Abstract
Asthma is related to various cardiovascular (CV) risk. Whether a history of asthma from childhood contributes to arterial stiffness in adulthood, a non-invasive surrogate for CV events, is unknown. Prospective analyses were performed among 1746 Bogalusa Heart Study participants aged 20 to 51 years with data on self-report asthma collected since childhood. Aorta-femoral pulse wave velocity (af-PWV, m/s) was repeatedly assessed among adults aged 18 years and over. Generalized linear mixed models and generalized linear models were fitted for the repeated measurements of af-PWV and its changes between the last and the first measurements, respectively. After a median follow-up of 11.1 years, participants with a history of asthma from childhood had a higher af-PWV (6.78 vs. 6.20, p=0.048) and a greater increase in af-PWV (8.99 vs. 2.95, p=0.043) than those without asthma, adjusted for age, gender, race, smoking status, heart rate, body mass index (BMI), systolic blood pressure (SBP), lipids, and glycemia. In addition, we found significant interactions of asthma with BMI and SBP on af-PWV and its changes (p for interaction<0.01). The associations of asthma with af-PWV and its changes appeared to be stronger among participants who were overweight and obese (BMI≥25 kg/m2) or with prehypertension and hypertension (SBP≥120 mm Hg), compared with those with a normal BMI or SBP. Our findings indicate that a history of asthma from childhood is associated with higher af-PWV and greater increases in af-PWV, and such associations are stronger among young adults who are overweight or with elevated blood pressure.
Keywords: asthma, arterial stiffness, pulse wave velocity, systolic blood pressure, obesity, childhood, longitudinal analysis
Introduction
Arterial stiffness expressed as pulse wave velocity (PWV) is a functional marker of arterial vulnerability and recognized as organ damage, resulting from aging, arteriosclerosis, and elevated blood pressure.1–4 In hypertensive patients or those with established diseases (e.g., type 2 diabetes, heart disease, chronic kidney and pulmonary diseases), persistent elevation of aortic PWV is related to high risk for future death and adverse cardiovascular (CV) events.5–7 Growing evidence has shown that accelerated stiffening of central elastic arteries, especially in children and young adults, is a strong independent predictor for CV events, cardiac disease, renal disease, and future death.2,8–11 Recently, a growing body of evidence has linked increased PWV with chronic inflammation,12–15 independent of traditional CV risk factors.
Asthma is a chronic inflammatory disorder characterized by inflamed and narrowed airways, affecting 8.4% (6.2 million) of children and 7.6% (18.4 million) of adults in the US in 2015.16 Adult patients with asthma have been found to have an increased risk of incident stroke and total CV events.17,18 However, there are limited data linking asthma with subclinical vessel changes and cardiac dysfunction, and the findings are inconsistent.19–21 Several cross-sectional studies of both pediatric and adult populations have found a positive association between asthma and PWV.19,21–23 To our knowledge, there have been no prospective studies assessing the relationship between a history of asthma from childhood and aortic PWV and its changes in young adults.
In this study, we evaluated the association between a history of asthma from childhood and aorta-femoral PWV (af-PWV) in young adulthood using data from the Bogalusa Heart Study, an ongoing, community-based, epidemiologic study begun in 1973.24 In addition, we particularly investigated potential interactions between asthma and major CV risk factors.
Methods
Study Participants and Data Source
The Bogalusa Heart Study (BHS) is a community-based, biracial (65% white and 35% black), series of surveys to determine the natural history of CVD and its risk factors, established by Dr. Gerald S. Berenson in 1973.24 A total of ~16,000 individuals have been recruited in BHS surveys, including nine surveys of children and adolescents aged 4–19 years from 1973 till 1994 and 11 surveys of adults aged 20 to 52 years who were examined during childhood and remained accessible from 1977 till now.25 It now consists of series of surveys and is ongoing still, with examinations every 2 to 3 years from childhood through adulthood. Thus, the repeated measurements of both CV function and CVD risk factors enabled longitudinal analyses in a unique childhood-to-adulthood cohort setting. A detailed description of study design and participant recruitment in BHS was provided elsewhere.26 Data, analytic methods, and study materials are available to other researchers through National Heart, Lung, and Blood Institute BioLINCC repository at https://biolincc.nhlbi.nih.gov/studies/bhs/ , or through ClinicalTrials.gov at https://clinicaltrials.gov/ct2/show/NCT00005129.
In the current study, baseline information on a history of asthma since childhood was collected among 8289 individuals using a self-administered questionnaire from September 27, 1983 to December 29, 2010. Non-invasive cardiac subclinical markers derived from echocardiography were measured as the outcomes in the follow-ups from February 14, 2001 to December 29, 2010. We excluded subjects who only had baseline information on asthma, or who didn’t have subclinical cardiac measurements in the follow-ups, or whose self-reported asthma was reported after cardiac markers were measured. In addition, we excluded subjects who had a physician-diagnosed or self-reported CVD from baseline to follow-up, including arteritis, angina pectoris, angioplasty, blockage in ventricle, bypass surgery, congenital heart disease, congestive cardiac failure, enlarged left ventricle, heart murmur, heart attack, mitral valve prolapse, mitral valve stenosis, stroke, tachyarrhythmia, ventricular arrhythmia, valve replacement, and unspecified mitral disease.27
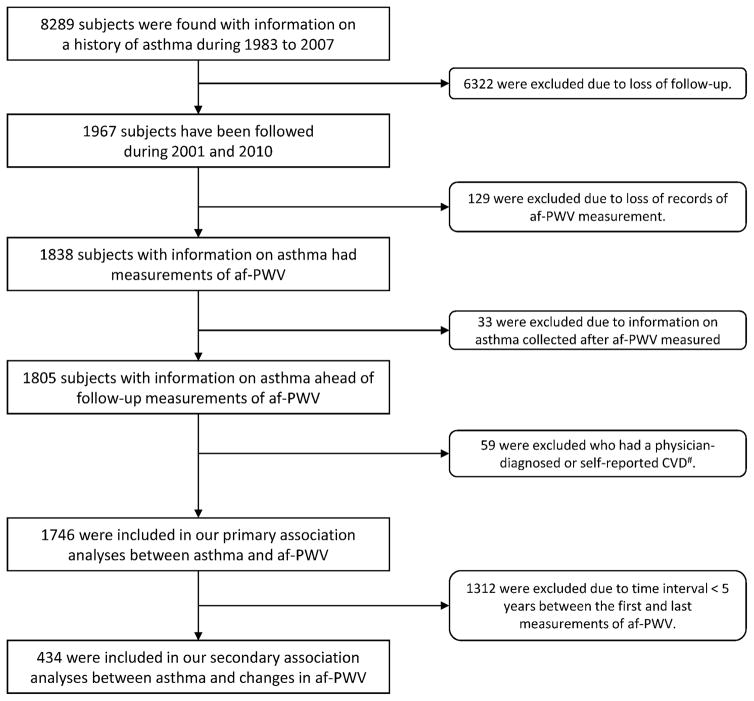
After applying the exclusion criteria, a total of 1746 participants formed the study cohort (Figure 1), with one to four follow-ups apiece. In order to examine the impact of asthma on longitudinal changes in af-PWV, a minimum of 5 years’ time interval between the first and last echocardiographs was required for included participants (434 subjects included). Written informed consent was obtained from adult participants and from parent/guardian of children and adolescent at each examination circle. The study protocol was approved by the biomedical committee of the Tulane University Institutional Review Board.
Figure 1. Flowchart of the study sample.
af-PWV, aorta-femoral pulse wave velocity; CVD, cardiovascular disease; #, CVD from baseline to follow-up, including arteritis, angina pectoris, angioplasty, blockage in ventricle, bypass surgery, congenital heart disease, congestive cardiac failure, enlarged left ventricle, heart murmur, heart attack, mitral valve prolapse, mitral valve stenosis, stroke, tachyarrhythmia, ventricular arrhythmia, valve replacement, and unspecified mitral disease.
History of Asthma
Individual information on a history of asthma was collected by questionnaire. Asthma prevalence was measured by the child’s parent/guardian’s responding to the question “does your child now have asthma or has your child had asthma in the past?”. The survey time was approximately considered as the age of asthma diagnosis for children and teenagers. Adults aged over 18 years of old were asked: “do you now have asthma or have you had asthma in the past?”. For participants who reported having asthma in the past, they were further asked: “when were you first diagnosed with asthma?”. If this information was missing, the survey time then became the proxy time of diagnosis. For those with multiple times of reported asthma, the first report of asthma was considered as the baseline for asthmatic subjects, and the time when firstly reporting of no asthma was used for those who never reported asthma from 1983 till 2010.27
Assessment of Aorta-femoral Pulse Wave Velocity
Aorta-femoral pulse wave velocity (af-PWV) were measured using the Toshiba Power Vision SSH-380 (Toshiba America Medical Systems, Tustin, CA), with one non-directional transcutaneous Doppler flow probe (Toshiba PSK25AT, 2.5 MHz) positioned at the suprasternal notch, and another probe (Toshiba PCK703AT, 7.5 MHz) at left femoral artery in a supine position.28 The arterial flow waves from the two arterial sites were recorded. The distance between the aorta and femoral arteries was measured with a caliper instrument.29 The difference in timing between the two waves represents the time component of the velocity equation. Then af-PWV was calculated by dividing the distance traveled by the time differential between 2 waveforms, and the mean value of af-PWV from 3 repeated measurements was used for each participant.30 Additionally, a random 6% sample of participants were selected for repeat measurements 10 to 12 days apart. As described elsewhere30,31, the coefficient of variation for inter-reader and intra-reader variability for all measures of cardiac anatomy was less than 10%. The echocardiography was performed among adults aged 18 years and over, with height ranged from 133 to 196 cm.
Assessment of Covariates
Demographic information were all self-reported or reported by parents or guardians on self-administrated questionnaires, including age in years at screening, gender (male/female), race (white/black), smoking status (current/non-current smokers), use of antihypertensive medicine (Yes/No), medication for dyslipidemia (Yes/No), and medication for glycemia (Yes/No). Physical and laboratory examinations followed rigid protocols performed by trained examiners. Body mass index (BMI, weight in kilograms divided by the square of the height in meters) was repeatedly measured, and the mean values were used for analysis. Resting heart rate (beats per min) was counted at the radial pulse in a relaxed sitting position, and three measurements were obtained separated by brief intervals. Systolic and diastolic blood pressures (the 4th Korotkoff phase for children and the 5th for adults) were measured using a mercury sphygmomanometer by two well-trained observers (3 replicates each). The cutoff value of 120 mm Hg for systolic blood pressure (SBP), according to the update in the 2017 Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults,32 was used to define prehypertension and hypertension in the current study The mean value of the six readings of blood pressure was used for analysis. Fasting overnight was required for laboratory examination. Total cholesterol (TC) and low-density lipoprotein cholesterol (LDL-C) levels (mg/dL) were determined by enzymatic procedures on the Abbott VP instrument (Abbott Laboratories, Chicago, IL) from 1987 to 1996, and on the Hitachi 902 Automatic Analyzer (Roche Diagnostics, Indianapolis, IN) after 1996. Fasting plasma glucose (mg/dL) was measured by a glucose oxidase method using a Beckman Glucose Analyzer (Beckman Instruments, Palo Alto, CA) from 1981 to 1991, and measured enzymatically as part of a multichemistry (SMA20) profile since 1991.33 Information on covariates was obtained during the same day when the echocardiography was completed in the examining center.
Statistical Analyses
Characteristics of the subjects were compared between asthma and non-asthma groups, using the chi-square test for categorical variables, and the Student’s t-test or Kruskal-Wallis test for continuous variables with normal or skewed distributions, respectively. We fitted a generalized linear mixed model (GLMM) to examine the association of asthma with repeated af-PWV measures. Statistical adjustments were made for age, gender, race, smoking status, use of antihypertensive medicine, medication for dyslipidemia, medication for glycemia, total cholesterol, LDL-C, fasting glucose, and heart rate as fixed effects, as well as individual ID as random effects. Further, we estimated the least-squares means and confidence intervals of af-PWV with adjustment for covariates. To examine the potential interactions, we categorized age (<35 and ≥35 years), heart rate (<70 and ≥70 beats per min), SBP (<120 and ≥120 mm Hg), total cholesterol (<200 and ≥200 mg/dL), LDL-C (<130 and ≥130 mg/dL), fasting glucose (<100 and ≥100 mg/dL) into two groups, and BMI (<25, 25–30, and ≥30 kg/m2) into three groups (Table 3), and then included the interaction term of asthma and each covariate (considered as both categorical and continuous variables) in the GLMM model. Similarly, we fitted a generalized linear model (GLM) to assess the association between asthma and the changes in af-PWV (Δaf-PWV) with additional adjustments of the first af-PWV measurement and the time interval between the first and last af-PWV measurement. A two-sided P-value <0.05 was considered as statistically significant, and false discovery rate (FDR) from p values was computed for multiple testing corrections using the Benjamine & Hochberg. All data management and analyses were conducted using R version 3.3.0 released on May 3, 2016.
Table 3.
Adjusted means (95% CI) of aorta-femoral pulse wave velocity and its changes by asthma according to demographics and covariates
| Subgroups | af-PWV*, m/s (95% CI)
|
PFDR for interaction | Δaf-PWV#, m/s (95% CI)
|
PFDR for interaction | ||
|---|---|---|---|---|---|---|
| Without Asthma (n=1370) | With Asthma (n=129) | Without Asthma (n=402) | With Asthma (n=32) | |||
| Age, yrs | 0.219† | <0.001† | ||||
| <35 | 5.37 (3.86, 6.89) | 6.50 (4.69, 8.32) | 2.83 (−0.48, 6.14) | 11.28 (2.02, 20.53) | ||
| ≥35 | 6.74 (6.07, 7.40) | 6.95 (5.95, 7.96) | 2.99 (0.84, 5.15) | 5.13 (1.15, 9.11) | ||
| Race | 0.937† | 0.290† | ||||
| Whites | 5.83 (5.03, 6.63) | 6.50 (5.37, 7.63) | 2.90 (0.46, 5.34) | 11.11 (3.17, 19.06) | ||
| Blacks | 6.67 (5.89, 7.45) | 7.33 (6.29, 8.37) | 4.51 (1.96, 7.05) | 6.36 (2.44, 10.29) | ||
| Gender | 0.213† | 0.075† | ||||
| Male | 6.49 (5.75, 7.24) | 6.71 (5.60, 7.82) | 3.68 (−2.99, 10.35) | 2.05 (−7.93, 12.03) | ||
| Female | 5.81 (4.85, 6.77) | 6.98 (5.70, 8.26) | −0.55 (−11.43, 10.33) | 12.41 (−1.2, 26.02) | ||
| Current smoking | 0.004† | 0.090† | ||||
| Yes | 5.59 (3.63, 7.56) | 7.82 (5.27, 10.38) | 10.24 (−7.16, 27.65) | 24.48 (−0.74, 49.71) | ||
| No | 6.17 (5.68, 6.66) | 6.14 (5.43, 6.85) | 2.47 (−0.33, 5.27) | 3.71 (0.11, 7.31) | ||
| Heart Rate, beats/m | 0.303‡ | 0.373‡ | ||||
| <70 | 5.97 (5.46, 6.48) | 6.33 (5.67, 6.98) | 3.02 (0.46, 5.58) | 9.90 (2.59, 17.22) | ||
| ≥70 | 6.70 (5.60, 7.80) | 8.08 (6.22, 9.95) | 2.29 (0.80, 3.77) | 1.42 (−3.49, 6.33) | ||
| BMI, kg/m2 | 0.007‡ | <0.001‡ | ||||
| <25 | 5.39 (1.27, 9.52) | 5.16 (0.64, 9.67) | 4.10 (0.22, 7.98) | −0.19 (−13.45, 13.07) | ||
| 25~30 | 6.73 (5.79, 7.67) | 7.60 (6.38, 8.81) | 2.13 (1.12, 3.15) | 1.81 (−3.21, 6.82) | ||
| ≥30 | 6.34 (5.65, 7.02) | 7.21 (6.15, 8.26) | 3.13 (−2.65, 8.91) | 17.05 (5.84, 28.25) | ||
| SBP, mm Hg | 0.002‡ | 0.023‡ | ||||
| <120 | 5.64 (4.75, 6.53) | 5.40 (4.27, 6.53) | 4.17 (−4.33, 12.67) | 4.91 (−5.79, 15.60) | ||
| ≥120 | 6.75 (5.88, 7.62) | 9.01 (7.64, 10.38) | 3.89 (−4.45, 12.23) | 17.01 (4.03, 29.99) | ||
| Total cholesterol, mg/dL | 0.138‡ | 0.207‡ | ||||
| <200 | 5.99 (5.27, 6.71) | 7.07 (6.07, 8.08) | 3.67 (−5.36, 12.69) | 12.21 (0.72, 23.71) | ||
| ≥200 | 6.52 (5.61, 7.43) | 6.36 (4.84, 7.88) | 2.04 (1.17, 2.92) | 0.63 (−3.51, 4.78) | ||
| LDL-C, mg/dL | 0.048‡ | 0.361‡ | ||||
| <130 | 5.85 (5.15, 6.56) | 7.06 (6.08, 8.03) | 5.52 (−9.70, 20.74) | 11.67 (−5.18, 28.51) | ||
| ≥130 | 6.81 (5.84, 7.79) | 6.14 (4.54, 7.74) | 2.75 (0.41, 5.09) | 0.06 (−5.69, 5.81) | ||
| Fasting glucose, mg/dL | 0.766‡ | 0.821‡ | ||||
| <100 | 5.84 (4.73, 6.96) | 6.40 (5.15, 7.65) | 2.05 (−5.23, 9.34) | 8.23 (−1.28, 17.75) | ||
| ≥100 | 7.63 (6.57, 8.68) | 8.26 (5.88, 10.65) | 2.98 (−10.90, 16.86) | 4.20 (−25.39, 33.80) | ||
af-PWV, aorta-femoral pulse wave velocity; Δaf-PWV, longitudinal changes in aorta-femoral pulse wave velocity; BMI, Body mass index; SBP, systolic blood pressure; LDL-C, low-density lipoprotein cholesterol; 95% CI, 95% confidence interval.
Stratified analysis was performed according to different groups of each covariate. Generalized linear mixed models (GLMM) were used to adjust for age (yrs), gender (male/female), race (black/white), smoking status (current smokers/non-current smokers), use of antihypertensive medicine (Yes/No), medication for dyslipidemia (Yes/No), medication for glycemia (Yes/No), total cholesterol (mg/dL), low-density lipoprotein cholesterol (mg/dL), fasting glucose (mg/dL), and heart rate (beats per min) as fixed effects, as well as individual ID as random effects. The interaction term of asthma and according to covariate was included in the GLMMs.
P values with FDR correction of the interaction term in each GLMM/GLM models were given, considering both asthma and the covariates as binary variables.
P values with FDR correction of the interaction term in each GLMM/GLM models were given, considering both asthma and the covariates as continuous variables.
Stratified analysis was performed according to different groups of each covariate. Generalized linear models (GLM) were used to adjust for age (yrs), follow-up period (yrs), gender (male/female), race (black/white), smoking status (current smokers/non-current smokers), use of antihypertensive medicine (Yes/No), total cholesterol (mg/dL), low-density lipoprotein cholesterol (mg/dL), fasting glucose (mg/dL), heart rate (beats per min), and PWV at baseline (m/s). The interaction term of asthma and according to covariate was included in the GLMs.
Results
Table 1 summarizes the characteristics of the study subjects at both baseline and follow-up by asthma status. With a median follow-up of 11.1 years, 1746 participants were included, with a total of 3944 follow-up measurements of af-PWV, and 56.2% of them were followed 2~4 times. Of the longitudinal cohort (58.2% were women, and 28.9% were blacks), 9.7% had a self-reported history of asthma. The median (interquartile range) of age was 29.0 (15.0, 34.0) and 37.0 (29.0, 43.0) years of old at baseline and in the last follow-up, respectively. Compared with participants without asthma, subjects who had a history of asthma were younger at both baseline (22.5 vs. 29.5, p<0.01) and last follow-up (36.0 vs. 37.0, p=0.02) and had a higher BMI (30.8 vs. 29.3, p=0.02). No significant difference was found in gender, race, smoking, SBP, heart rate, TC, LDL-C, fasting glucose, and medication for hypertension, dyslipidemia, and diabetes.
Table 1.
Characteristics of the study cohorts by asthma
| Variables | Without Asthma (n=1577) | With Asthma (n=169) | P# |
|---|---|---|---|
| Baseline† | |||
| Age, yrs | 29.5 (15.0, 34.0) | 22.5 (14.0, 32.0) | <0.01 |
| Female, n (%) | 920 (58.3) | 97 (57.4) | 0.81 |
| Blacks, n (%) | 465 (29.5) | 65 (23.1) | 0.02 |
| Follow-up observations‡, n (%) | 3606 (91.4) | 338 (8.6) | |
| Individuals with # times of follow-ups‡, n (%) | <0.01 | ||
| 1 time | 675 (42.8) | 89 (52.7) | |
| 2 times | 189 (12.0) | 22 (13.0) | |
| 3 times | 299 (19.0) | 27 (16.0) | |
| 4 times | 414 (26.2) | 31 (18.3) | |
| Follow-up‡, years | 11.1 (5.5, 13.0) | 11.2 (3.1, 16.9) | 0.51 |
| Follow-up† (last measurement) | |||
| Age, yrs | 37.0 (30.0, 43.0) | 36.0 (26.0, 41.0) | 0.02 |
| Current smokers, n (%) | 483 (30.6) | 46 (27.2) | 0.36 |
| BMI, kg/m2 | 29.3 (7.7) | 30.8 (8.1) | 0.02 |
| SBP, mm Hg | 115.3 (14.3) | 116.0 (13.9) | 0.57 |
| Heart rate, beats per min | 70.9 (9.2) | 70.7 (9.3) | 0.75 |
| Total cholesterol, mg/dL | 189.2 (39.7) | 185.9 (38.4) | 0.31 |
| Low-density lipoprotein cholesterol, mg/dL | 121.9 (34.3) | 118.0 (31.9) | 0.13 |
| Fasting glucose, mg/dL | 84.0 (78.0, 92.0) | 86.0 (78.0, 92.0) | 0.47 |
| Medication for high blood pressure, n (%) | 207 (13.1) | 31 (18.3) | 0.08 |
| Medication for dyslipidemia, n (%) | 87 (5.5) | 9 (5.3) | 1.00 |
| Medication for diabetes, n (%) | 57 (3.6) | 7 (4.1) | 0.90 |
Continuous variables were described as either means (standard deviation) if normal distribution satisfied, or medians (Interquartile range) if normal distribution is unsatisfied.
In the follow-ups, 764 participants were with just one-time follow-up measurement, and the rest of 982 participants were with 2 to 4 repeated follow-up af-PWV measurements, which added up to a total of 3180 observations. The follow-up years were calculated starting from the baseline questionnaire until the last af-PWV measurement.
Two groups’ characteristics were compared using the Student’s t-test or Kruskal-Wallis test for continuous variables, and chi-square test for categorical variables.
BMI, body mass index; SBP, systolic blood pressure.
In Table 2, participants with a history of asthma since childhood had significantly higher af-PWV in their young adulthood, compared with those without asthma after full adjustment (6.78 vs. 6.13, p=0.048). We also examined the association of asthma with the changes in af-PWV (Δaf-PWV, m/s), measured during a median of 11.1 years (interquartile range: 5.4–13.0 years) among 434 subjects. Of note, participants with a history of asthma had almost a threefold greater af-PWV compared with those without asthma (adjusted means of Δaf-PWV: 9.52 vs. 3.60, p=0.032). Further adjustment for SBP or/and BMI did not appreciably change the associations (p<0.05).
Table 2.
Adjusted means (95% CI) of aorta-femoral pulse wave velocity and its changes by asthma
| Outcomes | Models | Without Asthma | With Asthma | P |
|---|---|---|---|---|
| af-PWV, m/s | ||||
| Model 1† | 6.20 (5.61, 6.79) | 6.89 (6.06, 7.73) | 0.04 | |
| Model 1† + SBP | 6.19 (5.60, 6.78) | 6.87 (6.04, 7.70) | 0.04 | |
| Model 1† + BMI | 6.08 (5.48, 6.68) | 6.73 (5.89, 7.57) | 0.05 | |
| Model 1† + SBP + BMI | 6.13 (5.53, 6.72) | 6.78 (5.95, 7.62) | 0.05 | |
| Δaf-PWV‡, m/s | ||||
| Model 1‡ | 3.60 (−0.91, 8.11) | 9.52 (2.82, 16.22) | 0.03 | |
| Model 1‡ + SBP | 2.98 (−2.67, 8.62) | 9.06 (1.22, 16.90) | 0.04 | |
| Model 1‡ + BMI | 2.95 (−2.70, 8.60) | 8.99 (1.15, 16.83) | 0.04 | |
| Model 1‡ + SBP + BMI | 2.95 (−2.73, 8.63) | 8.99 (1.11, 16.88) | 0.04 | |
af-PWV, aorta-femoral pulse wave velocity; Δaf-PWV, longitudinal changes in aorta-femoral pulse wave velocity; BMI, body mass index; SBP, systolic blood pressure.
Model 1 used a generalized linear mixed model (GLMM) in participants with asthma (n=169) or without asthma (n=1577), controlled for age (yrs), gender (male/female), race (black/white), smoking status (current smokers/non-current smokers), use of antihypertensive medicine (Yes/No), medication for dyslipidemia (Yes/No), medication for glycemia (Yes/No), total cholesterol (mg/dL), low-density lipoprotein cholesterol (mg/dL), fasting glucose (mg/dL), and heart rate (beats per min) as fixed effects, as well as individual ID as random effects.
The time interval between the first and last PWV measurement was ranged from 5.0 to 16.1 years (median=11.5). Model 1 used a generalized linear model (GLM) in participants with asthma (n=32) or without asthma (n=402), controlled for age (yrs), interval time between the first and the last af-PWV measurements (yrs), gender (male/female), race (black/white), smoking status (current smokers/non-current smokers), use of antihypertensive medicine (Yes/No), total cholesterol (mg/dL), low-density lipoprotein cholesterol (mg/dL), fasting glucose (mg/dL), heart rate (beats per min), and PWV at baseline (m/s).
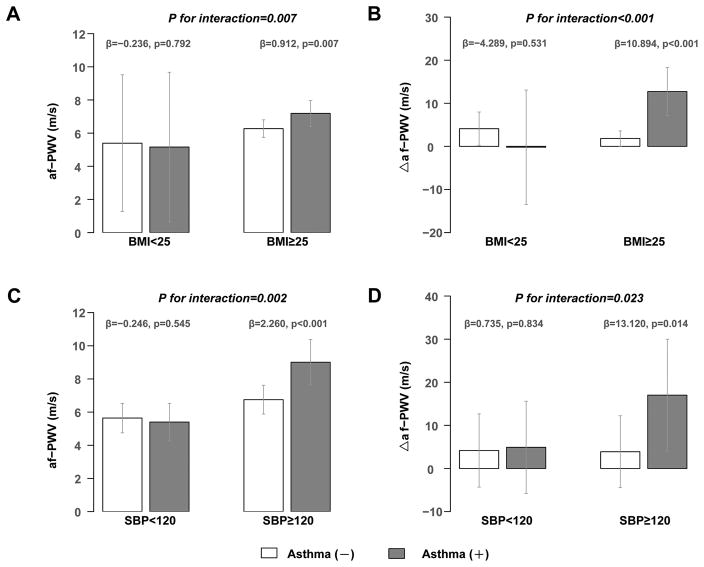
We further analyzed the potential interactions between a history of asthma and major cardiovascular risk factors on af-PWV and its changes, using the Benjamini & Hochberg FDR procedure for multiple-testing correction (Table 3). We found that both BMI and SBP significantly modified the association of asthma with af-PWV and Δaf-PWV (PFDR for interaction < 0.05). For young adults who were overweight or obese (BMI≥25 kg/m2), significant differences in af-PWV and Δaf-PWV were both observed between asthma and non-asthma group (adjusted means: 7.19 vs. 6.27 for af-PWV in Figure 2a, 12.73 vs. 1.83 for Δaf-PWV in Figure 2b, p values for both regression coefficients (β) <0.01). For participants who were with prehypertension or hypertension (SBP≥120 mmHg), similar differences were also shown between two groups (β=2.26 for af-PWV in Figure 2c, p<0.01; β=13.12 for Δaf-PWV in Figure 2d, p=0.01), whereas among subjects with normal weight (BMI<25 kg/m2) or SBP less than 120 mmHg, the difference in af-PWV and Δaf-PWV between asthma and non-asthma groups was not significant (β=−0.236 and −4.289, p=0.792 and 0.531, respectively, for af-PWV in Figure 2a and Δaf-PWV in Figure 2b; β= −0.246 and 0.735, p=0.545 and 0.834, respectively, for af-PWV in Figure 2c and Δaf-PWV in Figure 2d). No significant interactions were observed between asthma and other risk factors.
Figure 2. Interaction of Asthma with BMI and SBP on aorta-femoral Pulse Wave Velocity and its longitudinal Changes.
Adjusted means and 95% confident intervals of the repeated measurements of aorta-femoral pulse wave velocity (af-PWV) were calculated using generalized linear mixed models (GLMM) stratified by BMI (Figure 2a) and SBP (Figure 2c), controlled for age, gender, race, smoking status, use of antihypertensive medicine, medication for dyslipidemia, medication for glycaemia, total cholesterol, low-density lipoprotein cholesterol, fasting glucose, and heart rate as fixed effects, as well as individual ID as random effects.
Adjusted means and 95% confident intervals of the 11-year changes in af-PWV (Δaf-PWV) were calculated using generalized linear models (GLM) stratified by BMI (Figure 2b) and SBP (Figure 2d), controlled for age, gender, race, smoking status, use of antihypertensive medicine, total cholesterol, low-density lipoprotein cholesterol, fasting glucose, and heart rate.
β was the regression coefficient of asthma on af-PWV (the left) and its changes (the right) in stratified BMI and SBP groups in GLMMs or GLMs, followed by its p values for statistical significance. P values with FDR correction for interaction term were given by including the interaction term in the GLMMs or GLMs.
Discussion
In this longitudinal cohort of a median follow-up of 11-year, a history of asthma from childhood was significantly associated with a greater af-PWV and accelerated the increase in Δaf-PWV among asymptomatic young adults, independent of traditional CV risk factors. We observed significant interactions of asthma with SBP and BMI on af-PWV and Δaf-PWV; the positive associations of asthma with af-PWV and its change appeared to be stronger among participants with higher BMI (overweight and obese) or higher SBP (prehypertension and hypertension), compared with those who had a lower BMI and SBP.
To our knowledge, the current study is the first to prospectively analyze the association between a history of asthma from childhood and arterial stiffness in young adults. Results from a meta-analysis of 17 prospective cohort studies showed 1.0 m/s increase in aortic PWV was associated with 14%, 15%, and 15% increase in total CV events, CV mortality, and all-cause mortality, respectively.11 Of note, our study participants are young adults, a small increase in af-PWV is a reliable marker for an abnormally accelerated stiffing in central arteries in the early adulthood.34 In addition, previous studies have indicated that significant clinical difference in cardiovascular risks in middle age might originate from a small difference of PWV in early adulthood.11,35 Our results were in line with several previous cross-sectional studies performed in middle-aged adults, in which asthma and its severity were related to a higher risk of elevated arterial stiffness.21,23 However, such associations were inconsistent in children and adolescents.19,22 Our findings showing asthma associated with PWV indicate that a history of asthma from childhood may contribute to the acceleration of central arterial stiffness and subsequently increased CV risk. While our previous findings have suggested an adverse impact of asthma on left ventricular (LV) mass,27 a structural measure of myocardial vulnerability, the current study provides incremental evidence of its detrimental impact on impaired vessel function.
In the current study, a history asthma from childhood is independently associated with higher af-PWV and a greater increase in Δaf-PWV. However, the potential mechanisms underlying these associations remain unclear. Given the fact that asthmatic patients were accompanied by elevated levels of inflammatory biomarkers,36–38 as well as increased arterial stiffness in chronic inflammatory disorders,13,14 the shared pathogenesis of systemic inflammation could play an important role in linking asthma and arterial stiffness. Pulmonary function decline in asthmatics might also partly account for this association. Wu and colleagues39 demonstrated higher brachial-ankle PWV with restrictive spirometry patterns, a 116% and 95% increase in the risk of arterial stiffness in men and women, respectively. In addition, medicine for asthma (including oral corticosteroids, short-acting β agonists, and their combinations) has been reported to increase the risk of incident CV events and death.40,41 Moreover, patients with asthma demonstrate a more sedentary lifestyle,42–44 and such unhealthy lifestyle may also adversely affect aortic stiffness.45–47
Intriguingly, we found the significant interactions of asthma with BMI and SBP on af-PWV and Δaf-PWV—the associations of a history of asthma from childhood on central arterial stiffness and its progress in a later life were stronger among young adults who were overweight and obese, or who had pre-hypertension or hypertension. Supportively, two longitudinal population-based studies showed similar interactions between asthma and hypertension on coronary vasospastic angina and peripheral artery disease (p for interaction=0.062 and 0.142, respectively).48,49 Despite the lack of direct evidence, such interactions were biologically possible. Considered the most common comorbidities in the asthmatics,50–53 both obesity54 and high BP55,56 may worsen the existing risk profiles, including systematic inflammation,5,51,57,58 leptin and adiponectin regulation,59–61 and vascular trophic factors,62,63 which leads to acceleration of arterial stiffness during the aging process.
In the current study, the community-based prospective cohort provided us a unique opportunity to examine the association of a history of asthma from childhood with arterial stiffness among asymptomatic young adults. Repeated echocardiographic measurements during a long follow-up period enabled us to evaluate the impact of asthma on the progress of aortic stiffness over time. The availability of covariate information enabled us to control for potential confounders and performing interaction analyses. In addition, correction for multiple testing was adopted in our interaction analyses to reduce type I error. However, several limitations should be considered. First, data on asthma was self-reported from questionnaires, rather than physician diagnosed or through clinical records. Second, the relatively small sample size yielded wide 95% confidence intervals in subgroups. Third, although a variety of covariates was carefully controlled for in our analyses, residual confounders remained (e.g., childhood af-PWV at baseline, the severity of asthma and its medication, pulmonary function, physical activity, excessive alcohol consumption, and diet). Forth, it would be better to include systemic inflammatory biomarkers at baseline in our major analyses to elucidate the presumed mechanism between systematic inflammation/oxidative stress at baseline and increased arterial stiffness in follow-ups.
Perspectives
Our results for the first time provided evidence that a history of asthma from childhood was significantly associated with higher af-PWV and a greater increase in af-PWV over time in young adults, independent of traditional CV risk factors. Additionally, such associations were more prominent among participants who are overweight or with elevated SBP. Our data underscore the importance for the prevention and control of asthma in pediatric and young adult population, and the need for lifestyle interventions among asthmatic patients, especially those with a high BMI or elevated SBP, to mitigate CV complications.
Novelty and Significance.
What Is New?
A history of asthma from childhood was associated with accelerated aortic stiffness among asymptomatic young adults, independent of traditional cardiovascular risk factors.
The detrimental impact of asthma on aortic stiffness were stronger among participants who were overweight and obese, or with elevated systolic blood pressure.
What Is Relevant?
Elevated systolic blood pressure precedes large-artery stiffening in middle-aged adults.
Elevated systemic inflammation, declined pulmonary function, and more sedentary lifestyle in asthmatic patients may contribute to development and progression of hypertension.
Summary
A history of asthma from childhood may contribute to accelerated aortic stiffness in adulthood, and such detrimental impact is stronger in participants who are overweight or with elevated blood pressure, which underscores the importance of targeted interventions among asthmatic patients.
Acknowledgments
Sources of Funding
The study was supported by grants from the National Heart, Lung, and Blood Institute (HL071981, HL034594, HL126024), the National Institute of Diabetes and Digestive and Kidney Diseases (DK091718, DK100383, DK078616), the Boston Obesity Nutrition Research Center (DK46200), and United States–Israel Binational Science Foundation Grant2011036. Dr. Qi was a recipient of the American Heart Association Scientist Development Award (0730094N).
Abbreviations list (in alphabetical order)
- af-PWV
aorta-femoral pulse wave velocity
- BMI
body mass index
- LDL-C
low-density lipoprotein cholesterol
- PWV
pulse wave velocity
- SBP
systolic blood pressure
Footnotes
Conflict-of-interest/disclosure
None.
References
- 1.Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: The Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC) European Heart Journal. 2013;34:2159–2219. doi: 10.1093/eurheartj/eht151. [DOI] [PubMed] [Google Scholar]
- 2.Townsend RR, Wilkinson IB, Schiffrin EL, Avolio AP, Chirinos JA, Cockcroft JR, Heffernan KS, Lakatta EG, McEniery CM, Mitchell GF, Najjar SS, Nichols WW, Urbina EM, Weber T. Recommendations for Improving and Standardizing Vascular Research on Arterial Stiffness: A Scientific Statement from the American Heart Association. 2015;66 doi: 10.1161/HYP.0000000000000033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hansen TW, Staessen JA, Torp-Pedersen C, Rasmussen S, Thijs L, Ibsen H, Jeppesen J. Prognostic value of aortic pulse wave velocity as index of arterial stiffness in the general population. Circulation. 2006;113:664–670. doi: 10.1161/CIRCULATIONAHA.105.579342. [DOI] [PubMed] [Google Scholar]
- 4.Chen W, Li S, Fernandez C, Sun D, Lai C-C, Zhang T, Bazzano L, Urbina EM, Deng H-W. Temporal Relationship Between Elevated Blood Pressure and Arterial Stiffening Among Middle-Aged Black and White Adults: The Bogalusa Heart Study. American Journal of Epidemiology. 2016;183:599–608. doi: 10.1093/aje/kwv274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Payne RA, Wilkinson IB, Webb DJ. Arterial stiffness and hypertension: Emerging concepts. Hypertension. 2010;55:9–14. doi: 10.1161/HYPERTENSIONAHA.107.090464. [DOI] [PubMed] [Google Scholar]
- 6.Orlova I, Nuraliev EY, Yarovaya EB, Ageev FT. Prognostic value of changes in arterial stiffness in men with coronary artery disease. Vascular Health and Risk Management. 2010;6:1015. doi: 10.2147/VHRM.S13591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.London GM, Pannier B, Guerin AP, Marchais SJ, Safar ME, Cuche JL. Cardiac hypertrophy, aortic compliance, peripheral resistance, and wave reflection in end-stage renal disease. Comparative effects of ACE inhibition and calcium channel blockade. Circulation. 1994;90:2786–2796. doi: 10.1161/01.cir.90.6.2786. [DOI] [PubMed] [Google Scholar]
- 8.Chow B, Rabkin SW. The relationship between arterial stiffness and heart failure with preserved ejection fraction: a systemic meta-analysis. Heart Failure Reviews. 2015;20:291–303. doi: 10.1007/s10741-015-9471-1. [DOI] [PubMed] [Google Scholar]
- 9.Ben-Shlomo Y, Spears M, Boustred C, et al. Aortic Pulse Wave Velocity Improves Cardiovascular Event Prediction. Journal of the American College of Cardiology. 2014;63:636–646. doi: 10.1016/j.jacc.2013.09.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van Sloten TT, Sedaghat S, Laurent S, London GM, Pannier B, Ikram MA, Kavousi M, Mattace-Raso F, Franco OH, Boutouyrie P, Stehouwer CDA. Carotid stiffness is associated with incident stroke: a systematic review and individual participant data meta-analysis. Journal of the American College of Cardiology. 2015;66:2116–2125. doi: 10.1016/j.jacc.2015.08.888. [DOI] [PubMed] [Google Scholar]
- 11.Vlachopoulos C, Aznaouridis K, Stefanadis C. Prediction of Cardiovascular Events and All-Cause Mortality With Arterial Stiffness. A Systematic Review and Meta-Analysis. Journal of the American College of Cardiology. 2010;55:1318–1327. doi: 10.1016/j.jacc.2009.10.061. [DOI] [PubMed] [Google Scholar]
- 12.Sabit R, Bolton CE, Edwards PH, Pettit RJ, Evans WD, McEniery CM, Wilkinson IB, Cockcroft JR, Shale DJ. Arterial Stiffness and Osteoporosis in Chronic Obstructive Pulmonary Disease. American Journal of Respiratory and Critical Care Medicine. 2007;175:1259–1265. doi: 10.1164/rccm.200701-067OC. [DOI] [PubMed] [Google Scholar]
- 13.Booth AD, Wallace S, McEniery CM, Yasmin J, Brown J, Jayne DRW, Wilkinson IB. Inflammation and arterial stiffness in systemic vasculitis: A model of vascular inflammation. Arthritis & Rheumatism. 2004;50:581–588. doi: 10.1002/art.20002. [DOI] [PubMed] [Google Scholar]
- 14.Arroyo-Espliguero R, Mollichelli N, Avanzas P, Zouridakis E, Newey VR, Nassiri DK, Kaski JC. Chronic inflammation and increased arterial stiffness in patients with cardiac syndrome X. European Heart Journal. 2003;24:2006–2011. doi: 10.1016/j.ehj.2003.09.029. [DOI] [PubMed] [Google Scholar]
- 15.Liang Y-L, Shiel LM, Teede H, Kotsopoulos D, McNeil J, Cameron JD, McGrath BP, Sammaritano L, Levine DM, Shankar B-A, Moeller E, Salmon JE. Effects of Blood Pressure, Smoking, and Their Interaction on Carotid Artery Structure and Function. Hypertension (Dallas, Tex: 1979) 2001;37:6–11. doi: 10.1161/01.hyp.0000168055.89955.db. [DOI] [PubMed] [Google Scholar]
- 16.(US) NC for HS. Health, United States, 2015. National Center for Health Statistics (US); 2016. [Accessed November 27, 2017]. http://www.ncbi.nlm.nih.gov/pubmed/27308685. [Google Scholar]
- 17.Xu M, Xu J, Yang X. Asthma and Risk of Cardiovascular Disease or All-Cause Mortality: A Meta-Analysis. Annals of Saudi Medicine. 2017;37:99–105. doi: 10.5144/0256-4947.2017.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wen L, Ni H, Li K, Yang H, Cheng J, Wang X, Zhao D, Xie M, Su H. Asthma and Risk of Stroke: A Systematic Review and Meta-analysis. Journal of Stroke and Cerebrovascular Diseases. 2016;25:497–503. doi: 10.1016/j.jstrokecerebrovasdis.2015.11.030. [DOI] [PubMed] [Google Scholar]
- 19.Ulger Z, Gulen F, Ozyurek AR. Abdominal aortic stiffness as a marker of atherosclerosis in childhood-onset asthma: a case-control study: cardiovascular topics. Cardiovascular Journal Of Africa. 2015;26:8–12. doi: 10.5830/CVJA-2014-046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bobrov LL, Obrezan AG, Sereda VP. Left ventricular diastolic function in patients with bronchial asthma. [Accessed November 28, 2017];Klinicheskaia meditsina. 2003 81:35–40. http://www.ncbi.nlm.nih.gov/pubmed/12856567. [PubMed] [Google Scholar]
- 21.Tuleta I, Skowasch D, Aurich F, Eckstein N, Schueler R, Pizarro C, Schahab N, Nickenig G, Schaefer C, Pingel S. Asthma is associated with atherosclerotic artery changes. In: Feng Y-M, editor. PloS one. Vol. 12. 2017. p. e0186820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Steinmann M, Abbas C, Singer F, Casaulta C, Regamey N, Haffner D, Fischer D-CC, Simonetti GD. Arterial stiffness is increased in asthmatic children. European Journal of Pediatrics. 2015;174:519–523. doi: 10.1007/s00431-014-2423-2. [DOI] [PubMed] [Google Scholar]
- 23.Sun W-X, Jin D, Li Y, Wang R-T. Increased arterial stiffness in stable and severe asthma. Respiratory medicine. 2014;108:57–62. doi: 10.1016/j.rmed.2013.10.021. [DOI] [PubMed] [Google Scholar]
- 24.Srinivasan SR, Frerichs RR, Webber LS, Berenson GS. Serum lipoprotein profile in children from a biracial community: the Bogalusa Heart Study. Circulation. 1976;54:309–318. doi: 10.1161/01.cir.54.2.309. [DOI] [PubMed] [Google Scholar]
- 25.Du Y, Zhang T, Sun D, Li C, Bazzano L, Qi L, Krousel-Wood M, He J, Whelton PK, Chen W, Li S. Effect of Serum Adiponectin Levels on the Association Between Childhood Body Mass Index and Adulthood Carotid Intima-Media Thickness. American Journal of Cardiology. 2018;121:579–583. doi: 10.1016/j.amjcard.2017.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Berenson GS. Bogalusa Heart Study: A Long-Term Community Study of a Rural Biracial (Black/White) Population. The American Journal of the Medical Sciences. 2001;322:267–274. doi: 10.1097/00000441-200111000-00007. [DOI] [PubMed] [Google Scholar]
- 27.Sun D, Wang T, Heianza Y, Lv J, Han L, Rabito F, Kelly T, Li S, He J, Bazzano L, Chen W, Qi L. A History of Asthma From Childhood and Left Ventricular Mass in Asymptomatic Young Adults: The Bogalusa Heart Study. JACC: Heart Failure. 2017:5. doi: 10.1016/j.jchf.2017.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bhuiyan AR, Srinivasan SR, Chen W, Paul TK, Berenson GS. Correlates of vascular structure and function measures in asymptomatic young adults: the Bogalusa Heart Study. Atherosclerosis. 2006;189:1–7. doi: 10.1016/j.atherosclerosis.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 29.Chen W, Srinivasan SR, Berenson GS. Differential impact of heart rate on arterial wall stiffness and thickness in young adults: The Bogalusa Heart Study. Journal of the American Society of Hypertension : JASH. 2008;2:152–157. doi: 10.1016/j.jash.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 30.Bazzano LA, Belame SN, Patel DA, Chen W, Srinivasan S, McIlwain E, Berenson GS. Obesity and left ventricular dilatation in young adulthood: The Bogalusa Heart Study. Clinical Cardiology. 2011;34:153–159. doi: 10.1002/clc.20896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yun M, Li S, Sun D, Ge S, Lai C-C, Fernandez C, Chen W, Srinivasan SR, Berenson GS. Tobacco smoking strengthens the association of elevated blood pressure with arterial stiffness: the Bogalusa Heart Study. Journal of hypertension. 2015;33:266–274. doi: 10.1097/HJH.0000000000000410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Whelton PK, Committee W, Carey RM, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults. Journal of the American College of Cardiology. 2017:735–1097. doi: 10.1016/j.jacc.2017.11.006. [DOI] [Google Scholar]
- 33.Sun D, Li S, Zhang X, Fernandez C, Chen W, Srinivasan SR, Berenson GS. Uric acid is associated with metabolic syndrome in children and adults in a community: the Bogalusa Heart Study. PloS one. 2014;9:e89696. doi: 10.1371/journal.pone.0089696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mattace-Raso FUS, Hofman A, Verwoert GC, et al. Determinants of pulse wave velocity in healthy people and in the presence of cardiovascular risk factors: “Establishing normal and reference values. ” European Heart Journal. 2010;31:2338–2350. doi: 10.1093/eurheartj/ehq165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Najjar SS, Scuteri A, Shetty V, Wright JG, Muller DC, Fleg JL, Spurgeon HP, Ferrucci L, Lakatta EG. Pulse wave velocity is an independent predictor of the longitudinal increase in systolic blood pressure and of incident hypertension in the Baltimore Longitudinal Study of Aging. Journal of the American College of Cardiology. 2008;51:1377–1383. doi: 10.1016/j.jacc.2007.10.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Silvestri M, Bontempelli M, Giacomelli M, Malerba M, Rossi GA, Di Stefano A, Rossi A, Ricciardolo FLM. High serum levels of tumour necrosis factor-? and interleukin-8 in severe asthma: markers of systemic inflammation? Clinical & Experimental Allergy. 2006;36:1373–1381. doi: 10.1111/j.1365-2222.2006.02502.x. [DOI] [PubMed] [Google Scholar]
- 37.Jensen ME, Gibson PG, Collins CE, Wood LG. Airway and systemic inflammation in obese children with asthma. The European respiratory journal. 2013;42:1012–1019. doi: 10.1183/09031936.00124912. [DOI] [PubMed] [Google Scholar]
- 38.HIGASHIMOTO Y, YAMAGATA Y, TAYA S, IWATA T, OKADA M, ISHIGUCHI T, SATO H, ITOH H. Systemic inflammation in chronic obstructive pulmonary disease and asthma: Similarities and differences. Respirology. 2007;0:071023220449015. doi: 10.1111/j.1440-1843.2007.01170.x. -??? [DOI] [PubMed] [Google Scholar]
- 39.Wu I-H, Sun Z-J, Lu F-H, Yang Y-C, Chou C-Y, Chang C-J, Wu J-S. Restrictive Spirometry Pattern Is Associated With Increased Arterial Stiffness in Men and Women. Chest. 2017;152:394–401. doi: 10.1016/j.chest.2017.03.039. [DOI] [PubMed] [Google Scholar]
- 40.Singh S, Loke YK, Furberg CD. Inhaled Anticholinergics and Risk of Major Adverse Cardiovascular Events in Patients With Chronic Obstructive Pulmonary Disease. Jama. 2008;300:1439. doi: 10.1001/jama.300.12.1439. [DOI] [PubMed] [Google Scholar]
- 41.Iribarren C, Tolstykh IV, Miller MK, Sobel E, Eisner MD. Adult asthma and risk of coronary heart disease, cerebrovascular disease, and heart failure: A prospective study of 2 matched cohorts. American Journal of Epidemiology. 2012;176:1014–1024. doi: 10.1093/aje/kws181. [DOI] [PubMed] [Google Scholar]
- 42.Williams B, Powell A, Hoskins G, Neville R. Exploring and explaining low participation in physical activity among children and young people with asthma: a review. BMC Family Practice. 2008;9:40. doi: 10.1186/1471-2296-9-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lang DM, Butz AM, Duggan AK, Serwint JR. Physical activity in urban school-aged children with asthma. Pediatrics. 2004;113:e341–6. doi: 10.1542/PEDS.113.4.E341. [DOI] [PubMed] [Google Scholar]
- 44.McPherson AC, Glazebrook C, Forster D, James C, Smyth A, Newbould R, Smyth A. A Randomized, Controlled Trial of an Interactive Educational Computer Package for Children With Asthma. PEDIATRICS. 2006;117:1046–1054. doi: 10.1542/peds.2005-0666. [DOI] [PubMed] [Google Scholar]
- 45.Yun M, Li S, Sun D, Ge S, Lai C-C, Fernandez C, Chen W, Srinivasan SR, Berenson GS. Tobacco smoking strengthens the association of elevated blood pressure with arterial stiffness: the Bogalusa Heart Study. Journal of hypertension. 2015;33:266–274. doi: 10.1097/HJH.0000000000000410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.van de Laar RJJ, Stehouwer CDA, van Bussel BCT, te Velde SJ, Prins MH, Twisk JWR, Ferreira I. Lower lifetime dietary fiber intake is associated with carotid artery stiffness: the Amsterdam Growth and Health Longitudinal Study. The American journal of clinical nutrition. 2012;96:14–23. doi: 10.3945/ajcn.111.024703. [DOI] [PubMed] [Google Scholar]
- 47.O’Donovan C, Lithander FE, Raftery T, Gormley J, Mahmud A, Hussey J. Inverse Relationship between Physical Activity and Arterial Stiffness in Adults with Hypertension. Journal of Physical Activity and Health. 2014;11:272–277. doi: 10.1123/jpah.2012-0075. [DOI] [PubMed] [Google Scholar]
- 48.Yao C-W, Shen T-C, Lu C-R, Wang Y-C, Lin C-L, Tu C-Y, Hsia T-C, Shih C-M, Hsu W-H, Sung F-C. Asthma Is Associated With a Subsequent Risk of Peripheral Artery Disease: A Longitudinal Population-Based Study. Medicine. 2016;95:e2546. doi: 10.1097/MD.0000000000002546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hung M-J, Mao C-T, Hung M-Y, Chen T-H. Impact of Asthma on the Development of Coronary Vasospastic Angina: A Population-Based Cohort Study. Medicine. 2015;94:e1880. doi: 10.1097/MD.0000000000001880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Christiansen SC, Schatz M, Yang SJ, Ngor E, Chen W, Zuraw BL. Hypertension and Asthma: A Comorbid Relationship. Journal of Allergy and Clinical Immunology: In Practice. 2016;4:76–81. doi: 10.1016/j.jaip.2015.07.009. [DOI] [PubMed] [Google Scholar]
- 51.Pauletto P, Rattazzi M. Inflammation and hypertension: The search for a link. Nephrology Dialysis Transplantation. 2006;21:850–853. doi: 10.1093/ndt/gfl019. [DOI] [PubMed] [Google Scholar]
- 52.Flaherman V. A meta-analysis of the effect of high weight on asthma. Archives of Disease in Childhood. 2006;91:334–339. doi: 10.1136/adc.2005.080390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Beuther DA, Sutherland ER. Overweight, obesity, and incident asthma: A meta-analysis of prospective epidemiologic studies. American Journal of Respiratory and Critical Care Medicine. 2007;175:661–666. doi: 10.1164/rccm.200611-1717OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kravitz L. The growing problem of obesity. Journal of the American Society of Hypertension. 2015;9:44–97. doi: 10.1161/ATVBAHA.115.305753. [DOI] [PubMed] [Google Scholar]
- 55.Dominiczak AF, Kuo D. Hypertension: Update 2017. Hypertension. 2017;69:3–4. doi: 10.1161/HYPERTENSIONAHA.116.08683. [DOI] [PubMed] [Google Scholar]
- 56.Humphrey JD, Harrison DG, Figueroa CA, Lacolley P, Laurent S. Central Artery stiffness in hypertension and aging a problem with cause and consequence. Circulation Research. 2016;118:379–381. doi: 10.1161/CIRCRESAHA.115.307722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Safar ME. Obesity, Arterial Stiffness, and Cardiovascular Risk. Journal of the American Society of Nephrology. 2006;17:S109–S111. doi: 10.1681/ASN.2005121321. [DOI] [PubMed] [Google Scholar]
- 58.Shore SA. Obesity and asthma: Possible mechanisms. Journal of Investigational Allergology and Clinical Immunology. 2008;18:420–425. doi: 10.1016/j.jaci.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 59.Bruno A, Pace E, Chanez P, Gras D, Vachier I, Chiappara G, La Guardia M, Gerbino S, Profita M, Gjomarkaj M. Leptin and leptin receptor expression in asthma. Journal of Allergy and Clinical Immunology. 2009;124:230–237. e4. doi: 10.1016/j.jaci.2009.04.032. [DOI] [PubMed] [Google Scholar]
- 60.Sood A. Association between leptin and asthma in adults. Thorax. 2006;61:300–305. doi: 10.1136/thx.2004.031468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ma W, Huang T, Wang M, Zheng Y, Wang T, Heianza Y, Sun D, Smith SR, Bray GA, Sacks FM, Qi L. Two-year changes in circulating adiponectin, ectopic fat distribution and body composition in response to weight-loss diets: the POUNDS Lost Trial. International Journal of Obesity. 2016;40:1723–1729. doi: 10.1038/ijo.2016.128. [DOI] [PubMed] [Google Scholar]
- 62.Drazner MH. The progression of hypertensive heart disease. Circulation. 2011;123:327–334. doi: 10.1161/CIRCULATIONAHA.108.845792. [DOI] [PubMed] [Google Scholar]
- 63.Williams AS, Chen L, Kasahara DI, Si H, Wurmbrand AP, Shore SA. Obesity and airway responsiveness: Role of TNFR2. Pulmonary Pharmacology & Therapeutics. 2013;26:444–454. doi: 10.1016/j.pupt.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]