Abstract
Most tumors are unresponsive to immune checkpoint blockade, especially if deep immunosuppression in the tumor develops prior to and prevents T cell immunosurveillance. Failed or frustrated T-cell priming often needs repair before successful sensitization to PD-1/PD-L1 blockade. CD40 activation plays a critical role in generating T cell immunity, by activating dendritic cells, and converting cold tumors to hot. In preclinical studies, agonistic CD40 antibodies demonstrate T cell-dependent anti-tumor activity, especially in combination with chemotherapy, checkpoint inhibitory antibodies, and other immune modulators. With the advent of multiple CD40 agonists with acceptable single-agent toxicity, clinical evaluation of CD40 combinations has accelerated.
Introduction
The Immune Revolution in cancer is upon us. Deep tumor regressions achievable in multiple cancers with checkpoint inhibitory antibodies and remissions in refractory leukemia from chimeric antigen receptor (CAR) T cells have prompted a series of FDA approvals that have begun to change the face of cancer care. Still, there is a bittersweet quality to these successes: most patients do not respond to current renditions of checkpoint or CAR T cell therapy, and many patients too quickly relapse after an initial response. The PD-1 antibody pembrolizumab, for example, is approved for use as first-line therapy for patients with metastatic non-small cell lung cancer that overexpresses PD-L1 – outperforming chemotherapy in a manner thought impossible 10 years ago. Yet, nearly 30% of such patients are found to be refractory to therapy at the first restaging studies and another 25% have tumor progression at one year (Reck et al., 2016).
Cold tumors
The extent of intratumoral T cell infiltration positively predicts overall survival across many cancer types, and is also considered a major predictor of clinical response to checkpoint therapy with PD-1 and PD-L1 monoclonal antibodies (mAb). These so-called “hot” tumors stand in contrast to “cold” tumors that do not respond to single-agent PD-1 or PD-L1 therapy (Sharma and Allison, 2015; Teng et al., 2015). Pancreatic ductal adenocarcinoma (PDA), which now accounts for more deaths in the United States than breast cancer (Siegel et al., 2018), is an example of a tumor which remains almost entirely refractory to single-agent PD-1 or CTLA-4 antibody therapy. The only exception appears to be in <1% of PDA patients with high microsatellite instability (Le et al., 2017). Studies of the adaptive immune response in PDA, based on the KPC genetically engineered mouse model of this disease KRasLSL-G12D/+, Trp53LSL-R172H/+, Pdx1-Cre (Hingorani et al., 2005), highlight the extent to which macrophages dominate the spontaneous tumor microenvironment (TME) and the extent to which T cells are classically excluded from the TME even at the earliest stages of the disease (Clark et al., 2007). These observations are consistent with a model of a “cold” tumor (Sharma and Allison, 2015) and “immune privilege” (acquired or not) that requires a novel approach for therapy (Vonderheide and Bayne, 2013). T cell exclusion in KPC mice is a prominent feature not only in primary tumors but also metastatic lesions (Aiello et al., 2016; Clark et al., 2007). KPC tumors exhibit a low mutational burden, near absence of classically defined neo-epitopes, scant T cell infiltration and resistance to checkpoint therapy (Beatty et al., 2011; Evans et al., 2016; Feig et al., 2013; Winograd et al., 2015). In humans, T cell exclusion in PDA – with marked surrounding desmoplasia – is also well-described; however, a subset of primary pancreatic tumors do exhibit moderate infiltration of CD8+ T cells and other immune cells (Bailey et al., 2016; Balachandran et al., 2017; Fukunaga et al., 2004; Ino et al., 2013b; Wormann et al., 2014), and this phenotype correlates with expression of functional cytotoxicity genes and overall survival (Balachandran et al., 2017; Balli et al., 2017; Fukunaga et al., 2004; Ino et al., 2013a). In addition, human pancreatic tumors express a moderate range of non-synonymous mutations that are predicted to function as neo-epitopes (Balachandran et al., 2017; Balli et al., 2017; Rech et al., 2018). Recently, an in-depth evaluation of neo-epitopes in human PDA revealed special qualities of neopeptides, such as high differential agretopicity index (DAI) (Duan et al., 2014; Rech et al., 2018), that predicts long-term survival (Balachandran et al., 2017). High DAI reflects a mutated peptide that binds with high affinity to MHC whereas the wild type counterpart peptide does not. Yet, even in the setting of neoepitopes, checkpoint antibody therapy nearly universally fails in PDA. Thus, there is a disconnect, at least in PDA, in applying the notion that CD8+ T cell infiltration and non-synonymous mutations are sufficient to confer responsiveness to checkpoint blockade.
Revisiting cancer immunosurveillance
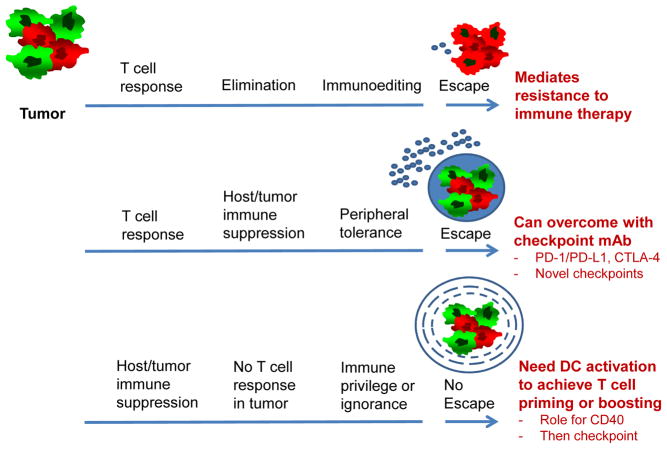
In the prevailing view of cancer immunosurveillance, T cell recognition of tumor antigens leads to tumor clearance unless the tumor undergoes immunoediting (e.g. loss of MHC or the antigen) or immunosuppressive pathways arise to dampen T cell reactivity (e.g. PD-L1) (Schreiber et al., 2011) (Figure 1). In each case, immune suppression follows as a consequence of T cell recognition. These observations were deduced from landmark studies in the highly mutated 3-methylcholanthrene (MCA) carcinogen-induced model of mouse sarcoma (Matsushita et al., 2012; Shankaran et al., 2001). For PDA and other cold tumors, however, it has been hypothesized that immunosuppression is an early (not secondary) event, and T cell reactivity fails to fully unfold during the entire natural course of the tumor (Vonderheide and Bayne, 2013) (Figure 1). In such tumors, poor T cell priming – or even immunological ignorance – may be linked to downstream mediators of oncogenes such as mutant Kras and others. Thus, in one scenario (classic MCA model), checkpoint blockade is successful; in another scenario (KPC model), checkpoint blockade alone is ineffective.
Fig. 1. Potential manifestations of cancer immunosurveillance.
In situations of an active T cell response to cancer (illustrated in the Figure by small round blue cells), a heterogenous tumor (illustrated in the upper left by a mix of red and green cells) may respond by immunoediting (top pathway) or by invoking peripheral tolerance pathways such as PD-1 and CTLA-4 (middle pathway) – each an example of immune escape. In some settings (bottom pathway), however, there is poor T cell reactivity from the start of tumor formation – potentially related to immune ignorance or immune privilege (illustrated by concentric circles around the tumor) – and thus there is no classic “escape” as noted in the first two scenarios. Rather priming or boosting of T cell responses, potentially enabled by CD40 or other means of dendritic cell activation, is required for therapeutic effect and sensitization to checkpoint blockade.
To understand immune surveillance further in cold tumors, the class MCA experiments were redone in the KPC model (Evans et al., 2016). The development of spontaneous tumors was followed in KPC mice that were either replete or depleted of T cells. There was no difference in overall survival nor time to diagnosis. Tumor cell lines harvested from T cell replete or depleted KPC mice grow equally upon reimplantation into syngeneic normal mice and are never rejected regardless of whether host mice are T cell-depleted. These observations are in contrast to those from similarly designed experiments in the MCA model.
Upon expression of the strong model antigen ovalbumin (OVA), KPC tumors are uniformly rejected unless (i) CD8+ T cells were depleted in host mice or (ii) host mice were made tolerant to OVA. OVA in these experiments is a neoantigen. When OVA-negative and OVA-expressing KPC tumor clones are injected together, only the OVA-negative cells grow out in T cell competent mice – a variation of immunediting in which only antigen-negative tumor cells emerge.
These observations (Evans et al., 2016), and similar findings in other genetically engineered mice (Casanovas et al., 2005; Ciampricotti et al., 2012; Ciampricotti et al., 2011; DeNardo et al., 2009), suggest that (i) the cardinal features of immunoediting are not universally observed, (ii) in such cases, this lack of immunoediting may be a consequence, and not a cause, of poor antigenicity, and (iii) antigen strength dictates the outcome of immune surveillance and can overcome even deep immmunosuppression in the TME. These conclusions are similar to other experimental models that highlight the dependency of cancer immunosurveillance on antigenic strength (DuPage et al., 2012). These observations carry an important implication for the design of novel clinical strategies: namely, in the absence of strong antigens, there may be no Darwinian-like pressure from T cells; thus, the underlying tumor cells may remain susceptible to T cells, but only if these T cells can be boosted or provoked.
CD40 and T cell priming
Increasing attention has turned toward evaluating and repairing insufficient T cell priming as a root cause of cold tumors and checkpoint unresponsiveness. Antigen presenting cells (APC), particularly BATF3-dependent type I classic dendritic cells (DC), are critical in driving T cell priming and function in tumor-bearing mice (Broz et al., 2014; Byrne and Vonderheide, 2016; Durai and Murphy, 2016; Engelhardt et al., 2012; Roberts et al., 2016; Salmon et al., 2016; Sanchez-Paulete et al., 2016; Spranger et al., 2017), reinforcing a long-standing appreciation of DC dysfunction in the TME (Aspord et al., 2007; Gabrilovich, 2004). Impaired T cell trafficking into the tumor as well as hostile TME factors that limit T cell persistence in the TME are additional likely factors that drive a T cell-poor tumor (Spranger et al., 2017; Stromnes et al., 2015; Vonderheide and Bayne, 2013).
In KPC mice, classic DCs are a rare but definite population in the TME. Notably, these cells express CD40 (Winograd et al., 2015). CD40 is a cell-surface member of the TNF receptor superfamily that is most prominently expressed on DC, B cells, and myeloid cells (van Kooten and Banchereau, 2000). It is a well-known regulator of T cell immunity including in cancer (Grewal and Flavell, 1997). CD40-ligand, primarily expressed on activated T cells, interacts with CD40 on APCs resulting in a ‘licensed’ state (Lanzavecchia, 1998). Licensed DC exhibit upregulation of cytokines (such as IL-12), antigen-presenting molecules (such as MHC), costimulatory molecules (such as CD80 and CD86), adhesion molecules (such as ICAM-1) and an array of other TNF receptor family ligands that then engage receptors of the TNF superfamily on T cells. CD40 signaling mechanisms have been summarized previously (Vonderheide, 2007). The TNF ligand-receptor orientation is uniquely reversed for CD40/CD40-ligand in the DC:T cell synapse, and as such, CD40 is as a proximal regulator of other TNF family signaling receptors on T cells. Thus, upon activation of CD40 on DCs, multiple other agonistic pathways such as OX40, GITR, and 41BB are engaged. Importantly, CD40 is also prominently expressed by B cells and CD40 activation massively upregulates APC function (Coughlin et al., 2004; Schultze et al., 1997). Thus, it is possible that CD40 activation will also influence B cell function in the TME, including B regulatory cells and tertiary lymphoid structures (Gunderson et al., 2016; Lutz et al., 2014; Poschke et al., 2016).
Both loss-of-function and gain-of-function studies in KPC model systems point to a critical role for the CD40 pathway in regulating T cell priming in tumors. OVA-expressing KPC tumor clones are rejected in wild type mice but grow progressively in CD40 knock out mice or BATF3 knock out mice that do not have CD103+ DCs (Byrne and Vonderheide, 2016). KPC tumor cells, which do not express CD40, are poor APC. Agonistic CD40 mAb triggers T cell-dependent tumor rejection in certain experimental models with antigenic tumors (Sandin et al., 2014; van Mierlo et al., 2002), and CD40 mAb used in combination with blocking PD-L1 or PD-1 mAb functions to promote tumor regression further (Zippelius et al., 2015). CD40 can drive an IL-12-dependent downregulation of PD-1 expression on T cells, reversing T-ccell exhaustion and permitting tumor response in otherwise PD-1 mAb refractory tumors (Ngiow et al., 2016).
In KPC mice bearing spontaneous tumors, CD40 therapy leads to non-durable tumor regression and transient involution of tumor stroma in a fraction of mice, each dependent on the presence of CD40-activated macrophages (Beatty et al., 2011). T cells are not required for this effect. Although tumor regressions were observed with agonistic CD40 mAb in a clinical trial of patients with metastatic PDA (Beatty et al., 2011; Beatty et al., 2013), neither tumor-bearing patients nor mice demonstrate durable tumor regressions from this approach.
Prior to activation, but not after, DCs have uniquely enhanced capacity to take up antigen (Albert et al., 1998; Heath and Carbone, 2001). This paradigm explains how chemotherapy followed by CD40 activation (but not CD40 followed by chemotherapy) results in the establishment of effective, T-cell dependent immunity and memory in tumor-bearing mice for which CD40 alone is insufficient (Nowak et al., 2003). The rate-limiting step of chemotherapy/CD40 effectiveness appears to be the extent to which the chemotherapy is cytotoxic against the tumor and presumably “spills antigen” (Byrne and Vonderheide, 2016). In the KPC model, gemcitabine (Gem) followed by agonistic CD40 is effective in generating ‘hot’ T cell-inflamed tumors and tumor regression only in a subcutaneously implanted tumors that have a far less complicated TME (Beatty et al., 2015). However, the addition of nab-paclitaxel (nP) to gemcitabine, which is a combination more effective in patients than Gem alone, synergizes to trigger tumor regression, establish T cell memory, and improve survival in subcutaneous, orthotopic, and spontaneous KPC tumors (Byrne and Vonderheide, 2016). This phenotype is entirely dependent on T cells, and not macrophages. In each case, the combination of Gem/nP plus CD40 leads to a marked increase in T cell infiltration in the TME, with a skewing toward IFNγ and TNFα secreting T cells, an increase in activated DCs, and loss of M2 macrophages. In addition, Tregs are cleared from the TME with therapy. Importantly, Gem/nP/CD40 completely fails in BATF3 or CD40 knockout mice, suggesting a critical therapeutic dependence on DCs (Byrne and Vonderheide, 2016).
The anti-tumor effect of Gem/nP chemotherapy with agonistic CD40 antibody is independent of toll-like receptor pathways and other innate signaling mechanisms because tumor regressions are observed in MyD88, TLR4, TLR3, TRIF, Casp 11, IL-1R, and P2X7R knock out mice as well as STING mutant and IFNAR knockout mice (Byrne and Vonderheide, 2016). Thus, CD40 activation with chemotherapy obviates the need for STING or type I interferon activation; however, these observations also suggest that the addition of CD40 mAb with STING agonists, for example, may be synergistic, given the non-redundant aspects of these pathways on DC activation.
From an immune surveillance perspective, these data in KPC mice are important for two reasons. First, the use of chemotherapy plus CD40 agonists has the capacity to sensitize tumors to checkpoint blockade (Figure 1). Combination therapy with Gem/nP/CD40 plus PD-1 antibody further extends the activity and durability of response to chemo/CD40 alone (Winograd et al., 2015). Similarly, agonistic CD40 mAb also cooperate with radiation therapy and checkpoint blockade for tumor regression (Verbrugge et al., 2012). Second, T cell infiltration, tumor regressions, and immunological memory in response to chemotherapy/CD40 is accomplished in a murine model system that does not express classically defined nor alternatively defined (i.e. high DAI) neopeptides (Evans et al., 2016). Although further investigations are ongoing to identify potential neoantigens in the KPC model beyond those arising from non-synonymous tumor mutations, there is evidence that shared antigens are responsible for the effect of Gem/nP/CD40. Cured mice are protected against subsequent challenge with unrelated KPC tumors (Byrne and Vonderheide, 2016; Evans et al., 2016). These data suggest that that are likely other types of tumor rejection antigens beyond those derived from non-synonymous mutations in the tumor.
It is not yet clear if CD40 chemoimmunotherapy, as described above, boosts weak preexisting responses and/or truly primes new anti-tumor T cells. In KPC mice bearing spontaneous tumors, implantation of syngeneic KPC tumor cells subcutaneously (i.e. two tumors) results in CD8+ T cell infiltration into the implanted tumor, and chemotherapy/CD40 triggers regression of subcutaneous tumors (Beatty et al., 2015). In contrast, KPC tumor cells implanted subcutaneously in wild type mice do not trigger the same level of CD8+ T cell infiltration, suggesting that the presence of a spontaneous KPC tumor is associated with a prior priming event in KPC mice (Beatty et al., 2015). In other studies, T cell reactivity against KPC tumors, including against shared KPC antigens, is evident among splenic T cells isolated from of tumor-bearing KPC mice, although the level of this reactivity is very low (Feig et al., 2013). In these experiments, the combination of CXCR4 inhibitor with PD-1 and CTLA-4 mAb – delivered without chemotherapy – results in T cell infiltration and modest, transient regressions (Feig et al., 2013). These observations are consistent with a low-level pre-existing T cell immunity in KPC mice, insufficient to infiltrate or persist in the spontaneous tumor. On the other hand, TCR deep sequencing shows that Gem/nP/CD40 treatment of subcutaneous KPC tumors leads to expansion of certain, pre-existing T cell clones but also is able to recruit new T cell clones specifically to the TME (Byrne and Vonderheide, 2016).
CD40 with vaccines
It is thought that chemotherapy and radiation cooperate with CD40 largely by spilling antigen and as such, function as “vaccines”. CD40 mAb also exhibit capacity to enhance the activity of conventional vaccination in both tumor and non-tumor experimental systems in mice (Li and Ravetch et al, 2011; Vonderheide, 2007). In some studies, CD40 activation substitutes for T cell help. Thus, any reagent that activates adaptive immunity, especially while sparing systemic immune suppression, may be considered a logical partner for CD40 mAb. These possibilities include true “vaccine” approaches (such neoepitope-based vaccines) but also anti-tumor antibodies, oncolytic viruses, and targeted therapy (Vonderheide and Glennie, 2013). In nearly every case studied, the addition of CD40 activation enhances the activity of the “vaccine”. There is strong preclinical data that CD40 activation and TLR agonists may synergize for APC activation (Ahonen et al., 2004; Ahonen et al., 2008; Carpenter et al., 2009; Scarlett et al., 2009). It is also possible that certain chemotherapies or hypofractionated radiation therapy themselves have adjuvant properties and trigger inflammatory or immunogenic signals beyond simply antigen release from dying tumor cells (Demaria et al., 2015; Gandhi et al., 2015; Harding et al., 2017). Finally, STING agonists along with T cell agonists (e.g. CD137 or OX40 agonists) are additional strategies now entering clinical trials aimed at enhancing tumor vaccine activity even further (Broomfield et al., 2009; Uno et al., 2006).
Role for blocking PD-1/PD-L1 and other checkpoints
If T cell vaccination overcomes privilege or ignorance to generate an anti-tumor adaptive response, it is likely that anti-PD-1/PD-L1 will be needed to address T cell exhaustion that subsequently develops after priming. Cancer vaccines developed more than 15 years were shown to break tolerance to tumor antigens, but objective tumor responses in patients were unusual. Yet, none of these vaccines had the benefit of combination with anti-PD-1 or anti-PD-L1. In both humans and mice with PDA, the potential role of anti-PD-1/PD-L1 as an immunological assist after vaccination has been highlighted (Lutz et al., 2014; Winograd et al., 2015) as well as in other tumor models (Zamarin et al., 2014). There are of course many other negative immune “checkpoints” in the TME and stroma beyond PD-1/PD-L1 that may also need to be blocked at the cellular or molecular level to fully enable vaccine therapy (Coussens et al., 2013; Kraman et al., 2011).
Clinical translation
Activation of the CD40 pathway in cancer is therapeutically tractable, as has been previously extensively reviewed (Remer et al., 2017; Vonderheide and Glennie, 2013). Three main approaches have been: (i) recombinant multimeric CD40-ligand (Vonderheide et al., 2001), (ii) gene therapeutic delivery of CD40-ligand (Messmer and Kipps, 2005), and (iii) agonistic CD40 mAb, for which there is the largest clinical experience (Vonderheide and Glennie, 2013). Like all agonists in medicine, dose and schedule of CD40 agonists have been difficult to define, and there remains no consensus on the optimal route of administration. For at least two CD40 agents, a transient, moderate cytokine release syndrome has defined the maximum tolerated dose following intravenous infusion. Typical symptoms include fever, chills, and fatigue that resolve with supportive care over 1 hr to 24 hr in the outpatient setting (Johnson et al., 2017; Vonderheide et al., 2007). Agonistic CD40 mAb infusion has been associated with mild-to-moderate, transient liver function test abnormalities and transient decreases in platelets. There have no reports of autoimmune events involving colitis, hypophysitis, pneumonitis, or uveitis which are characteristic of checkpoint antibodies (Vonderheide and Glennie, 2013). The serum half-life of agonistic CD40 mAb is less than 24 hr, decidedly shorter than typical for human IgG, yet understood in relation to the large sink of CD40 molecules found on, for example, B cells and endothelial cells. Whether CD40 activation of B cells and endothelial cells contributes to treatment-related cytokine release syndrome, or to the mechanism of anti-tumor action for that matter, remains poorly understood.
Objective tumor responses with single-agent agonistic CD40 mAb therapy have been observed, but the rate has been <20% in advanced, metastatic patients with solid tumors (Vonderheide and Glennie, 2013). In the absence of chemotherapy, radiation therapy or another immune combination partner, preclinical studies predict a low single-agent response rate in cold tumors. One patient with refractory metastatic melanoma was treated with repeated doses of CD40 for one year and remains in complete remission for more than a decade without other therapy (Bajor et al., 2014). The adaptive immune response in this patient has been extensively documented (Bajor et al., 2014). Response rates are higher in clinical trials of agonistic CD40 mAb combined with chemotherapy in solid tumors (Beatty et al., 2011; Nowak et al., 2015; Vonderheide et al., 2013), but the CD40 contribution to tumor regressions over and above chemotherapy has not been definitively discerned in randomized studies. The response rate of Gem/CD40 was 23.8% in treatment-naive patients with metastatic pancreatic cancer, with a progression free survival of 5.6 months (Beatty et al., 2011), higher than typically reported for Gem. In patients with metastatic melanoma previously untreated with PD-1 or PD-L1 mAb, the combination of CD40 and CTLA-4 mAb produced a response rate of 27.3% and a 1-year overall survival of 26.1 months (Bajor et al., 2015). Clinical trials are now underway with at least five different CD40 mAb across multiple cancers and in various combinations, including with PD-1, PD-L1, and CSF1R mAb.
Immune pharmacodynamics studies have provided evidence of CD40-induced activation of B cells and DC (Johnson et al., 2015; McDonnell et al., 2017; Ruter et al., 2010; Vonderheide et al., 2007). These effects are dose dependent and transient. From these studies, it seems unlikely that the maximum tolerated dose of agonistic CD40 mAb is the maximum biological dose.
Role of CD40 mAb crosslinking
The bulk of preclinical investigations using agonistic CD40 mAb utilize reagents that require Fc crosslinking by host Fc receptors. Activity of these CD40 mAb is minimal in vitro and lost in vivo in FcR deficient mice (Li and Ravetch, 2011; Richman and Vonderheide, 2014; White et al., 2013; White et al., 2011). Nevertheless, the CD40 therapeutic agent for which there is the largest clinical experience – selicrelumab (formally known as CP-870,893 or RO7009789) – is a fully human IgG2 for which crosslinking is not necessary for activity in vitro (Richman and Vonderheide, 2014). Although selective enhancement of FcγRIIB-binding remains possible and can increase in vivo activity (Dahan et al., 2016), FcR-independent activity of human IgG2 CD40 mAb is also provided by a conformationally distinct subfraction characterized by a unique arrangement of hinge disulfide bonds (White et al., 2015). CP-870,893 also does not compete with the CD40-ligand site on CD40. Each of these features (i.e., lack of required crosslinking, distinction from the CD40-ligand binding site) contrasts those of the anti-CD40 mAb used in the vast majority of murine studies, which absolutely require crosslinking and bind the CD40-ligand binding site. Considering that not all CD40 antibodies are “the same” – and to explore whether this translates into clinical reagents with important distinguishing clinical manifestations – newer CD40 clinical reagents have been designed to mimic the pharmacology of murine reagents on which the large body of preclinical data is based (Remer et al., 2017). APX005M, for example, is an Fc-mutated, humanized IgG1 that requires crosslinking for activity and competes with the CD40-ligand binding site (Johnson et al., 2017). In the first-in-human study, infusional side effects of APX005M were dose dependent and manageable, and immune pharmacodynamic studies revealed strong activation of APC, increased systemic levels of IL-12 and T cell activation after treatment (Johnson et al., 2017). Multiple combination studies with APX005M are underway, including one for treatment-naive patients with metastatic PDA who receive Gem/nP/APX005M with or without PD-1 mAb nivolumab (NCT03214250).
Conclusions and Perspectives
Immune privilege – manifesting with tumor T cell exclusion – is a biology without immunoediting. It may be especially notable in oncogene-driven carcinomas in which tumor-driven immunosuppression establishes in the earliest stages of the disease. Addressing deficient T cell priming, therefore, represents a large opportunity in cancer immunotherapy particularly for PD-1/PD-L1 refractory cancers. CD40 activation is one therapeutically tractable approach with multiple new reagents available to exploit this. Mechanistically, CD40 activation is a proximal event in T cell priming and thus, CD40 mAb may be critical in converting cold tumors to hot and generating effective T cell immunity. In the clinic, concerns that the therapeutic index of CD40 mAb is too narrow to permit clinical activity at tolerable doses have fortunately not been realized. Current efforts are aimed at using CD40 mAb in combination with non-redundant immune modulators, which is likely the best route to success.
Acknowledgments
The author acknowledges support from NIH grants R01 CA169123, P01 CA210944, and P30 CA016520, as well as support from the Parker Institute for Cancer Immunotherapy, Stand Up 2 Cancer-Lustgarten Foundation, the Breast Cancer Research Foundation, and Penn’s Basser Center for BRCA and the Abramson Cancer Center. Helpful discussions were with Drs. Katelyn Byrne, Mark Diamond, Jeffrey Lin, and Ben Stanger.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahonen CL, Doxsee CL, McGurran SM, Riter TR, Wade WF, Barth RJ, Vasilakos JP, Noelle RJ, Kedl RM. Combined TLR and CD40 triggering induces potent CD8+ T cell expansion with variable dependence on type I IFN. J Exp Med. 2004;199:775–784. doi: 10.1084/jem.20031591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahonen CL, Wasiuk A, Fuse S, Turk MJ, Ernstoff MS, Suriawinata AA, Gorham JD, Kedl RM, Usherwood EJ, Noelle RJ. Enhanced efficacy and reduced toxicity of multifactorial adjuvants compared with unitary adjuvants as cancer vaccines. Blood. 2008;111:3116–3125. doi: 10.1182/blood-2007-09-114371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aiello NM, Bajor DL, Norgard RJ, Sahmoud A, Bhagwat N, Pham MN, Cornish TC, Iacobuzio-Donahue CA, Vonderheide RH, Stanger BZ. Metastatic progression is associated with dynamic changes in the local microenvironment. Nat Commun. 2016;7:12819. doi: 10.1038/ncomms12819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert ML, Sauter B, Bhardwaj N. Dendritic cells acquire antigen from apoptotic cells and induce class I-restricted CTLs. Nature. 1998;392:86–89. doi: 10.1038/32183. [DOI] [PubMed] [Google Scholar]
- Aspord C, Pedroza-Gonzalez A, Gallegos M, Tindle S, Burton EC, Su D, Marches F, Banchereau J, Palucka AK. Breast cancer instructs dendritic cells to prime interleukin 13-secreting CD4+ T cells that facilitate tumor development. J Exp Med. 2007;204:1037–1047. doi: 10.1084/jem.20061120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey P, Chang DK, Nones K, Johns AL, Patch AM, Gingras MC, Miller DK, Christ AN, Bruxner TJ, Quinn MC, et al. Genomic analyses identify molecular subtypes of pancreatic cancer. Nature. 2016;531:47–52. doi: 10.1038/nature16965. [DOI] [PubMed] [Google Scholar]
- Bajor DL, Mick R, Riese MJ, Richman LP, Xu X, Torigian DA, Stelekati E, Sweeney M, Sullivan B, Schuchter LM, et al. Combination of agonistic CD40 monoclonal antibody CP-870,893 and anti-CTLA-4 antibody tremelimumab in patients with metastatic melanoma. Cancer Res. 2015;75(15 Suppl):CT137. [Google Scholar]
- Bajor DL, Xu X, Torigian DA, Mick R, Garcia LR, Richman LP, Desmarais C, Nathanson KL, Schuchter LM, Kalos M, Vonderheide RH. Immune activation and a 9-year ongoing complete remission following CD40 antibody therapy and metastasectomy in a patient with metastatic melanoma. Cancer Immunol Res. 2014;2:1051–1058. doi: 10.1158/2326-6066.CIR-14-0154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balachandran VP, Luksza M, Zhao JN, Makarov V, Moral JA, Remark R, Herbst B, Askan G, Bhanot U, Senbabaoglu Y, et al. Identification of unique neoantigen qualities in long-term survivors of pancreatic cancer. Nature. 2017;551:512–516. doi: 10.1038/nature24462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balli D, Rech AJ, Stanger BZ, Vonderheide RH. Immune cytolytic activity stratifies molecular subsets of human pancreatic cancer. Clin Cancer Res. 2017;23:3129–3138. doi: 10.1158/1078-0432.CCR-16-2128. [DOI] [PubMed] [Google Scholar]
- Beatty GL, Chiorean EG, Fishman MP, Saboury B, Teitelbaum UR, Sun W, Huhn RD, Song W, Li D, Sharp LL, et al. CD40 agonists alter tumor stroma and show efficacy against pancreatic carcinoma in mice and humans. Science. 2011;331:1612–1616. doi: 10.1126/science.1198443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beatty GL, Torigian DA, Chiorean EG, Saboury B, Brothers A, Alavi A, Troxel AB, Sun W, Teitelbaum UR, Vonderheide RH, O’Dwyer P. A phase I study of an agonist CD40 monoclonal antibody (CP-870,893) in combination with gemcitabine in patients with advanced pancreatic ductal adenocarcinoma. Clin Cancer Res. 2013;19:6286–95. doi: 10.1158/1078-0432.CCR-13-1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beatty GL, Winograd R, Evans RA, Long KB, Luque SL, Lee JW, Clendenin C, Gladney WL, Knoblock DM, Guirnalda PD, Vonderheide RH. Exclusion of T cells from pncreatic carcinomas in mice is regulated by Ly6C(low) F4/80(+) extratumoral macrophages. Gastroenterology. 2015;149:201–210. doi: 10.1053/j.gastro.2015.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broomfield SA, van der Most RG, Prosser AC, Mahendran S, Tovey MG, Smyth MJ, Robinson BW, Currie AJ. Locally administered TLR7 agonists drive systemic antitumor immune responses that are enhanced by anti-CD40 immunotherapy. J Immunol. 2009;182:5217–5224. doi: 10.4049/jimmunol.0803826. [DOI] [PubMed] [Google Scholar]
- Broz ML, Binnewies M, Boldajipour B, Nelson AE, Pollack JL, Erle DJ, Barczak A, Rosenblum MD, Daud A, Barber DL, et al. Dissecting the tumor myeloid compartment reveals rare activating antigen-presenting cells critical for T cell immunity. Cancer Cell. 2014;26:938. doi: 10.1016/j.ccell.2014.11.010. [DOI] [PubMed] [Google Scholar]
- Byrne KT, Vonderheide RH. CD40 stimulation obviates innate sensors and drives T cell immunity in cancer. Cell Rep. 2016;15:2719–2732. doi: 10.1016/j.celrep.2016.05.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter EL, Mick R, Ruter J, Vonderheide RH. Activation of human B cells by the agonist CD40 antibody CP-870,893 and augmentation with simultaneous toll-like receptor 9 stimulation. J Transl Med. 2009;7:93. doi: 10.1186/1479-5876-7-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casanovas O, Hicklin DJ, Bergers G, Hanahan D. Drug resistance by evasion of antiangiogenic targeting of VEGF signaling in late-stage pancreatic islet tumors. Cancer Cell. 2005;8:299–309. doi: 10.1016/j.ccr.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Ciampricotti M, Hau CS, Doornebal CW, Jonkers J, de Visser KE. Chemotherapy response of spontaneous mammary tumors is independent of the adaptive immune system. Nat Med. 2012;18:344–346. doi: 10.1038/nm.2652. [DOI] [PubMed] [Google Scholar]
- Ciampricotti M, Vrijland K, Hau CS, Pemovska T, Doornebal CW, Speksnijder EN, Wartha K, Jonkers J, de Visser KE. Development of metastatic HER2(+) breast cancer is independent of the adaptive immune system. J Pathol. 2011;224:56–66. doi: 10.1002/path.2837. [DOI] [PubMed] [Google Scholar]
- Clark CE, Hingorani SR, Mick R, Combs C, Tuveson DA, Vonderheide RH. Dynamics of the immune reaction to pancreatic cancer from inception to invasion. Cancer Res. 2007;67:9518–9527. doi: 10.1158/0008-5472.CAN-07-0175. [DOI] [PubMed] [Google Scholar]
- Coughlin CM, Vance BA, Grupp SA, Vonderheide RH. RNA-transfected CD40-activated B cells induce functional T-cell responses against viral and tumor antigen targets: implications for pediatric immunotherapy. Blood. 2004;103:2046–2054. doi: 10.1182/blood-2003-07-2379. [DOI] [PubMed] [Google Scholar]
- Coussens LM, Zitvogel L, Palucka AK. Neutralizing tumor-promoting chronic inflammation: a magic bullet? Science. 2013;339:286–291. doi: 10.1126/science.1232227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahan R, Barnhart BC, Li F, Yamniuk AP, Korman AJ, Ravetch JV. Therapeutic activity of agonistic, human anti-CD40 monoclonal antibodies requires selective FcgammaR engagement. Cancer Cell. 2016;29:820–831. doi: 10.1016/j.ccell.2016.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demaria S, Golden EB, Formenti SC. Role of local radiation therapy in cancer immunotherapy. JAMA Oncol. 2015;1:1325–1332. doi: 10.1001/jamaoncol.2015.2756. [DOI] [PubMed] [Google Scholar]
- DeNardo DG, Barreto JB, Andreu P, Vasquez L, Tawfik D, Kolhatkar N, Coussens LM. CD4(+) T cells regulate pulmonary metastasis of mammary carcinomas by enhancing protumor properties of macrophages. Cancer Cell. 2009;16:91–102. doi: 10.1016/j.ccr.2009.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan F, Duitama J, Al Seesi S, Ayres CM, Corcelli SA, Pawashe AP, Blanchard T, McMahon D, Sidney J, Sette A, et al. Genomic and bioinformatic profiling of mutational neoepitopes reveals new rules to predict anticancer immunogenicity. J Exp Med. 2014;211:2231–2248. doi: 10.1084/jem.20141308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DuPage M, Mazumdar C, Schmidt LM, Cheung AF, Jacks T. Expression of tumour-specific antigens underlies cancer immunoediting. Nature. 2012;482:405–409. doi: 10.1038/nature10803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durai V, Murphy KM. Functions of Murine Dendritic Cells. Immunity. 2016;45:719–736. doi: 10.1016/j.immuni.2016.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelhardt JJ, Boldajipour B, Beemiller P, Pandurangi P, Sorensen C, Werb Z, Egeblad M, Krummel MF. Marginating dendritic cells of the tumor microenvironment cross-present tumor antigens and stably engage tumor-specific T cells. Cancer Cell. 2012;21:402–417. doi: 10.1016/j.ccr.2012.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans RA, Diamond MS, Rech AJ, Chao T, Richardson MW, Lin JH, Bajor DL, Byrne KT, Stanger BZ, Riley JL, et al. Lack of immunoediting in murine pancreatic cancer reversed with neoantigen. JCI Insight. 2016;1:e88328. doi: 10.1172/jci.insight.88328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feig C, Jones JO, Kraman M, Wells RJ, Deonarine A, Chan DS, Connell CM, Roberts EW, Zhao Q, Caballero OL, et al. Targeting CXCL12 from FAP-expressing carcinoma-associated fibroblasts synergizes with anti-PD-L1 immunotherapy in pancreatic cancer. Proc Natl Acad Sci U S A. 2013;110:20212–20217. doi: 10.1073/pnas.1320318110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukunaga A, Miyamoto M, Cho Y, Murakami S, Kawarada Y, Oshikiri T, Kato K, Kurokawa T, Suzuoki M, Nakakubo Y, et al. CD8+ tumor-infiltrating lymphocytes together with CD4+ tumor-infiltrating lymphocytes and dendritic cells improve the prognosis of patients with pancreatic adenocarcinoma. Pancreas. 2004;28:e26–31. doi: 10.1097/00006676-200401000-00023. [DOI] [PubMed] [Google Scholar]
- Gabrilovich D. Mechanisms and functional significance of tumour-induced dendritic-cell defects. Nat Rev Immunol. 2004;4:941–952. doi: 10.1038/nri1498. [DOI] [PubMed] [Google Scholar]
- Gandhi SJ, Minn AJ, Vonderheide RH, Wherry EJ, Hahn SM, Maity A. Awakening the immune system with radiation: Optimal dose and fractionation. Cancer Lett. 2015;368:185–190. doi: 10.1016/j.canlet.2015.03.024. [DOI] [PubMed] [Google Scholar]
- Grewal IS, Flavell RA. The CD40 ligand. At the center of the immune universe? Immunol Res. 1997;16:59–70. doi: 10.1007/BF02786323. [DOI] [PubMed] [Google Scholar]
- Gunderson AJ, Kaneda MM, Tsujikawa T, Nguyen AV, Affara NI, Ruffell B, Gorjestani S, Liudahl SM, Truitt M, Olson P, et al. Bruton tyrosine kinase-dependent immune cell cross-talk drives pancreas cancer. Cancer Discov. 2016;6:270–285. doi: 10.1158/2159-8290.CD-15-0827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding SM, Benci JL, Irianto J, Discher DE, Minn AJ, Greenberg RA. Mitotic progression following DNA damage enables pattern recognition within micronuclei. Nature. 2017;548:466–470. doi: 10.1038/nature23470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath WR, Carbone FR. Cross-presentation, dendritic cells, tolerance and immunity. Annu Rev Immunol. 2001;19:47–64. doi: 10.1146/annurev.immunol.19.1.47. [DOI] [PubMed] [Google Scholar]
- Hingorani SR, Wang L, Multani AS, Combs C, Deramaudt TB, Hruban RH, Rustgi AK, Chang S, Tuveson DA. Trp53R172H and KrasG12D cooperate to promote chromosomal instability and widely metastatic pancreatic ductal adenocarcinoma in mice. Cancer Cell. 2005;7:469–483. doi: 10.1016/j.ccr.2005.04.023. [DOI] [PubMed] [Google Scholar]
- Ino Y, Yamazaki-Itoh R, Oguro S, Shimada K, Kosuge T, Zavada J, Kanai Y, Hiraoka N. Arginase II expressed in cancer-associated fibroblasts indicates tissue hypoxia and predicts poor outcome in patients with pancreatic cancer. PLoS One. 2013a;8:e55146. doi: 10.1371/journal.pone.0055146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ino Y, Yamazaki-Itoh R, Shimada K, Iwasaki M, Kosuge T, Kanai Y, Hiraoka N. Immune cell infiltration as an indicator of the immune microenvironment of pancreatic cancer. Br J Cancer. 2013b;108:914–923. doi: 10.1038/bjc.2013.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson M, Fakih M, Bendell J, Bajor D, Cristea M, Tremblay T, Trifan O, Vonderheide R. First in human study with the CD40 agonistic monoclonal antibody APX005M in subjects with solid tumors. J Immuno Ther Cancer. 2017;5(Suppl 3):89. [Google Scholar]
- Johnson P, Challis R, Chowdhury F, Gao Y, Harvey M, Geldart T, Kerr P, Chan C, Smith A, Steven N, et al. Clinical and biological effects of an agonist anti-CD40 antibody: a Cancer Research UK phase I study. Clin Cancer Res. 2015;21:1321–1328. doi: 10.1158/1078-0432.CCR-14-2355. [DOI] [PubMed] [Google Scholar]
- Kraman M, Bambrough PJ, Arnold JN, Roberts EW, Magiera L, Jones JO, Gopinathan A, Tuveson DA, Fearon DT. Suppression of antitumor immunity by stromal cells expressing fibroblast activation protein-alpha. Science. 2011;330:827–830. doi: 10.1126/science.1195300. [DOI] [PubMed] [Google Scholar]
- Lanzavecchia A. Immunology. Licence to kill. Nature. 1998;393:413–414. doi: 10.1038/30845. [DOI] [PubMed] [Google Scholar]
- Le DT, Durham JN, Smith KN, Wang H, Bartlett BR, Aulakh LK, Lu S, Kemberling H, Wilt C, Luber BS, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. 2017;357:409–413. doi: 10.1126/science.aan6733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F, Ravetch JV. Inhibitory Fcgamma receptor engagement drives adjuvant and anti-tumor activities of agonistic CD40 antibodies. Science. 2011;333:1030–1034. doi: 10.1126/science.1206954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz ER, Kinkead H, Jaffee EM, Zheng L. Priming the pancreatic cancer tumor microenvironment for checkpoint-inhibitor immunotherapy. Oncoimmunology. 2014;3:e962401. doi: 10.4161/21624011.2014.962401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushita H, Vesely MD, Koboldt DC, Rickert CG, Uppaluri R, Magrini VJ, Arthur CD, White JM, Chen YS, Shea LK, et al. Cancer exome analysis reveals a T-cell-dependent mechanism of cancer immunoediting. Nature. 2012;482:400–404. doi: 10.1038/nature10755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonnell AM, Cook A, Robinson BWS, Lake RA, Nowak AK. Serial immunomonitoring of cancer patients receiving combined antagonistic anti-CD40 and chemotherapy reveals consistent and cyclical modulation of T cell and dendritic cell parameters. BMC Cancer. 2017;17:417. doi: 10.1186/s12885-017-3403-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messmer D, Kipps TJ. CD154 gene therapy for human B-cell malignancies. Ann N Y Acad Sci. 2005;1062:51–60. doi: 10.1196/annals.1358.008. [DOI] [PubMed] [Google Scholar]
- Ngiow SF, Young A, Blake SJ, Hill GR, Yagita H, Teng MW, Korman AJ, Smyth MJ. Agonistic CD40 mAb-driven IL12 reverses resistance to anti-PD1 in a T-cell-rich tumor. Cancer Res. 2016;76:6266–6277. doi: 10.1158/0008-5472.CAN-16-2141. [DOI] [PubMed] [Google Scholar]
- Nowak AK, Cook AM, McDonnell AM, Millward MJ, Creaney J, Francis RJ, Hasani A, Segal A, Musk AW, Turlach BA, et al. A phase 1b clinical trial of the CD40-activating antibody CP-870,893 in combination with cisplatin and pemetrexed in malignant pleural mesothelioma. Ann Oncol. 2015;26:2483–2490. doi: 10.1093/annonc/mdv387. [DOI] [PubMed] [Google Scholar]
- Nowak AK, Robinson BW, Lake RA. Synergy between chemotherapy and immunotherapy in the treatment of established murine solid tumors. Cancer Res. 2003;63:4490–4496. [PubMed] [Google Scholar]
- Poschke I, Faryna M, Bergmann F, Flossdorf M, Lauenstein C, Hermes J, Hinz U, Hank T, Ehrenberg R, Volkmar M, et al. Identification of a tumor-reactive T-cell repertoire in the immune infiltrate of patients with resectable pancreatic ductal adenocarcinoma. Oncoimmunology. 2016;5:e1240859. doi: 10.1080/2162402X.2016.1240859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rech AJ, Balli D, Mantero A, Ishwaran H, Nathanson KL, Stanger BZ, Vonderheide RH. Tumor immunity and survival as a function of alternative neopeptides in human cancer. Cancer Immunol Res. 2018 doi: 10.1158/2326-6066.CIR-17-0559. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reck M, Rodriguez-Abreu D, Robinson AG, Hui R, Csoszi T, Fulop A, Gottfried M, Peled N, Tafreshi A, Cuffe S, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. 2016;375:1823–1833. doi: 10.1056/NEJMoa1606774. [DOI] [PubMed] [Google Scholar]
- Remer M, White A, Glennie M, Al-Shamkhani A, Johnson P. The use of anti-CD40 mAb in cancer. Curr Top Microbiol Immunol. 2017;405:165–207. doi: 10.1007/82_2014_427. [DOI] [PubMed] [Google Scholar]
- Richman LP, Vonderheide RH. Role of crosslinking for agonistic CD40 monoclonal antibodies as immune therapy of cancer. Cancer Immunol Res. 2014;3:e28610. doi: 10.1158/2326-6066.CIR-13-0152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts EW, Broz ML, Binnewies M, Headley MB, Nelson AE, Wolf DM, Kaisho T, Bogunovic D, Bhardwaj N, Krummel MF. Critical Role for CD103(+)/CD141(+) dendritic cells bearing CCR7 for tumor antigen trafficking and priming of T cell immunity in melanoma. Cancer Cell. 2016;30:324–336. doi: 10.1016/j.ccell.2016.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruter J, Antonia SJ, Burris HA, 3rd, Huhn RD, Vonderheide RH. Immune modulation with weekly dosing of an agonist CD40 antibody in a phase I study of patients with advanced solid tumors. Cancer Biol Ther. 2010;10:983–993. doi: 10.4161/cbt.10.10.13251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmon H, Idoyaga J, Rahman A, Leboeuf M, Remark R, Jordan S, Casanova-Acebes M, Khudoynazarova M, Agudo J, Tung N, et al. Expansion and activation of CD103(+) dendritic cell progenitors at the tumor site enhances tumor responses to therapeutic PD-L1 and BRAF inhibition. Immunity. 2016;44:924–938. doi: 10.1016/j.immuni.2016.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Paulete AR, Cueto FJ, Martinez-Lopez M, Labiano S, Morales-Kastresana A, Rodriguez-Ruiz ME, Jure-Kunkel M, Azpilikueta A, Aznar MA, Quetglas JI, et al. Cancer immunotherapy with immunomodulatory anti-CD137 and anti-PD-1 monoclonal antibodies requires BATF3-dependent dendritic cells. Cancer Discov. 2016;6:71–79. doi: 10.1158/2159-8290.CD-15-0510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandin LC, Orlova A, Gustafsson E, Ellmark P, Tolmachev V, Totterman TH, Mangsbo SM. Locally delivered CD40 agonist antibody accumulates in secondary lymphoid organs and eradicates experimental disseminated bladder cancer. Cancer Immunol Res. 2014;2:80–90. doi: 10.1158/2326-6066.CIR-13-0067. [DOI] [PubMed] [Google Scholar]
- Scarlett UK, Cubillos-Ruiz JR, Nesbeth YC, Martinez DG, Engle X, Gewirtz AT, Ahonen CL, Conejo-Garcia JR. In situ stimulation of CD40 and Toll-like receptor 3 transforms ovarian cancer-infiltrating dendritic cells from immunosuppressive to immunostimulatory cells. Cancer Res. 2009;69:7329–7337. doi: 10.1158/0008-5472.CAN-09-0835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science. 2011;331:1565–1570. doi: 10.1126/science.1203486. [DOI] [PubMed] [Google Scholar]
- Schultze JL, Michalak S, Seamon MJ, Dranoff G, Jung K, Daley J, Delgado JC, Gribben JG, Nadler LM. CD40 activated human B cells: an alternative source of highly efficient antigen presenting cells to generate autologous antigen-specific T cells for adoptive immunotherapy. J Clin Invest. 1997;100:2757–2765. doi: 10.1172/JCI119822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankaran V, Ikeda H, Bruce AT, White JM, Swanson PE, Old LJ, Schreiber RD. IFNgamma and lymphocytes prevent primary tumour development and shape tumour immunogenicity. Nature. 2001;410:1107–1111. doi: 10.1038/35074122. [DOI] [PubMed] [Google Scholar]
- Sharma P, Allison JP. The future of immune checkpoint therapy. Science. 2015;348:56–61. doi: 10.1126/science.aaa8172. [DOI] [PubMed] [Google Scholar]
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7–30. doi: 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- Spranger S, Dai D, Horton B, Gajewski TF. Tumor-residing Batf3 dendritic cells are required for effector T cell trafficking and adoptive T cell therapy. Cancer Cell. 2017;31:711–723. e714. doi: 10.1016/j.ccell.2017.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stromnes IM, Schmitt TM, Hulbert A, Brockenbrough JS, Nguyen H, Cuevas C, Dotson AM, Tan X, Hotes JL, Greenberg PD, Hingorani SR. T cells engineered against a native antigen can surmount immunologic and physical barriers to treat pancreatic ductal adenocarcinoma. Cancer Cell. 2015;28:638–652. doi: 10.1016/j.ccell.2015.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng MW, Ngiow SF, Ribas A, Smyth MJ. Classifying cancers based on T-cell infiltration and PD-L1. Cancer Res. 2015;75:2139–2145. doi: 10.1158/0008-5472.CAN-15-0255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uno T, Takeda K, Kojima Y, Yoshizawa H, Akiba H, Mittler RS, Gejyo F, Okumura K, Yagita H, Smyth MJ. Eradication of established tumors in mice by a combination antibody-based therapy. Nat Med. 2006;12:693–698. doi: 10.1038/nm1405. [DOI] [PubMed] [Google Scholar]
- van Kooten C, Banchereau J. CD40-CD40 ligand. J Leukoc Biol. 2000;67:2–17. doi: 10.1002/jlb.67.1.2. [DOI] [PubMed] [Google Scholar]
- van Mierlo GJ, den Boer AT, Medema JP, van der Voort EI, Fransen MF, Offringa R, Melief CJ, Toes RE. CD40 stimulation leads to effective therapy of CD40(−) tumors through induction of strong systemic cytotoxic T lymphocyte immunity. Proc Natl Acad Sci U S A. 2002;99:5561–5566. doi: 10.1073/pnas.082107699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbrugge I, Hagekyriakou J, Sharp LL, Galli M, West A, McLaughlin NM, Duret H, Yagita H, Johnstone RW, Smyth MJ, Haynes NM. Radiotherapy increases the permissiveness of established mammary tumors to rejection by immunomodulatory antibodies. Cancer Res. 2012;72:3163–3174. doi: 10.1158/0008-5472.CAN-12-0210. [DOI] [PubMed] [Google Scholar]
- Vonderheide RH. Prospect of targeting the CD40 pathway for cancer therapy. Clin Cancer Res. 2007;13:1083–1088. doi: 10.1158/1078-0432.CCR-06-1893. [DOI] [PubMed] [Google Scholar]
- Vonderheide RH, Bayne LJ. Inflammatory networks and immune surveillance of pancreatic carcinoma. Curr Opin Immunol. 2013;25:200–205. doi: 10.1016/j.coi.2013.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vonderheide RH, Burg JM, Mick R, Trosko JA, Li D, Shaik MN, Tolcher AW, Hamid O. Phase I study of the CD40 agonist antibody CP-870,893 combined with carboplatin and paclitaxel in patients with advanced solid tumors. Oncoimmunology. 2013;2:e23033. doi: 10.4161/onci.23033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vonderheide RH, Dutcher JP, Anderson JE, Eckhardt SG, Stephans KF, Razvillas B, Garl S, Butine MD, Perry VP, Armitage RJ, et al. Phase I study of recombinant human CD40 ligand in cancer patients. J Clin Oncol. 2001;19:3280–3287. doi: 10.1200/JCO.2001.19.13.3280. [DOI] [PubMed] [Google Scholar]
- Vonderheide RH, Flaherty KT, Khalil M, Stumacher MS, Bajor DL, Hutnick NA, Sullivan P, Mahany JJ, Gallagher M, Kramer A, et al. Clinical activity and immune modulation in cancer patients treated with CP-870,893, a novel CD40 agonist monoclonal antibody. J Clin Oncol. 2007;25:876–883. doi: 10.1200/JCO.2006.08.3311. [DOI] [PubMed] [Google Scholar]
- Vonderheide RH, Glennie MJ. Agonistic CD40 antibodies and cancer therapy. Clin Cancer Res. 2013;19:1035–1043. doi: 10.1158/1078-0432.CCR-12-2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White AL, Chan HT, French RR, Beers SA, Cragg MS, Johnson PW, Glennie MJ. FcgammaRIotaIotaB controls the potency of agonistic anti-TNFR mAbs. Cancer Immunol Immunother. 2013;62:941–948. doi: 10.1007/s00262-013-1398-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White AL, Chan HT, French RR, Willoughby J, Mockridge CI, Roghanian A, Penfold CA, Booth SG, Dodhy A, Polak ME, et al. Conformation of the human immunoglobulin G2 hinge imparts superagonistic properties to immunostimulatory anticancer antibodies. Cancer Cell. 2015;27:138–148. doi: 10.1016/j.ccell.2014.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White AL, Chan HT, Roghanian A, French RR, Mockridge CI, Tutt AL, Dixon SV, Ajona D, Verbeek JS, Al-Shamkhani A, et al. Interaction with FcgammaRIIB is critical for the agonistic activity of anti-CD40 monoclonal antibody. J Immunol. 2011;187:1754–1763. doi: 10.4049/jimmunol.1101135. [DOI] [PubMed] [Google Scholar]
- Winograd R, Byrne KT, Evans RA, Odorizzi PM, Meyer AR, Bajor DL, Clendenin C, Stanger BZ, Furth EE, Wherry EJ, Vonderheide RH. Induction of T-cell immunity overcomes complete resistance to PD-1 and CTLA-4 blockade and improves survival in pancreatic carcinoma. Cancer Immunol Res. 2015;3:399–411. doi: 10.1158/2326-6066.CIR-14-0215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wormann SM, Diakopoulos KN, Lesina M, Algul H. The immune network in pancreatic cancer development and progression. Oncogene. 2014;33:2956–2967. doi: 10.1038/onc.2013.257. [DOI] [PubMed] [Google Scholar]
- Zamarin D, Holmgaard RB, Subudhi SK, Park JS, Mansour M, Palese P, Merghoub T, Wolchok JD, Allison JP. Localized oncolytic virotherapy overcomes systemic tumor resistance to immune checkpoint blockade immunotherapy. Sci Transl Med. 2014;6:226ra232. doi: 10.1126/scitranslmed.3008095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zippelius A, Schreiner J, Herzig P, Muller P. Induced PD-L1 expression mediates acquired resistance to agonistic anti-CD40 treatment. Cancer Immunol Res. 2015;3:236–244. doi: 10.1158/2326-6066.CIR-14-0226. [DOI] [PubMed] [Google Scholar]