Abstract
Sirtuin 1 (SIRT1) is an NAD+-dependent protein deacetylase that plays a critical role in controlling energy metabolism, stress response and aging. Hence, enhancing SIRT1 activity could be a potential therapeutic strategy to treat metabolic diseases such as diabetes. However, pharmacological activators for SIRT1 are scarce to date. In this study, using the optimized high throughput screening, we identified E6155, a piperazine 1, 4- diamide compound, as a new small molecular activator of SIRT1. We further found that E6155 significantly upregulated glucose uptake in cultured mouse liver cells and skeletal muscle cells through increasing SIRT1 deacetylase activity. In type 2 diabetic KKAy mice, E6155 treatment markedly decreased the level of fasting glucose. Moreover, E6155 improved oral glucose tolerance and insulin tolerance. Euglycemic clamp and the homeostasis model assessment of insulin resistance index showed that E6155 ameliorated the insulin resistance and increased insulin sensitivity in diabetic mice. Mechanistically, we observed that the antidiabetic effects of E6155 were involved in SIRT1 dependent activation of LKB1/AMPK and IRS1/AKT pathways. In conclusion, our findings identified E6155 as a novel SIRT1 activator and suggested that E6155 could be a promising drug candidate for treating insulin resistance and diabetes.
Keywords: SIRT1, AMPK, AKT, glucose metabolism, insulin sensitivity, diabetes
Introduction
Calorie restriction has pleiotropic effects on cell metabolism and extended lifespan in various species[1]. It has been proposed that the beneficial effects of calorie restriction is partially through silent information regulator 2 (Sir2), a NAD+-dependent histone deacetylase [2]. Sirtuin 1 (SIRT1) belongs to mammals’ sirtuins family, which shares the highest sequence homology with yeast silent information regulator-2 (ySir2), is the most extensively studied member of the class III histone deacetylase [3].
Recently, many studies have demonstrated that SIRT1 activation could improve insulin sensitivity and modulate glucose metabolism [4–6]. SIRT1 has been shown to have benefits in improving insulin resistance and regulating insulin-stimulated glucose uptake by AMP-activated kinase (AMPK)-dependent and insulin receptor substrate 1 (IRS1)/AKT-dependent pathways [7, 8]. Accumulating evidence suggested that AMPK activation could ameliorate insulin resistance and prevent the pathologies of type 2 diabetes [9]. In addition, overexpression of SIRT1 could activate liver kinase B1 (LKB1) and subsequently activate AMPK by decreasing lysine acetylation of LKB1, which caused the translocation of LKB1 from the nucleus to the cytoplasm [10]. The IRS1/AKT pathway is important in mediating Glut4 translocation and glucose transport, however, the signaling is impaired in the conditions of insulin resistance [7, 11]. Also, it was demonstrated that resveratrol, a SIRT1 activator, promotes SIRT1 through LKB1-dependent AMPK activation [12] and IRS1/AKT activation by increasing the phosphorylation of IRS1 and AKT [13]. Therefore, SIRT1 was thought to be a promising therapeutic target for the treatment of metabolic disorders and insulin resistance-related diseases.
In this study, through high throughput screening (HTS), we discovered a novel SIRT1 activator E6155, which is a piperazine 1, 4- diamide compound. We further evaluated the beneficial effects of E6155 on glucose metabolism and explored the possible mechanism in vitro and in vivo. Our findings demonstrated that E6155 improved insulin sensitivity and prevented insulin resistance in KKAy mouse model of type 2 diabetes, and thus E6155 might be potentially used in treating diabetes caused by insulin resistance.
Materials and methods
Materials
The small-molecule compound library was maintained by the National Laboratory for Screening New Microbial Drugs, CAMS&PUMC (Beijing, China). E6155 for the animal experiments was ordered from J&K Scientific (Beijing, China). 2-(N-(7-Nitrobenz-2-oxa-1,3-diazol-4-yl)Amino)-2-Deoxyglucose (2-NBDG) was ordered from Invitrogen (MA, USA). EX527 was purchased from Selleckchem (TX, USA). Insulin, Doxorubicin and Palmitate were obtained from Sigma Aldrich (MO, USA). Dulbecco’s modified Eagle’s medium (DMEM) and fetal bovine serum (FBS) were purchased from Gibco (MA, USA).
Cell culture
HepG2 cells, L02 cells and L6 cells were purchased from National Infrastructure of Cell Line Resource (Beijing, China). HepG2 cells, L02 cells and L6 cells were cultured in DMEM with 10% FBS (v/v) in 37°C, 5% CO2 incubator. For L6 myoblast differentiation, after the cells met 80% confluence, the medium was changed to DMEM with 2% FBS for 7 days.
HTS assay for SIRT1 activators
In order to identify SIRT1 activators from the synthetic and natural compound library of our laboratory, we established an HTS assay using the purified recombinant human SIRT1 and HTRF SIRT1 assay purchased from Cisbio bioassays (Parc Marcel Boiteux, France). Reactions were conducted in 384-well white-bottom plates which contains 0.025 U purified SIRT1 enzyme, 1 mM dithiothreitol, 150 μM NAD+, and 6 nM SIRT1-specific deacetylation substrate-d2, DMSO (final concentration 1%, negative control) or a test compound (final DMSO concentration 1%) each well. Enzyme control wells did not contain SIRT1 enzyme and negative control wells did not contain test compound. After mixing the reaction components, plates were incubated at room temperature for 2 h. Then Anti-acetyl MAb-cryptate was added for additional 5 h. Fluorescence at 620 nm and 665 nm of each well was measured using a fluorometric plate reader (PerkinElmer). Sample activity was defined as the percentage of signal increase relative to the signal window, according to the following formula: ratio = (absorbance at 665 nm/absorbance at 620 nm) ×104; deacetylation of substrate (%) = 100− (ratiosample / ratioEnzyme control × 100); upregulating activity regulatory= ratiosample/rationegative control × 100%. A value ≥ 150% was considered to have a potential activation effect. After validated as potential activators, EC50 values were calculated by dose-dependent curve using GraphPad Prism 5 software.
Western blotting
Western blotting was performed as previously described [14]. The primary antibodies include Acetyl-p53 (#2570, Cell Signaling Technology), p53 (#2524, Cell Signaling Technology), Phospho-AMPKα (#2531, Cell Signaling Technology), AMPKα (#5832, Cell Signaling Technology), Phospho-AKT (#9271, Cell Signaling Technology), AKT (#4691, Cell Signaling Technology), and GAPDH (#AB2302, EMD Millipore). All western blot experiments were repeated at least three times and representative images were shown.
Glucose uptake assay
Glucose uptake activity of the cells after the treatment of E6155 with or without EX527 was assayed by 2-NBDG as described previously [15]. L02 or L6 myotubes were incubated with E6155 (0.1 μM) with or without EX527 (10 μM) in 37 °C for 18 h. After that, cells were stimulated with 100 nM insulin for 30 min. Then, insulin was replaced with 50 μM of 2-NBDG. After 1h, the cells were washed three times and lysed in lysis buffer for 10 min. The fluorescence intensity of supernatants which containing equal amount of protein were measured using a fluorometric plate reader (PerkinElmer) at 485 nm excitation and 535 nm emission, respectively. Each glucose uptake assay was performed three times.
Glucose homeostasis analysis in type 2 diabetic KKAy mice
Female KKAy mice (16-weeks old, 30–35 g) were purchased from the Institute of Laboratory Animal Sciences, CAMS, PUMC. Animal care and experimental procedures were performed in accordance with the regulations of the Institutional Animal Care and Use Committee of the Institute of Medicinal Biotechnology Institute. In all groups, the mice were fed a high-fat diet to render the mice diabetic (fasting blood glucose>11.1 mmol/L). The model group mice were treated with 0.5% carboxymethylcellulose sodium (CMC-Na). E6155 group were treated with E6155 (10 mg/kg) in CMC-Na by oral gavage once daily for 3 weeks. At day 0, 4, 7, 11, 17 and 20 of the treatment, the mice were fasted for 12 h and the fasting blood glucose was measured using the blood samples from the tail vein. At the end of the experiment, the mice were anesthetized and blood samples were collected from the retro-orbital plexus after fasted for 12 h. Serum insulin, glucose, leptin and adiponectin were measured using commercially available kits (Nanjing SenBeiJia, China) according to manufacturers’ protocol.
Oral glucose tolerance test (OGTT)
After 2 weeks of E6155 or vehicle treatment, mice were fasted for 6 h but not limited to the water. 2 g/kg body weight glucose was given by gavage and glucose levels were detected at 0, 30, 60, 90 and 120 min after the glucose treatment [16].
Insulin tolerance test (ITT)
After 2 weeks of E6155 or vehicle treatment, another cohort of mice was fasted for 12 h but have free access to water. Then the mice were subcutaneous injected with insulin solution at 0.75 IU/kg body weight. After 0, 40, 90 and 120 min injection, the blood samples from the tail vein of each mouse were used to measure blood glucose levels [17].
Hyperinsulinemic–euglycemic clamp analysis
After E6155 or vehicle treated for 3 weeks, hyperinsulinemic–euglycemic clamp experiments were performed [18]. Mice were fasted for 12 h and anesthetized before the study. The right jugular vein was catheterized the Y shape pipe for the infusion of glucose and insulin. After the adaptation period, human insulin was infused by syringe infusion pump (KD Scientific, USA) at the rate of 20 mIU/kg/min. When the blood glucose levels decreased to below 6.0 mmol/L, infusion of glucose was started and monitored the blood glucose level every 5 min. The glucose infusion rate (GIR) of five time points after the glucose levels maintaining at 6.0 ± 0.5 mmol/L represents the GIR of the steady state and represents whole-body insulin sensitivity.
Statistical analysis
Data are presented as mean ± SEM and analyzed with 2-tailed, paired t-test or One-way ANOVA with appropriate Bonferroni’s multiple comparison test using GraphPad Prim 5 software. p < 0.05 was considered as statistically significant.
Results
Identification of E6155 as a SIRT1 activator
To identify potential SIRT1 activators, an HTS assay suitable for SIRT1 activator screening was successfully established. Using the established SIRT1 activator HTS assay, we identified E6155 (Figure 1A) as a positive hit compound among 12,000 compounds. E6155 named (Z)-1-(4-(3-benzylidene-2,3-dihydro-1H-cyclopenta[b]quinoline-9-carbonyl)piperazin-1-yl)ethan-1-one is a piperazine 1,4-diamide compound that has not previously been reported to have any bioactivity. In our assay, we observed that E6155 activated SIRT1 at a maximum deacetylation of substrate (%) value of 93.69% and with an EC50 of 0.88 μM (Figure 1B).
Figure 1. High throughput screening identified E6155 as a new small molecular activator of SIRT1 and augmented insulin-stimulated glucose uptake.
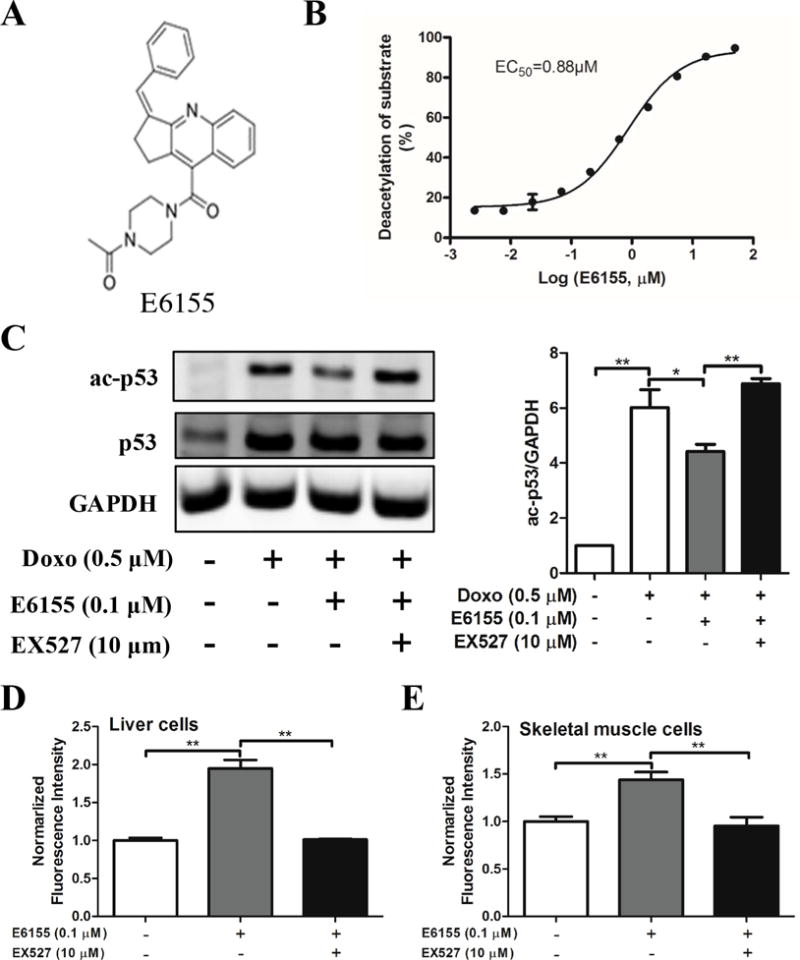
(A) The chemical structure of E6155. (B) Dose-dependent curve of E6155 (in log scale) in SIRT1 activators screening model. (C) Western blotting analysis of ac-p53 and total p53 of whole HepG2 cell lysates. Values are presented as mean±SEM, One-way ANOVA (nonparametric, Bonferroni’s multiple comparison test), *p<0.05, **p<0.01, n=4. (D) E6155 improves the glucose uptake in normal liver L02 cells and (E) skeletal muscle L6 cells in a SIRT1 dependent manner. (D–E) Data are expressed as mean ± SEM, 2-tailed, paired t-test, *p < 0.05, **p < 0.01, vs Control group, n=3.
p53 is a well-established deacetylation substrate for SIRT1 [19]. To further validate the activity of E6155 in cultured cells, we analyzed p53 acetylation level in HepG2 cells. Expectedly, E6155 decreased the acetylation level of acetylated p53 significantly (Figure 1C). Moreover, the effect of E6155 on p53 deacetylation was blocked by a SIRT1 specific inhibitor EX527 [20] (Figure 1C). These results demonstrate that E6155 is a bona fide SIRT1 activator.
E6155 augmented insulin-induced glucose uptake in vitro
SIRT1 has beneficial effects on glucose homeostasis and insulin sensitivity [21]. Since glucose uptake and utilization in liver and skeletal muscle was critical for glucose homeostasis in vivo, we evaluated the effect of E6155 on glucose uptake in normal human normal liver L02 cells and L6 myotubes.
The glucose uptake assay showed that E6155 significantly increased the glucose uptake in L02 cells and this effect was reduced when cells were simultaneously treated with SIRT1 specific inhibitor EX527 (Figure 1D). L6 myotubes was formed after 2% FBS stimulated for 7 days. Similarly, the glucose uptake in L6 myotubes was markedly increased after E6155 treatment (Figure 1E). Consistent with the result in L02 cells, this upregulation of glucose uptake by E6155 in L6 myotubes could be blocked by SIRT1 specific inhibitor EX527 (Figure 1E).
E6155 improved whole-body glucose homeostasis in type 2 diabetic KKAy mice
Since E6155 displays augmented glucose uptake in normal liver and skeletal muscle cells, we further asked whether E6155 has positive effects on diabetes in vivo. To this end, 16 week-old female KKAy mice fed a high-fat diet were used to establish the obesity and hyperglycemia model [22]. The mice were administrated with E6155 (10 mg/kg) or vehicle for 3 weeks. As shown in Figure 2A, compared with vehicle control, the fasting blood glucose levels of the mice in E6155 treated group significantly decreased at day 4, 7, 10, 17 and 20.
Figure 2. E6155 improved whole-body glucose homeostasis and oral glucose tolerance and insulin tolerance in type 2 diabetic KKAy mice.
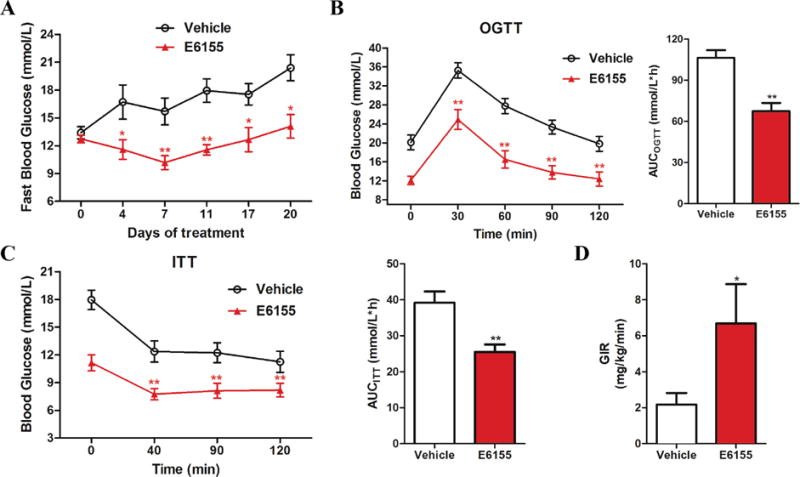
(A) The fasting blood glucose during the course of treatment with vehicle or E6155. (B) Blood glucose levels during the OGTT or (C) ITT and the areas under the curve (AUC) of the blood glucose after two weeks of treatment with vehicle or E6155. (A–C) Data are expressed as mean ± SEM, 2-tailed, paired t-test, *p < 0.05, **p < 0.01, vs vehicle group, n=12. (D) GIR in Hyperinsulinemic–Euglycemic clamp. Values are expressed as mean ± SEM, 2-tailed, paired t-test, *p < 0.05, **p < 0.01, vs vehicle group, 4 mice for each group.
To further assess whether E6155 regulate glucose homeostasis through improving glucose tolerance and insulin tolerance in type 2 diabetic KKAy mice, OGTT and ITT were performed after E6155 treatment for two weeks. OGTT data showed that after the glucose injection, E6155 treatment significantly accelerated the glucose metabolism at four time points examined (Figure 2B). The decreased area under curve (AUC) also showed the improved glucose tolerance and glucose homeostasis after E6155 treatment (Figure 2B). Also, another cohort of mice was treated with E6155 or vehicle for two weeks before performing ITT experiment. As shown in Figure 2C, compared with vehicle control, E6155 significantly lowered blood glucose level and reduced AUC after insulin injection, indicating E6155 improves insulin sensitivity in vivo.
In addition, at day 21 (the end of E6155 treatment), four mice were randomly selected to perform the hyperinsulinemic– euglycemic clamp experiments. And the rest mice were anesthetized and blood samples were collected from the retro-orbital plexus. At the end of the clamp, compared with vehicle treated mice, GIRs of the clamp were significantly increased in E6155 treated mice which indicating improved insulin sensitivity after E6155 administration (Figure 2D). These findings demonstrate that E6155 controls the glucose homeostasis by improving glucose tolerance and insulin tolerance in type 2 diabetic KKAy mice.
Then Homeostasis Model Assessment of Insulin Resistance (HOMA-IR) index was calculated to evaluate the insulin resistance effect of E6155. At day 21, the mice were fasted overnight and serum insulin (Figure 3A) and serum glucose (Figure 3B) levels were determined by commercial kits and HOMA-IR index values were calculated. HOMA-IR index in E6155 treated mice was significantly smaller than that in vehicle treated mice (Figure 3C). These results indicate that E6155 improves insulin resistance in diabetic KKAy mice.
Figure 3. E6155 increased insulin sensitivity in type 2 diabetic KKAy mice.
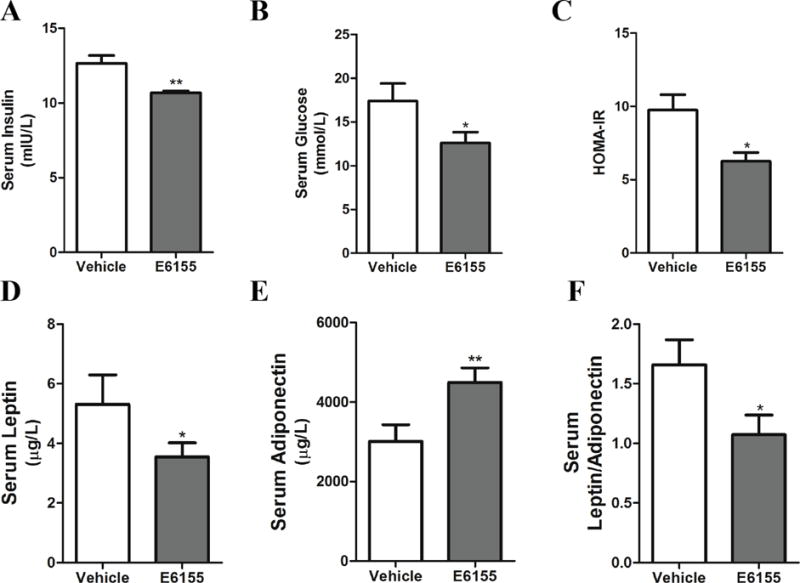
(A) Insulin, (B) glucose, (D) leptin and (E) adiponectin levels in the serum of KKAy mice administered either vehicle or E6155. (C) HOMA-IR index and (F) ratio of leptin/adiponectin of vehicle or E6155 group. (A–F) Data are expressed as mean ± SEM, 2-tailed, paired t-test, *p < 0.05, **p < 0.01, vs vehicle group, n=8.
The leptin level in the serum is closely related to insulin resistance and obesity and reflects the severity of insulin resistance [23]. Adiponectin, released from adipocytes, plays an important role in insulin sensitizing, inflammation and cell apoptotic in multiple cell types. The concentration of adiponectin was dramatically decreased in severe metabolic disorders [24]. The increased ratio of leptin/adiponectin indicates the insulin resistance in obesity [25] and type 2 diabetes mellitus [26, 27]. Our data showed that leptin level was significantly decreased while the adiponectin was upregulated after E6155 treatment (Figure 3D–E) at the end of the experiment. Thus the ratio of leptin/adiponectin markedly decreased by the E6155 treatment (Figure 3F). Collectively, these results demonstrate that E6155 significantly improves whole-body glucose homeostasis by ameliorating insulin resistance in type 2 diabetic KKAy mice.
E6155 enhanced phosphorylation of AKT and AMPK in type 2 diabetic KKAy mice and cultured cells
LKB1/AMPK and IRS1/AKT pathways are involved in SIRT1 regulated insulin resistance and insulin-induced glucose uptake [7, 8, 13]. To elucidate the antidiabetic effect of E6155, we first examined the total AMPK and phospho-AMPK protein levels in the liver of diabetic KKAy mice after E6155 treatment. As shown in Figure 4A, E6155 significantly increased liver AMPK activity by upregulating the phosphorylation of AMPK. In vitro studies also showed that E6155 significantly activated AMPK by increasing the phosphorylation of AMPK in HepG2 cells (Figure 4B). Then the IRS1/AKT pathway was examined to test whether E6155 administration could affect it or not. It was found that the phosphorylation level of AKT was increased in the liver of mice treated with E6155 (Figure 4A), indicating that E6155 also enhances the activation of the IRS1/AKT signaling pathway in vivo. Similarly, in the case of insulin resistance which induced by Palmitate [28] in HepG2 cells, E6155 increased the level of phosphorylated-AKT significantly (Figure 4C). These results suggested that the antidiabetic effect of E6155 was at least involved in the activation of AMPK-dependent and IRS1/AKT-dependent pathways.
Figure 4. E6155 enhanced phosphorylation of AMPK and AKT in type 2 diabetic KKAy mice and cultured cells.
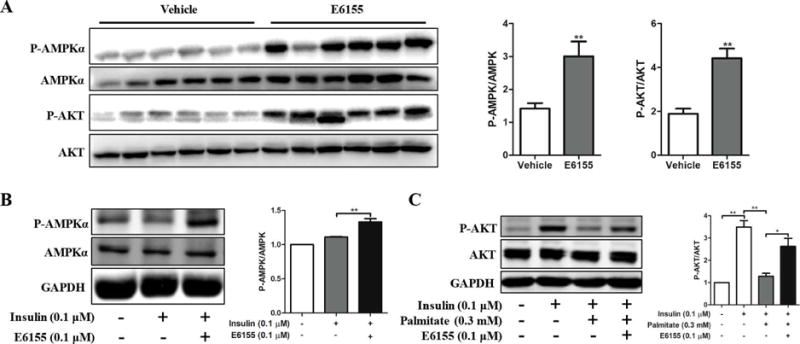
(A) Western Blots analysis of P-AMPKα, AMPKα, P-AKT and AKT in liver from KKAy mice treated with or without E6155. Representative immunoblots are shown. Values are expressed as means ± SEM, 2-tailed, paired t-test, *p < 0.05, **p < 0.01, vs vehicle group, n=12. (B) Protein levels of P-AMPKα and AMPKα in HepG2 cells. Cells were pretreated with E6155 (0.1μM) for 6 h, then insulin (0.1μM) was added for 15 min, respectively. (C) P-AKT and AKT levels were determined by Western blotting in HepG2 cells. Cells were pretreated with Palmitate (0.3mM) for 18 h, then cells were treated with E6155 (0.1μM) for 6 h, and insulin was added for 15 min finally. (B–C) Data are presented as mean±SEM, One-way ANOVA (nonparametric, Bonferroni’s multiple comparison test), *p<0.05, **p<0.01, n=3.
Discussion
Diabetes is a major cause of mortality and disability worldwide. World Health Organization reported that the number of people with diabetes has risen from 108 million in 1980 to 422 million in 2014 [29]. An accumulating number of studies have demonstrated that SIRT1 activation may improve insulin sensitivity and modulate glucose metabolism [4–6, 30]. Nonetheless, pharmacological and molecular activators for SIRT1 remain lacking. Resveratrol and SRT1720, which could increase SIRT1 activity, have been reported as a promising therapeutics for type 2 diabetes [21]. Therefore, the efforts for identifying SIRT1 activator have raised a lot of interest these years. In the present study, using HTS assay for SIRT1 activators, we have identified E6155, a piperazine 1, 4- diamide compound, as a novel SIRT1 activator that improve glucose homeostasis.
In the present study, in contemplation of discovering SIRT1 activators, we established an HTS assay for SIRT1 activators using human SIRT1 fragments containing N- and C- terminal catalytic regions (160–665 amino acids). To confirm the upregulation of the bioactive compound, we performed the p53 acetylation assay and predictably found that E6155 increased the effect of SIRT1 deacetylation significantly, which was blocked by SIRT1 specific inhibitor. Previous studies showed that SIRT1 could activate AMPK by activate LKB1 through decreasing lysine acetylation of LKB1 [10]. Also, there are several publications showed that SIRT1 activation could improve insulin resistance and regulating insulin-stimulated glucose uptake through activating AMPK and IRS1/AKT pathway [7–9]. Based on these evidences, we assumed that E6155 exerts the effect of improving insulin sensitivity by SIRT1/LKB1/AMPK and SIRT1/IRS1/AKT pathway in vivo. Consistently, the activation of AMPK and AKT by E6155 in cultured cells affirmed our hypothesis. Further studies are needed to clarify the importance of SIRT1/AMPK-dependent pathway and SIRT1/AKT-dependent pathway in mediating E6155 beneficial effect on glucose uptake and insulin sensitivity.
In summary, we have identified a novel SIRT1 activator E6155 regulates glucose metabolism in vitro and in vivo. Our findings also suggest that SIRT1 could be a novel pharmacological target for modulating insulin sensitivity and glucose metabolism related disorders.
Supplementary Material
Highlights.
E6155, a piperazine 1, 4-diamide compound, was first found to be a SIRT1 activator.
E6155 regulated glucose uptake dependent on SIRT1 in L02 and L6 cells.
E6155 improved glucose tolerance and insulin sensitivity in type 2 diabetic mice.
The antidiabetic effect of E6155 was involved in activation of AMPK and AKT pathways.
Acknowledgments
The authors thank Dr. Quan Liu in Professor Zhufang Shen Lab (Chinese Academy of Medical Sciences) for her technical support in hyperinsulinemic– euglycemic clamp experiment.
This work was supported by Health and Medical Creation Program of CAMS [grant No. 2016-I2M-1-011], CAMS Innovation Fund for Medical Sciences (CIFMS), National Natural Science Foundation of China [grant No. 81573482, 81703503, 81273515 and 81621064], the Key New Drug Creation and Manufacturing Program (grant No. 2018ZX09711001-003-006), National Institutes of Health [grant No. HL09502, HL114570, HL128363 and HL130167 to ZGJ], American Heart Association Grant-In-Aid [17GRNT33660671 to ZGJ] and Chinese Government Scholarship [to YX].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interests
The authors have declared no conflict of interests.
References
- 1.Wood JG, Rogina B, Lavu S, Howitz K, Helfand SL, Tatar M, et al. Sirtuin activators mimic caloric restriction and delay ageing in metazoans. Nature. 2004;430:686–689. doi: 10.1038/nature02789. [DOI] [PubMed] [Google Scholar]
- 2.Blander G, Guarente L. The Sir2 family of protein deacetylases. Annu Rev Biochem. 2004;73:417–435. doi: 10.1146/annurev.biochem.73.011303.073651. [DOI] [PubMed] [Google Scholar]
- 3.Frye RA. Characterization of five human cDNAs with homology to the yeast SIR2 gene: Sir2-like proteins (sirtuins) metabolize NAD and may have protein ADP-ribosyltransferase activity. Biochem Biophys Res Commun. 1999;260:273–279. doi: 10.1006/bbrc.1999.0897. [DOI] [PubMed] [Google Scholar]
- 4.Zhang J. Resveratrol inhibits insulin responses in a SirT1-independent pathway. Biochem J. 2006;397:519–527. doi: 10.1042/BJ20050977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Feige JN, Lagouge M, Canto C, Strehle A, Houten SM, Milne JC, et al. Specific SIRT1 activation mimics low energy levels and protects against diet-induced metabolic disorders by enhancing fat oxidation. Cell Metab. 2008;8:347–358. doi: 10.1016/j.cmet.2008.08.017. [DOI] [PubMed] [Google Scholar]
- 6.Banks AS, Kon N, Knight C, Matsumoto M, Gutierrez-Juarez R, Rossetti L, et al. SirT1 gain of function increases energy efficiency and prevents diabetes in mice. Cell Metab. 2008;8:333–341. doi: 10.1016/j.cmet.2008.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Knight ZA, Gonzalez B, Feldman ME, Zunder ER, Goldenberg DD, Williams O, et al. A pharmacological map of the PI3-K family defines a role for p110alpha in insulin signaling. Cell. 2006;125:733–747. doi: 10.1016/j.cell.2006.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ruderman NB, Xu XJ, Nelson L, Cacicedo JM, Saha AK, Lan F, et al. AMPK and SIRT1: a long-standing partnership? Am J Physiol Endocrinol Metab. 2010;298:E751–760. doi: 10.1152/ajpendo.00745.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ruderman NB, Carling D, Prentki M, Cacicedo JM. AMPK, insulin resistance, and the metabolic syndrome. J Clin Invest. 2013;123:2764–2772. doi: 10.1172/JCI67227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lan F, Cacicedo JM, Ruderman N, Ido Y. SIRT1 modulation of the acetylation status, cytosolic localization, and activity of LKB1. Possible role in AMP-activated protein kinase activation. J Biol Chem. 2008;283:27628–27635. doi: 10.1074/jbc.M805711200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Asano T, Fujishiro M, Kushiyama A, Nakatsu Y, Yoneda M, Kamata H, et al. Role of phosphatidylinositol 3-kinase activation on insulin action and its alteration in diabetic conditions. Biol Pharm Bull. 2007;30:1610–1616. doi: 10.1248/bpb.30.1610. [DOI] [PubMed] [Google Scholar]
- 12.Hou X, Xu S, Maitland-Toolan KA, Sato K, Jiang B, Ido Y, et al. SIRT1 regulates hepatocyte lipid metabolism through activating AMP-activated protein kinase. J Biol Chem. 2008;283:20015–20026. doi: 10.1074/jbc.M802187200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen S, Li J, Zhang Z, Li W, Sun Y, Zhang Q, et al. Effects of resveratrol on the amelioration of insulin resistance in KKAy mice. Can J Physiol Pharmacol. 2012;90:237–242. doi: 10.1139/y11-123. [DOI] [PubMed] [Google Scholar]
- 14.Xu Y, Liu P, Xu S, Koroleva M, Zhang S, Si S, et al. Tannic acid as a plant-derived polyphenol exerts vasoprotection via enhancing KLF2 expression in endothelial cells. Sci Rep. 2017;7:6686. doi: 10.1038/s41598-017-06803-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blodgett AB, Kothinti RK, Kamyshko I, Petering DH, Kumar S, Tabatabai NM. A fluorescence method for measurement of glucose transport in kidney cells. Diabetes Technol Ther. 2011;13:743–751. doi: 10.1089/dia.2011.0041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sung MM, Kim TT, Denou E, Soltys CM, Hamza SM, Byrne NJ, et al. Improved Glucose Homeostasis in Obese Mice Treated With Resveratrol Is Associated With Alterations in the Gut Microbiome. Diabetes. 2017;66:418–425. doi: 10.2337/db16-0680. [DOI] [PubMed] [Google Scholar]
- 17.Liu C, Feng T, Zhu N, Liu P, Han X, Chen M, et al. Identification of a novel selective agonist of PPARgamma with no promotion of adipogenesis and less inhibition of osteoblastogenesis. Sci Rep. 2015;5:9530. doi: 10.1038/srep09530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu Q, Liu S, Gao L, Sun S, Huan Y, Li C, et al. Anti-diabetic effects and mechanisms of action of a Chinese herbal medicine preparation JQ-R in vitro and in diabetic KKAy mice. Acta Pharm Sin B. 2017;7:461–469. doi: 10.1016/j.apsb.2017.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vaziri H, Dessain SK, Ng Eaton E, Imai SI, Frye RA, Pandita TK, et al. hSIR2(SIRT1) functions as an NAD-dependent p53 deacetylase. Cell. 2001;107:149–159. doi: 10.1016/s0092-8674(01)00527-x. [DOI] [PubMed] [Google Scholar]
- 20.Peck B, Chen CY, Ho KK, Di Fruscia P, Myatt SS, Coombes RC, et al. SIRT inhibitors induce cell death and p53 acetylation through targeting both SIRT1 and SIRT2. Mol Cancer Ther. 2010;9:844–855. doi: 10.1158/1535-7163.MCT-09-0971. [DOI] [PubMed] [Google Scholar]
- 21.Milne JC, Lambert PD, Schenk S, Carney DP, Smith JJ, Gagne DJ, et al. Small molecule activators of SIRT1 as therapeutics for the treatment of type 2 diabetes. Nature. 2007;450:712–716. doi: 10.1038/nature06261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Castle CK, Colca JR, Melchior GW. Lipoprotein profile characterization of the KKA(y) mouse, a rodent model of type II diabetes, before and after treatment with the insulin-sensitizing agent pioglitazone. Arterioscler Thromb. 1993;13:302–309. doi: 10.1161/01.atv.13.2.302. [DOI] [PubMed] [Google Scholar]
- 23.Sahin-Efe A, Katsikeris F, Mantzoros CS. Advances in adipokines. Metabolism. 2012;61:1659–1665. doi: 10.1016/j.metabol.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 24.Turer AT, Scherer PE. Adiponectin: mechanistic insights and clinical implications. Diabetologia. 2012;55:2319–2326. doi: 10.1007/s00125-012-2598-x. [DOI] [PubMed] [Google Scholar]
- 25.Tajtakova M, Petrasova D, Pidanicova A, Gallovicova A, Blanarova C, Petrovicova J. Serum levels of leptin, adiponectin, retinol binding protein 4 and leptin/adiponectin molar ratio as another possible marker of insulin resistance in obese. Bratisl Lek Listy. 2010;111:212–215. [PubMed] [Google Scholar]
- 26.Zaletel J, Barlovic DP, Prezelj J. Adiponectin-leptin ratio: a useful estimate of insulin resistance in patients with Type 2 diabetes. J Endocrinol Invest. 2010;33:514–518. doi: 10.1007/BF03346639. [DOI] [PubMed] [Google Scholar]
- 27.Inoue M, Maehata E, Yano M, Taniyama M, Suzuki S. Correlation between the adiponectin-leptin ratio and parameters of insulin resistance in patients with type 2 diabetes. Metabolism. 2005;54:281–286. doi: 10.1016/j.metabol.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 28.Nakamura S, Takamura T, Matsuzawa-Nagata N, Takayama H, Misu H, Noda H, et al. Palmitate induces insulin resistance in H4IIEC3 hepatocytes through reactive oxygen species produced by mitochondria. J Biol Chem. 2009;284:14809–14818. doi: 10.1074/jbc.M901488200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006;3:e442. doi: 10.1371/journal.pmed.0030442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang RH, Kim HS, Xiao C, Xu X, Gavrilova O, Deng CX. Hepatic Sirt1 deficiency in mice impairs mTorc2/Akt signaling and results in hyperglycemia, oxidative damage, and insulin resistance. J Clin Invest. 2011;121:4477–4490. doi: 10.1172/JCI46243. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


