- Wu YW, Rong TY, Li HH, et al. Analysis of Lingo1 variant in sporadic and familial essential tremor among Asians. Acta Neurol Scand. 2011;124:264–268. doi: 10.1111/j.1600-0404.2010.01466.x. [DOI] [PubMed] [Google Scholar]
- Zuo X, Jiang H, Guo JF, et al. Screening for two SNPs of LINGO1 gene in patients with essential tremor or sporadic Parkinson’s disease in Chinese population. Neurosci Lett. 2010;481:69–72. doi: 10.1016/j.neulet.2010.06.041. [DOI] [PubMed] [Google Scholar]