Synopsis
The recent, widespread success of mechanical circulatory support has ushered in a new era of cardiovascular medicine in which numerous implantable devices exist to treat advanced heart failure. As cardiac assist devices gain prevalence in the clinical management of cardiovascular disease, it is increasingly important to raise awareness of novel device systems, the unique mechanisms by which they function, and implications for patient management. In this article, we present state-of-the-art devices that are currently under development or in clinical trials. Devices are categorized as Standard Full-Support (HeartMate III, CorAide, Evaheart LVAS), Less-Invasive Full-Support (MVAD), Partial-Support (CircuLite Synergy Pocket Micro-Pump, Reitan Catheter Pump, Procyrion CAD, C-Pulse, Symphony Counterpulsation Device) Right Ventricular Assist Device (RVAD; DexAide, Impella RD Recover, Impella RP), and Total Artificial Heart (TAH; CardioWest, AbioCor II, Continuous-Flow TAH, Continuous-Flow BiVAD). Implantation strategy, mechanism of action, durability, efficacy, hemocompatibility, and human factors such as quality of life during device support are considered. The feasibility of novel strategies for unloading the failing heart is examined.
Keywords: heart failure, left ventricular assist device (LVAD), right ventricular assist device (RVAD), total artificial heart (TAH), pulsatile flow, continuous flow, full-support, partial-support
Introduction
Adult patients with advanced heart failure that is refractory to pharmacological and electrical resynchronization therapies have a limited prognosis. For select patients, heart transplantation offers the best opportunity for long-term survival. However, the number of available donor hearts (~3,500 annually worldwide) is inadequate to meet the needs of more than 30,000 patients listed for heart transplantation worldwide each year1. As a result, cardiac transplant waiting lists have the highest mortality rate (30%) of any of the solid organ waiting lists2. If a patient does receive a donor heart, chronic rejection and sequelae of long-term immunosupression limit post-transplant survival to 55% at 10 years3. Furthermore, many patients are not considered for transplantation due to age, comorbidities, or even inadequate insurance coverage. As a result, more patients are being considered for destination-therapy with a permanent, implantable cardiac assist device. Indeed, over the past decade mechanical circulatory support therapies have emerged as a standard, long-term therapy for adult patients with advanced, intractable heart failure.
Currently, more than 20 novel cardiac assist devices are under development or in clinical trials with nearly a dozen new systems poised to begin clinical trials during the first half of the decade4–18. Each device entails unique surgical and physiological considerations and offers benefits and drawbacks for the patient and the physician. For example, currently available left ventricular assist devices (LVADs) require extensive surgery but can replace cardiac function. These “full-support” devices are reserved as a final treatment option only for patients with life-threatening heart failure. Consequently, physicians may be reluctant to refer less-sick patients for invasive, full-support LVAD therapy.
In order to expand the role of mechanical circulatory support for the treatment of heart failure, investigators and industry partners are miniaturizing LVADs for less-invasive and earlier therapy. Small devices that are designed to provide moderate or “partial-support” in heart failure patients with less advanced disease may be employed before the onset of irreversible myocardial damage and end organ dysfunction. It has been speculated that partial unloading of the failing left ventricle will interrupt the progressive hemodynamic deterioration observed in heart failure7 as well as increase functional capacity, improve quality of life, and promote myocardial recovery. Although there is limited data to support this hypothesis, initial clinical results with partial-support devices are encouraging7,17,19,20.
Right ventricular failure remains a major concern after orthotopic heart transplantation or the implantation of an LVAD and accounts for half of early mortality with an LVAD21–23. As the number of patients with LVADs increases, the incidence of right ventricular failure will also increase. As such, novel right ventricular assist devices (RVADs) that are easy to place and remove are gaining attention for the treatment of right ventricular failure.
In parallel, the total artificial heart (TAH) has gained further acceptance as a therapy for patients with irreversible biventricular failure24. The success of current TAHs has prompted industry to reduce the size of approved TAHs to fit in a broader range of body sizes. More recently, concurrent implantation of two continuous-flow, full-support devices has emerged as a potential long-term therapy for patients with biventricular failure25. If proven safe and efficacious, therapy with dual, intracorporeal rotary pumps adds an additional treatment modality for end-stage, biventricular failure.
As mechanical circulatory support gains prevalence in the clinical management of cardiovascular disease, it is increasingly important to raise awareness of novel systems, the unique physiological mechanisms by which each functions, and implications for patient management. In this review article, we present state-of-the-art devices that are currently under development or in clinical trials. Devices are categorized as 1) Full-Support, 2) Less-Invasive Full-Support, 3) Partial-Support, 4) RVAD, and 5) TAH. Implantation strategy, mechanism of action, durability, efficacy, hemocompatibility, and quality of life during device support are considered. The feasibility of novel strategies for unloading the failing heart are examined.
Standard Full-Support LVADs
Over the past two decades, full-support LVADs have evolved into a standard therapy for patients with end-stage heart failure26–28 as a bridge to cardiac transplantation29, as destination-therapy29, or as a bridge to myocardial recovery30,31. In 2002, the milestone REMATCH trial demonstrated clinical success with pulsatile LVADs as a long-term therapy for end-stage heart failure patients27. Subsequently, the United States Food and Drug Administration (FDA) approved use of the pulsatile HeartMate XVE as a destination-therapy device in patients ineligible for cardiac transplantation32.
More recently, first generation pulsatile LVADs which mimic native cardiac function have essentially been replaced by rotary blood pumps which continuously unload the failing left ventricle33. In 2008 and 2010 the FDA approved the HeartMate II continuous-flow LVAD for patients as a bridge to transplant34 and then as a destination-therapy35, respectively. Compared to first-generation pulsatile devices, the HeartMate II and other second-generation continuous-flow devices are smaller, more reliable and durable, more energy efficient, less thrombogenic, and less surgically traumatic to implant. Importantly, superior clinical outcomes have been established with continuous-flow devices—with nearly 3,000 patients logged, the Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS) documented a recent 1-year survival rate of 75%36.
Yet, better clinical outcomes are necessary before prolonged LVAD support is more widely accepted. For example, currently approved devices contain parts that wear down such as polymeric valves and diaphragms or mechanical contact bearings. To counter this limitation and increase device durability, third-generation full-support devices include a bearingless, magnetically suspended impeller that does not wear over time or generate frictional heat6. Lack of pulsatile blood flow, excessive anticoagulation, device-induced coagulopathy, pump thrombosis, and morbidity related to driveline infections have also raised concerns. Consequently, continuous-flow pulsation algorithms are being developed to generate pulsatility37,38, textured interior surfaces have been revisited to minimize anticoagulation requirements and clotting39, and totally implantable systems with transcutaneous energy transfer (TET) technology are gaining attention40. As device companies incorporate these features into new systems, full-support LVAD therapy is likely to further increase in clinical success and prevalence. Close on the horizon, the Thoratec HeartMate III, Cleveland Heart CorAide, and EVAHEART left ventricular assist system (LVAS) hold promise as novel, full-support LVADs.
HeartMate III
The HeartMate III (Thoratec Corporation, Pleasanton, CA) is a compact (69 × 30 mm; 535 g; 175 ml displacement), bearingless centrifugal-flow LVAD with a magnetically levitated impeller that is designed for long-term destination-therapy4. Via median sternotomy, the flexible inflow cannula (Figure 1A) is inserted in the left ventricular apex. The outflow graft is sewn end-to-side to the ascending aorta (Figure 1B). As blood is drawn into the device, the impeller imparts angular acceleration to the blood flow as it is delivered into the aorta. The patient wears an extracorporeal driver and electronics.
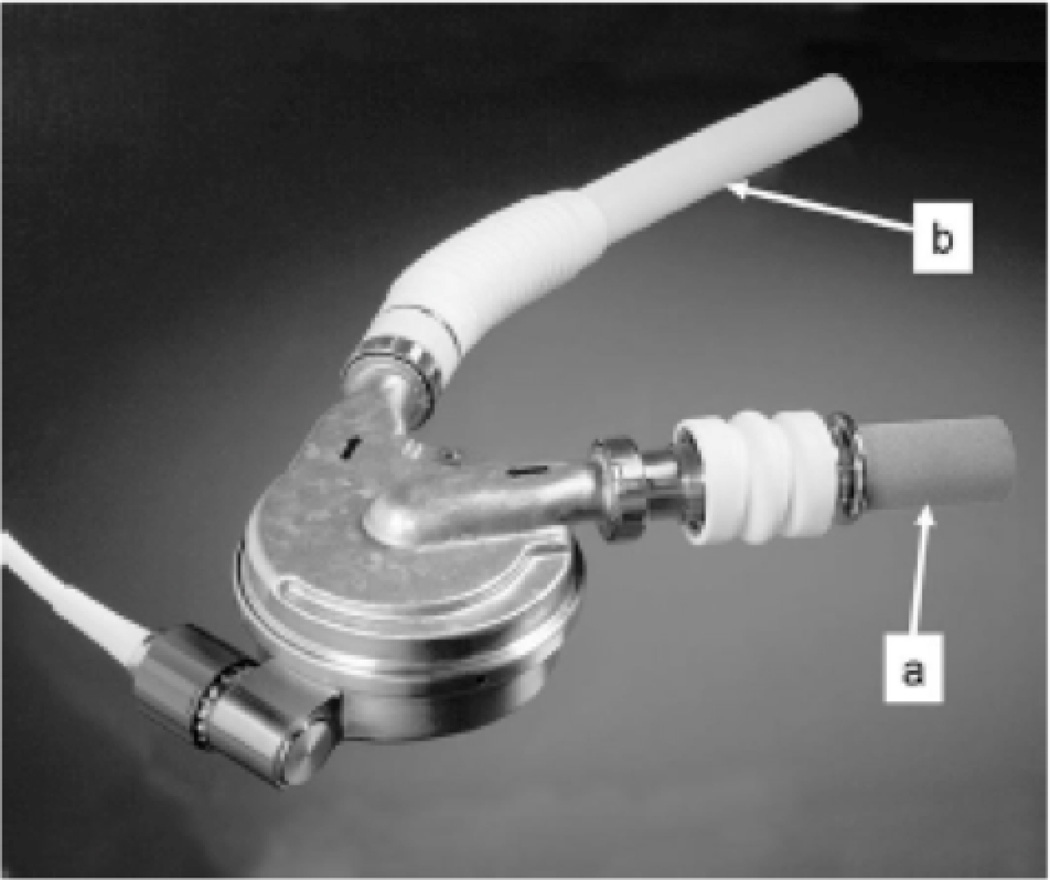
Figure 1.
The HeartMate III, third generation continuous-flow centrifugal device, is shown with inflow cannula (a) and outflow graft (b).
The HeartMate III includes a number of attractive features. The magnetically suspended bearing eliminates friction wear and may permit continuous support of up to 11 L/min for more than a decade4,41. At 4,000 to 5,000 rpm, the HeartMate III can generate 7 L/min of flow against a pump head pressure of 135 mmHg while consuming less than 10 W of power. Control of the device is achieved with an autoresponsive algorithm based on motor parameters. As afterload and preload change, the device performance parameters are automatically adjusted to maintain the same flow. Magnetic levitation permits precise control of the impeller. Radial suspension and rotation are actively controlled. Tilting and axial motion are passively controlled without power consumption. In the event of a device component failure, the electronics are designed with single fault-tolerant redundancy to ensure uninterrupted flow4. Similar to the Thoratec HeartMate II, a sensorless algorithm based on the relationship between power consumption, rotor speed, and electrical current estimates device flow42.
The driveline of the HeartMate III attaches to the device with a hermetically sealing quick-connector. Such a modular approach separates cable reliability from pump reliability and permits an upgrade from a percutaneously powered system to a TET power source4. The current percutaneous version includes a belt-mounted driver and pair of batteries, which allow untethered mobility for up to 6 hours4. A tethered configuration provides uninterrupted power from a standard wall electrical socket to the device. The totally implantable version is currently under development and is expected to minimize device-related infections and improve psychological and physical quality of life.
To minimize the potential for thromboembolism and to decrease anticoagulant pharmacotherapy, sintered titanium coats all blood-contacting surfaces except the smooth titanium impeller4. In the Thoratec HeartMate XVE, a textured surface successfully promoted in-growth of a fibrocellular, pseudoneointimal lining which lowered the risk of thromboembolic complications39. In these patients, daily aspirin alone provided sufficient anticoagulation. Additionally, relatively large gaps between the HeartMate III impeller and impeller housing provide channels outside of the main blood-flow path to wash internal device components.
An artificial pulse mode was developed to generate permanent or intermittent pulsatile blood flow37. In an acute ovine model with cardiectomy, by rapidly increasing and decreasing the rotational speed of the magnetically suspended impeller, a pulse pressure of up to 33 mmHg was achieved37,43. The clinical relevance of pulsatility during mechanical circulatory support is unclear33. However, pulsatile blood flow is an important component of cardiovascular homeostasis that may have important implications for endothelial function, cardiac and arterial architecture and remodeling, end-organ perfusion, and weaning from a device. It remains to be determined whether induced pulsatility with an LVAD will improve long-term clinical outcomes.
Extensive preclinical testing is underway. Mock circulatory loop testing is ongoing with a goal of 80% reliability with 60% confidence at 5 years4. In a chronic bovine model that included a subset of animals studied under a Good Laboratory Practices (GLP) standard4, the HeartMate III generated flow of up to 11 L/min during 90 day experiments. Hemolysis and tissue heating did not occur. End organs were functionally normal and free of infarction.
Additional preclinical testing in an acute ovine model demonstrated successful bi-ventricular assist device (BiVAD) therapy after native cardiectomy with and without induced pulsation37,43. Importantly, in a chronic bovine model, dual HeartMate III implantation successfully supported a calf for 20 days and established the feasibility of long-term continuous-flow BiVAD therapy44. The potential for dual intracorporeal support with native cardiectomy is significant and is discussed later in this article.
CorAide
The CorAide Blood Pump (Cleveland Heart Incorporated, Charlotte, NC) is a magnetically levitated, centrifugal continuous-flow LVAD14. The CorAide LVAD is implanted via median sternotomy with extracorporeal circulation. The inflow cannula resides within the left ventricular apex, and the outflow graft is anastomosed end-to-side to the aorta. The pump contains a cast-titanium volute housing (84 ml volume displacement, 293 g), a rotating magnetic ring with impeller veins, and a fluorinated ethylene-propolene (FEP)-coated titanium stator (Figure 2, Left). Plans are being made to replace the FEP-coated stator with BioMedFlex, a hard carbon coating45. The rotating ring spins around the stator post suspended in the axial direction by magnetic forces and in the radial direction by a thin layer of blood. This innovative blood-lubricated film bearing eliminates blood stagnation, mechanical wear, and heat generation, and has demonstrated significant tolerance to motion and mechanical impact46. A driveline is externalized to a portable electronics module powered through a standard wall socket or two nickel-metal hydride batteries, which may provide up to 6 hours of support47. The lightweight controller (1.35 kg) facilitates patient mobility14. At a flow of 5.5 L/min, the CorAide consumes approximately 6 W48.
Figure 2.
The CorAide LVAD is a third generation continuous-flow device. Internal components are shown.
Extensive preclinical testing has been performed in 53 animals for 30 or 90 days47–49. In 18 chronic animals, hemodynamics were stable, and there were no instances of bleeding, organ dysfunction, or mechanical failure49. Of note, six implants have been successfully conducted without anticoagulation50.
In a European trial, an earlier version of the CorAide was implanted in two male patients with a follow-up of more than six months. Both patients had an uneventful postoperative course, and no thromboembolic events, hemolysis, infection, or mechanical failures occurred. In both patients, preoperative cardiac output increased from less than 3.25 L/min to 5.6 L/min, cardiac index doubled, and pulmonary capillary wedge pressure was reduced by half14. Both patients were anticoagulated due to atrial fibrillation. Despite excellent pump performance and biocompatibility, post-explant analysis demonstrated delamination of the FEP coating. Subsequently, BioMedFlex has been introduced into the CorAide (and DexAide RVAD, described later in this review) as a new journal-bearing material for cardiac assist devices. In chronic animal testing with this material, delamination was not observed, and biological deposition did not occur45.
EVAHEART LVAS
The EVAHEART LVAS (Evaheart Medical Incorporated, Pittsburgh, PA; Sun Medical Technology Research Corporation, Nagano, Japan) is a full-support centrifugal pump (55 × 76 mm, 420 g) that is designed for long-term use (Figure 3A, B)51. Through a median sternotomy, the inflow cannula is placed in the left ventricular apex, and the outflow graft is sewn to the ascending aorta. A sensorless, brushless direct-current motor drives the impeller (40 mm) at 1,900 to 2,600 rpm. The EVAHEART LVAS was designed with a relatively flat pressure-flow relationship that enables a peak flow of up to 20 L/min with the low-pressure head. Relatively large inflow and outflow cannulas (16 mm) as well as the wide cross-sectional area of the pump provide a low resistance flow path within the device. A portable external driver powers the device (Figure 3A).
Figure 3.
The EVAHEART LVAS is a full-support centrifugal pump. A) The device and the control unit are shown. B) A schematic diagram of device design and implant position is illustrated.
The EVAHEART LVAS contains a number of attractive features. The innovative combination of a flat pressure-flow relationship and a wide flow pathway generates a significant increase in flow during native ventricular systole that results in a wide pulse pressure. To improve hemocompatibility, the blood-contacting surfaces are coated with 2-methylacryloyloxyethyl phosphorylcholine (MPC), an antithrombogenic organic zwitterion found on the surface of erythrocytes52,53. A low temperature mechanical seal fuses the shaft54. Recirculating sterile water continuously flushes the inner faces of the seal to improve convective heat transfer and prevent heat denaturation of serum proteins. An extended durability of up to ten years of support is expected.
In an acute bovine model, during exercise the EVAHEART LVAS automatically increased pump flow without a change in rpm55. At a constant pump speed, exercise increased systolic pump flow by a factor of three and indicated a sensitive responsiveness to preload and changing heart rate during increased total-body metabolic demands.
In a chronic bovine model, through a left thoracotomy the EVAHEART LVAS successfully supported calves for 3 to 7 months51. At 3 to 5 L/min, the pump consumed 8 to 10 W. During treadmill exercise, pump flow exceeded 10 L/min. No hemolysis occurred, and six of ten animals survived to elective termination. At necropsy, there was no evidence of infection or pump thrombus on the blood-contacting surfaces of the inflow cannula, outflow graft, or pump. A few small renal infarcts were noted51,53. In additional chronic calves, MPC coating of blood-contacting surfaces significantly reduced activated platelets as compared to a diamond-like carbon coating53. In this study, three animals were successfully supported without postoperative anticoagulation.
In May 2005, a clinical trial was initiated at four centers in Japan18. An initial report of two patients describes functional class improvements from NYHA class IV to class I without adverse events at 603 and 543 days of support56. Importantly, patients demonstrated an aortic pulse pressure of 20 to 30 mmHg. In December 2010, the Japanese Pharmaceutical and Medical Device Agency approved the EVAHEART LVAS for bridge to transplantation. A multicenter trial is currently underway in the United States under an Investigational Device Exemption (IDE). Importantly, this trial may help to determine if the potential advantages of the device (preserved pulse pressure, antithrombogenic coating, extended durability) may translate into improved, long-term patient outcomes.
Less-Invasive Full-Support Devices
If combined, the benefits of a full-support device implanted with a minimally invasive surgical approach may expand the potential patient population for LVAD therapy. As LVADs are miniaturized, minimally invasive implantation may increase acceptance by patients and physicians who are more likely to refer patients for less-invasive surgical therapies57. As a result, earlier intervention in less-sick patients may increase the public-health impact of mechanical circulatory support. Novel surgical approaches may include limited thoracotomy, subxiphoid access, thoracic keyhole access, placement of surface devices, or percutaneous implantation.
Furthermore, these operative approaches often do not require cardiopulmonary bypass58. As a result, less postoperative coagulopathy may reduce postoperative bleeding and blood transfusions which play a role in right ventricular dysfunction and infection with LVADs59. Close on the horizon, the HeartWare MVAD holds promise as a small, full-support rotary pump with multiple configurations that may be implanted without a sternotomy or extracorporeal circulation.
Miniature Ventricular Assist Device
The miniature ventricular assist device (MVAD; HeartWare® Incorporated, Miami Lakes, FL) platform technology revolves around a small (15 ml displacement), bearingless, axial-flow LVAD5. Within a cylindrical titanium housing, an electromagnetic motor stator powers a magnetically levitated, wide-blade impeller with a large surface area to produce hydrodynamic thrust bearings. Small gaps minimize the distance between magnetic poles and the stator and provide impeller stability. The combination of hydrodynamic thrust bearings and an axial suspended impeller eliminate upstream and downstream support structures. This innovative design produces a platform for multiple device configurations that may be implanted through less-invasive surgical approaches yet still provide full-support therapy. At 12,000 to 20,000 rpm, the MVAD generates up to 8 L/min of flow. Hydrodynamic thrust bearings that are larger than the main blood-flow paths passively suspend the rotor. The patient wears an extracorporeal power source and electronics.
The small housing is one-third the size of HeartWare’s predicate HVAD (Figure 4A #3) and can be manufactured in three configurations which are currently under investigation60. Two configurations employ a transapical approach, and a third configuration employs a transmitral approach. The first transapical MVAD configuration is implanted with a left thoracotomy or median sternotomy. The cylindrical inflow/pump housing of the device is implanted through the left ventricular apex (Figure 4A #2, 4B), and the base of the device resides within the pericardium. A 10 mm double woven outflow graft angled at 90° from the base of the device is anastomosed end-to-side to the descending aorta.
Figure 4.
The HeartWare HVAD (A #3) and various configurations of the HeartWare MVAD in development (A #1, 2, 4) are shown. The first transapical MVAD configuration is implanted through the left ventricular apex (A #2, B) with the outflow graft sewn to the descending aorta. The second, transapical MVAD configuration is implanted with a subxiphoid approach (A #4, C). The transmitral MVAD configuration is implanted through a small, right-sided thoracotomy (A #1, D).
A second, transapical MVAD configuration is implanted with a subxiphoid approach60. After entering the pericardium through a small incision below the xiphoid process, the device is inserted through the left ventricular apex to reside within the left ventricular chamber (Figure 4A #4, 4C). The inflow of the device unloads up to 8 L/min of blood volume from within the left ventricle through an outflow cannula that is permanently positioned across the aortic valve (Figure 4C). This novel approach places a continuous-flow LVAD in series (rather than in parallel) with the circulation, obviates an outflow graft anastomosis, and dramatically reduces the invasiveness and length of the implantation procedure. Intraventricular placement may also eliminate device pocket infections5. The long-term effect on the aortic valve and the incidence of thromboembolism remain to be defined.
The transmitral MVAD configuration is implanted through a small, right-sided thoracotomy (Figure 4A #1, 4D). A flexible inflow cannula is implanted in the left atrium through Waterson’s groove between the right superior and inferior pulmonary veins. The tip of the inflow cannula is positioned across the mitral valve into the left ventricle. The outflow graft is sewn to the ascending aorta.
In a chronic bovine model, the first transapical MVAD configuration was implanted successfully for 30 days. Measurement of cardiac output with the thermodilution technique demonstrated that pump speeds of 19,500 to 21,3000 rpm generated an average flow of 4.25±0.75 L/min, which was approximately 80% of cardiac output. After euthanasia, the explanted device was free of thrombus, no thermal damage was present, and end organs were free of infarction5. Preclinical testing of the other two MVAD configurations is currently underway.
Partial-Support Devices: Rotary Pumps
If a patient on a cardiac transplant waiting list is worsening and an appropriate organ is not available, a full-support LVAD may be the only option for survival. Yet many heart failure patients are not ill enough or have contraindications to placement of a full-support LVAD. In these patients, the notion of combining the benefits of partial support with a minimally invasive and short operation without cardiopulmonary bypass may be feasible. A recent National Heart, Lung, and Blood Institute (NHLBI) mission statement included the pursuit of long-term hemodynamic support with minimally invasive surgery to provide moderate levels of mechanical assistance earlier in the progression of heart failure (NHLBI, Clinical Use of Ventricular Assist Devices Working Group, March 27–28, 2008 Crystal City, VA)61. The long-term benefits of chronic, partial unloading of the failing left ventricle are unknown but may soon be characterized by present clinical trials7. Significant clinical benefit has been predicted62 and may decrease the number of patients that require a transplant.
For example, a large gap in available therapies exists for patients in NYHA class III that have not responded to biventricular pacing. If these patients are transplant ineligible or have not met hemodynamic and clinical criteria to justify the risks and comorbidities of sternotomy and a full-support LVAD, limited options exist. In these patients, partial support with a less-invasive device is an attractive option. If partial support is administered early enough, favorable reverse myocardial remodeling may occur and permit explantation of the device63. This hypothesis has not been rigorously tested but is conceptually appealing.
Indeed, mounting evidence suggests that full support of the failing left ventricle with an LVAD can promote reverse myocardial remodeling and LVAD explantation in select patients30,31. In these instances, functional recovery has been accompanied by favorable changes at the molecular, histological, and functional levels64,65. Yet, strategies to promote myocardial recovery with a full-support LVAD have demonstrated limited success. Furthermore, over time maximum volume unloading of the left ventricle may actually be detrimental to the cardiovascular system. For example, as the level of full-support therapy increases, variation in end-systolic and end-diastolic volumes diminishes and eliminates the native workload of the heart33. As a result, myocyte atrophy66,67 and ventricular stiffening68 may occur and preclude myocardial recovery. Simultaneously, complete volume unloading of the heart decreases peak systolic left ventricular pressures to the point where the aortic valve remains chronically closed and may result in fused valve leaflets, acquired aortic stenosis, or total occlusive thrombosis of prosthetic aortic valves69. Excessive ventricular unloading with both pulsatile70 and continuous71 LVADs can also result in suction events and ventricular collapse which may trigger episodes of ventricular tachyarrhythmias. Of additional concern, during therapy with a full-support LVAD, approximately 30% of patients develop right ventricular dysfunction22 with an associated mortality rate of 43%23. An abrupt increase in cardiac output can acutely overload the right ventricle and cause right ventricular failure. Early clinical data suggest that partial support does not dramatically increase cardiac output and right-sided overload is unlikely7. For these reasons, complete volume unloading of the left ventricle may cause adverse consequences and limit recovery with a full-support LVAD.
Furthermore, patients in NYHA class III or early class IV heart failure with less-advanced disease may not require full support with an LVAD, and these patients may have a higher likelihood of myocardial recovery62. Accordingly, rather than completely unloading blood volume from the failing left ventricle, earlier and partial volume unloading may reduce but not eliminate native ventricular workload, preserve myocardial structure, and prevent myocardial atrophy and stiffening. By reducing ventricular workload and augmenting myocardial blood flow while still allowing the heart to fill and empty over a controlled range of ventricular volumes, partial support may be an effective strategy to augment hemodynamics and promote favorable myocardial remodeling in hearts with less disease. With this goal in mind, the CircuLite, Synergy Pocket Micro-Pump was designed. Recent clinical results are encouraging.
CircuLite Synergy Pocket Micro-Med Pump
The CircuLite Synergy Pocket Micro-Pump (CircuLite Incorporated, Saddle Brook, NJ) is a small continuous-flow device the size of an AA battery (Figure 5A, 49 mm, outer diameter 14 mm, 25 g)19. The CircuLite LVAD is implanted via a miniature right-sided thoracotomy without extracorporeal circulation. Via modified Seldinger technique, the inflow cannula is implanted between the right superior and inferior pulmonary veins and positioned in the left atrium. Left atrial versus left ventricular cannulation with a continuous-flow LVAD provide similar flow rates, and left ventricular volumes and energetic parameters decrease with increasing pump speed irrespective of cannulation site72. These data and the clinical experience described below support the use of the left atrium as a cannulation site for continuous-flow devices. The outflow graft is anastomosed to the subclavian artery. The pump is implanted subcutaneously in a pacemaker pocket in the right infra-clavicular groove anterior to the pectoralis major muscle (Figure 5B–D).
Figure 5.
The CircuLite Synergy Pocket Micro-Pump is a small, partial-support LVAD (A). Radiograph and CT scans show the position of pump in vivo (B–D).
The CircuLite Synergy Pump is designed to continuously, partially unload 2.5 to 3.0 L/min of blood from the left atrium into the subclavian artery. A brushless, microelectric motor powers a magnetically stabilized, hydrodynamically levitated single-stage impeller, which rotates at 20,000 to 28,000 rpm. Axial, centrifugal, and orthogonal blood flow paths ensure continuous washing of internal components to reduce the risk of impeller thrombosis. A nitinol-reinforced silicone inflow cannula (length 20.5 cm, inner diameter 6 mm) includes a Dacron cuff with titanium tip designed to facilitate implantation and healing in the left atrium. A polytetrafluoroethylene (PTFE) outflow cannula (inner diameter 8 mm) is trimmed to the appropriate length during the implantation procedure. A percutaneous lead exits the body from the right upper quadrant and connects to a controller and dual battery pack (1.5 kg), which permit approximately 16 to 18 hours of untethered mobility73.
Original theoretical work suggested that continuous, partial support of a failing ventricle may increase cardiac output and lower ventricular filling pressures62. The greatest hemodynamic benefits were predicted for less-dilated and less-dysfunctional hearts. Computer simulations were validated in an acute bovine model of cardiac dysfunction in which continuous partial support at a rate of 3 L/min decreased left atrial pressure by 6 to 7 mmHg and increased cardiac output by greater than 1 L/min62. Ongoing bench testing has suggested long-term durability. Nine Synergy pumps that have run in a mock circulatory loop for 30 months have not demonstrated mechanical wear at the pivot bearing7.
In an ongoing multicenter European clinical trial7, ionotrope-independent ambulatory patients on the transplant list with NYHA class IIIB or IVA heart failure and preserved end-organ function demonstrated hemodynamic improvements over a 3-month follow-up7,19,73 with an ongoing maximum support duration of eight months. Partial hemodynamic recovery included significant increases in cardiac index from 2.0±0.4 to 2.8±0.6 L/min/m2, an increase in mean arterial pressure from 67±8 to 80±9 mmHg, and a reduction in pulmonary capillary wedge pressure from 30±5 to 18±5 mmHg19. N-terminal fragment pro-brain natriuretic peptide was significantly reduced from 6,452±5,470 to 3,209±2,379 pg/ml73 and suggested decreased myocyte mechanical stress.
As predicted, right heart failure was not a clinical challenge with the CircuLite pump. During support, the acute increase in cardiac output ranged from 1.0 to 1.5 L/min and did not overload the right ventricle7. However, this clinical trial was not free of adverse events. During the first 30 days of support, serious adverse events which included bleeding, infection, stroke, pump thrombosis, and pump exchange occurred at approximately half the rate reported for the HeartMate II7.
Pump thrombosis and pump exchange accounted for more than one-third of the adverse events reported after 30 days of support7. As a result, modifications were made that included an increased size of the washout channels and redesign of the impeller and control algorithms, which have reduced the rate of pump thrombosis73. Notwithstanding, a great advantage is that the Synergy pump may be easily and rapidly exchanged through the original infraclavicular incision without entering the chest.
An additional and unique advantage of the CircuLite pump is that the implantation procedure involves a right-sided thoracotomy in which it is unlikely that adhesions will form in the anterior mediastinum and complicate median sternotomy if heart transplantation is indicated. A future design that obviates entrance into the thorax will include a percutaneous inflow graft placed through the subclavian vein and advanced through the interatrial septum into the left atrium. As with the current design, the outflow graft will be sewn to the subclavian artery. With this approach, the need for a thoracotomy will be eliminated, and the infraclavicular incision will be the only incision necessary to implant the entire system73. This approach will likely further increase acceptance by patients and referring physicians.
Reitan Catheter Pump
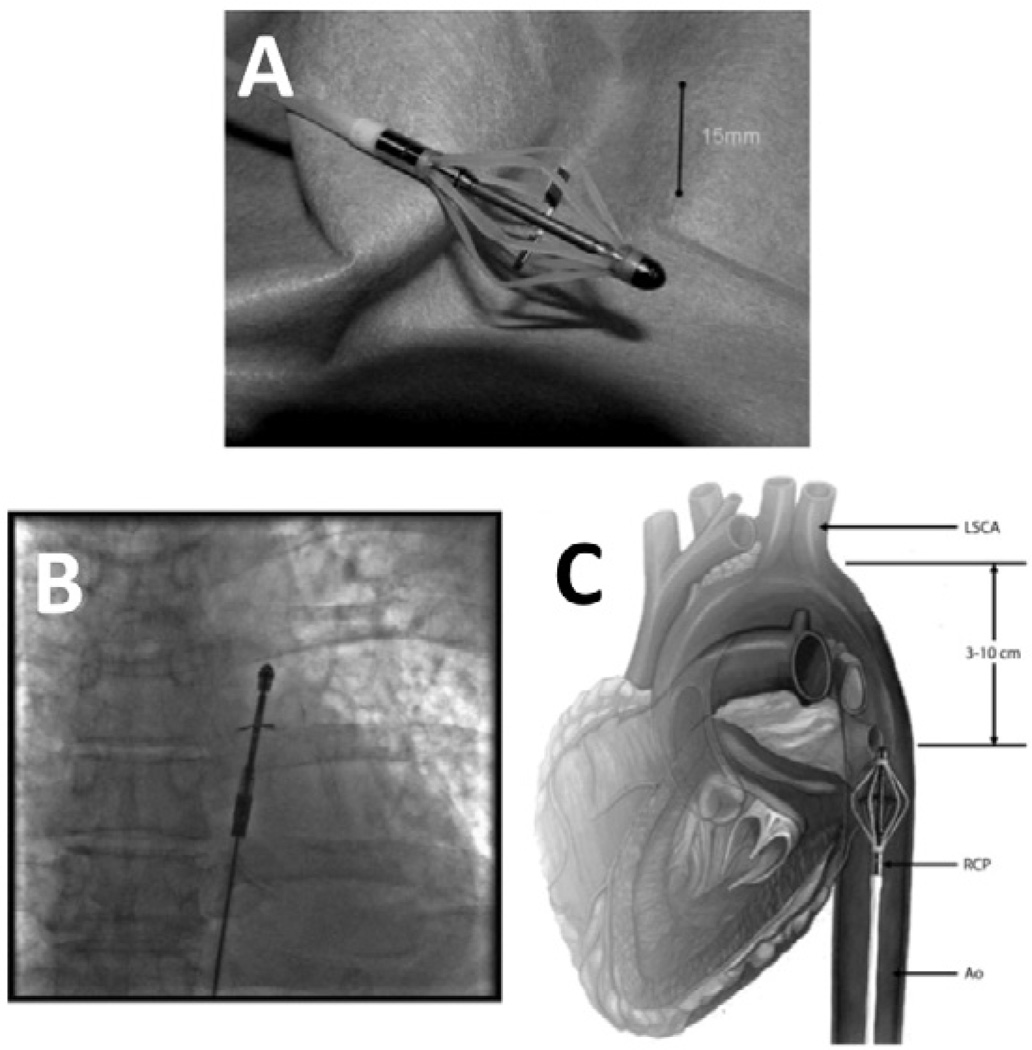
The Reitan Catheter Pump (CardioBridge, Hechingen, Germany) is a novel, intraaortic propeller-based catheter pump10 (Figure 6A). In patients with cardiogenic shock or patients undergoing high-risk PCI in whom an IABP is contraindicated due to tachyarrhythmia or aortic valve incompetence, the Reitan Catheter Pump may provide an alterative strategy for short-term cardiac support. A collapsible cage surrounds a retractable propeller that is loaded on a flexible catheter (collapsed outer diameter 4.6 mm). Via modified Seldinger technique, the catheter is introduced percutaneously into the high ascending aorta (Figure 6B, C). In vivo, the cage is deployed and the propeller blades are extended and rapidly rotated. With rotational speeds of 10,000 to 14,000 rpm, a pressure gradient of up to 25 mmHg is produced across the propeller, which unloads the left ventricle by reducing pressure in the aortic arch proximal to the pump. Distal to the pump, augmented blood pressure increases perfusion of the abdominal organs and lower half of the body. The proximal end of the catheter is connected to an external drive unit and user console.
Figure 6.
The Reitan Catheter Pump shown ex vivo (A), in vivo (B), and in a schematic diagram (C).
The hydraulic properties of the Reitan catheter pump have been evaluated in mock circulatory loops74. The generated pressure reduction was related to the size of the mock aorta. Of great importance, the aortic diameter influenced the pressure gradient that developed across the propeller. In larger aortas, a deceased pressure gradient was likely due to backflow around the tips of the rotating propeller blades. Clinically, this finding suggests that the Reitan Catheter Pump may be most effective in patients with smaller aortas.
In normal pigs, the Reitan Catheter Pump increased cardiac output and reduced proximal aortic pressure75. In a bovine model of acute mitral regurgitation, the Reitan Catheter Pump caused significant rpm-dependent reductions in mean ascending aortic pressure that reached −10 mmHg and in left ventricular peak systolic pressure, an indirect index of myocardial metabolic demand. A significant increase in abdominal aortic pressure was observed. However, cardiac output did not improve, and negative pressure in the ascending aorta decreased carotid artery flow and mean diastolic coronary artery flow (net coronary blood flow was unaffected due to an increased contribution in systolic coronary flow)76. Similarly, in a bovine model of pharmacologically-induced acute heart failure, the Reitan Catheter Pump significantly decreased left ventricular systolic pressure by approximately 20 mmHg and left atrial pressure by 5 mmHg and increased femoral pressure by 19 mmHg. A 15% reduction in carotid artery blood flow was observed. Coronary artery, renal, and femoral blood flow were unchanged77.
In a randomized clinical trial in 10 patients, the Reitan Catheter Pump safely provided cardiac support during PCI10. No deaths or strokes occurred. No significant hemolysis occurred, and platelet function was unchanged. At rotational speeds of 10,500 rpm, an aortic pressure gradient of approximately 10 mmHg was maintained across the pump. Importantly, an improvement in renal function suggested increased abdominal and lower body perfusion. In the setting of PCI, increased renal perfusion may protect against contrast nephropathy. However, this hypothesis remains to be tested with this device.
Procyrion CAD
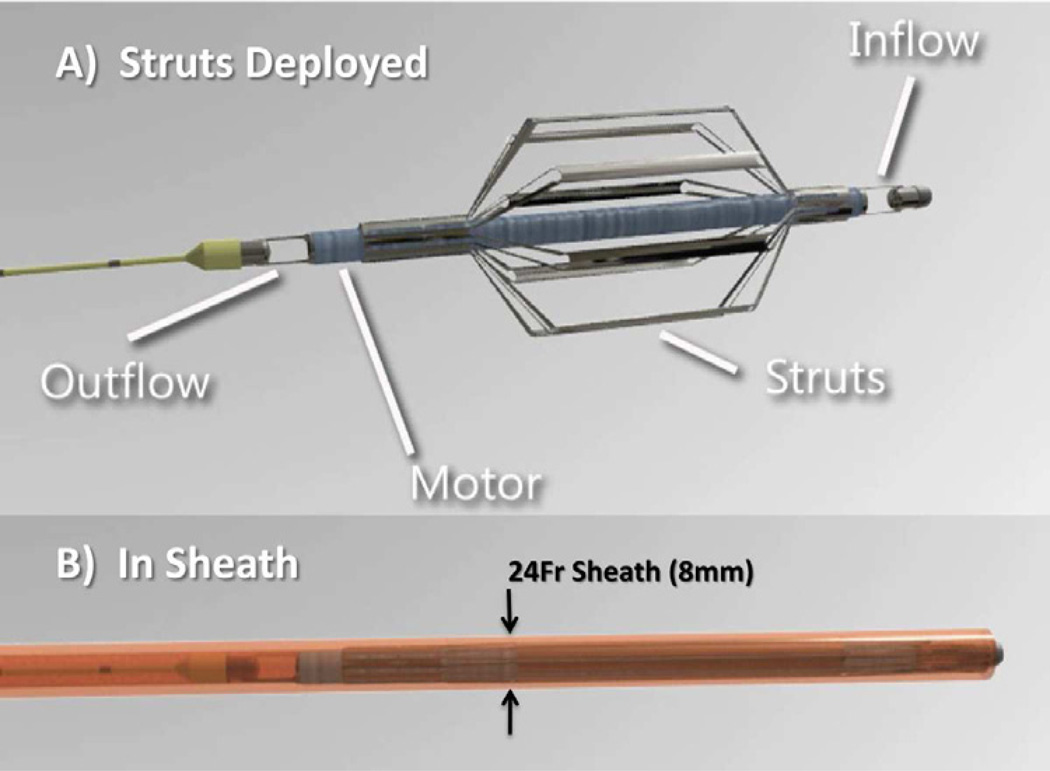
The Procyrion Circulatory Assist Device (Procyrion CAD; Procyrion Incorporated, Houston, TX) is a catheterdeployed intraaortic, continuous-flow LVAD designed for long-term partial circulatory support. The Procyrion CAD consists of a miniature rotary pump caged within a catheter-based nitinol strut system (Figure 7A – Deployed, 7B - Collapsed). Via a percutaneous approach, the catheter-based pump is advanced and permanently deployed in the descending aorta. As with the Reitan catheter pump, the Procyrion CAD unloads the left ventricle by decreasing proximal aortic resistance while providing distal aortic flow augmentation.
Figure 7.
The Procyrion Circulatory Assist Device is shown with struts deployed (A) and collapsed prior to deployment (B).
In a mock circulatory loop, the Procyrion CAD improved mean arterial pressure by 5%, cardiac output by 6%, and decreased left ventricular external work by 15%. In an acute ovine model of pharmacologically-induced heart failure, the Procyrion CAD moderately increased dP/dt and increased cardiac output from 3.5 to 4.6 L/min (unpublished results, Reynolds Delgado, M.D.)
Partial-Support Devices: Counterpulsation Devices
Counterpulsation with an intraaortic balloon pump (IABP) is the most common mechanical circulatory support strategy for a wide variety of cardiovascular pathologies. Although IABP implantation does not require major surgery, during IABP support the patient must remain supine and is thereby immobilized and susceptible to numerous complications. Biocompatibility issues typically limit IABP therapy to short durations of hours to days. When IABP therapy is employed for prolonged periods of greater than 20 days, the frequency of vascular complications, bleeding, and infection is high and is associated with increased mortatlity78,79.
Nevertheless, decades of widespread success with the IABP for short-term indications have generated interest in long-term counterpulsation strategies. Multiple surgical approaches (aortomyoplasty80, skeletal muscle ventricle81), and invasive devices (Paraaortic Counterpulsation Device82, CardioVAD17) aimed to supplement cardiac function for long-term therapy. However, these approaches all required sternotomy or thoracotomy.
In patients with severe hemodynamic impairment, chronic counterpulsation would not provide the same degree of support as an LVAD and is not a suitable option. However, less-invasive chronic counterpulsation may be a practical partial-support strategy to expand treatment options and improve quality of life in a large cohort of patients with less advanced heart failure.
As discussed previously, early partial support may promote reverse myocardial remodeling. The objectives of counterpulsation are to 1) generate an ancillary pressure pulse during native ventricular diastole to augment diastolic pressure and increase coronary and systemic blood flow (increase supply), and 2) decrease aortic pressure during native ventricular systole to reduce vascular afterload and ventricular work (decrease demand). Both of these mechanisms favorably affect myocardial mechanoenergetics. Consequently, long-term counterpulsation may promote myocardial recovery. However, chronic ambulatory counterpulsation has not been rigorously evaluated, and this hypothesis remains to be tested. With this goal in mind, two novel devices, the Sunshine Heart C-Pulse and the SCR Symphony device have been developed for less-invasive, long-term counterpulsation.
C-Pulse
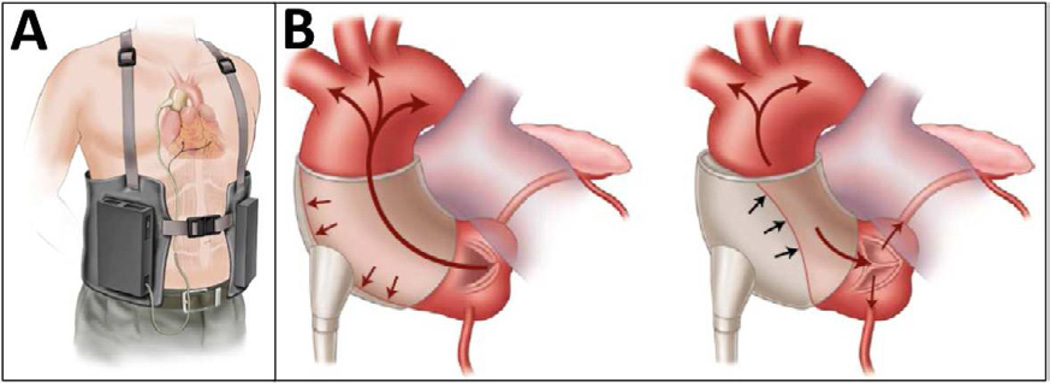
The C-Pulse (Sunshine Heart Incorporated, Tustin, CA) is an implantable, extraaortic counterpulsation device9. Via median sternotomy or right anterior thoracotomy without cardiopulmonary bypass, a polyester-coated polyurethane balloon cuff is installed circumferentially around the ascending aorta between the sinotubular junction and the brachiocephalic artery. Cuffs accommodate ascending aortic diameters of 28 to 40 mm. A minimum length of 70 mm of aorta is needed. A bipolar epicardial electrocardiographic lead (Capsure Epi 65 cm, Medtronic Incorporated, Stillwater, MN) is attached to the right ventricular outflow tract to trigger the device. A driveline is passed from the thorax subcutaneously through the abdominal wall. At the percutaneous exit site, a connector fastens the driveline to a portable pneumatic driver (Figure 8A).
Figure 8.
The C-Pulse implantable, extraaortic counterpulsation device is shown in a schematic diagram with portable driver (A), in position around the aorta with balloon deflated (B) and inflated (C).
The C-Pulse functions by classic counterpulsation in series with the cardiovascular system. Just after closure of the aortic valve during native diastole, the balloon cuff inflates and compresses the ascending aortic wall. The “thumb-print” deflection of the outer curvature of the aorta displaces 20 to 30 ml of aortic root blood volume toward the heart to increase diastolic coronary artery blood flow, and toward the body to augment end-organ perfusion (Figure 8B). The R-wave on the electrocardiogram triggers the balloon cuff to deflate. As a result, central aortic end-diastolic blood pressure falls and reduces the metabolic demands of the heart necessary to eject through the aortic valve (Figure 8C).
In an acute porcine model, extra-aortic balloon counterpulsation with a 7 ml balloon cuff outperformed a 25 ml IABP in diastolic coronary blood-flow augmentation and performed comparably in diastolic pressure augmentation, afterload reduction, and augmentation of cardiac output83. Histopathological changes were not observed in the ascending aortic tunica intima or tunica media. However, mild hemorrhagic inflammatory changes were observed in the tunica adventitia.
A short-term intraoperative safety study was performed in six patients with normal ventricular function undergoing off-pump, first-time coronary artery bypass grafting. During C-Pulse support, transesophageal echocardiography demonstrated a 31% reduction in left ventricular wall stress, a 16% reduction in left ventricular end-systolic area, and a 13% increase in fractional area change. Diastolic coronary blood flow increased by 67%84.
More recently, a prospective multi-center feasibility trial was initiated in the United States to evaluate the safety and efficacy of the C-Pulse in 20 NYHA class III and IV heart failure patients. Initial success was reported20,85. All patients improved by at least one NYHA class. In three patients, right heart catheterization at one month demonstrated an improvement in cardiac index and reduction in pulmonary artery pressures. Infection complicated device therapy in 60% of patients20. Aortic tissue retrieved at autopsy demonstrated macroscopically normal aortic walls with an intact tunica intima and tunica media. Arterial remodeling was observed in some patients and included thickening of the adventitia, foreign-body response, neutrophilic infiltrate, and/or necrotic adventitia.
Perhaps the best feature of the C-Pulse is the absence of blood contact. As a result, anticoagulation is unnecessary. Additionally, the device may be turned off safely at any point during support, which has been well received by patients who have reported feeling less encumbered. Better mobility may translate into improved ability to perform activates of daily living, less device-related stress, and improved quality of life scores (personal communication, Sanjeev Aggarwal, M.D.). The limited operative approach and no postoperative anticoagulation may favorably impact patient outcomes by decreasing the incidence of thromboembolism, hemorrhage, and device related coagulopathy.
The C-Pulse is contraindicated in patients with aortic regurgitation, severe disease of the ascending aorta, or patent aortocoronary bypass grafts. The long-term histopathological effects to the aortic root and surrounding tissues have not been determined. Additionally, the ease with which the device may be removed if operative therapies are needed (such as heart transplantation or full-support LVAD implantation) has not been reported. In the event of myocardial recovery, it may be possible to sever the driveline and leave the C-Pulse in situ to avoid a repeat, invasive procedure.
Symphony Counterpulsation Device
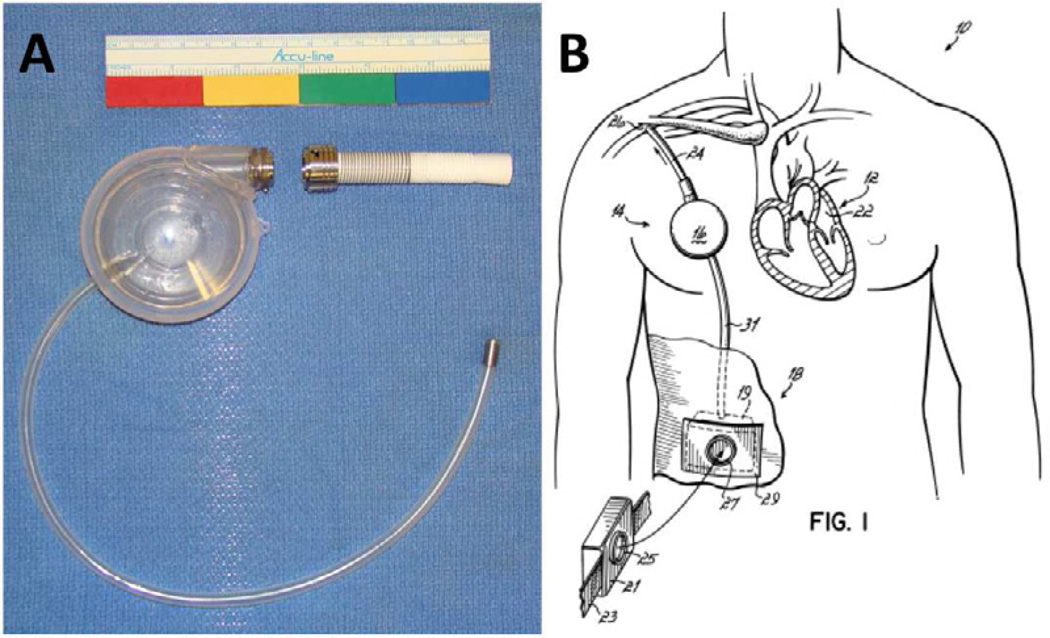
The Symphony Counterpulsation Device (SCR Incorporated, Louisville, KY) is a peripheral counterpulsation device designed to deliver long-term, partial support without entering the thorax. In a simple surgical procedure, a valveless inflow/outflow cannula is anastomosed to the subclavian artery and attached to a pump with a 32-ml stroke volume (Figure 9A). The pump is placed above the pectoralis major in a subcutaneous pocket similar to a pacemaker (Figure 9B). To minimize complications associated with blood stasis and thrombosis, a constant but shifting vortex within the reservoir continuously “washes” the inside of the device. A percutaneous driveline exits in the right upper quadrant and attaches to a lightweight, portable pneumatic driver (20 × 10 × 10 cm, 1.5 kg), which is triggered by the patient ECG.
Figure 9.
The Symphony Counterpulsation Device is shown ex vivo (A) and in a schematic diagram (B).
The Symphony functions by counterpulsation with a peripheral capacitance chamber. Blood is removed from the circulation into the pump during systole and returned to the circulation during diastole. As with standard counterpulsation devices that employ volume displacement (rather than volume removal), the Symphony device results in afterload reduction during systole and coronary and systemic flow augmentation during diastole8. By these mechanisms, the Symphony improves the myocardial oxygen supply/demand ratio8.
Although the Symphony operates similarly to an IABP, the delivery of support is fundamentally different86,87. The surgical configuration does not influence the internal impedance of the aorta, and device filling and ejection are less dependent on timing than an IABP. With an IABP, inflation occurs immediately after aortic valve closure, and deflation must begin before the end of ventricular diastole to ensure that the balloon deflates and aortic resistance is low88. In contrast, theoretical work suggests that adjustment of the timing of filling and ejection of the Symphony may permit modest tradeoffs between improved coronary flow and left ventricular workload86,87. Consequently, subtle variations in the delivery of support may have important utility for incremental patient management on an individualized basis. For example, ejection of the Symphony may be delayed until after isovolumetric relaxation when the coronary resistance is lowest, which may result in a modest additional increase in coronary artery flow as compared to an IABP86,87. If filled prior to the beginning of ventricular systole before the aortic valve opens, a large decrease in left ventricular ejection pressure translates into a large reduction in ventricular work. However, filling later during systole may result in less afterload reduction and may permit gradual reloading of the heart. Therefore, after an initial period of maximal counterpulsation therapy, left ventricular workload may be gradually increased to strengthen the myocardium and facilitate weaning of the device. Importantly, if explantation is indicated, the Symphony may be surgically removed without entrance into the thorax.
The feasibility of effective counterpulsation via peripheral volume displacement with the Symphony has been validated in silico86 and demonstrated in vitro in a mock circulatory loop86,87. Efficacy has been confirmed in an acute bovine model8,87,89,90. These studies suggested that hemodynamic benefits with the Symphony are comparable to or better than an IABP in normal animals87 and in animals with acute heart failure8, hypotension8, hypertension8, and chronic heart failure90. In preparation for clinical trials, long-term implants are currently underway.
Of note, the implantation, operation, and management of an LVAD are cost-prohibitive for many health care systems, including China and India. As a result, the use of mechanical circulatory support in these markets is limited91. Importantly, the Symphony device has a lower manufacturing cost and can be implanted (and explanted) with a limited operative approach without extracorporeal circulation. Therefore, the Symphony may be a desirable alternative to the more invasive, expensive, and complex LVAD systems and may expand the use of mechanical circulatory support devices to these under-served markets.
Right Ventricular Assist Devices
Right ventricular failure is a serious complication with a high mortality rate. Approximately 50% of all morbidity and 20% of early mortality after orthotopic heart transplantation relate to right ventricular failure21. Similarly, the acute increase in left ventricular output and septal shift with an LVAD produce right ventricular dysfunction in 30% of patients22 with an associated mortality rate of 43%23. Although most patients recover with ionotropes and pulmonary vasodilators, right ventricular dysfunction remains a clinical challenge.
Few recent reports of next-generation implantable RVADs exist. The clinically-approved Jarvik 2000 Flow Maker has been used off-label for right ventricular support92. In 2004, the pneumatically-driven Thoratec IVAD was approved by the FDA for use as an implantable RVAD93. However, the large IVAD is not ideal for patient mobility outside of the hospital setting. Anatomical compatibility of an RVAD with a preexisting LVAD is an additional and important consideration due to space constraints if an LVAD is present and because implantation of an isolated RVAD is rare94. Consequently, a small, next-generation implantable RVAD is still needed. The recent success of novel LVAD systems has bolstered interest in the development of next-generation RVAD systems.
DexAide
The DexAide Blood Pump (Cleveland Heart, Charlotte, NC) is a magnetically levitated, centrifugal pump95 designed in parallel with the CorAide LVAD14. The DexAide contains a cast-titanium volute housing (44 × 48 mm, 69 ml volume displacement, 280 g), a rotating magnetic ring with impeller veins, and a zirconia (zirconium oxide) ceramic stator96 (Figure 2, Right). The rotating assembly spins around the stator post suspended in the axial direction by magnetic forces and in the radial direction by a blood lubricated film97. Various inflow cannula designs and surgical implantation sites were rigorously tested for biocompatibility98. An open-ended titanium inflow cannula with two side openings positioned through the diaphragmatic surface of the right ventricle resulted in the least tissue deposition. The outflow cannula is anastomosed end-to-side to the trunk of the pulmonary artery. A driveline is externalized to a portable electronics module powered through a standard wall socket or two nickel-metal hydride batteries, which may provide up to 12 hours of support96. Currently, Cleveland Heart is seeking an FDA IDE for the DexAide RVAD13.
In mock circulatory loops, at 2,450 rpm the DexAide generated 4 L/min of flow and 40 mmHg of pressure while consuming 3 W97. An 18-month in vitro endurance test has been completed13. In an acute bovine model, pump flow correlated with pump speed during high pulmonary arterial pressures but was limited during low volume conditions. A right atrial pressure of at least 5 mmHg was necessary to maintain sufficient pump flow.
In a chronic bovine model, an early version of the DexAide with a cast-titanium stator provided approximately 5 L/min of blood flow and consumed 3 W during 14 to 90-day studies95,98. Subsequent in vitro96 and 14 to 91-day experiments99 demonstrated that the new zirconia ceramic stator reduced power consumption by 20% with similar hemodynamic performance to the original cast-titanium stator. No biological deposition was observed on the new stator, which suggests that zirconia ceramic may be a useful material in the journal bearings of novel LVADs. In these experiments, a fixed-flow mode was successfully introduced to maintain target flow while preventing right ventricular suction events100.
Human-fit studies have been performed to simulate implantation of the DexAide in a patient with a preexisting HeartMate I or II, CorAide LVAD, or Novacor LVAD95,101,102. Findings indicated that implantation of the DexAide in the preperitoneal space interfered with the outflow conduit of the LVAD. As an alternative, implantation of the DexAide RVAD in the right chest cavity fit well with each LVAD.
Importantly, the Cleveland Heart DexAide RVAD and CorAide LVAD have been developed in parallel for use as a continuous-flow BiVAD system103. The potential for dual intracorporeal support with small continuous-flow devices is significant and is discussed later in this review.
Impella Recover RD
The Impella Recover RD (Abiomed, Inc, Danvers, MA) is an implantable RVAD designed for short-term support104. The inflow cage is implanted in the right atrium. The flexible, ring-reinforced PTFE outflow graft is anastomosed end-to-side to the pulmonary artery. A microaxial-flow pump with a miniaturized propeller located within the inflow cannula (outer diameter 6.4 mm, 12 ml inner volume, 65 cm2 blood contacting surface, 11 g) rotates at up to 32,000 rpm to generate up to 6 L/min of blood flow (Figure 10). An external purger continuously delivers in situ anticoagulation of the pump via heparinized rinsing fluid (10 to 20% glucose, 2,500 IU heparin at 200 to 600 IU heparin/hour) from an external 50 ml syringe. The implantation technique is rapid, does not require cardiopulmonary bypass, additional cutaneous incisions for cannulas or a driveline, and the pump fits within the pericardium. Maximum recommended use is 10 days15. A pressure sensor which measures the pressure gradient across the impeller and the rotational speed are used to estimate pump flow104. The driveline is externalized and connected to a mobile console.
Figure 10.
The Impella RD right ventricular assist device. The arrow points to the inflow cannula that is positioned in the right atrium. The outflow graft is sewn to the pulmonary artery.
In an acute porcine model of delayed orthotopic heart transplantation-induced right ventricular failure (24 hour cold ischemic time), the Impella RD provided continuous blood flow for 5 hours105. Mechanical support of the left ventricle was not necessary. After weaning and explanting the device, hemodynamics remained stable. In a chronic ovine model without anticoagulation, the Impella RD successfully supported normal animals for 7 days without hemodynamic or end-organ complications106. Small clots were observed on the right atrial inflow cage and indicated that anticoagulation therapy is necessary with this device.
Multiple case studies and a few small case series have documented successful implementation of the Impella Recover RD as a bridge to right ventricular recovery. Although a large clinical trial has not been performed, the device has been used to support right ventricular failure after post-transplant cardiogenic shock, left ventricular rupture, repeat mitral valve replacement, LVAD implantation, and during myocardial revascularization with and without cardiopulmonary bypass15,104,107–111.
Impella RP
Perhaps the most exciting advance in right ventricular support is the development of the Impella Right Peripheral (Impella RP; Abiomed Incorporated, Danvers, MA), a catheter-based continuous-flow device placed percutaneously across the pulmonic valve via the femoral vein. The Impella RP functions similar to the predicate catheter-based, Abiomed Impella LP that was designed for left heart support during high-risk PCI112. The inflow portion of the catheter resides within the right atrium. The outflow portion of the device resides within the main pulmonary artery.
The Impella RP has been used successfully for right-sided support after an orthotopic heart transplantation (personal communication, Anson Cheung, M.D.). After percutaneous placement, the Impella RP provided right ventricular support for six days and facilitated right ventricular recovery and explantation of the device.
Advantages of a catheter-based approach to right ventricular support include percutaneous implantation, no anastomosis, no concern for device fit, and the ability to remove the device without (re)-entering the chest. If late right ventricular failure occurs, the Impella RP may be placed at the bedside without the need to transport an ill patient back to the operating theater.
Total Artificial Hearts
Despite recent advancements in mechanical circulatory support devices, the management of biventricular failure continues to be a challenge. Though survival with LVADs continues to improve, there remains significant early morbidity and mortality due to right ventricular failure22,23. In select patients, a TAH may be the best treatment option. Over the past four decades, 14 different TAHs have been implanted globally at more than 30 centers in 1,108 patients24. Currently, two TAHs are available for clinical use in the United States, the SynCardia CardioWest and the Abiomed AbioCor. However, a major limitation is the large size of these devices, which cannot be implanted in a substantial portion of the population. CardioWest implantation is limited to adult patients with a body surface area (BSA) between 1.7 and 2.5 m2 113, and the AbioCor will not fit in patients with a BSA less than 2.0 m2 114. Consequently, modifications to the CardioWest and development of the AbioCor II are underway to reduce the size of each device and expand the potential patient population.
The shift from volume displacement pumps to continuous-flow devices has progressively decreased the size and increased the durability of LVADs. Not surprisingly, the development of a continuous-flow TAH (CFTAH) is underway. As an alternative, concurrent implantation of small, right- and left-sided continuous-flow devices has evolved into a practicable therapy. The remainder of this review will focus on changes to the clinically approved CardioWest, the AbioCor II, and the Cleveland Heart CFTAH. Preliminary success and long-term feasibility of continuous-flow BiVAD therapy is examined as an emerging treatment for end-stage biventricular failure.
CardioWest
The CardioWest TAH (SynCardia Systems Incorporated, Tucson, AZ) is the world’s first and only TAH approved by FDA, Health Canada, and Conformite Europeene (CE). Available in the United States as a bridge-to-transplant therapy, the CardioWest TAH has demonstrated better survival to transplantation than with medical management alone113.
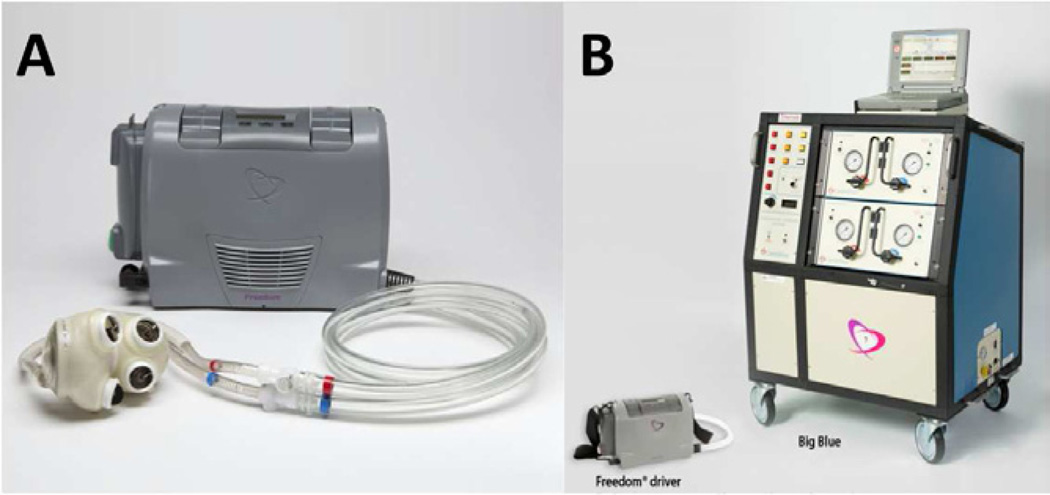
The recent development of the 13.5 lb Freedom® portable driver (Figure 11A), which is substantially smaller than the original Big Blue driver (Figure 11B), has expanded the utility of the CardioWest in the United States. An IDE clinical trial is currently underway in which patients may now be discharged from the hospital. Additionally, a smaller device that will fit in the majority of adult patients is currently under development. Tri-leaflet polymer, central-flow valves have replaced current tilting-disk, Medtronic-Hall valves. Also, the pneumatic membranes have been replaced by Elast-Eon™, a more durable elastic polymer. The smaller CardioWest 2 will be available in two sizes: 70 cc for patients with a BSA of 1.7 m2 or greater, and 50 cc for women and patients of smaller stature with a BSA of 1.2 to 1.7 m2. SynCardia plans to make submissions to the FDA and CE during 2011. Pre-clinical studies to support an IDE are underway.
Figure 11.
The SynCardia TAH shown with the new Freedom portable driver (A). The Freedom portable driver is placed next to Big Blue, the old pneumatic driver (B).
AbioCor
The AbioCor TAH (Abiomed Incorporated, Danvers, MA) is approved for destination-therapy in transplant-ineligible patients with severe biventricular failure. In the initial trial, 15 patients demonstrated 30-day survival of 87% and 60-day survival of 73%24. The AbioCor II, a hybrid design of the AbioCor and the Penn State TAH, has been developed. The device is significantly smaller than the AbioCor and will likely have improved durability. Clinical trials have not been planned.
Importantly, the AbioCor TAH is totally implantable. A major lesson learned from this experience was that TET was simple, improved patient sense-of-freedom, and did not present technical or clinical challenges. The power requirements of the AbioCor are greater than current continuous-flow LVADs, which suggests that TET technology plus a next-generation internal battery may provide prolonged periods of untethered mobility, improved quality of life, and increased acceptance by patients and referring physicians.
Continuous-Flow Total Artificial Heart
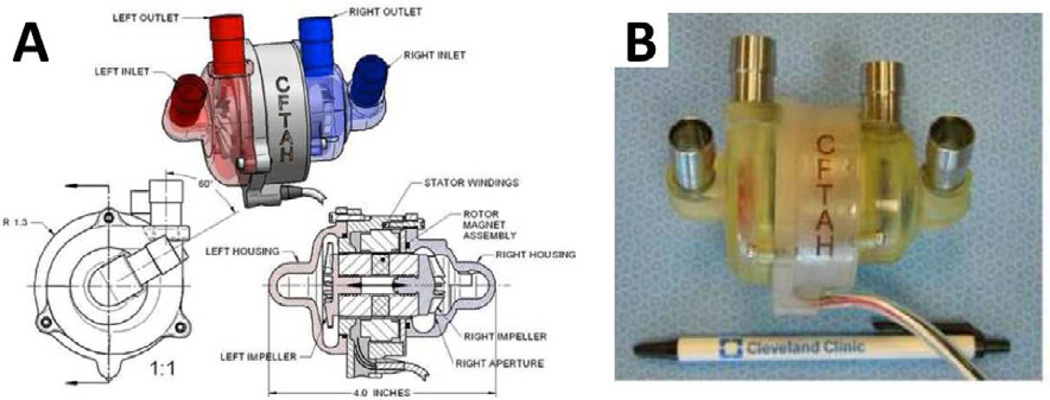
The CFTAH (Cleveland Heart, Charlotte, NC) is a small (60 × 100 mm, 37 ml priming volume), valveless, sensorless, biventricular support device (Figure 12A, B). The double centrifugal pump includes a single continuously rotating, brushless DC motor and a single rotor supported by hydrodynamic bearings. Two separate impellers are mounted on opposite ends of the rotor and create opposing forces at opposite ends of the device. A pressure regulator passively balances right- and left-sided pressures and flow. Within the housing, the motor’s magnetic assembly is shorter than the housing laminations and allows free axial movement of the rotor from left to right. As a result, atrial pressure differences move the rotor and change the aperture at the outer diameter of the right-sided impeller to affect relative right-left output in a direction to correct right-left imbalance115. The control algorithm permits active speed modulation as an additional method to maintain left-right balance and induce pulsatility.
Figure 12.
The Continuous-Flow Total Artificial Heart shown as a schematic (A) and ex vivo (B).
The CFTAH is designed to provide 3 to 8 L/min of flow against an SVR of 700 to 2,000 dyne•sec•cm−5 and PVR of 100–500 dyne•sec•cm−5. In a mock circulatory loop, passive self-regulation maintained balanced pump flows with atrial pressure differences of less than 10 mmHg at physiologic and supraphysiologic pulmonary vascular resistance (PVR) and systemic vascular resistance (SVR)115. The CFTAH consumed 13 W at 8 L/min with 20 mmHg and 80 mmHg of right and left afterload, respectively12. The magnitude of induced-pulsatility permitted active control to adjust right-left balance and pump flows and achieve a pulse pressure of 38 mmHg. The additional power consumption to generate a physiological pulse pressure was 16.2%116.
In an acute bovine model, with rotational speeds of 2,000 to 3,000 rpm, the CFTAH balanced right and left flows and maintained a maximum atrial pressure difference of 10 mmHg. By varying the amplitude of the speed waveform, the CFTAH achieved a pulse pressure of 9 mmHg in the pulmonary artery and 18 mmHg in the aorta12.
Continuous-Flow BiVAD
The use of continuous-flow BiVADs as total heart replacement is gaining momentum as an attractive therapy for end-stage biventricular failure. Small, continuous-flow devices address the size and durability limitations of pulsatile systems. Importantly, left-right balance may not be an issue with this approach.
Preliminary acute animal studies have demonstrated the feasibility of continuous-flow BiVAD with or without native cardiectomy. In an ovine model of biventriculectomy, two HeartMate III centrifugal assist devices satisfactorily preserved a physiologic circulation43. The inflow sewing rings were attached to a rim of right and left ventricular tissue. Outflow grafts were anastomosed end-to-end to the aorta and pulmonary artery. In order to balance different right and left ventricular preload and output, a large atrial-septal window was created surgically. At low RVAD speeds, the interatrial shunt was right to left. As the right-sided VAD increased speed, the interatrial shunt became bidirectional and then reversed to left to right. At no pump speed did either atrium collapse.
Subsequent experiments have demonstrated than an interatrial shunt is unnecessary to maintain right-left balance during continuous-flow BiVAD support103,117. Unlike pulsatile flow devices, rotary pumps operate with a Starling-like response. Pump output is sensitive to both preload and afterload55,117. Consequently, modifications must be made to existing LVADs for use as an RVAD. A device implanted in the left ventricle and right ventricle and set at the same speed will generate different flow rates due to differences in right and left ventricular filling pressures and differences in vascular resistance. Undoubtedly, decreasing the speed of the RVAD will decrease right-sided output. However, low speeds may promote thrombosis and are not suggested25. A better solution may be to equalize left and right device resistance. Narrowing of the right-sided outflow graft with Prolene suture elevates the resistance of the right pump to a value comparable to SVR25 in order to balance the pressure/flow relationship. Similarly, caution must be used when increasing the rotational speed of the RVAD. Left atrial pressure is especially sensitive to RVAD speeds, whereas right atrial pressure is much less sensitive to a change in LVAD speed103.
Chronic large animal studies have been performed to investigate the feasibility and long-term effects of a totally pulseless circulation during total heart replacement with dual intracorporeal continuous-flow devices. In a chronic bovine model, after excision of the native heart, two HeartMate II axial flow LVADs were implanted to replace the right and left ventricles (Figure 13)117. During a seven week study, non-pulsatile systemic arterial and pulmonary arterial blood flow did not adversely affect homeostasis, end-organ and vasomotor function, or the ability to exercise117. Right- and left-sided balance was achieved with right atrial pressures from 5 to 15 mmHg. In a similar experiment, after native cardiectomy, two Jarvik 2000 pumps adequately maintained systemic and pulmonary circulation in a calf for 20 days44. Systemic and pulmonary resistances were maintained pharmacologically to preserve a mean arterial pressure of 100 mmHg, mean pulmonary pressure of 20 mmHg, and atrial pressures of 15 mmHg. The left pump, set at a fixed speed of 14,000 rpm, autoregulated in response to changes in right-sided output, pulmonary and systemic pressures, activity level, and fluid status. Hemodynamics, end-organ function, and neurohormonal status remained normal throughout the study.
Figure 13.
A schematic diagram of two HeartMate II devices placed after cardiectomy for full circulatory support is shown.
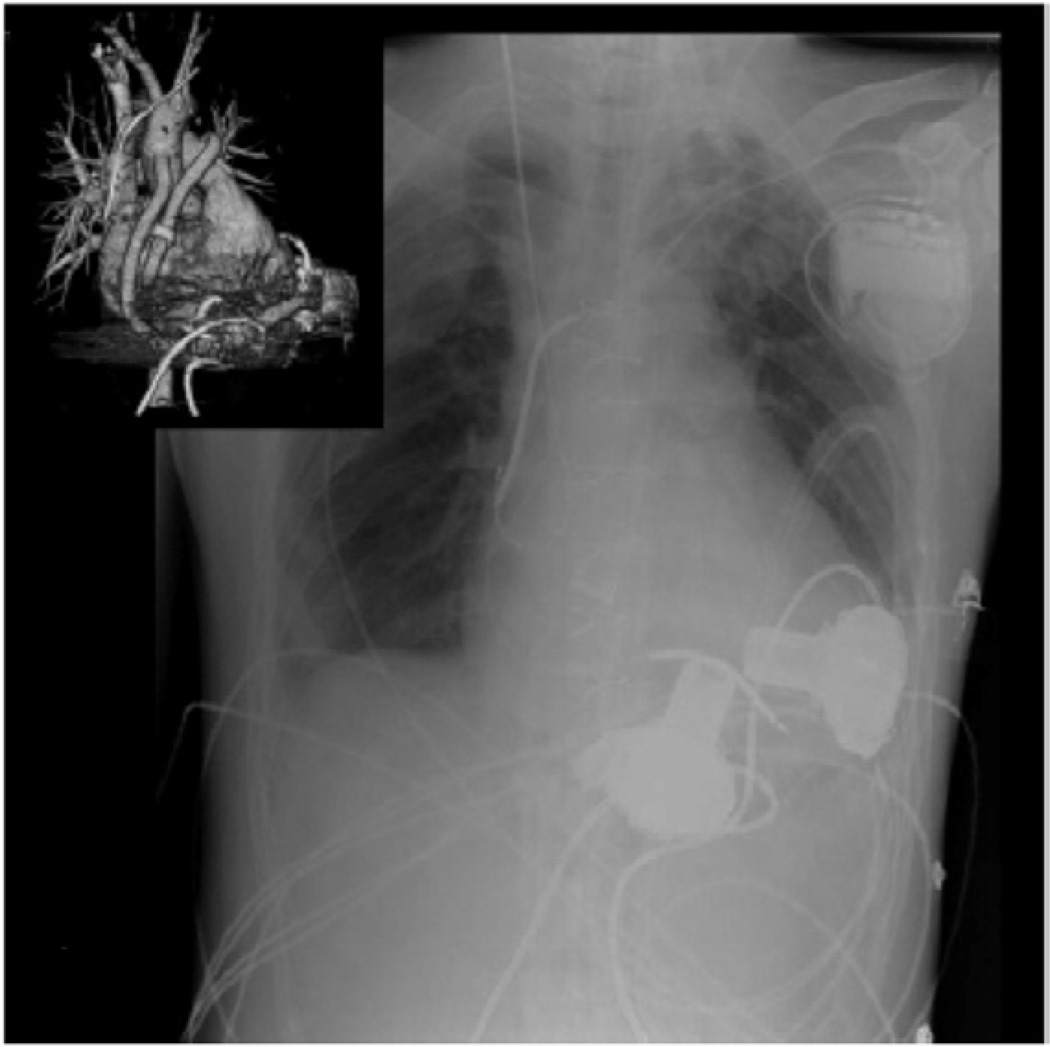
Initial human experiences with dual intracorporeal rotary pumps for the treatment of biventricular failure are encouraging25,92,118–120 (Figure 14). Multiple recent reports document successful implantation of dual HeartWare HVADs in patients for periods of up to 180 days without adverse events118. In these patients, in order to avoid overflowing the pulmonary circuit, the outflow graft of the right-sided pump was narrowed from 10 mm to 5 mm. In one case, a small male patient (BSA1.6 m2 and small chest size) was discharged home with an ongoing follow-up of 189 days119.
Figure 14.
This radiograph demonstrates the in vivo placement of dual HeartWare HVAD devices as an RVAD and an LVAD for long-term bi-ventricular support.
Importantly, in patients with pulmonary hypertension (and an elevated PVR), the RVAD outflow graft should be narrowed to a lesser degree to ensure comparable PVR and SVR25. Of additional concern, it remains to be determined whether dual continuous-flow pumps may increase the susceptibility over a single continuous-flow LVAD to acquired von Willebrand disease and bleeding events121. The development of one controller and one battery power source remain to be achieved.
Conclusions
Recent international experience with continuous-flow devices has progressively improved clinical outcomes and the quality of life of patients that require mechanical circulatory support. New databanks for implantable cardiac devices such as INTERMACS will increase this trend. As devices are miniaturized for earlier support with less-invasive operative approaches, the incidence and prevalence of long-term mechanical circulatory support is likely to increase globally.
Novel LVADs, RVADs, BiVADs, and TAHs should reduce the problems of present systems. The implantation, operation, and management profiles of next-generation devices will be different from each other and different from predicate devices. Less-invasive surgical approaches and strategies of long-term partial unloading of the heart must be further investigated and refined. Invasive full-support devices may still be reserved as a final treatment option for patients with life-threatening, end-stage heart failure as a bridge to heart transplantation or as a destination-therapy. In contrast, less-invasive partial-support devices may prove successful for the management of less-severe heart failure and relieve heart transplantation waiting lists. Partial-support devices may interrupt the progressive hemodynamic deterioration of heart failure, improve symptoms and quality of life, promote reverse myocardial remodeling, and allow for device explantation in select patients. Smaller TAHs and dual intracorporeal rotary pumps may expand the treatment options for patients with end-stage biventricular failure.
Proper patient selection is critical to achieving success with any device and should be tailored to patient needs. Ultimately, careful examination of experiences with future generations of implantable devices will determine the relative utility for each device.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing Interest Statement
Dr. Dowling is the Medical Director of SCR Inc., Louisville, KY and medical consultant for CircuLite Inc., Saddle Brook, NJ, Abiomed Inc., Danvers, MA, and Evaheart Medical Inc., Pittsburgh, PA.
References
- 1.Mielniczuk L, et al. Patient selection for left ventricular assist devices. Artif Organs. 2004;28:152–157. doi: 10.1111/j.1525-1594.2003.47333.x. [DOI] [PubMed] [Google Scholar]
- 2.Eurotransplant. Eurotransplant Annual Report. 2009 accessible at www.eurotransplant.nl.
- 3.Stehlik J, et al. The Registry of the International Society for Heart and Lung Transplantation: twenty-seventh official adult heart transplant report--2010. J Heart Lung Transplant. 2010;29:1089–1103. doi: 10.1016/j.healun.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 4.Farrar DJ, Bourque K, Dague CP, Cotter CJ, Poirier VL. Design features, developmental status, and experimental results with the Heartmate III centrifugal left ventricular assist system with a magnetically levitated rotor. Asaio J. 2007;53:310–315. doi: 10.1097/MAT.0b013e3180536694. [DOI] [PubMed] [Google Scholar]
- 5.Slaughter MS, et al. HeartWare miniature axial-flow ventricular assist device: design and initial feasibility test. Tex Heart Inst J. 2009;36:12–16. [PMC free article] [PubMed] [Google Scholar]
- 6.Hoshi H, Shinshi T, Takatani S. Third-generation blood pumps with mechanical noncontact magnetic bearings. Artif Organs. 2006;30:324–338. doi: 10.1111/j.1525-1594.2006.00222.x. [DOI] [PubMed] [Google Scholar]
- 7.Meyns BP, et al. Clinical benefits of partial circulatory support in New York Heart Association Class IIIB and Early Class IV patients. Eur J Cardiothorac Surg. 2010 doi: 10.1016/j.ejcts.2010.07.049. [DOI] [PubMed] [Google Scholar]
- 8.Bartoli CR, et al. A novel subcutaneous counterpulsation device: acute hemodynamic efficacy during pharmacologically induced hypertension, hypotension, and heart failure. Artif Organs. 2010;34:537–545. doi: 10.1111/j.1525-1594.2010.01009.x. [DOI] [PubMed] [Google Scholar]
- 9.Sales VL, McCarthy PM. Understanding the C-pulse device and its potential to treat heart failure. Curr Heart Fail Rep. 2010;7:27–34. doi: 10.1007/s11897-010-0007-7. [DOI] [PubMed] [Google Scholar]
- 10.Smith EJ, Reitan O, Keeble T, Dixon K, Rothman MT. A first-in-man study of the Reitan catheter pump for circulatory support in patients undergoing high-risk percutaneous coronary intervention. Catheter Cardiovasc Interv. 2009;73:859–865. doi: 10.1002/ccd.21865. [DOI] [PubMed] [Google Scholar]
- 11.Baldwin JT, et al. The National Heart, Lung, and Blood Institute Pediatric Circulatory Support Program. Circulation. 2006;113:147–155. doi: 10.1161/CIRCULATIONAHA.105.571422. [DOI] [PubMed] [Google Scholar]
- 12.Fumoto H, et al. In vivo acute performance of the Cleveland Clinic self-regulating, continuous-flow total artificial heart. J Heart Lung Transplant. 2010;29:21–26. doi: 10.1016/j.healun.2009.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fukamachi K, et al. Development of DexAide right ventricular assist device: update II. Asaio J. 2008;54:589–593. doi: 10.1097/MAT.0b013e31818a30f1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gazzoli F, et al. Arrow CorAide left ventricular assist system: initial experience of the cardio-thoracic surgery center in Pavia. Ann Thorac Surg. 2007;83:279–282. doi: 10.1016/j.athoracsur.2006.05.026. [DOI] [PubMed] [Google Scholar]
- 15.Sugiki H, Nakashima K, Vermes E, Loisance D, Kirsch M. Temporary right ventricular support with Impella Recover RD axial flow pump. Asian Cardiovasc Thorac Ann. 2009;17:395–400. doi: 10.1177/0218492309338121. [DOI] [PubMed] [Google Scholar]
- 16.Krishnamani R, DeNofrio D, Konstam MA. Emerging ventricular assist devices for long-term cardiac support. Nat Rev Cardiol. 2010;7:71–76. doi: 10.1038/nrcardio.2009.222. [DOI] [PubMed] [Google Scholar]
- 17.Jeevanandam V, et al. Circulatory assistance with a permanent implantable IABP: initial human experience. Circulation. 2002;106:I183–I188. [PubMed] [Google Scholar]
- 18.Yamazaki K, et al. Next Generation LVAD "EVAHEART": Current Status of Japanese Clinical Trial. Journal of Cardiac Failure. 2006;12(Suppl) [Google Scholar]
- 19.Meyns B, et al. Proof of concept: hemodynamic response to long-term partial ventricular support with the synergy pocket micro-pump. J Am Coll Cardiol. 2009;54:79–86. doi: 10.1016/j.jacc.2009.04.023. [DOI] [PubMed] [Google Scholar]
- 20.Hayward CS, et al. Chronic extra-aortic balloon counterpulsation: First-in-human pilot study in end-stage heart failure. J Heart Lung Transplant. 2010;29:1427–1432. doi: 10.1016/j.healun.2010.06.014. [DOI] [PubMed] [Google Scholar]
- 21.Kaul TK, Fields BL. Postoperative acute refractory right ventricular failure: incidence, pathogenesis, management and prognosis. Cardiovasc Surg. 2000;8:1–9. doi: 10.1016/s0967-2109(99)00089-7. [DOI] [PubMed] [Google Scholar]
- 22.Potapov EV, et al. Experience with over 1000 implanted ventricular assist devices. J Card Surg. 2008;23:185–194. doi: 10.1111/j.1540-8191.2008.00606.x. [DOI] [PubMed] [Google Scholar]
- 23.Kavarana MN, et al. Right ventricular dysfunction and organ failure in left ventricular assist device recipients: a continuing problem. Ann Thorac Surg. 2002;73:745–750. doi: 10.1016/s0003-4975(01)03406-3. [DOI] [PubMed] [Google Scholar]
- 24.Bartoli CR, Anderson M, Dowling RD. The Total Artificial Heart: Bridge-to-Transplant and Destination-Therapy for End-Stage Biventricular Heart Failure. Lippincott Williams & Wilkins; 2010. [Google Scholar]
- 25.Hetzer R, Krabatsch T, Stepanenko A, Hennig E, Potapov EV. Long-term biventricular support with the heartware implantable continuous flow pump. J Heart Lung Transplant. 2010;29:822–824. doi: 10.1016/j.healun.2010.02.012. [DOI] [PubMed] [Google Scholar]
- 26.Goldstein DJ, Oz MC, Rose EA. Implantable left ventricular assist devices. N Engl J Med. 1998;339:1522–1533. doi: 10.1056/NEJM199811193392107. [DOI] [PubMed] [Google Scholar]
- 27.Rose EA, et al. Long-term mechanical left ventricular assistance for end-stage heart failure. N Engl J Med. 2001;345:1435–1443. doi: 10.1056/NEJMoa012175. [DOI] [PubMed] [Google Scholar]
- 28.Slaughter MS, et al. Advanced Heart Failure Treated with Continuous-Flow Left Ventricular Assist Device. N Engl J Med. 2009 doi: 10.1056/NEJMoa0909938. [DOI] [PubMed] [Google Scholar]
- 29.Birks EJ, Yacoub MH, Banner NR, Khaghani A. The role of bridge to transplantation: should LVAD patients be transplanted? Curr Opin Cardiol. 2004;19:148–153. doi: 10.1097/00001573-200403000-00015. [DOI] [PubMed] [Google Scholar]
- 30.Birks EJ, et al. Left ventricular assist device and drug therapy for the reversal of heart failure. N Engl J Med. 2006;355:1873–1884. doi: 10.1056/NEJMoa053063. [DOI] [PubMed] [Google Scholar]
- 31.Frazier OH, Myers TJ. Left ventricular assist system as a bridge to myocardial recovery. Ann Thorac Surg. 1999;68:734–741. doi: 10.1016/s0003-4975(99)00801-2. [DOI] [PubMed] [Google Scholar]
- 32.Reitz BA. Mechanical devices and US Food and Drug Administration(FDA) approval. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu. 2006:123–127. doi: 10.1053/j.pcsu.2006.02.018. [DOI] [PubMed] [Google Scholar]
- 33.Bartoli CR, et al. Hemodynamic Responses to Continuous versus Pulsatile Mechanical Unloading of the Failing Left Ventricle. Asaio J. 2010 doi: 10.1097/MAT.0b013e3181e7bf3c. [DOI] [PubMed] [Google Scholar]
- 34.Thoratec. Thoratec Announces Filing of PMA Seeking Destination Therapy Approval for HeartMate II. 2009 [press release] [Google Scholar]
- 35.FDA. Thoratec HeartMate II LVAS - P060040/S005. 2010 [Press Release] [Google Scholar]
- 36.Kirklin JK, et al. Third INTERMACS Annual Report: The evolution of destination therapy in the United States. J Heart Lung Transplant. 2011;30:115–123. doi: 10.1016/j.healun.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 37.Bourque K, et al. In vivo assessment of a rotary left ventricular assist device-induced artificial pulse in the proximal and distal aorta. Artif Organs. 2006;30:638–642. doi: 10.1111/j.1525-1594.2006.00276.x. [DOI] [PubMed] [Google Scholar]
- 38.Khalil HA, et al. Induced pulsation of a continuous-flow total artificial heart in a mock circulatory system. J Heart Lung Transplant. 2010;29:568–573. doi: 10.1016/j.healun.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 39.Menconi MJ, et al. Properties of blood-contacting surfaces of clinically implanted cardiac assist devices: gene expression, matrix composition, and ultrastructural characterization of cellular linings. J Cell Biochem. 1995;57:557–573. doi: 10.1002/jcb.240570320. [DOI] [PubMed] [Google Scholar]
- 40.Slaughter MS, Myers TJ. Transcutaneous energy transmission for mechanical circulatory support systems: history, current status, and future prospects. J Card Surg. 2010;25:484–489. doi: 10.1111/j.1540-8191.2010.01074.x. [DOI] [PubMed] [Google Scholar]
- 41.Bourque K, et al. HeartMate III: pump design for a centrifugal LVAD with a magnetically levitated rotor. Asaio J. 2001;47:401–405. doi: 10.1097/00002480-200107000-00020. [DOI] [PubMed] [Google Scholar]
- 42.Slaughter MS, et al. Intraoperative Evaluation of the HeartMate II Flow Estimator. J Heart Lung Transpl. 2009;28:39–43. doi: 10.1016/j.healun.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 43.Frazier OH, Tuzun E, Cohn W, Tamez D, Kadipasaoglu KA. Total heart replacement with dual centrifugal ventricular assist devices. Asaio J. 2005;51:224–229. doi: 10.1097/01.mat.0000160400.84250.87. [DOI] [PubMed] [Google Scholar]
- 44.Frazier OH, Tuzun E, Cohn WE, Conger JL, Kadipasaoglu KA. Total heart replacement using dual intracorporeal continuous-flow pumps in a chronic bovine model: a feasibility study. Asaio J. 2006;52:145–149. doi: 10.1097/01.mat.0000196827.61241.07. [DOI] [PubMed] [Google Scholar]
- 45.Takaseya T, et al. In vivo biocompatibility evaluation of a new resilient, hard-carbon, thin-film coating for ventricular assist devices. Artif Organs. 2010;34:1158–1163. doi: 10.1111/j.1525-1594.2009.00985.x. [DOI] [PubMed] [Google Scholar]
- 46.Gerhart RL, Horvath DJ, Ochiai Y, Krogulecki AY, Golding LA. The effects of impact on the CorAide ventricular assist device. Asaio J. 2002;48:449–452. doi: 10.1097/00002480-200207000-00021. [DOI] [PubMed] [Google Scholar]
- 47.Doi K, et al. Preclinical readiness testing of the Arrow International CorAide left ventricular assist system. Ann Thorac Surg. 2004;77:2103–2110. doi: 10.1016/j.athoracsur.2003.07.048. [DOI] [PubMed] [Google Scholar]
- 48.Fukamachi K. New technologies for mechanical circulatory support: current status and future prospects of CorAide and MagScrew technologies. J Artif Organs. 2004;7:45–57. doi: 10.1007/s10047-004-0256-x. [DOI] [PubMed] [Google Scholar]
- 49.Fukamachi K, et al. Chronic evaluation of the Cleveland Clinic CorAide left ventricular assist system in calves. Artif Organs. 2002;26:529–533. doi: 10.1046/j.1525-1594.2002.06994.x. [DOI] [PubMed] [Google Scholar]
- 50.Ochiai Y, et al. Cleveland clinic CorAide blood pump circulatory support without anticoagulation. Asaio J. 2002;48:249–252. doi: 10.1097/00002480-200205000-00009. [DOI] [PubMed] [Google Scholar]
- 51.Yamazaki K, et al. EVAHEART: an implantable centrifugal blood pump for long-term circulatory support. Jpn J Thorac Cardiovasc Surg. 2002;50:461–465. doi: 10.1007/BF02919636. [DOI] [PubMed] [Google Scholar]
- 52.Kihara S, et al. In vivo evaluation of a MPC polymer coated continuous flow left ventricular assist system. Artif Organs. 2003;27:188–192. doi: 10.1046/j.1525-1594.2003.t01-2-06993.x. [DOI] [PubMed] [Google Scholar]
- 53.Snyder TA, et al. Preclinical biocompatibility assessment of the EVAHEART ventricular assist device: coating comparison and platelet activation. J Biomed Mater Res A. 2007;81:85–92. doi: 10.1002/jbm.a.31006. [DOI] [PubMed] [Google Scholar]
- 54.Yamazaki K, et al. The cool seal system: a practical solution to the shaft seal problem and heat related complications with implantable rotary blood pumps. Asaio J. 1997;43:M567–M571. [PubMed] [Google Scholar]
- 55.Akimoto T, et al. Rotary blood pump flow spontaneously increases during exercise under constant pump speed: results of a chronic study. Artif Organs. 1999;23:797–801. doi: 10.1046/j.1525-1594.1999.06426.x. [DOI] [PubMed] [Google Scholar]
- 56.Yamazaki K, Saito S, Kihara S, Tagusari O, Kurosawa H. Completely pulsatile high flow circulatory support with a constant-speed centrifugal blood pump: mechanisms and early clinical observations. Gen Thorac Cardiovasc Surg. 2007;55:158–162. doi: 10.1007/s11748-007-0098-6. [DOI] [PubMed] [Google Scholar]
- 57.Casula R, Athanasiou T, Foale R. Recent advances in minimal-access cardiac surgery using robotic-enhanced surgical systems. Expert Rev Cardiovasc Ther. 2004;2:589–600. doi: 10.1586/14779072.2.4.589. [DOI] [PubMed] [Google Scholar]
- 58.Frazier OH. Implantation of the Jarvik 2000 left ventricular assist device without the use of cardiopulmonary bypass. Ann Thorac Surg. 2003;75:1028–1030. doi: 10.1016/s0003-4975(02)04304-7. [DOI] [PubMed] [Google Scholar]
- 59.Hunt SA. Mechanical circulatory support: new data, old problems. Circulation. 2007;116:461–462. doi: 10.1161/CIRCULATIONAHA.107.715300. [DOI] [PubMed] [Google Scholar]
- 60.HeartWare. MVAD and HVAD Update - HeartWare International, Inc. 2010 http://202.66.146.82/listco/au/heartware/cpresent/cpr081120.pdf. [Google Scholar]
- 61.Baldwin JT, Mann DL. NHLBI's program for VAD therapy for moderately advanced heart failure: the REVIVE-IT pilot trial. J Card Fail. 2010;16:855–858. doi: 10.1016/j.cardfail.2010.06.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Morley D, et al. Hemodynamic effects of partial ventricular support in chronic heart failure: results of simulation validated with in vivo data. J Thorac Cardiovasc Surg. 2007;133:21–28. doi: 10.1016/j.jtcvs.2006.07.037. [DOI] [PubMed] [Google Scholar]
- 63.Burkhoff D, Klotz S, Mancini DM. LVAD-induced reverse remodeling: basic and clinical implications for myocardial recovery. J Card Fail. 2006;12:227–239. doi: 10.1016/j.cardfail.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 64.Entwistle JW., 3rd Short- and long-term mechanical ventricular assistance towards myocardial recovery. Surg Clin North Am. 2004;84:201–221. doi: 10.1016/S0039-6109(03)00213-5. [DOI] [PubMed] [Google Scholar]
- 65.Birks EJ, George RS. Molecular changes occurring during reverse remodelling following left ventricular assist device support. J Cardiovasc Transl Res. 2010;3:635–642. doi: 10.1007/s12265-010-9220-8. [DOI] [PubMed] [Google Scholar]
- 66.Kinoshita M, et al. Cardiac disuse atrophy during LVAD pumping. ASAIO Trans. 1988;34:208–212. [PubMed] [Google Scholar]
- 67.Kinoshita M, Takano H, Takaichi S, Taenaka Y, Nakatani T. Influence of prolonged ventricular assistance on myocardial histopathology in intact heart. Ann Thorac Surg. 1996;61:640–645. doi: 10.1016/0003-4975(95)01087-4. [DOI] [PubMed] [Google Scholar]
- 68.Klotz S, et al. Mechanical unloading during left ventricular assist device support increases left ventricular collagen cross-linking and myocardial stiffness. Circulation. 2005;112:364–374. doi: 10.1161/CIRCULATIONAHA.104.515106. [DOI] [PubMed] [Google Scholar]
- 69.Rose AG, Park SJ. Pathology in patients with ventricular assist devices: a study of 21 autopsies, 24 ventricular apical core biopsies and 24 explanted hearts. Cardiovasc Pathol. 2005;14:19–23. doi: 10.1016/j.carpath.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 70.Ziv O, et al. Effects of left ventricular assist device therapy on ventricular arrhythmias. J Am Coll Cardiol. 2005;45:1428–1434. doi: 10.1016/j.jacc.2005.01.035. [DOI] [PubMed] [Google Scholar]
- 71.Vollkron M, Voitl P, Ta J, Wieselthaler G, Schima H. Suction events during left ventricular support and ventricular arrhythmias. J Heart Lung Transplant. 2007;26:819–825. doi: 10.1016/j.healun.2007.05.011. [DOI] [PubMed] [Google Scholar]
- 72.Vandenberghe S, Nishida T, Segers P, Meyns B, Verdonck P. The impact of pump speed and inlet cannulation site on left ventricular unloading with a rotary blood pump. Artif Organs. 2004;28:660–667. doi: 10.1111/j.1525-1594.2004.07374.x. [DOI] [PubMed] [Google Scholar]
- 73.Klotz S, et al. Partial mechanical long-term support with the CircuLite Synergy pump as bridge-to-transplant in congestive heart failure. Thorac Cardiovasc Surg. 2010;58(Suppl 2):S173–S178. doi: 10.1055/s-0029-1240687. [DOI] [PubMed] [Google Scholar]
- 74.Reitan O, Sternby J, Ohlin H. Hydrodynamic properties of a new percutaneous intra-aortic axial flow pump. Asaio J. 2000;46:323–329. doi: 10.1097/00002480-200005000-00016. [DOI] [PubMed] [Google Scholar]
- 75.Reitan O, et al. Initial tests with a new cardiac assist device. Asaio J. 1999;45:317–321. doi: 10.1097/00002480-199907000-00012. [DOI] [PubMed] [Google Scholar]
- 76.Dekker A, et al. Efficacy of a new intraaortic propeller pump vs the intraaortic balloon pump: an animal study. Chest. 2003;123:2089–2095. doi: 10.1378/chest.123.6.2089. [DOI] [PubMed] [Google Scholar]
- 77.Reitan O, Steen S, Ohlin H. Hemodynamic effects of a new percutaneous circulatory support device in a left ventricular failure model. Asaio J. 2003;49:731–736. doi: 10.1097/01.mat.0000093964.33468.ca. [DOI] [PubMed] [Google Scholar]
- 78.Manord JD, et al. Implications for the vascular surgeon with prolonged(3 to 89 days) intraaortic balloon pump counterpulsation. J Vasc Surg. 1997;26:511–515. doi: 10.1016/s0741-5214(97)70044-2. discussion 515–516. [DOI] [PubMed] [Google Scholar]
- 79.Freed PS, Wasfie T, Zado B, Kantrowitz A. Intraaortic balloon pumping for prolonged circulatory support. Am J Cardiol. 1988;61:554–557. doi: 10.1016/0002-9149(88)90763-1. [DOI] [PubMed] [Google Scholar]
- 80.Sherwood JT, Schomisch SJ, Thompson DR, George DT, Cmolik BL. Aortomyoplasty: Hemodynamics and comparison to intraaortic balloon pump. J Surg Res. 2003;110:315–321. doi: 10.1016/s0022-4804(02)00039-2. [DOI] [PubMed] [Google Scholar]
- 81.Patel BG, et al. Skeletal muscle ventricle aortic counterpulsation: function during chronic heart failure. Ann Thorac Surg. 2002;73:588–593. doi: 10.1016/s0003-4975(01)03458-0. [DOI] [PubMed] [Google Scholar]
- 82.Terrovitis JV, et al. Superior performance of a paraaortic counterpulsation device compared to the intraaortic balloon pump. World J Surg. 2003;27:1311–1316. doi: 10.1007/s00268-003-6928-5. [DOI] [PubMed] [Google Scholar]
- 83.Davies AN, et al. Extra-ascending aortic versus intra-descending aortic balloon counterpulsation-effect on coronary artery blood flow. Heart Lung Circ. 2005;14:178–186. doi: 10.1016/j.hlc.2005.03.018. [DOI] [PubMed] [Google Scholar]
- 84.Legget ME, et al. Extra-aortic balloon counterpulsation: an intraoperative feasibility study. Circulation. 2005;112:I26–I31. doi: 10.1161/CIRCULATIONAHA.104.521831. [DOI] [PubMed] [Google Scholar]
- 85.Mitnovetski S, et al. Extra-aortic implantable counterpulsation pump in chronic heart failure. Ann Thorac Surg. 2008;85:2122–2125. doi: 10.1016/j.athoracsur.2007.12.049. [DOI] [PubMed] [Google Scholar]
- 86.Giridharan GA, Pantalos GM, Litwak KN, Spence PA, Koenig SC. Predicted hemodynamic benefits of counterpulsation therapy using a superficial surgical approach. Asaio J. 2006;52:39–46. doi: 10.1097/01.mat.0000196522.29376.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Koenig SC, et al. Hemodynamic and left ventricular pressure-volume responses to counterpulsation in mock circulation and acute large animal models. Conf Proc IEEE Eng Med Biol Soc. 2004;5:3761–3764. doi: 10.1109/IEMBS.2004.1404055. [DOI] [PubMed] [Google Scholar]
- 88.Trost JC, Hillis LD. Intra-aortic balloon counterpulsation. Am J Cardiol. 2006;97:1391–1398. doi: 10.1016/j.amjcard.2005.11.070. [DOI] [PubMed] [Google Scholar]
- 89.Koenig SC, Spence PA, Pantalos GM, Dowling RD, Litwak KN. Development and early testing of a simple subcutaneous counterpulsation device. Asaio J. 2006;52:362–367. doi: 10.1097/01.mat.0000227729.70008.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Koenig SC, et al. Acute hemodynamic efficacy of a 32-ml subcutaneous counterpulsation device in a calf model of diminished cardiac function. Asaio J. 2008;54:578–584. doi: 10.1097/MAT.0b013e318186891f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Moskowitz AJWD, Tierney A, et al. Economic considerations of left ventricular assist device implantation. Cardiac Assist Devices. (Ist edition) 2006:183–193. [Google Scholar]
- 92.Frazier OH, Myers TJ, Gregoric I. Biventricular assistance with the Jarvik FlowMaker: a case report. J Thorac Cardiovasc Surg. 2004;128:625–626. doi: 10.1016/j.jtcvs.2004.02.023. [DOI] [PubMed] [Google Scholar]
- 93.Reichenbach SH, Farrar DJ, Hill JD. A versatile intracorporeal ventricular assist device based on the thoratec VAD system. Ann Thorac Surg. 2001;71:S171–S175. doi: 10.1016/s0003-4975(00)02616-3. discussion S183-174. [DOI] [PubMed] [Google Scholar]
- 94.Moazami N, et al. Mechanical support for isolated right ventricular failure in patients after cardiotomy. J Heart Lung Transplant. 2004;23:1371–1375. doi: 10.1016/j.healun.2003.09.022. [DOI] [PubMed] [Google Scholar]
- 95.Fukamachi K, et al. Progress in the development of the DexAide right ventricular assist device. Asaio J. 2006;52:630–633. doi: 10.1097/01.mat.0000240700.03478.d0. [DOI] [PubMed] [Google Scholar]
- 96.Saeed D, et al. Use of zirconia ceramic in the DexAide right ventricular assist device journal bearing. Artif Organs. 2010;34:146–149. doi: 10.1111/j.1525-1594.2009.00757.x. [DOI] [PubMed] [Google Scholar]
- 97.Fukamachi K, et al. Development of a small implantable right ventricular assist device. Asaio J. 2005;51:730–735. doi: 10.1097/01.mat.0000181031.66900.b6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ootaki Y, et al. Development of the DexAide right ventricular assist device inflow cannula. Asaio J. 2008;54:31–36. doi: 10.1097/MAT.0b013e31815b3ea4. [DOI] [PubMed] [Google Scholar]
- 99.Saeed D, et al. In vivo evaluation of zirconia ceramic in the DexAide right ventricular assist device journal bearing. Artif Organs. 2010;34:512–516. doi: 10.1111/j.1525-1594.2009.00918.x. [DOI] [PubMed] [Google Scholar]
- 100.Saeed D, et al. Introduction of fixed-flow mode in the DexAide right ventricular assist device. J Heart Lung Transplant. 2010;29:32–36. doi: 10.1016/j.healun.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ootaki Y, et al. Cadaver fitting study of the DexAide right ventricular assist device. Artif Organs. 2007;31:646–648. doi: 10.1111/j.1525-1594.2007.00438.x. [DOI] [PubMed] [Google Scholar]
- 102.Ootaki Y, et al. Human clinical fitting study of the DexAide right ventricular assist device. Artif Organs. 2009;33:558–561. doi: 10.1111/j.1525-1594.2009.00741.x. [DOI] [PubMed] [Google Scholar]
- 103.Saeed D, et al. Acute in vivo evaluation of an implantable continuous flow biventricular assist system. Asaio J. 2008;54:20–24. doi: 10.1097/MAT.0b013e31815b2d1e. [DOI] [PubMed] [Google Scholar]
- 104.Christiansen S, Dohmen G, Autschbach R. Treatment of right heart failure with a new microaxial blood pump. Asian Cardiovasc Thorac Ann. 2006;14:418–421. doi: 10.1177/021849230601400515. [DOI] [PubMed] [Google Scholar]
- 105.Martin J, et al. The new "Impella" intracardiac microaxial pump for treatment of right heart failure after orthotopic heart transplantation. Transplant Proc. 2001;33:3549–3550. doi: 10.1016/s0041-1345(01)02428-9. [DOI] [PubMed] [Google Scholar]
- 106.Christiansen S, Perez-Bouza A, Reul H, Autschbach R. In vivo experimental testing of a microaxial blood pump for right ventricular support. Artif Organs. 2006;30:94–100. doi: 10.1111/j.1525-1594.2006.00187.x. [DOI] [PubMed] [Google Scholar]
- 107.Schmidt T, Siefker J, Spiliopoulos S, Dapunt O. New experience with the paracardial right ventricular axial flow micropump impella elect 600. Eur J Cardiothorac Surg. 2003;24:307–308. doi: 10.1016/s1010-7940(03)00250-1. [DOI] [PubMed] [Google Scholar]
- 108.Jurmann MJ, Siniawski H, Erb M, Drews T, Hetzer R. Initial experience with miniature axial flow ventricular assist devices for postcardiotomy heart failure. Ann Thorac Surg. 2004;77:1642–1647. doi: 10.1016/j.athoracsur.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 109.Christiansen S, Brose S, Demircan L, Autschbach R. A new right ventricular assist device for right ventricular support. Eur J Cardiothorac Surg. 2003;24:834–836. doi: 10.1016/s1010-7940(03)00512-8. [DOI] [PubMed] [Google Scholar]
- 110.Boening A, Friedrich C, Caliebe D, Cremer J. Efficacy of intracardiac right ventricular microaxial pump support during beating surgery. Interact Cardiovasc Thorac Surg. 2004;3:495–498. doi: 10.1016/j.icvts.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 111.Lamarche Y, et al. Comparative outcomes in cardiogenic shock patients managed with Impella microaxial pump or extracorporeal life support. J Thorac Cardiovasc Surg. 2010 doi: 10.1016/j.jtcvs.2010.07.075. [DOI] [PubMed] [Google Scholar]
- 112.Engstrom AE, Piek JJ, Henriques JP. Percutaneous left ventricular assist devices for high-risk percutaneous coronary intervention. Expert Rev Cardiovasc Ther. 2010;8:1247–1255. doi: 10.1586/erc.10.93. [DOI] [PubMed] [Google Scholar]
- 113.Copeland JG, et al. Cardiac replacement with a total artificial heart as a bridge to transplantation. N Engl J Med. 2004;351:859–867. doi: 10.1056/NEJMoa040186. [DOI] [PubMed] [Google Scholar]
- 114.Samuels LE, Dowling R. Total artificial heart: destination therapy. Cardiol Clin. 2003;21:115–118. doi: 10.1016/s0733-8651(02)00141-8. [DOI] [PubMed] [Google Scholar]
- 115.Fukamachi K, et al. An innovative, sensorless, pulsatile, continuous-flow total artificial heart: device design and initial in vitro study. Heart Lung Transplant. 2010;29:13–20. doi: 10.1016/j.healun.2009.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Shiose A, et al. Speed modulation of the continuous-flow total artificial heart to simulate a physiologic arterial pressure waveform. Asaio J. 2010;56:403–409. doi: 10.1097/MAT.0b013e3181e650f8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Frazier OH, Cohn WE, Tuzun E, Winkler JA, Gregoric ID. Continuous-flow total artificial heart supports long-term survival calf. Tex Heart Inst J. 2009;36:568–574. [PMC free article] [PubMed] [Google Scholar]
- 118.Strueber M, Meyer AL, Malehsa D, Haverich A. Successful use of the HeartWare HVAD rotary blood pump for biventricular support. J Thorac Cardiovasc Surg. 2010;140:936–937. doi: 10.1016/j.jtcvs.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 119.Loforte A, Lilla Della Monica P, Montalto A, Musumeci F. HeartWare third-generation implantable continuous flow pump as biventricular support: mid-term follow-up. Interact Cardiovasc Thorac Surg. 2010 doi: 10.1510/icvts.2010.250654. [DOI] [PubMed] [Google Scholar]
- 120.Loforte A, Montalto A, Della Monica PL, Contento C, Musumeci F. Biventricular support with the HeartWare implantable continuous flow pump: an additional contribution. J Heart Lung Transplant. 2010;29:1443–1444. doi: 10.1016/j.healun.2010.07.012. [DOI] [PubMed] [Google Scholar]
- 121.Geisen U, et al. Non-surgical bleeding in patients with ventricular assist devices could be explained by acquired von Willebrand disease. Eur J Cardiothorac Surg. 2008;33:679–684. doi: 10.1016/j.ejcts.2007.12.047. [DOI] [PubMed] [Google Scholar]